Abstract
This article reports a Glanzmann thrombasthenia (GT) patient, N.M., with a point mutation in the third cysteine-rich repeat of β3-integrin or platelet glycoprotein (GP) IIIa, leading to the expression of a constitutively activated fibrinogen receptor. The diagnosis of GT was based on a severely reduced platelet-aggregation response to a series of agonists and approximately 20% of surface-expressed GPIIb-IIIa. The patient's GPIIb-IIIa constitutively expressed epitopes recognized by antibodies to ligand-induced binding sites (LIBS) and also spontaneously bound the fibrinogen-mimetic antibody, PAC-1. Furthermore, significant amounts of bound fibrinogen were detected on his platelets ex vivo. No signs of platelet activation were observed on sections of unstimulated platelets from N.M. by electron microscopy. Immunogold labeling highlighted the presence of surface-bound fibrinogen but revealed platelet heterogeneity with regard to the surface density. When the patient's platelets were stimulated by thrombin-receptor activating peptide, amounts of surface-expressed GPIIb-IIIa increased and the aggregation response improved, although it failed to normalize. Platelets from N.M. were able to adhere and spread on immobilized fibrinogen. Sequence analysis of genomic DNA from N.M. revealed a homozygous g1776T>C mutation in GPIIIa, leading to a Cys560Arg amino acid substitution. A stable Chinese hamster ovary (CHO) cell line was prepared expressing surface GPIIb-Arg560IIIa. Like platelets from the patient, GPIIb-Arg560IIIa–transfected CHO cells constitutively bound LIBS antibodies and PAC-1. They also showed an enhanced ability to adhere on surface-bound fibrinogen. Overall, these data demonstrate that a gain-of-function mutation can still be associated with a thrombasthenic phenotype even though platelets show spontaneous fibrinogen binding.
Introduction
Integrins are heterodimeric transmembrane proteins that mediate cell adhesion and cell migration.1-3 They are involved in numerous fundamental biologic processes, including development, homing, inflammation, angiogenesis, and wound healing, all processes in which integrin function is subjected to a highly sophisticated regulation.4-8 The platelet fibrinogen receptor, the αIIbβ3 integrin (glycoprotein [GP] IIb-IIIa), has constituted an ideal model for the study of an integrin's role in cell contact interactions.9-13 This receptor has the capacity to shift through conformational changes from a low-affinity state unable to bind macromolecular ligands to a ligand-competent state on activated platelets. This process of affinity modulation is an essential part of integrin function. Furthermore, ligand binding to GPIIb-IIIa itself modifies the conformation of this integrin, exposing neoepitopes known as ligand-induced binding sites (LIBS) and leading to postreceptor occupancy events such as clustering of GPIIb-IIIa complexes, generation of secondary signals, and cytoskeletal rearrangement, all of which contribute to maximal platelet aggregation.13-15
The biologic importance of platelet GPIIb-IIIa is underscored by an inherited bleeding disorder, Glanzmann thrombasthenia (GT). The hallmark of this disease is absent or severely reduced platelet aggregation because of absence or dysfunction of the fibrinogen receptor GPIIb-IIIa.16-19 In recent years, the molecular bases of numerous GPIIb-IIIa defects have been elucidated, leading to the creation of a database for GT available on the Internet (www.med.mssm.edu/glanzmanndb or www.med.unc.edu/isth). In addition to deletions, insertions, or rearrangements, several point mutations have been identified in the GPIIb and GPIIIa genes of GT patients.20 Among point mutations occurring in the GPIIIa gene, some alter the function of the GPIIb-IIIa complex by disrupting ligand binding sites (Cam or Asp119Tyr, ET or Arg214Gln, and Stras I and CM or Arg214Trp variants).21-25 Others in the cytoplasmic tail result in the integrin's being locked in a low-affinity state (Ser752Pro and Arg724Ter mutations).26,27 In contrast to naturally occurring mutations, GPIIIa mutations leading to gain of function have been experimentally induced and studied in recombinant GPIIb-IIIa–transfected cells. Replacement of the ligand binding site of β3-integrin (residues 129-133) by the corresponding sequence of β1 resulted in increased fibrinogen binding.28 Deletions or mutations in conserved sequences within GPIIb and GPIIIa membrane-proximal cytoplasmic domains also have been found to result in activated complexes locked in a high-affinity state.29 30
Recent studies suggest that the cysteine-rich domain of GPIIIa might be involved in GPIIb-IIIa activation. First, it has been known for a long time that a mild reduction of disulfide bonds by dithiothreitol can activate GPIIb-IIIa.31 Second, truncation of the cysteine-rich repeats (CRRs) of GPIIIa led to recombinant GPIIb-IIIa complexes exhibiting enhanced fibrinogen binding capacities.32 Third, antibodies directed against LIBS in the cysteine-rich domain of GPIIIa increased the affinity of GPIIb-IIIa for its ligands and induced fibrinogen binding to platelets.33 Fourth, disruption of the Cys5-Cys435 long-range disulfide bond by replacing cysteine with Ala resulted in constitutively activated GPIIb-IIIa integrin complexes showing high-affinity binding to immobilized fibrinogen.34,35Fifth, the conformational changes that facilitate the shift of GPIIb-IIIa from a resting state to an activated state involved the regions in GPIIIa brought into proximity by the Cys5-Cys435 bond.36 Furthermore, the resting conformation of GPIIb-IIIa displays 2 to 3 unpaired cysteines, whereas the activated conformation has 4 to 5 free cysteines.37 Sixth, a Thr562Asn mutation located in the cysteine-rich core of GPIIIa identified during a screening of Chinese hamster ovary (CHO) cell transfectants gave rise to the expression of an activated GPIIb-IIIa receptor.38 The rationale for the implication of the GPIIIa CRR region in integrin activation is based on the emerging concept that the redox state of cysteine-containing molecules could regulate their biologic activity. Protein disulfide isomerase (PDI) is well known for assisting proteins in folding during biosynthesis by catalyzing disulfide rearrangements in intracellular compartments.39 PDI has also been reported to participate in rearrangements of disulfide bonds in adjacent proteins on the external surface of platelets and to be involved in β1- and β3-integrin–dependent adhesion of platelets to fibrinogen, collagen, and fibronectin.40,41 PDI was shown to interact with GPIb on activated platelets and to modulate the binding of von Willebrand factor to GPIb.42 PDI was not found to colocalize with GPIIb-IIIa, but the integrin itself may possess an endogenous thiol isomerase activity associated with the CRRs of GPIIIa.43
In this context, we report a novel case of GT in which a homozygous point mutation of Cys560 within the GPIIIa cysteine-rich domain resulted in a GPIIb-IIIa receptor locked in a high-affinity state.
Patient, materials, and methods
Case report
The propositus is a 40-year-old French male, N.M. He has a clinical history of very mild bleeding, with rare gum bleeding but neither epistaxis nor bruising. GT was diagnosed before his receiving a kidney transplant in 1986. His bleeding time was prolonged, and the analysis of plasma hemostatic factors and standard platelet functions was consistent with GT. His plasma fibrinogen level was normal (approximately 3 g/L). The patient has a history of moderate thrombocytopenia, with a platelet count of 100 to 150 × 109/L. Despite several transfusions, no evidence for antiplatelet antibodies was found. On his father's side, mild bleeding symptoms have been reported in his grandmother and a first cousin, but they were not available for further study. His mother is asymptomatic and has normal hemostatic test results and platelet function. However, genetic study of her GPIIb-IIIa confirmed that she is heterozygous for the GT mutation. Consanguinity is denied in the family.
Antibodies
Anti-GPIIb and anti-GPIIIa polyclonal antibodies, and monoclonal antibodies AP2 (anti–GPIIb-IIIa), AP3 (anti-GPIIIa), and AP5 (LIBS anti-GPIIIa) were from the Blood Research Institute (Milwaukee, WI). D3 (LIBS anti-GPIIIa) was obtained from Dr Jennings (University of Tennessee, Memphis).44 NaM28-7D6 (LIBS anti-GPIIIa) and NaM12-6E1 (anti–GPIIb-IIIa complex) (abbreviated 7D6 and 6E1, respectively) were provided by Dr Blanchard (BioAtlantic, Nantes, France).45,46 CRC54 (LIBS anti-GPIIIa) was obtained from Dr Mazurov (Cardiology Research Center, Moscow, Russian Federation).47 SZ1 (anti–GPIb-IX) and SZ22 (anti-GPIIb) were from Immunotech (Marseille, France). PAC-1 (fibrinogen mimetic IgM) was provided by Dr Shattil (Scripps Research Institute, La Jolla, CA).48 49 Fluorescein isothiocyanate (FITC)–PAC-1 was from Becton Dickinson (Le Pont-de-Claix, France).
Platelet glycoprotein analysis by flow cytometry
Platelet-rich plasma (PRP) was obtained by centrifugation of acid-citrate-dextrose–anticoagulated blood at 240g for 10 minutes, and platelets were isolated from PRP supplemented with 10−7 M prostaglandin E1 (PGE1) by centrifugation at 2500g for 8 minutes. The pellet was washed twice with RCD buffer (108 mM NaCl, 3.8 mM KCl, 1.7 mM NaHCO3, 21.2 mM sodium citrate, 27.8 mM glucose, pH 6.5, containing 10−7 M PGE1). For platelet activation studies, no PGE1 was added to the final wash.
Surface expression of GPIIb-IIIa was assessed by incubating 106 washed platelets with AP3, SZ22, 6E1, and SZ1, followed by FITC-conjugated goat anti–mouse IgG F(ab′)2(BioAtlantic). The cells were washed twice and analyzed on a FACScan (Becton Dickinson).
To study the adhesive properties of the patient's GPIIb-IIIa, we incubated FITC-fibrinogen50 and FITC–PAC-1 with platelets in Tyrode-HEPES buffer51 supplemented with 2 mM CaCl2 and 1 mM MgCl2. Platelets were fixed in phosphate-buffered saline (PBS)–1% formaldehyde and subjected to flow cytometry. Ligand binding was expressed as an activation index (AI), defined as (Fx−Fix)/(Fm−Fim). Fx is the mean fluorescence intensity (MFI) of ligand binding to resting platelets. Fm is the MFI of the maximal ligand binding to platelets stimulated with either 25 μM ADP (for PAC-1 binding) or 100 μM ADP (for fibrinogen binding). These concentrations of ADP were defined to produce the maximum binding of the tested ligands. Fix and Fim are the MFIs of ligand binding in the presence of the inhibitor RGDS peptide to resting and ADP-activated platelets, respectively.
To assess LIBS exposure on N.M.'s platelets, we incubated PRP supplemented with 10−7 M PGE1 with 2 mM RGDS or RGES peptide (Bachem, Voisins-le-Bretonneux, France) and AP5, D3, and 7D6. After washes, binding to platelets was detected using FITC-conjugated anti–mouse IgG F(ab′)2 and analyzed by flow cytometry.
To assess fibrinogen binding to platelets in vivo, we incubated 5 μL freshly drawn citrated blood with FITC-conjugated rabbit anti–human fibrinogen (Dako, Trappes, France). Binding was studied in the presence of saturating concentrations of anti-FcγRII blocking antibody, IV.3 (Medarex, Annandale, NJ), as described previously by Warkentin et al.52
Semiquantitative analysis of total platelet GPIIb-IIIa content by Western blotting
Sodium dodecyl sulfate (SDS) platelet lysates were resolved by SDS–polyacrylamide gel electrophoresis under reducing conditions. Proteins were transferred to polyvinylidene difluoride (PVDF) membrane, and the blot was incubated with polyclonal antibody specific for GPIIb or GPIIIa.
Electron microscopy
Platelet preparation.
Blood from the patient or a control donor was obtained by venipuncture and placed in 3.8% (wt/vol) sodium citrate. The initial 3 mL of blood was discarded. PRP was prepared by centrifugation at 120gfor 10 minutes at room temperature. One part of the PRP was stimulated with 50 μM thrombin receptor activating peptide 6 (TRAP6) (Bachem) for 5 minutes at 37°C; incubations were not stirred after the initial mixing. Samples were then fixed in 1.25% wt/vol glutaraldehyde diluted in 0.1 M phosphate buffer.
Immunogold labeling before embedding.
Glutaraldehyde-fixed platelets were incubated with rabbit antifibrinogen antibody (Dako, Copenhagen, Denmark). After washing, the samples were incubated with affinity-purified goat anti–rabbit IgG coupled to 10-nm gold particles (Amersham, Les Ulis, France). Platelets were postfixed, dehydrated, and finally embedded in Epon (Taab Laboratories, Reading Berks, United Kingdom). Ultrathin sections were cut with an Ultracut E ultramicrotome (Reichert, Vienna, Austria) and stained before being observed in a Jeol JEM-1010 electron microscope (Jeol, Croissy-sur-Seine, France) at 80 kV.53
Platelet aggregation following TRAP-induced secretion and mobilization of the internal GPIIb-IIIa pool
Platelet aggregation was performed using citrated PRP (2 × 108 platelets/mL), which was stirred in the aggregometer before addition of TRAP6. After maximal aggregation, samples were removed and centrifuged for 30 minutes at 2500g. Release of platelet factor-4 (PF4, a platelet α-granule protein) into the supernatant was quantified by enzyme-linked immunosorbent assay (ELISA) using an anti-PF4 antibody (Asserachrom kit; Asnières-sur-Seine, France). To evaluate GPIIb-IIIa expression at the surface, we incubated 106TRAP6-stimulated platelets with FITC-conjugated 6E1 and analyzed them by flow cytometry.
Platelet adhesion and spreading on fibrinogen
Coating was performed with 100 μg/mL purified fibrinogen (Kordia, Leiden, The Netherlands) or 5 mg/mL bovine serum albumin (BSA) in 3 mL PBS per dish (Cofralab, Gradignan, France) overnight at 4°C; dishes were then blocked with 5 mg/mL PBS-BSA at room temperature for 2 hours. Platelets were isolated by gel-filtration as described previously.27 To prevent platelet activation, 10−7 M PGE1 and 1 U/mL apyrase were present during the gel-filtration. One milliliter volume (3 × 108 platelets) was added to each dish and incubated for 90 minutes at 37°C. After 2 washes with cold PBS to discard nonadherent platelets, platelet adhesion and spreading were observed by phase microscopy.
Nucleotide sequence analysis of GPIIb and GPIIIa from genomic DNA
All 30 exons and immediate flanking sequences of the GPIIb gene, and all 15 exons and immediate flanking sequences of the GPIIIa gene, were amplified by polymerase chain reaction (PCR) using well-established methods. Amplified DNA fragments were purified and subjected to direct cycle sequence analysis using automated sequencing (Applied Biosystems, Foster City, CA).
Site-directed mutagenesis of GPIIIa cDNA
The mutation corresponding to that found in the GPIIIa gene of the patient was introduced into full-length GPIIIa cDNA using a mismatched primer corresponding to the GPIIIa cDNA sequence for Arg at position 560 (5′-TACTACTGCAACCGTACCACGCGTA-3′). After secondary PCR, the cDNA fragment containing the mutation was cloned into mammalian expression vector pcDNA3, which contains a neomycin resistance gene (Invitrogen, San Diego, CA). The sequence of all constructs was analyzed using automated sequencing (Applied Biosystems).
Cell line transfections
CHO cells were cultured in alpha-minimum essential medium (MEM) with ribo- and deoxyribonucleotides containing 10% fetal calf serum. CHO cells were cotransfected with GPIIb cDNA in the mammalian expression vector EMC-3 and wild-type GPIIIa (WT GPIIb-IIIa) or Arg560GPIIIa (GPIIb-Arg560IIIa) in pcDNA3 using the calcium phosphate method. EMC-3, which contains a methotrexate resistance gene, was a gift of Dr Larsen (Genetics Institute, Boston, MA). After 48 hours, transfected CHO cells were selected in alpha-MEM without ribo- or deoxyribonucleotides containing 600 μg/mL geneticin (Gibco, Gaithersburg, MD) for 2 weeks. To obtain stable cell lines with high levels of expression, transfected CHO cells were sorted in a FACStar (Becton Dickinson) using AP3, cloned by limiting dilutions, and maintained in selection media containing 5 nM methotrexate.
Flow cytometric analysis of recombinant GPIIb-Arg560IIIa
CHO cells were harvested, washed, and incubated on ice with AP3 and AP2, then washed and incubated with FITC-conjugated goat anti–mouse IgG (Jackson, West Grove, PA). LIBS and PAC-1 antibodies were incubated for 45 minutes at room temperature, and then binding to cells was assessed using FITC-conjugated anti-IgG or anti-IgM (Jackson) for 30 minutes and subjected to flow cytometric analysis.
Results
Initial screening showed that N.M. has a GT-like phenotype
The patient's bleeding time is typically markedly prolonged (more than 20 minutes; Ivy method) despite his mild bleeding tendency. The closure time measured with the platelet-function analyzer 100 (PFA-100; Dade Behring, Paris La Défense, France) was infinite with both ADP or epinephrine cartridges. Platelet aggregation in PRP was markedly impaired in response to usual concentrations of physiologic agonists, including 1 to 5 μM ADP (0% aggregation), 0.25 μg/mL arachidonic acid (30%), 100 μM epinephrine (0%), and 1.5 and 3 μg/mL collagen (0 and 10%, respectively) (data not shown). However, ristocetin (0.8-2 mg/mL) induced normal aggregation. Whole blood and plasma clot retraction were markedly reduced (data not shown).
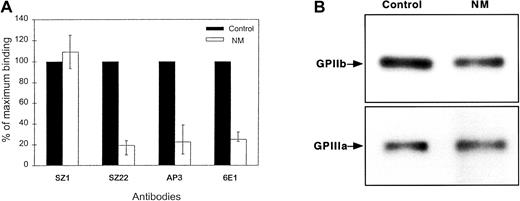
The presence of glycoproteins on N.M.'s platelets was analyzed by flow cytometry. The surface expression of GPIIb-IIIa was about 20% of normal, whereas GPIb (93%) was normally expressed (Figure1A). Because the binding of the complex-specific antibody, 6E1, was similar to that of subunit-specific antibodies, GPIIb and GPIIIa in N.M.'s platelets appeared to associate correctly to form an integrin complex. Furthermore, the complexes appeared stable because attempts to dissociate them with increasing concentrations of EDTA at room temperature failed to prematurely separate the GPIIb and GPIIIa subunits (data not shown). To estimate the total platelet content of GPIIb and GPIIIa, we performed Western blot analysis with polyclonal antibodies specific for each subunit. Interestingly, N.M.'s platelets contained more GPIIb and GPIIIa than would have been predicted from the surface expression, with levels ranging from 50% to subnormal compared with control platelets (Figure1B). The platelet fibrinogen content was similar to that of control platelets, as determined by immunostaining with antifibrinogen antibody (data not shown).
Expression of GPIIb-IIIa in the patient's platelets.
(A) Cell-surface expression was assessed using monoclonal antibodies (10 μg/mL) directed against the GPIIb-IIIa complex (6E1), GPIIb (SZ22), and GPIIIa (AP3) subunits. Bound IgG was assessed by flow cytometry using FITC-conjugated goat anti–mouse antibody. Results are given as the percentage (mean + range from 4 separate experiments) of the binding of the same antibodies to control platelets, assigned as 100%. N.M.'s platelets expressed about 20% GPIIb-IIIa compared with normal platelets. Surface expression of GPIb was similar on both N.M.'s and control platelets as shown by the binding of SZ1. (B) Total platelet GPIIb-IIIa was evaluated by immunoblotting. SDS-soluble platelet proteins (12 μg) from both control and N.M.'s platelets were subjected to electrophoresis on a 7% polyacrylamide gel under reducing conditions, transferred to PVDF membrane, and incubated with 20 μg/mL rabbit polyclonal antibodies specific for GPIIb and GPIIIa. Bound antibodies were detected using alkaline phosphatase–conjugated goat anti–rabbit IgG, followed by color development using the nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrates.
Expression of GPIIb-IIIa in the patient's platelets.
(A) Cell-surface expression was assessed using monoclonal antibodies (10 μg/mL) directed against the GPIIb-IIIa complex (6E1), GPIIb (SZ22), and GPIIIa (AP3) subunits. Bound IgG was assessed by flow cytometry using FITC-conjugated goat anti–mouse antibody. Results are given as the percentage (mean + range from 4 separate experiments) of the binding of the same antibodies to control platelets, assigned as 100%. N.M.'s platelets expressed about 20% GPIIb-IIIa compared with normal platelets. Surface expression of GPIb was similar on both N.M.'s and control platelets as shown by the binding of SZ1. (B) Total platelet GPIIb-IIIa was evaluated by immunoblotting. SDS-soluble platelet proteins (12 μg) from both control and N.M.'s platelets were subjected to electrophoresis on a 7% polyacrylamide gel under reducing conditions, transferred to PVDF membrane, and incubated with 20 μg/mL rabbit polyclonal antibodies specific for GPIIb and GPIIIa. Bound antibodies were detected using alkaline phosphatase–conjugated goat anti–rabbit IgG, followed by color development using the nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrates.
The patient's platelets express a constitutively activated form of GPIIb-IIIa
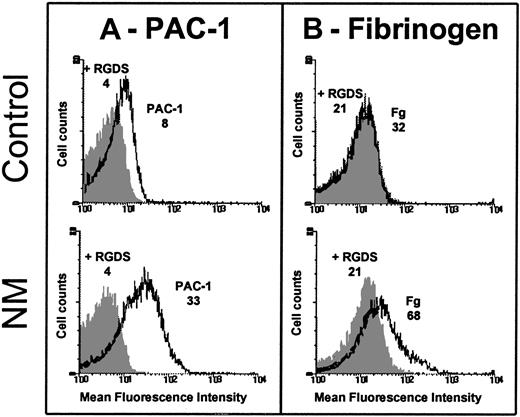
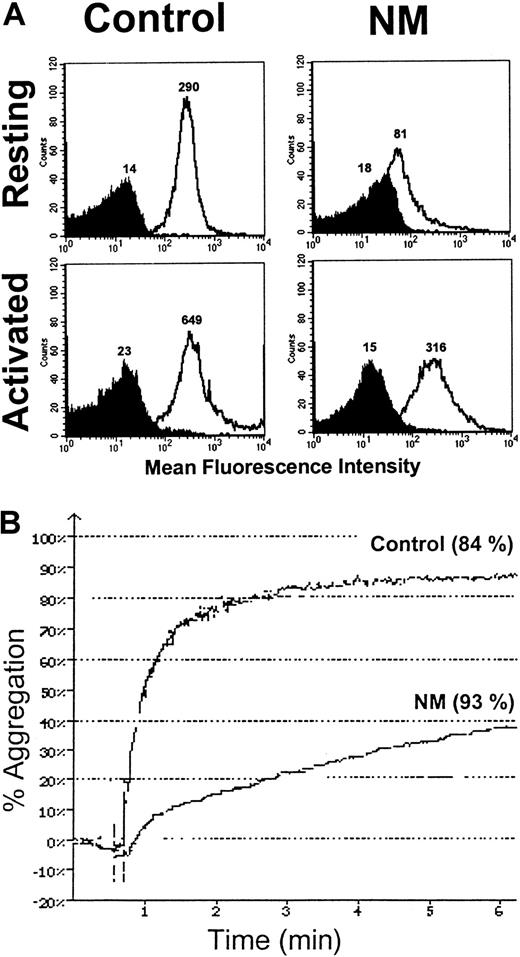
Because some GPIIb-IIIa was detectable at the platelet surface, we assessed its ability to bind FITC-conjugated PAC-1. Although care was taken to prevent platelet activation during the preparation procedures, we observed a spontaneous binding of FITC-labeled PAC-1 to resting patient's platelets, but not to resting control platelets (Figure 2A). The AI was calculated as follows: 0.270 ± 0.067 (n = 3) for N.M. versus 0.040 ± 0.029 (n = 3) for the control. Platelets from N.M. also spontaneously bound FITC-fibrinogen (Figure 2B); the AI was calculated to be 0.220 ± 0.140 (n = 3) for the patient versus 0.027 ± 0.005 (n = 3) for the control. Because the patient's platelets expressed only about 20% of the normal amount of GPIIb-IIIa, PAC-1 and fibrinogen binding would be much greater with a normal platelet density of mutated GPIIb-IIIa.
Spontaneous binding of PAC-1 and fibrinogen to resting platelets.
Platelets were washed and resuspended (2 × 106) in Tyrode-HEPES buffer containing 10−7 M PGE1 and incubated without stimulation with 20 μg/mL FITC–PAC-1 (A) or 250 nM FITC-fibrinogen (B) for 20 and 40 minutes, respectively, at room temperature (RT). Samples were diluted and fixed in 1% formaldehyde and subjected to flow cytometry. The mean fluorescence intensities (MFIs) of PAC-1 and fibrinogen binding in the presence (dark histogram) or in the absence of 2 mM RGDS peptide (clear histogram) obtained for the patient and control are indicated on the histograms. The fact that the patient's platelets expressed only 20% of the usual levels of GPIIb-IIIa reinforces the difference in ligand binding between N.M. and control platelets.
Spontaneous binding of PAC-1 and fibrinogen to resting platelets.
Platelets were washed and resuspended (2 × 106) in Tyrode-HEPES buffer containing 10−7 M PGE1 and incubated without stimulation with 20 μg/mL FITC–PAC-1 (A) or 250 nM FITC-fibrinogen (B) for 20 and 40 minutes, respectively, at room temperature (RT). Samples were diluted and fixed in 1% formaldehyde and subjected to flow cytometry. The mean fluorescence intensities (MFIs) of PAC-1 and fibrinogen binding in the presence (dark histogram) or in the absence of 2 mM RGDS peptide (clear histogram) obtained for the patient and control are indicated on the histograms. The fact that the patient's platelets expressed only 20% of the usual levels of GPIIb-IIIa reinforces the difference in ligand binding between N.M. and control platelets.
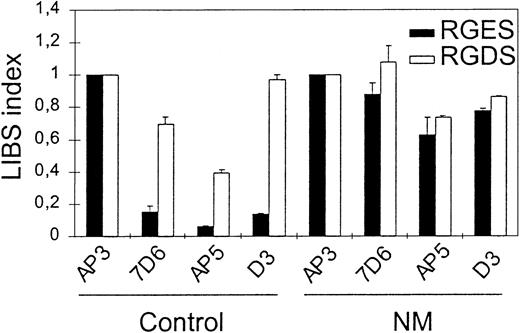
To evaluate further the competent conformation of the patient's GPIIb-IIIa for ligand, we performed flow cytometric analysis for LIBS exposure by incubating anti-LIBS antibodies 7D6, D3, and AP5 with N.M.'s platelets in PRP and in the presence of 2 mM RGDS or RGES peptides. The LIBS index obtained with 7D6, AP5, or D3 in the absence of RGDS peptide was much greater for N.M. (0.88, 0.63, and 0.78, respectively) compared with the corresponding values obtained for control platelets (0.16, 0.06, and 0.14, respectively) (Figure3). This demonstrates that LIBS are constitutively exposed on the patient's GPIIb-IIIa without a requirement for agonist stimulation. Incubation of N.M.'s platelets with RGDS peptide did not cause further LIBS expression. This suggests that all of the surface GPIIb-IIIa of the patient's platelets is already in a ligand binding competent state.
Evaluation of LIBS exposure on the patient's platelets.
To assess spontaneous LIBS exposure on platelets, PRP (2 × 106 platelets) with 10−7 M PGE1 was incubated with 10 μg/mL anti-LIBS antibodies 7D6, AP5, and D3 in the presence of 2 mM RGDS or RGES peptides for 60 minutes at RT. After 2 washes, platelets were incubated with FITC-labeled goat anti–mouse F(ab′)2 for 30 minutes, then samples were washed once and bound antibodies were analyzed by flow cytometry. LIBS antibody binding was expressed as a LIBS index (LI) by standardizing the MFI for each LIBS monoclonal antibody (mAb) to that obtained using AP3, the binding of which was not affected by the patient's mutation. LI = LIBS mAb MFI/AP3 MFI for the patient and for the control separately.
Evaluation of LIBS exposure on the patient's platelets.
To assess spontaneous LIBS exposure on platelets, PRP (2 × 106 platelets) with 10−7 M PGE1 was incubated with 10 μg/mL anti-LIBS antibodies 7D6, AP5, and D3 in the presence of 2 mM RGDS or RGES peptides for 60 minutes at RT. After 2 washes, platelets were incubated with FITC-labeled goat anti–mouse F(ab′)2 for 30 minutes, then samples were washed once and bound antibodies were analyzed by flow cytometry. LIBS antibody binding was expressed as a LIBS index (LI) by standardizing the MFI for each LIBS monoclonal antibody (mAb) to that obtained using AP3, the binding of which was not affected by the patient's mutation. LI = LIBS mAb MFI/AP3 MFI for the patient and for the control separately.
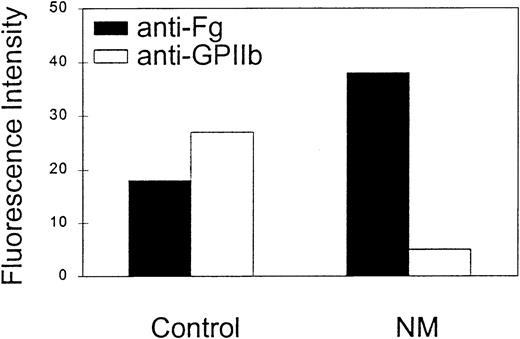
The spontaneously activated state of N.M.'s GPIIb-IIIa receptor would predict that some fibrinogen might be present on the surface of his circulating platelets in vivo. To assess the presence of fibrinogen on N.M.'s platelets, we used the micromethod described by Warkentin et al.52 An antifibrinogen antibody was used to detect bound fibrinogen in the presence of excess of soluble plasma fibrinogen. At least twice the amount of bound fibrinogen was detected on N.M.'s platelets compared with control platelets (Figure4). The MFIs indicated in Figure 4 do not take into account the reduced GPIIb-IIIa expression on N.M.'s platelets. GPIIb-IIIa expression at the cell surface was assessed in parallel with SZ22, an anti-GPIIb antibody, and is also shown in Figure4.
Detection of platelet-bound fibrinogen in whole blood.
A volume (5 μL) of freshly drawn citrated blood was incubated for 20 minutes at RT with 6.3 μg rabbit FITC-conjugated polyclonal rabbit anti–human fibrinogen. Saturating concentrations of anti-FcγRII blocking antibody, IV.3, were added to prevent platelet activation through the Fc receptors by immune complexes formed between soluble fibrinogen and antifibrinogen antibody. In parallel, 5 μL whole blood was incubated with 5 μg/mL FITC-conjugated anti-GPIIb antibody SZ22 to assess the GPIIb-IIIa expression at the cell surface. The samples were analyzed by flow cytometry and expressed as the MFI. These data are representative of 4 separate experiments.
Detection of platelet-bound fibrinogen in whole blood.
A volume (5 μL) of freshly drawn citrated blood was incubated for 20 minutes at RT with 6.3 μg rabbit FITC-conjugated polyclonal rabbit anti–human fibrinogen. Saturating concentrations of anti-FcγRII blocking antibody, IV.3, were added to prevent platelet activation through the Fc receptors by immune complexes formed between soluble fibrinogen and antifibrinogen antibody. In parallel, 5 μL whole blood was incubated with 5 μg/mL FITC-conjugated anti-GPIIb antibody SZ22 to assess the GPIIb-IIIa expression at the cell surface. The samples were analyzed by flow cytometry and expressed as the MFI. These data are representative of 4 separate experiments.
The presence of a spontaneously activated GPIIb-IIIa receptor at the surface of N.M.'s platelets was further demonstrated when washed platelets were incubated with fibrinogen in an aggregometer cuvette. In the absence of stirring, no aggregates were observed; however, when stirring was initiated, microaggregates started to form, although a full macroscopic platelet aggregation was not achieved (data not shown). This observation indicates that fibrinogen is able to bind to its receptor without previous platelet activation and that this interaction could mediate a primary platelet-to-platelet interaction under stirring. In vivo, we were not able to find any microaggregates in the patient's PRP or on his blood smears. Taken together, these data demonstrate that N.M.'s platelets constitutively display a receptor expressed in a competent conformation for ligand binding.
Platelet morphology and immunocytochemical studies
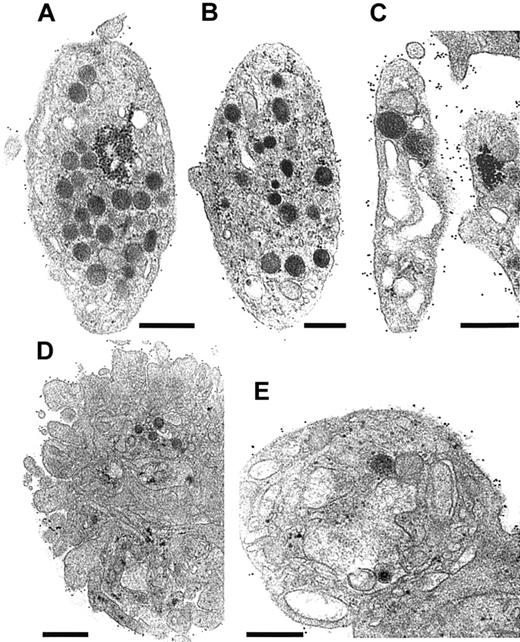
N.M.'s platelets mostly showed normal morphology, although increased size heterogeneity was noted. They were discoid with a full granule content (Figure 5B). When viewed on a broad field, immunogold staining for fibrinogen was globally increased at the platelet surface compared with control platelets. Although the labeling of some platelets (Figure 5B) resembled the control, others had an impressive concentration of gold particles at their surface (Figure 5C). The pattern of labeling for N.M. was distinctive, with clusters of gold particles at discrete intervals. Such a pattern was never seen for unstimulated control platelets (Figure 5A). In the illustrated platelet, we also noted the presence of enlarged vacuoles or distended channels (Figure 5C). Their presence was not a prerequisite for fibrinogen labeling at the surface. Note that the channels themselves did not contain gold beads. Although this may mean that the antibodies did not access this compartment in pre-embedded platelets, no signs of fibrinogen accumulation in the surface-connected canalicular system were seen when labeling was performed on frozen-thin sections (data not shown). After activation of N.M.'s platelets with TRAP6, shape change and secretion normally occurred. The quantity of fibrinogen detected on the surface of N.M.'s platelets increased globally. Some platelet aggregates were formed for the control even in the absence of continuous stirring (Figure 5D). For the patient, in contrast, only groups of 2 or 3 platelets were seen, despite immunogold labeling for fibrinogen on the surface (Figure 5E).
Ultrastructural analysis of N.M.'s platelets.
All studies were performed on platelets fixed in PRP and incubated with rabbit antifibrinogen antibody (dilution 1:50) for 1 hour at RT. Bound antibodies were detected using a species-specific anti-IgG antibody (dilution 1:10) coupled to 10-nm gold particles overnight at 4°C before embedding. (A,D) Platelet sections are from a control donor. (B,C,E) Selected sections of platelets from patient N.M. (A) A nonstimulated control platelet is illustrated; few gold particles are present on the surface. (B) A platelet from N.M. shows a normal discoid shape and internal organization. Few gold particles are present at the platelet surface. (C) Another discoid platelet with larger vacuoles or a distended surface canalicular system is shown. The labeling for fibrinogen on the surface of this platelet was much more intense. (D) An example of TRAP6-activated control platelets (5 minutes). Even without continuous stirring, some aggregates have formed in the PRP, and labeling for fibrinogen is seen at the periphery of the aggregate. (E) TRAP6-activated platelets from the patient remained isolated or as small aggregates composed of 2 or 3 platelets; inside the platelet, large vacuoles are present and the granules have disappeared. Labeling at the platelet surface continues to be seen. Bars = 0.5 μm
Ultrastructural analysis of N.M.'s platelets.
All studies were performed on platelets fixed in PRP and incubated with rabbit antifibrinogen antibody (dilution 1:50) for 1 hour at RT. Bound antibodies were detected using a species-specific anti-IgG antibody (dilution 1:10) coupled to 10-nm gold particles overnight at 4°C before embedding. (A,D) Platelet sections are from a control donor. (B,C,E) Selected sections of platelets from patient N.M. (A) A nonstimulated control platelet is illustrated; few gold particles are present on the surface. (B) A platelet from N.M. shows a normal discoid shape and internal organization. Few gold particles are present at the platelet surface. (C) Another discoid platelet with larger vacuoles or a distended surface canalicular system is shown. The labeling for fibrinogen on the surface of this platelet was much more intense. (D) An example of TRAP6-activated control platelets (5 minutes). Even without continuous stirring, some aggregates have formed in the PRP, and labeling for fibrinogen is seen at the periphery of the aggregate. (E) TRAP6-activated platelets from the patient remained isolated or as small aggregates composed of 2 or 3 platelets; inside the platelet, large vacuoles are present and the granules have disappeared. Labeling at the platelet surface continues to be seen. Bars = 0.5 μm
Patient N.M. possesses a novel mutation within the cysteine-rich domain of GPIIIa
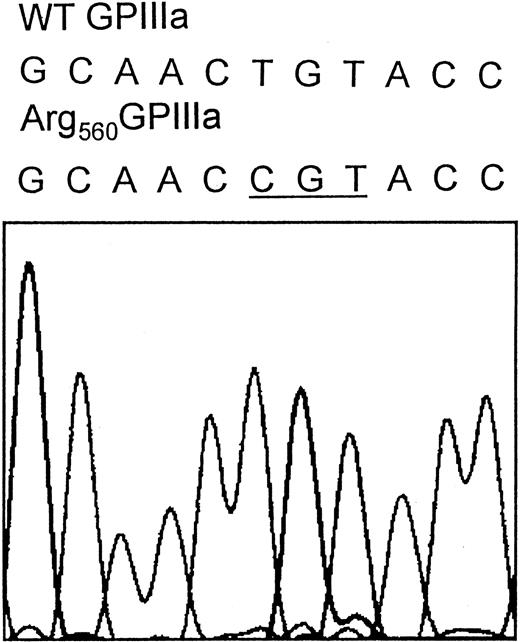
Direct sequence analysis of PCR-amplified fragments representing the coding sequences of the GPIIb and GPIIIa genes and the splice sites from patient N.M. revealed a homozygous g1776T>C substitution within exon 10 of the GPIIIa gene, which results in a Cys560Arg mutation located in the cysteine-rich domain of GPIIIa (Figure 6).
The Cys560Arg mutation in N.M.'s GPIIIa.
DNA sequence analysis was performed using N.M.'s genomic DNA. All exons, including intron-exon junctions, of both GPIIb and GPIIIa genes were amplified using PCR primer pairs hybridizing in the intron sequence flanking each exon. The resulting PCR fragments were purified and subjected to direct cycle sequencing. The nucleotide sequence of exon 10 of N.M.'s GPIIIa gene reveals a g1776T>C substitution (underlined codon), which results in the Cys560Arg mutation. The nucleotide substitution was also confirmed by sequence analysis performed by using reverse primer (not shown).
The Cys560Arg mutation in N.M.'s GPIIIa.
DNA sequence analysis was performed using N.M.'s genomic DNA. All exons, including intron-exon junctions, of both GPIIb and GPIIIa genes were amplified using PCR primer pairs hybridizing in the intron sequence flanking each exon. The resulting PCR fragments were purified and subjected to direct cycle sequencing. The nucleotide sequence of exon 10 of N.M.'s GPIIIa gene reveals a g1776T>C substitution (underlined codon), which results in the Cys560Arg mutation. The nucleotide substitution was also confirmed by sequence analysis performed by using reverse primer (not shown).
Expression of recombinant GPIIb-Arg560IIIa on the surface of transfected CHO cells
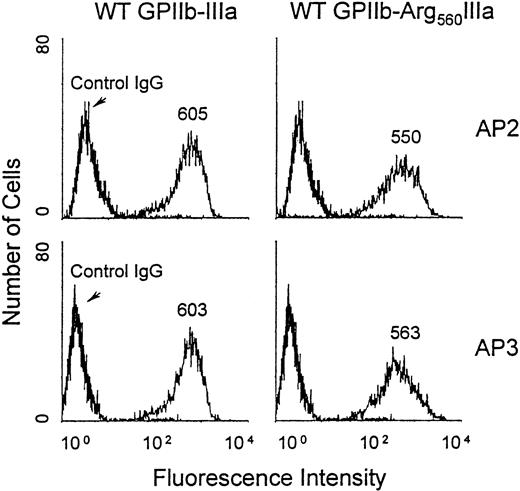
To further examine the effect of the Cys560Arg mutation on the processing and the function of GPIIb-IIIa, we constructed an expression vector encoding Arg560GPIIIa using site-directed mutagenesis and cotransfected it with wild-type (WT) GPIIb cDNA into CHO cells. After cell sorting, a stable cell line was obtained expressing the mutated complex (see “Patient, materials, and methods”). The expression of the GPIIb-Arg560IIIa complex on the cell surface was analyzed by flow cytometry using the anticomplex antibody AP2 and the antisubunit GPIIIa AP3 (Figure 7). These 2 antibodies specifically stained CHO cells expressing WT GPIIb-IIIa and GPIIb-Arg560IIIa, and the MFIs were similar for both antibodies. These data confirm that the Cys560Arg mutation does not affect the ability of GPIIIa to associate with GPIIb.
Flow cytometric analysis of GPIIb-Arg560IIIa expressed on the transfected CHO cell surface.
CHO cells were incubated with 40 μg/mL AP3 (lower), AP2 (upper), or IgG as a negative control for 60 minutes at 4°C; washed; and further incubated with 1:100 dilution of FITC-conjugated anti-IgG for 60 minutes before flow cytometric analysis using a FACScan. MFIs are indicated on the histograms.
Flow cytometric analysis of GPIIb-Arg560IIIa expressed on the transfected CHO cell surface.
CHO cells were incubated with 40 μg/mL AP3 (lower), AP2 (upper), or IgG as a negative control for 60 minutes at 4°C; washed; and further incubated with 1:100 dilution of FITC-conjugated anti-IgG for 60 minutes before flow cytometric analysis using a FACScan. MFIs are indicated on the histograms.
Assessment of LIBS exposure on recombinant GPIIb-IIIa
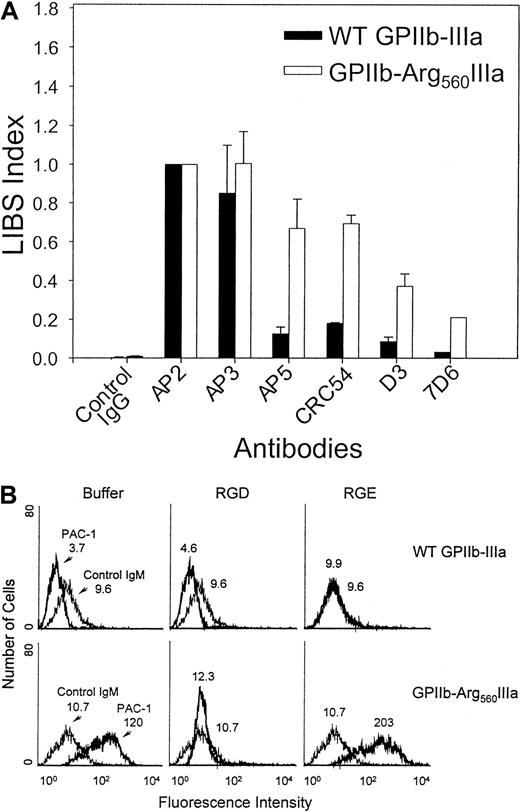
To examine whether the Cys560Arg mutation produces spontaneous LIBS exposure on GPIIb-IIIa at the CHO cell surface, as was observed for the patient's platelets, we incubated transfected CHO cells with AP5, CRC54, D3, and 7D6 LIBS antibodies and then performed flow cytometric analysis. As shown in Figure8A, the binding of LIBS antibodies to CHO cells expressing WT GPIIb-IIIa was low relative to that observed for the non-LIBS antibody AP3. However, the binding of LIBS antibodies AP5, D3, CRC54, and 7D6 to CHO cells expressing GPIIb-Arg560IIIa was increased (LIBS index [LI] = 0.7, 0.4, 0.75, and 0.2, respectively) compared with WT transfected cells (LI = 0.15, 0.1, 0.2, and 0.02, respectively). These results confirm that the conformation of the GPIIb-Arg560IIIa complex is modified by the Cys560Arg mutation.
Flow cytometric analysis of LIBS exposure on surface-expressed GPIIb-Arg560IIIa.
(A) Transfected CHO cells were incubated with 20 μg/mL LIBS antibodies for 45 minutes at RT, followed by FITC-conjugated goat anti–mouse IgG. The binding of LIBS mAbs to GPIIb-IIIa was expressed as a LIBS index (LI) by normalizing the MFI of each LIBS antibody to that obtained using AP2, a complex-specific mAb the binding of which is unaffected by the Cys560Arg mutation (LI = LIBS mAb MFI/AP2 MFI). (B) Flow cytometric analysis of the binding of the activation-dependent fibrinogen-mimetic antibody PAC-1 (20 μg/mL) to GPIIb-Arg560IIIa (bold histogram) was performed in the presence of buffer (left), 2 mM RGDW (middle), or RGEW (right). Peptides were synthesized at the Peptide Core Laboratory of the Blood Research Institute (Milwaukee, WI). The MFIs are indicated on the histograms.
Flow cytometric analysis of LIBS exposure on surface-expressed GPIIb-Arg560IIIa.
(A) Transfected CHO cells were incubated with 20 μg/mL LIBS antibodies for 45 minutes at RT, followed by FITC-conjugated goat anti–mouse IgG. The binding of LIBS mAbs to GPIIb-IIIa was expressed as a LIBS index (LI) by normalizing the MFI of each LIBS antibody to that obtained using AP2, a complex-specific mAb the binding of which is unaffected by the Cys560Arg mutation (LI = LIBS mAb MFI/AP2 MFI). (B) Flow cytometric analysis of the binding of the activation-dependent fibrinogen-mimetic antibody PAC-1 (20 μg/mL) to GPIIb-Arg560IIIa (bold histogram) was performed in the presence of buffer (left), 2 mM RGDW (middle), or RGEW (right). Peptides were synthesized at the Peptide Core Laboratory of the Blood Research Institute (Milwaukee, WI). The MFIs are indicated on the histograms.
To assess the ability of the GPIIb-Arg560IIIa complex to bind ligand, we examined the binding of PAC-1 to transfected CHO cells (Figure 8B). Although PAC-1 did not bind to CHO cells expressing WT GPIIb-IIIa (MFI = 3.7), PAC-1 binding to CHO cells expressing GPIIb-Arg560IIIa complex was dramatically increased (MFI = 120). Binding was specific because it was totally inhibited with excess RGDW peptide (Figure 8B). These data confirm that the ligand binding pocket is constitutively exposed in the GPIIb-Arg560IIIa complex.
Capacity for N.M.'s platelets to aggregate after secretion of the GPIIb-IIIa internal pool
Because we have shown that N.M.'s platelets contained significant total amounts of GPIIb-IIIa, we wondered whether aggregation was improved after TRAP6-induced α-granule secretion. Platelets in PRP were stimulated with 50 μM TRAP6, and expression of GPIIb-IIIa at the platelet surface was assessed with an anti–GPIIb-IIIa antibody (Figure9A). The patient's platelets expressed significantly more GPIIb-IIIa after stimulation, confirming the exposure of at least a part of the internal pool. This allowed the platelets to undergo a modified aggregation, which reached a maximum intensity of 40% despite a lower initial velocity (Figure 9B). Under similar conditions, the maximal aggregation of the control was 84%, a value also achieved when platelets were activated with lower amounts of TRAP6 (7 μM) (Figure 9B). When very high concentrations of other agonists were used, such as 100 μM ADP or 9 μg/mL collagen, N.M.'s platelets showed some residual aggregation but again with a reduced velocity (data not shown).
Expression of the internal pool of GPIIb-IIIa on the patient's platelets and subsequent aggregation after TRAP6 activation.
(A) A total of 2 × 106 platelets in PRP were activated with 50 μM TRAP6. Platelets were then incubated with anti–GPIIb-IIIa antibody 6E1 (clear histogram) and isotype control (dark histogram) for 30 minutes at RT. After the addition of 1% formaldehyde in PBS, samples were analyzed by flow cytometry. The MFIs are indicated on the histograms. (B) Aggregation of N.M.'s platelets in PRP after secretion of the internal GPIIb-IIIa pool. Citrated PRP (2 × 108platelets/mL) was stimulated with 7 μM and 50 μM TRAP6 for the control and the patient, respectively. The values (percent of maximal secretion) for PF4 release, as assessed with an ELISA, are in parentheses.
Expression of the internal pool of GPIIb-IIIa on the patient's platelets and subsequent aggregation after TRAP6 activation.
(A) A total of 2 × 106 platelets in PRP were activated with 50 μM TRAP6. Platelets were then incubated with anti–GPIIb-IIIa antibody 6E1 (clear histogram) and isotype control (dark histogram) for 30 minutes at RT. After the addition of 1% formaldehyde in PBS, samples were analyzed by flow cytometry. The MFIs are indicated on the histograms. (B) Aggregation of N.M.'s platelets in PRP after secretion of the internal GPIIb-IIIa pool. Citrated PRP (2 × 108platelets/mL) was stimulated with 7 μM and 50 μM TRAP6 for the control and the patient, respectively. The values (percent of maximal secretion) for PF4 release, as assessed with an ELISA, are in parentheses.
N.M.'s platelets and GPIIb-Arg560IIIa–transfected CHO cells are able to adhere to and spread on fibrinogen matrix
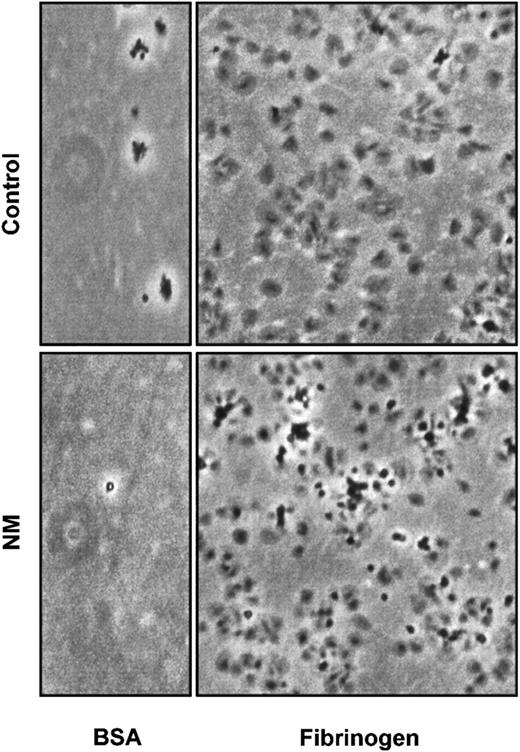
Previous studies have shown that normal platelets can adhere to immobilized fibrinogen without prior agonist activation.27 54 Adhesion assays with N.M.'s platelets were performed on dishes coated with 100 μg/mL fibrinogen during 90 minutes. After washing, adherent cells were examined by phase microscopy. N.M.'s platelets normally adhered to and spread on the fibrinogen matrix, adopting the typical fried-egg appearance (Figure 10).
Platelet adhesion and spreading on immobilized fibrinogen.
Adhesion and spreading of platelets were performed on dishes coated with 100 μg/mL fibrinogen or 5 mg/mL BSA as a control of adhesion. A total of 3 × 108 gel-filtered platelets were allowed to attach on a matrix for 90 minutes at 37°C.
Platelet adhesion and spreading on immobilized fibrinogen.
Adhesion and spreading of platelets were performed on dishes coated with 100 μg/mL fibrinogen or 5 mg/mL BSA as a control of adhesion. A total of 3 × 108 gel-filtered platelets were allowed to attach on a matrix for 90 minutes at 37°C.
In the same way, it has been shown previously that GPIIb-IIIa–expressing CHO cells can bind to a fibrinogen matrix.37 55 The adhesive properties of the GPIIb-Arg560IIIa complex were analyzed by examining the ability of transfected CHO cells to adhere to immobilized fibrinogen. The most striking difference in adhesion could be seen at an early time point (15 minutes), when twice as many CHO cells expressing the GPIIb-Arg560IIIa complex bound to 2 μg/mL immobilized fibrinogen compared with the WT transfected cells (data not shown). Cell spreading was also examined, and the Cys560Arg mutation imparted increased spreading capacities to GPIIb-IIIa–transfected CHO cells compared with CHO cells expressing WT GPIIb-IIIa at 15, 30, 60, and 90 minutes. The increased capacity for spreading was again most significant at 15 minutes, whereas it tended to decrease with time (data not shown).
Discussion
We report here the identification of a novel mutation in GPIIIa from a patient (N.M.) with a GT-like phenotype that produces a receptor locked in a high-affinity state and able to bind fibrinogen. We first diagnosed the patient as having GT on the basis of severely impaired platelet aggregation in PRP in response to physiologic agonists, abnormal clot retraction, and a prolonged bleeding time. Analysis of cell-surface GPIIb-IIIa showed an approximate 80% reduction in the expression of the integrin. Unexpectedly, the surface-expressed GPIIb-IIIa appeared to be locked in a high-affinity state constitutively expressing LIBS epitopes and spontaneously binding PAC-1 and fibrinogen. The molecular basis of this defect was elucidated. Sequence analysis of PCR fragments derived from N.M.'s genomic DNA revealed a homozygous g1776T>C nucleotide substitution leading to a Cys560Arg mutation in the GPIIIa subunit. This amino acid substitution occurs within the third CRR of GPIIIa.
Like other integrin β-subunits, GPIIIa contains 56 cysteines, of which 31 are located in 4 CRRs between residues 423 and 622 in a region proximal to the membrane.56 The data of Calvete et al56 suggested that all 56 cysteines of GPIIIa are paired together with no free thiol group. According to their model, Cys560 is thought to be paired with Cys567. Recently, however, Yan and Smith37 showed that the GPIIIa cysteines might not all be paired together and that a group of 2 to 3 cysteines in resting GPIIb-IIIa and up to 4 to 5 cysteines in activated GPIIb-IIIa might remain free, probably in the CRR-containing region. These data suggest that integrin activation might involve disulfide-bond rearrangements, as shown previously for functional regulation of other cysteine-containing molecules.41,42,57 58
We found that the Cys560Arg mutation leads to a GPIIb-IIIa receptor that exists in a high-affinity ligand binding state. It is tempting to speculate that replacement of Cys560 leads to disulfide-bond rearrangements that mimic activation of the integrin. Replacement of Cys560 might result in exposure of a free thiol at residue 567 or another cysteine elsewhere, increasing the association rate of GPIIb-IIIa with its ligand. It is noteworthy that neither Cys560 nor its putative Cys567 counterpart forms part of the CGXC consensus sequence associated with PDI-like activity43 or the CD/E motif recognized by NO in nitrosylations59,60; both processes are involved in regulation of redox states for integrin activation.41,42,57 58 Nonetheless, both the PDI and nitrosylation sites are relatively close to Cys560 and Cys567 in the linear sequence (Cys544-Gly-Asp-Cys547 and Cys581-Glu-Cys583, respectively), and they might be even closer in a tertiary structure, such that mutation of residue 560 could perturb disulfide rearrangements in this area and subsequently interfere with PDI and nitrosylation activities.
Not all mutations involving cysteine residues in the CRRs of GPIIIa lead to the production of an activated receptor. Arg636Cys and Cys655Tyr mutations have no apparent effect on GPIIb-IIIa biosynthesis or function.61,62 In contrast, Cys457Tyr, Cys506Tyr, and Cys542Arg mutations interfere with the expression of the GPIIb-IIIa complex on the platelet plasma membrane.63-65 Similarly, the Cys374Tyr mutation leads to a severely decreased expression of GPIIb-IIIa on the platelet surface, although the integrin complexes that become surface expressed appear to retain the ability to mediate adhesion to immobilized fibrinogen.66 These authors also found a decreased capacity of transiently transfected CHO cells to express the mutated integrin despite a normal transcription of the cDNA constructs. We also observed a reduced expression (approximate 60% reduction) of GPIIb-Arg560IIIa in stably transfected CHO cells before selection for high expression by sorting and cloning (C.-Y.L., Q.-H.S., and P.J.N., unpublished data, 1999). Significantly, we have also shown that the Cys5Ala and Cys435Ala forms of GPIIIa exhibit an increased affinity for immobilized fibrinogen.34,35 Involvement of the Cys5-Cys435 bond in GPIIb-IIIa activation has been reinforced lately by structural studies of resting and activated conformations of GPIIb-IIIa.36Interestingly, the neighboring Thr562Asn mutation does not target a cysteine but produces an active GPIIb-IIIa complex.38 Not all substitutions at residue 562 result in receptor activation, as neither Thr562Ala nor Thr562Gln forms of GPIIIa are “on,” supporting the notion that the nature of the amino acid substitution might be important. In this regard, Ambo et al67 reported a Phe residue substituted for a cysteine at the same position 560 as in patient N.M. It would be of interest to know whether this particular amino acid change results in the expression of a constitutively activated complex.
It is intriguing that expression of a constitutively activated GPIIb-IIIa receptor leads to a mild bleeding tendency instead of a prothrombotic phenotype. Similar observations have been made in the rare platelet-type von Willebrand disease (PT-vWD), in which bleeding symptoms and prolonged bleeding times are the result of mutations leading to heightened interaction of von Willebrand factor (vWF) with its receptor, GPIb.68 Thus, in both cases there is an up-regulation of the activation state of the receptor for its ligand. The parallel between N.M. and PT-vWD phenotypes could be extended because the mutations responsible for the increased vWF-GPIb interaction target residues located in disulfide-bonded loops in the N-terminal domain of GPIb known to be involved in the ligand binding itself or in its regulation.69-71 Nevertheless, whereas N.M.'s platelets are unable to aggregate normally even with high doses of suitable agonists, the platelets of PT-vWD patients display an enhanced aggregation in response to low doses of ristocetin, although leading to a bleeding phenotype by platelet adsorption of the high-molecular-mass vWF multimers that are the most effective for platelet adhesion.71
In the present study, it is important to note that despite a receptor constitutively expressed in a high-affinity conformation and occupied by fibrinogen, N.M.'s platelets did not display consistent signs of activation when examined by electron microscopy. A possible explanation for failure of this activated integrin to lead to cellular activation may be the reduced amount (approximately 20%) of GPIIb-IIIa present on the platelet surface. We observed that when N.M.'s platelets expressed increased amounts of GPIIb-IIIa, as after TRAP stimulation, the aggregation response improved. This is in agreement with the hypothesis that the low number of GPIIb-IIIa complexes on the circulating platelet surface is a limiting factor in the formation of the protein bridges necessary for platelet aggregation. Indeed, a facilitated binding of fibrinogen may lead to a high concentration of monovalently bound ligand unable to find an unoccupied counterreceptor. This would be particularly so in plasma where fibrinogen concentrations are high. Additionally, immunogold labeling with both antifibrinogen antibody (Figure 5) and antibody to GPIIb-IIIa complexes (data not shown) suggested that the residual surface-bound complexes are distributed heterogeneously within the total population of N.M.'s platelets. Thus, some platelets may not be able to support aggregation even when stimulated by a strong agonist.
The capacity of the patient's platelets as well as GPIIb-Arg560IIIa–transfected CHO cells to spread on a fibrinogen-covered surface demonstrates that despite the Cys560Arg mutation, the patient's GPIIb-IIIa complexes are still able to participate in outside-in signaling. The complexes are also able to transport fibrinogen, as the patient's α-granules contain a storage pool of this protein. Whether the mutation affects receptor trafficking in platelets will require further study; preliminary experiments have failed to show accumulated fibrinogen–GPIIb-IIIa complexes within the surface-connected canalicular system (data not shown). A misfolded extracellular region could have a number of consequences, including secondary effects on cytoplasmic tail interactions between GPIIb and GPIIIa themselves and with putative integrin-associated proteins, signaling molecules, and structural cytoskeletal proteins.13
In conclusion, the description of this novel case of Glanzmann thrombasthenia provides a new model to study the regulation of integrin activation through thiol bonding modifications in ectodomains independently from cytoplasmic inside-out signaling. Besides, the gain-of-binding mutation described in this GT patient may help to further understand complex molecular relations between GPIIb-IIIa receptor activation and integrin-mediated platelet aggregation and requirements for cellular adhesion events.
We are grateful to N.M. for generously donating his time to this project. We thank Drs Jennings, Mazurov, Shattil, and Blanchard for the generous supply of monoclonal antibodies. We also thank Drs Hourmant and Dantal as well as the transplantation unit in Nantes.
Supported by grant R01 HL-44612 from the National Institutes of Health (P.J.N.) and a grant from the Agence Française du Sang (FORTS 95000440744) (N.V.).
C.R. and C.-Y.L. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nathalie Valentin, Laboratoire d'Immunologie, Institut de Biologie, 9, quai Moncousu, 44093 Nantes Cedex 01, France; e-mail: nathalie.valentin@chu-nantes.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal