Abstract
CYP2C9 polymorphisms reported in Caucasians (Arg144Cys in exon 3 and Ile359Leu in exon 7) are extremely uncommon in Chinese persons. The genotype of CYP2C9 in this population was characterized to investigate its relation with the interindividual variation in warfarin dosages. Eighty-nine Chinese patients receiving warfarin were recruited. Target sequences inCYP2C9 in exons 1, 4, and 5 were amplified by polymerase chain reaction, followed by direct sequencing. Polymorphisms at 4 positions were demonstrated in exon 4. Heterozygosities for 608TTG>GTG (Leu208Val), 561CAG>CCG (Gln192Pro), 537CAT>CCT (His184Pro), and 527ATT>CTT (Ile181Leu) existed at frequencies 0.75, 0.20, 0.10, and 0.09, respectively. Seventeen patients (frequency, 0.19) were homozygous for Val208. The common genotypic combinations at these loci are Ile181/His184/Gln192/Leu208Val (n = 50), Ile181/His184/Gln192/Val208 (n = 15), Ile181/His184/Gln192/Leu208 (n = 4), Ile181/His184/Gln192Pro/Leu208Val (n = 6), Ile181/His184Pro/Gln192Pro/Leu208Val (n = 4), and Ile181Leu/His184/Gln192Pro/ Leu208Val (n = 4). At codon 208, heterozygous Leu208Val and homozygous Val208 appeared to have a lower warfarin dose requirement than the homozygous Leu208. Patients who are heterozygous for Ile181Leu had a higher warfarin dose requirement than the homozygous Ile181. Amplified sequences in exons 1 and 5 did not exhibit polymorphism. In conclusion, Chinese patients showed genetic polymorphisms of CYP2C9 in exon 4 and at codon 208; most were heterozygous Leu208Val and homozygous Val208. Homozygous Leu208, a common allele in Caucasians, is uncommon in this cohort. The significance of these CYP2C9 polymorphic alleles remains to be determined.
Introduction
Warfarin is widely used for prophylaxis and treatment of venous thromboembolism. Wide interindividual difference in warfarin sensitivity is a common problem.1 Although administering too little warfarin leads to inadequate anticoagulation, administering too much is a common cause of life-threatening hemorrhage; incidences range from 4.4% to 9.3% per patient year.2 Various factors have been shown to affect the maintenance dose of warfarin, including age, gender, body weight, and indications of anticoagulation.3
Genetic predisposition to warfarin resistance related to an increase in metabolic clearance has been reported in a patient who required a daily warfarin dose of 60 mg.4 Warfarin metabolism is catalyzed by cytochrome P450 (CYP) 2C9, which metabolizes S-warfarin to inactive S-7-hydroxywarfarin.5Polymorphisms in the coding region of the CYP2C9gene with variants at 416CGT>TGT (Arg144Cys) in exon 3 and 1061ATT>CTT (Ile359Leu) in exon 7 were found at frequencies of 0.125 and 0.085 in Caucasian patients.6 Amino acid substitutions at these sites impair enzymatic activities,7 and carriers of polymorphic alleles have reduced warfarin metabolism8and, hence, smaller dose requirements.9,10 However, both variants are extremely uncommon in Chinese.11 12 The current study was conducted using polymerase chain reaction (PCR) and automated DNA sequencing to characterize the genotype ofCYP2C9 in Chinese patients and to correlate the results with warfarin sensitivity in this population.
Study design
Patients
Eighty-nine patients receiving warfarin after prosthetic heart valve replacement were randomly recruited. Patients with severe congestive heart failure (NYHA class 3 or greater), liver cirrhosis, and thyroid disease were excluded from the study. All patients had been maintained on stable doses of warfarin for at least 3 months, and those with fluctuating warfarin requirements in the last 3 follow-ups (2 or more times the baseline dosage) were also excluded from the study. They had also been given dietary advice to avoid interference with warfarin pharmacokinetics. Anticoagulation was measured by International Normalized Ratio (INR), and the INR for all patients ranged between 1.5 and 3.0. There was no correlation between the target INR within this range and the warfarin dose required for anticoagulation (not shown).
Characterization of CYP 2C9 genotype
Buffy coat was obtained from 10-mL peripheral blood in each patient with informed consent. DNA was extracted by the QIAamp DNA Blood Minikit (Qiagen, Basel, Switzerland) and eluted with 200 μL buffer. DNA sequences in the CYP2C9 gene (GenBank accession numbers L16877, L16879, L16880) that shared least homology with otherCYP isoforms were amplified by PCR with the following primers, as previously described,6 and the PCR products were gel purified and directly sequenced (ABI Prism 377; PE Biosystems, Foster City, CA): exon 1—forward, 5′-ACG TGA ATT CAC TTT CCT AGC TCT CAA A-3′; reverse, 5′-TCA GGG ATC CGC ACA CCT ACC AAA TAA T-3′; exon 4—forward, 5′ACG TGA ATT CTC CTG GGC TGT GCT CCC TGC-3′; reverse, 5′-TCA GGG ATC CTT GGC CTT ACC TGG ATC CAG G-3′; exon 5—forward, 5′ACG TGA ATT CGC ACA ACC AAC CAT CTG AA-3′; reverse, 5′-TCA GGG ATC CAG TCA ACT GCA GTG TTT TC-3′.
Statistical analysis
Warfarin requirements in different CYP2C9 variants were compared by the Mann-Whitney U and the Kruskal-Wallis tests. The effects of different factors are evaluated by univariate regression analysis using SPSS (Chicago, IL). P < .05 were considered statistically significant.
Results and discussion
Clinical characteristics
The mean (± 1 SEM) dose of warfarin was 3.3 ± 0.13 mg/d (range, 1.0-6.7 mg/d). To obviate the variation caused by differences in body weight, the dose of warfarin per unit body weight was calculated and used in subsequent analysis. Weight-adjusted mean dose was 58.2 ± 2.6 μg/kg per day (range, 17.6-105.1 g/kg per day), and the mean INR achieved was 2.2 ± 0.04 (range, 1.9-2.9). Median age of the patients was 51 years (range, 26-82 years).
PCR amplification of CYP2C9 genes
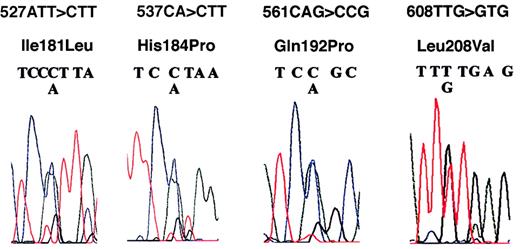
Figure 1 shows the automated sequence analyses at 4 positions of exon 4, and Table1 shows the frequencies of polymorphic alleles. Variants 608TTG>GTG (Leu208Val), 561CAG>CCG (Gln192Pro), 537CAT>CCT (His184Pro), and 527ATT>CTT (Ile181Leu) were found as heterozygotes at frequencies of 0.75, 0.20, 0.10, and 0.09, respectively. Seventeen patients (frequency, 0.19) were homozygous for Val208. Nucleotide sequences in exons 1 and 5 did not exhibit polymorphism and were identical to those in published cDNA sequences. Table2 shows the genotypic combination at these 4 alleles. Common associations at these loci are Ile181/His184/Gln192/Leu208Val (n = 50), Ile181/His184/Gln192/Val208 (n = 15), Ile181/His184/ Gln192/Leu208 (n = 4), Ile181/His184/Gln192Pro/Leu208Val (n = 6), Ile181/His184Pro/Gln192Pro/Leu208Val (n = 4), and Ile181Leu/His184/Gln192Pro/Leu208Val (n = 4). Nineteen patients carried polymorphic alleles at more than one locus, and 15 of them were heterozygous at both Gln192Pro and Leu208Val loci. The rarity of other combinations, however, precluded accurate analysis of linkage disequilibrium in this study.
DNA sequencing analysis in 4 patients in exon 4 of CYP 2C9.
Heterozygosities (shown by the presence of double peaking) were observed in codons 181, 184, 192, and 208, resulting in polymorphic alleles of 527ATT>CTT (Ile181Leu), 537CAT>CCT (His184Pro), 561CAG>CCG (Gln192Pro), and 608TTG>GTG (Leu208Val).
DNA sequencing analysis in 4 patients in exon 4 of CYP 2C9.
Heterozygosities (shown by the presence of double peaking) were observed in codons 181, 184, 192, and 208, resulting in polymorphic alleles of 527ATT>CTT (Ile181Leu), 537CAT>CCT (His184Pro), 561CAG>CCG (Gln192Pro), and 608TTG>GTG (Leu208Val).
Summary of the polymorphic alleles in exon 4 of cytochrome P450 2C9
| Locus . | No. of patients (%) . | ||
|---|---|---|---|
| Homozygotes . | Heterozygotes . | Homozygotes for variants . | |
| 527ATT>CTT Ile181Leu | 81 (91) | 8 (9) | 0 |
| 537CAT>CCT His184Pro | 80 (90) | 9 (10) | 0 |
| 561CAG>CCG Gln192Pro | 71 (80) | 18 (20) | 0 |
| 608TTG>GTG Leu208Val | 5 (5.6) | 67 (75.3) | 17 (19.1) |
| Locus . | No. of patients (%) . | ||
|---|---|---|---|
| Homozygotes . | Heterozygotes . | Homozygotes for variants . | |
| 527ATT>CTT Ile181Leu | 81 (91) | 8 (9) | 0 |
| 537CAT>CCT His184Pro | 80 (90) | 9 (10) | 0 |
| 561CAG>CCG Gln192Pro | 71 (80) | 18 (20) | 0 |
| 608TTG>GTG Leu208Val | 5 (5.6) | 67 (75.3) | 17 (19.1) |
Summary of genetic polymorphism in exon 4 of cytochrome P450 and mean daily warfarin dose (μg/kg per day) in 89 Chinese patients
| Codon 181 . | Codon 184 . | Codon 192 . | Codon 208 . | Mean dose ± SEM . | N . |
|---|---|---|---|---|---|
| Ile | His | Gln | Leu | 72.6 ± 14.0 | 4* |
| Ile | His | Gln | Leu/Val | 53.8 ± 2.9 | 50* |
| Ile | His | Gln | Val | 48.1 ± 3.5 | 15* |
| Ile | His | Gln/Pro | Leu/Val | 57.1 ± 5.5 | 6 |
| Ile | His | Gln/Pro | Val | 63.6 ± 0.0 | 1 |
| Ile | His/Pro | Gln/Pro | Leu/Val | 47.4 ± 16.5 | 4 |
| Ile | His/Pro | Gln/Pro | Val | — | 1 |
| Ile/Leu | His | Gln/Pro | Leu/Val | 87.6 ± 4.9 | 4 |
| Ile/Leu | His/Pro | Gln | Leu/Val | 95.3 ± 4.4 | 2 |
| Ile/Leu | His/Pro | Gln/Pro | Leu | 91.0 ± 0.0 | 1 |
| Ile/Leu | His/Pro | Gln/Pro | Leu/Val | 100.8 ± 0.0 | 1 |
| Codon 181 . | Codon 184 . | Codon 192 . | Codon 208 . | Mean dose ± SEM . | N . |
|---|---|---|---|---|---|
| Ile | His | Gln | Leu | 72.6 ± 14.0 | 4* |
| Ile | His | Gln | Leu/Val | 53.8 ± 2.9 | 50* |
| Ile | His | Gln | Val | 48.1 ± 3.5 | 15* |
| Ile | His | Gln/Pro | Leu/Val | 57.1 ± 5.5 | 6 |
| Ile | His | Gln/Pro | Val | 63.6 ± 0.0 | 1 |
| Ile | His/Pro | Gln/Pro | Leu/Val | 47.4 ± 16.5 | 4 |
| Ile | His/Pro | Gln/Pro | Val | — | 1 |
| Ile/Leu | His | Gln/Pro | Leu/Val | 87.6 ± 4.9 | 4 |
| Ile/Leu | His/Pro | Gln | Leu/Val | 95.3 ± 4.4 | 2 |
| Ile/Leu | His/Pro | Gln/Pro | Leu | 91.0 ± 0.0 | 1 |
| Ile/Leu | His/Pro | Gln/Pro | Leu/Val | 100.8 ± 0.0 | 1 |
Codons denoted by two amino acids represent heterozygosities at those loci. Warfarin dose of one patient (denoted by —) was unavailable.
At codon 208, heterozygous 608TTG > GTG (Leu208Val) and homozygous 608G > TG (Val208) appeared to require lower warfarin doses than the wild-type carriers (Leu208). However, the difference was not statistically significant (P = .2). N indicates number of patients in each category.
Correlation between warfarin dose requirement andCYP2C9 polymorphism and other clinical parameters
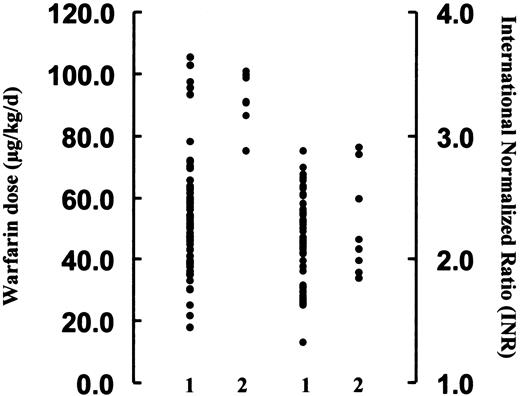
Patients carrying different polymorphic alleles were compared in terms of the warfarin requirement (Table 2). At codon 208, heterozygous Leu208Val (Ile181/His184/Gln192/Leu208Val) and homozygous Val208 (Ile181/His184/Gln192/Val208), which occurred at high frequencies in this cohort, appeared to require a lower warfarin dose than required for patients carrying the homozygous wild-type genotype (Ile181/His184/Gln192/Leu208). The latter occurred at a frequency of 0.04, with a warfarin dose requirement similar to that used in the Caucasian population.1 However, because of the small number of patients involved, the difference between the 3 groups of patients could not reach statistical significance (P = .2). At codon 181, heterozygous 527ATT>CTT (Ile181Leu) required a significantly higher warfarin dose than the homozygous 527ATT (Ile181) (Figure 2) (mean, 91.8 ± 3.1 vs 55.7 ± 2.9 μg/kg per day;P < .001). Interestingly, all patients carrying the heterozygous Ile181Leu alleles also carried at least one of the other polymorphic alleles; therefore, whether the higher warfarin dose requirement in this subgroup was related to leucine substitution at codon 181 or to the combined effects of amino acid substitutions at various loci could not be ascertained. To assess the contribution of other factors to the warfarin dose, age, gender, and INR were entered into univariate regression analysis together with the occurrence of polymorphic alleles. Only heterozygous 527ATT>CTT (Ile181Leu) (P = .002) was significantly associated with higher warfarin dose in the patients.
Correlation between warfarin dose (μg/kg per day), INR, and genotypes at codon 181 of exon 4.
The warfarin dose requirement (left axis) was significantly higher in patients carrying the heterozygous ATT/CTT alleles (lane 2) than in those carrying the homozygous ATT/ATT alleles (lane 1) (P < .001, Mann-Whitney U test). There was, however, no difference in INR between these 2 groups of patients (right axis).
Correlation between warfarin dose (μg/kg per day), INR, and genotypes at codon 181 of exon 4.
The warfarin dose requirement (left axis) was significantly higher in patients carrying the heterozygous ATT/CTT alleles (lane 2) than in those carrying the homozygous ATT/ATT alleles (lane 1) (P < .001, Mann-Whitney U test). There was, however, no difference in INR between these 2 groups of patients (right axis).
Hong Kong Chinese generally require a much lower warfarin dose than Caucasians, and the difference was not explainable entirely by variations in age, body weight, sex, dietary vitamin K intake, clinical indications for warfarin use, and target anticoagulation.1,13 We have previously shown that the common CYP2C9 variants in Caucasians, which are associated with reduced warfarin clearance and, hence, a lower warfarin dose requirement—namely, 416CGT>TGT (Arg144Cys) in exon 3 and 1061ATT>CTT (Ile359Leu) in exon 76-10,14 —were not found in our population.11
The current study demonstrated that exon 4 of the CYP2C9gene in Hong Kong Chinese patients exhibited genetic polymorphisms at 4 different sites—608TTG>GTG (Leu208Val), 561CAG>CCG (Gln192Pro), 537CAT>CCT (His184Pro), and 527ATT>CTT (Ile181Leu). To our knowledge, these polymorphic alleles have not been reported in the literature. In particular, the heterozygous Leu208Val and homozygous Val208 existed at high frequencies, and patients carrying these alleles appeared to have lower warfarin dose requirements than did carriers of the wild-type (Leu208) allele, who had warfarin requirements (72.6 ± 14.0 μg/kg per day) in the range used in the Caucasian population.1 We speculate that this may be an important polymorphic site that contributes to a lower warfarin dose requirement in this population. Structure-activity analysis ofCYP2C9 has recently been reported.15-17 Codon 208 is located in the F helix of the CYP2C9 and is directed to the active site of the enzyme (C. M. Masimirembwa et al, personal communication, 2001). As a result, amino acid substitution at this position may affect the catalytic activity of the enzyme and, hence, the metabolism of warfarin. This may explain the lower warfarin dose requirement in patients carrying these polymorphic alleles. In addition, some patients in this cohort exhibited heterozygosities at His184Pro and Gln192Pro. Codons 184 and 192 are located far from the active site, but proline substitutions at these locations may lead to helix breaking, thereby causing changes in the secondary structure and the enzymatic activity. However, warfarin dose requirements could not be interpreted easily because of the small number of patients involved. On the other hand, polymorphism at codon 181 (Ile181Leu) is associated with a higher warfarin dose requirement in this cohort. Codon 181 is located far from the active site ofCYP2C9. Given that all patients who were heterozygous for Ile181Leu also carried other polymorphic alleles, the higher warfarin dose requirement in these patients might be attributed to the combined effects of amino acid substitutions at these codons on the enzymatic activity of the CYP2C9. This speculation would have to be confirmed by in vitro studies. It is also interesting to note that 19 patients in this cohort carried more than one locus with polymorphic alleles, of whom 15 were heterozygous at both Gln192Pro and Leu208Val loci. Whether this represented true linkage disequilibrium would require confirmation with a larger number of patients.
The significance of CYP2C9 genetic polymorphism is uncertain. In routine clinical practice, the intensity of maintenance anticoagulation is often guided by INR, and knowledge of a patient's genotype is not a prerequisite for warfarin dosage adjustment. However, characterization of CYP2C9 polymorphic alleles may identify patients at risk, so that warfarin can be given more cautiously, especially during the induction phase of anticoagulation. In fact, CYP2C9genetic polymorphisms at the Arg144Cys and Ile359Leu loci have been associated with increased risk for bleeding complication during anticoagulation.9,18 In addition, becauseCYP2C9 is involved in the metabolism of various commonly used drugs,19 the demonstration of genetic polymorphisms of this enzyme may have implications on the interethnic and interindividual variations of drug pharmacokinetics.
In conclusion, novel polymorphic alleles at 4 positions (Ile181Leu, His184Pro, Gln192Pro, and Leu208Val) of exon 4 of the CYP2C9gene were identified in Hong Kong Chinese. At codon 208, polymorphic alleles existed at high frequency and appeared to have lower warfarin dose requirements. Further studies are needed to delineate the effects of these amino acid substitutions on the activity of the enzyme.
We thank Drs C. M. Masimirembwa, M. Ridderström, and T. B. Andersson (Department of Drug Metabolism and Pharmacokinetics and Bioanalytical Chemistry, AstraZeneca R&D, Mölndal, Sweden) for performing 3-dimensional analysis ofCYP2C9 at the reported loci.
Supported by the Kadoorie Charitable Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Raymond Liang, Department of Medicine, Queen Mary Hospital, Pok Fu Lam Rd, Hong Kong, People's Republic of China; e-mail: rliang@hkucc.hku.hk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal