Abstract
Ligation of the cell-surface Fas molecule by its ligand (Fas-L) or agonistic anti-Fas monoclonal antibodies results in the cleavage and activation of the cysteine protease procaspase 8 followed by the activation of procaspase 3 and by apoptosis. In some leukemia cell lines, cytotoxic drugs induce expression of Fas-L, which may contribute to cell killing through the ligation of Fas. The involvement of Fas, Fas-L, and caspase 8 was studied in the killing of B-cell chronic lymphocytic leukemia (B-CLL) cells by chlorambucil, fludarabine, or γ radiation. Spontaneous apoptosis was observed at 24-hour incubation, with additional apoptosis induced by each of the cytotoxic treatments. Although Fas mRNA expression was elevated after exposure to chlorambucil, fludarabine, or γ radiation, Fas protein levels only increased after irradiation. Therefore, Fas expression may be regulated by multiple mechanisms that allow the translation of Fas mRNA only in response to restricted cytotoxic stimuli. None of the cytotoxic stimuli studied here induced Fas-L expression. An agonistic anti-Fas monoclonal antibody (CH-11) did not significantly augment apoptosis induction by any of the death stimuli. A Fas-blocking antibody (ZB4) did not inhibit spontaneous, chlorambucil-, fludarabine-, or radiation-induced apoptosis. However, procaspase 8 processing was induced by all cytotoxic stimuli. These data suggest that the Fas/Fas-L signaling system does not play a major role in the induction of apoptosis in B-CLL cells treated with cytotoxic drugs or radiation. However, Fas-independent activation of caspase 8 may play a crucial role in the regulation of apoptosis in these cells.
Introduction
Apoptosis, a conserved mechanism of cell suicide, is responsible for the killing of cells by a wide variety of physiological and toxic stimuli.1 Although different apoptotic stimuli initially activate distinct pathways leading to cell death, these converge on a common execution mechanism involving the activation of a set of cysteine proteases—the caspases 3, 6, and 7. Cleavage of a restricted set of cellular substrates then results in the demise of the cell.2 3
The death receptor Fas (also known as APO-1 or CD95) contains an intracytoplasmic death domain that mediates interactions with the adapter molecule FADD.4 FADD associates in turn with procaspase 8. Ligation of Fas by Fas ligand (Fas-L) results in cleavage of procaspase 8, with the consequent generation of active caspase 8. Cleavage of caspase 3 by caspase 8 then results in apoptosis.4 Numerous cellular substrates are cleaved by caspase 3.3,5 These include poly(ADP ribose) polymerase (PARP), an enzyme involved in the regulation of DNA repair that is cleaved to a characteristic 85-kd fragment (p85 PARP).6
The mechanisms by which antineoplastic cytotoxic drugs kill leukemic cells is not well understood, though the activation of caspase 3 has been implicated.7 Recent observations have resulted in the suggestion that interactions between Fas and Fas-L may mediate cytotoxic killing of at least some leukemia cell lines.8First, cytotoxic drug treatment of leukemia cell lines can induce elevated expression of Fas-L9,10 or of Fas.11Drug-induced Fas expression may be dependent on p53-mediated transcriptional up-regulation of the Fasgene.12,13 Second, killing of target cells by cytotoxic drugs can be blocked by antibodies that interfere with Fas/Fas-L interactions,9 by antisense oligos against Fas-L mRNA, or by ectopic expression of dominant-negative FADD.14 Third, leukemia cell lines selected for resistance to Fas-dependent killing are also cross-resistant to cytotoxic drugs.9 Similar evidence has suggested a role for Fas-mediated signaling in the killing of solid tumor cell lines.12 15-17
Other studies, however, have failed to reveal a role for Fas/Fas-L interactions in the cytotoxic killing of leukemia cell lines. For example, cytotoxic drugs do not always result in the elevation of Fas or Fas-L expression.18,19 Blocking of Fas/Fas-L interactions by antibodies failed to block cytotoxic killing of leukemia cell lines in some studies.18-22 Fas resistant sublines of malignant human T cells22,23 or myeloma cells24 were found to be as susceptible to cytotoxic drugs as their Fas-sensitive parental lines.
Cytotoxic drugs also induce the release of cytochrome-c from mitochondria. The subsequent interaction of cytochrome-cwith the APAF-1 protein results in the recruitment of procaspase 9. Activation of procaspase 9 within this multiprotein complex, the apoptosome, results in the processing of downstream caspases, including caspase 3, and subsequent apoptotic death.3,5 An additional level of complexity is provided by recent observations that in some cell types, caspase 8 activation by death receptors can initiate cross-talk with the mitochondrial cell death pathway. This link is provided by caspase 8 cleavage of the proapoptotic BID protein, whose truncated form inserts into the mitochondrial outer membrane and promotes cytochrome-c release and consequent activation of the apoptosome.5,25 26
Killing of B-cell chronic lymphocytic leukemia (B-CLL) cells by cytotoxic agents is compromised by p53 mutations.27 In addition, we have observed that the killing of B-CLL cells by cytotoxic drugs or radiation is preceded by the up-regulation of p53 levels and of p53-mediated transcription. p53-mediated transcription and apoptosis were blocked by the p53 inhibitor pifithrin α,28suggesting that killing of B-CLL cells by cytotoxic agents is at least partially dependent on p53. Because theFas12,13,29 and Fas-L30genes are potential transcriptional targets of p53, we determined whether Fas or its ligand was induced at mRNA and protein levels after treatment with these agents. Given that cells can be sensitized to pre-existing Fas/Fas-L interactions by some apoptotic stimuli,31 we also determined whether cytotoxic killing of B-CLL cells could be blocked by an antagonistic Fas monoclonal antibody. Our data suggest that Fas/Fas-L signaling does not play a major role in the killing of B-CLL cells by cytotoxic agents. However, the activation of caspase 8 by a Fas-independent mechanism may contribute to apoptotic signaling.
Materials and methods
Preparation of B-CLL cells
B-CLL cells were obtained from 27 patients (21 men, 6 women) with informed consent. White blood cell counts ranged from 27.4 to 476 × 106 cells/mL. The peripheral blood of all patients contained more than 85% CD19+ cells and more than 90% CD5+ cells. Eleven patients were in Binet clinical stage A, 6 were in stage B, and 10 were in stage C. None of the patients had received treatment for at least 3 months before this study. Malignant B cells were obtained from heparinized peripheral blood after Ficoll-Hypaque centrifugation, plastic adherence to remove monocytes, and T-cell depletion by rosetting with neuraminidase-treated sheep red blood cells as described.32 Enriched cells were stained with monoclonal antibody CD2 (Becton Dickinson, San Jose, CA) and then analyzed by flow cytometry using Cell Quest software (Becton Dickinson). All samples studied contained less than 10% CD2+ T cells. On 5 occasions, we checked that more than 90% of the isolated cells were positive for both CD19 and CD5 by FACScan analysis.
Cell culture
B-CLL cells were cultured in RPMI 1640 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 10% heat-inactivated fetal calf serum. Cells were cultured for 24 hours after the addition of chlorambucil or fludarabine or after exposure to γ radiation. Concentrations of drugs used were selected in accordance with IC50 values previously observed by Silber et al.33 Anti–human Fas–blocking monoclonal immunoglobulin G1 (IgG1) antibody (ZB4; Upstate Biotechnology, Lake Placid, NY) was added at 250 ng/mL 1 hour before cytotoxic treatment. The agonistic anti-Fas IgM monoclonal (CH-11; Upstate Biotechnology) was added at 50 ng/mL. The Jurkat cell line, which expresses high levels of Fas, was used as a control for the actions of these antibodies.
Assessment of viability and apoptosis
Viable cells were estimated by quantitation of propidium iodide (PI)–excluding cells by FACScan analysis or by counting trypan blue–negative cells in a Neubauer counting chamber. Apoptotic cells were estimated by counting the proportion of cells with condensed or fragmented nuclei in May-Grünwald-Giemsa–stained cytospin preparations.34 At least 1000 cells in 4 randomly selected fields were counted on each slide. The ability of chlorambucil, fludarabine, and γ radiation to induce apoptosis of B-CLL cells was additionally verified by electron microscopy (not shown).
The actions of the ZB4 and CH-11 antibodies on the killing of B-CLL cells by drugs or radiation were also evaluated by quantifying their ability to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT).35 Apoptosis was additionally assessed by flow cytometric quantitation of cells with subdiploid DNA content36 and of cells with increased forward light scatter and decreased side scatter compared to normal cells.37
RNA extraction and cDNA synthesis
Total cellular RNA was isolated by the guanidinium–isothiocyanate method.38 cDNA was synthesized from 1 μg total RNA using 200 U Moloney murine leukemia virus reverse transcriptase (Life Technologies, Paisley, Scotland). The reaction was performed in a 20-μL volume containing 2.5 μM random hexadeoxynucleotide primers (Boehringer, Lewes, United Kingdom), 0.5 mM each dNTP (Amersham Pharmacia, Little Chalfont, United Kingdom), and 24 U RNasin (Promega, Madison, WI) for 60 minutes at 37°C. cDNA synthesis was stopped by incubation at 94°C for 5 minutes. Twenty microliters water was added, and the samples were stored at −70°C.
Polymerase chain reaction
In each polymerase chain reaction (PCR) reaction, 2 μL cDNA preparations were amplified using 2.5 U Taq polymerase (Promega) and 0.5 mM each dNTP. Primer sequences and amplification conditions are summarized in Table 1. All primers were used at 10 μg/mL. Thermal cycles consisted of 1 minute at 94°C, 1 minute at the annealing temperature (Table 1), and 1 minute at 72°C. In preliminary experiments, the cycle numbers used in conjunction with each set of primers were determined to be within the exponential range. PCR products were visualized on ethidium bromide–stained 3% agarose gels. Bands were quantified by densitometry using the Gel Doc video camera and Quantity One software (Bio-Rad, Hemel Hempstead, United Kingdom). Fas and Fas-L band intensities were normalized to the density of actin PCR bands generated from the same sample.
Oligonucleotide primer sequences
| Targets . | . | Primer sequence . | Annealing temperature . | Cycle no. . |
|---|---|---|---|---|
| β-actin | S | 5′-TGCTATCCAGGCTGTGCTAT-3′ | 55°C | 20 |
| AS | 5′-GATGGAGTTGAAGGTAGTTT-3′ | |||
| Fas | S | 5′-TAGAAGCTTCGGAGGATTGCTCAACAAC-3′ | 60°C | 28 |
| AS | 5′-TAGGAATTCTTGGTATTCTGGGTCCG-3′ | |||
| Fas-L | S | 5′-CAAGTCCAACTCAAGGTCCATGCC-3′ | 60°C | 30 |
| AS | 5′-CAGAGAGAGCTCAGATACGTTGAC-3′ |
| Targets . | . | Primer sequence . | Annealing temperature . | Cycle no. . |
|---|---|---|---|---|
| β-actin | S | 5′-TGCTATCCAGGCTGTGCTAT-3′ | 55°C | 20 |
| AS | 5′-GATGGAGTTGAAGGTAGTTT-3′ | |||
| Fas | S | 5′-TAGAAGCTTCGGAGGATTGCTCAACAAC-3′ | 60°C | 28 |
| AS | 5′-TAGGAATTCTTGGTATTCTGGGTCCG-3′ | |||
| Fas-L | S | 5′-CAAGTCCAACTCAAGGTCCATGCC-3′ | 60°C | 30 |
| AS | 5′-CAGAGAGAGCTCAGATACGTTGAC-3′ |
S indicates sense; AS, antisense orientation.
Flow cytometric analysis of Fas and Fas-L expression
Cell surface expression of Fas and Fas-L in B-CLL cells was quantified by flow cytometry. The expression of Fas was analyzed by incubating 106 cells/mL with monoclonal Fas–fluorescein isothiocyanate (FITC) antibody (UB2; Immunotech, Nottingham, United Kingdom) or control mouse IgG1 FITC antibody (Becton Dickinson) for 40 minutes at 4°C. Fas-L was quantified by incubation with monoclonal Fas-L antibody (G247-4; Pharmingen, Cowley, United Kingdom) or control mouse IgG1 antibody (Becton Dickinson) for 30 minutes at 4°C, followed by detection using FITC-labeled rabbit anti–mouse IgG (Dako, Ely, United Kingdom) for 30 minutes at 4°C. Data were analyzed using Cell Quest software and are presented as the ratio of Fas-FITC median cell fluorescence (MedCF)/control IgG1 FITC MedCF values.
Western blot analysis
B-CLL cells were washed with Hanks balanced salt solution (Life Technologies) and were resuspended in lysis buffer (20 mM Tris-HCl, pH 7.6, 165 mM KCl, 400 mM NaCl, 1 mM EDTA, 1% Triton X-100, 20% glycerol, 5 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, 100 U/mL aprotinin, 15 mM NaF, and 3 mM sodium vanadate). After 30-minute incubation at 4°C and 30-minute centrifugation at 12 000g, 20 μg protein from each supernatant was fractionated on Novex precast sodium dodecyl sulfate–polyacrylamide gradient gels (Invitrogen, Groningen, The Netherlands) and blotted onto enhanced chemiluminescence–Hybond filters (Amersham Pharmacia). The following primary antibodies were used: Fas (polyclonal C-20; Santa Cruz Biotechnology, Santa Cruz, CA); Fas-L (monoclonal NOK-1; Pharmingen); procaspase 8 (monoclonal B9-2; Pharmingen); caspase 8 p20 subunit (polyclonal sc-6136; Santa Cruz Biotechnology); procaspase 3 (polyclonal; Pharmingen); p85 PARP (monoclonal; Promega); β-actin (monoclonal AC15; Sigma). Bands were visualized using appropriate horseradish peroxidase–linked secondary antibodies (Dako) and the enhanced chemiluminescence system (Amersham International) and were analyzed using the GS700 densitometer (Bio-Rad).
Statistical analysis
Data obtained using multiple B-CLL isolates were evaluated using Student t test for paired samples.
Results
Reverse transcription–polymerase chain reaction analysis of Fas and Fas-L transcripts
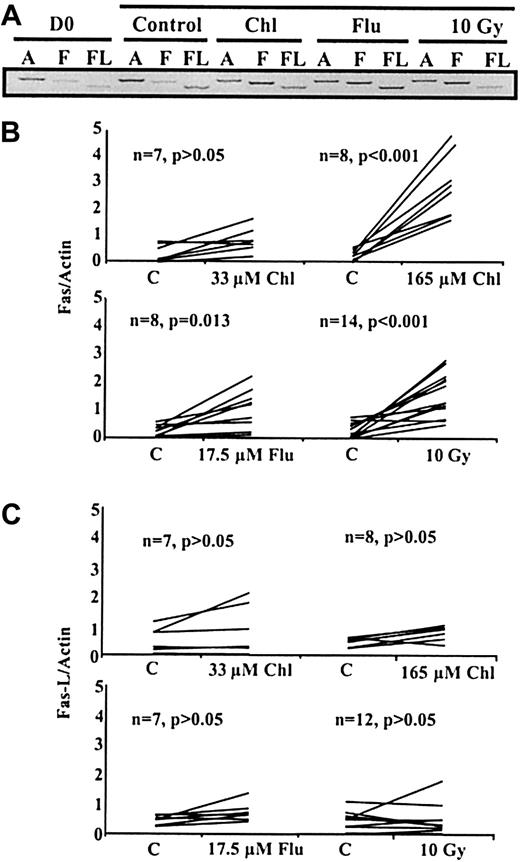
To determine whether Fas or Fas-L transcripts were up-regulated after treatment with chlorambucil, fludarabine, or γ irradiation, mRNA levels in B-CLL cells were quantified by reverse transcription (RT)–PCR. Analysis of transcripts from a representative experiment showed that treatment with all these cytotoxic agents resulted in augmented expression of Fas transcripts, but not of Fas-L transcripts, after 24 hours of culture (Figure1A). RT-PCR data obtained using multiple B-CLL isolates are summarized in Figure 1B-C. Relative to 24-hour controls, mean Fas mRNA levels increased by 2.3-fold (P > .05) after treatment with 33 μM chlorambucil, 11.3-fold (P < .001) with 165 μM chlorambucil, 4.0-fold (P = .013) with 17.5 μM fludarabine, and 4.7-fold (P < 0.001) after γ irradiation (Figure 1B). In contrast, Fas-L mRNA levels did not increase significantly after treatment with chlorambucil, fludarabine, or γ irradiation (Figure 1C).
Semiquantitative RT-PCR analysis of B-CLL transcripts.
(A) Analysis of actin (A), Fas (F), or Fas-L (FL) transcripts in B-CLL cells at day 0 (D0) or after 24-hour incubation with medium alone (control), 165 μM chlorambucil (Chl), 17.5 μM fludarabine (Flu), or 24 hours after irradiation (10 Gy). (B, C) Intensities of Fas, Fas-L, and actin PCR bands from multiple B-CLL samples were quantified. Fas/Actin and Fas-L/Actin ratios after treatment were compared to 24-hour controls and are presented in panels B and C, respectively.
Semiquantitative RT-PCR analysis of B-CLL transcripts.
(A) Analysis of actin (A), Fas (F), or Fas-L (FL) transcripts in B-CLL cells at day 0 (D0) or after 24-hour incubation with medium alone (control), 165 μM chlorambucil (Chl), 17.5 μM fludarabine (Flu), or 24 hours after irradiation (10 Gy). (B, C) Intensities of Fas, Fas-L, and actin PCR bands from multiple B-CLL samples were quantified. Fas/Actin and Fas-L/Actin ratios after treatment were compared to 24-hour controls and are presented in panels B and C, respectively.
Flow cytometric and Western blot analyses of Fas and Fas-L
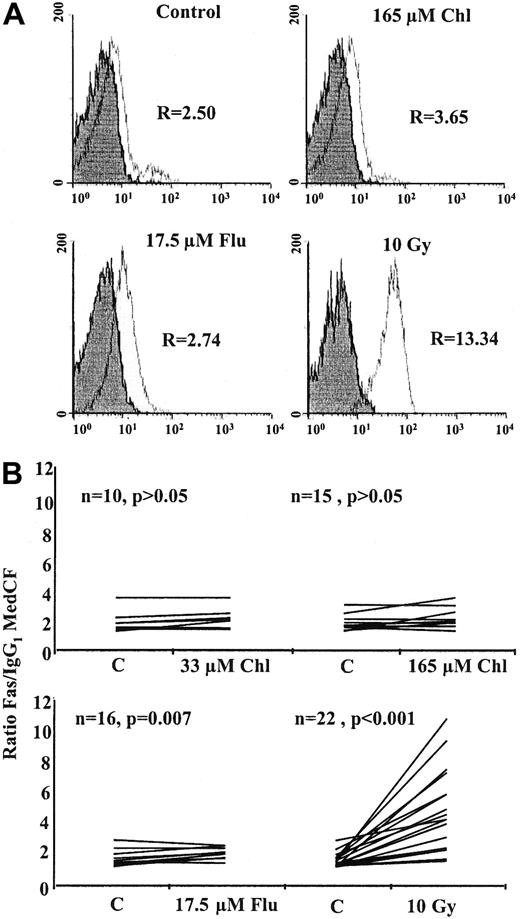
A representative histogram analysis of cell-surface Fas is shown in Figure 2A. Twenty-four–hour treatment with 165 μM chlorambucil or 17.5 μM fludarabine resulted in only marginal increases in Fas expression compared to the control. However, cells exposed to 10 Gy γ radiation showed enhanced expression of Fas. FACScan analysis of multiple B-CLL samples showed that cell-surface Fas expression, expressed as the ratio Fas FITC MedCF/IgG FITC MedCF, increased 24 hours after γ irradiation by a mean of 2.7-fold (P < .001), with 16 of 22 B-CLL samples showing increases of 1.5-fold or more (Figure 2B). Fludarabine increased surface Fas at 24 hours by a mean of 1.14-fold. Although this increase was statistically significant (P = .007), none of the 16 samples showed an increase greater than 1.5-fold. No significant increase in Fas protein was observed at 24-hour treatment with 33 or 165 μM chlorambucil.
Flow cytometric analysis of B-CLL cells.
(A) Analysis of cell-surface Fas expression in a representative B-CLL sample. R is the ratio of the MedCF of anti-Fas IgG FITC antibody-stained cells (open histogram) against IgG FITC isotype control-stained cells (shaded histogram). (B) Analysis of multiple B-CLL samples after 24-hour incubation with chlorambucil or fludarabine or after γ irradiation.
Flow cytometric analysis of B-CLL cells.
(A) Analysis of cell-surface Fas expression in a representative B-CLL sample. R is the ratio of the MedCF of anti-Fas IgG FITC antibody-stained cells (open histogram) against IgG FITC isotype control-stained cells (shaded histogram). (B) Analysis of multiple B-CLL samples after 24-hour incubation with chlorambucil or fludarabine or after γ irradiation.
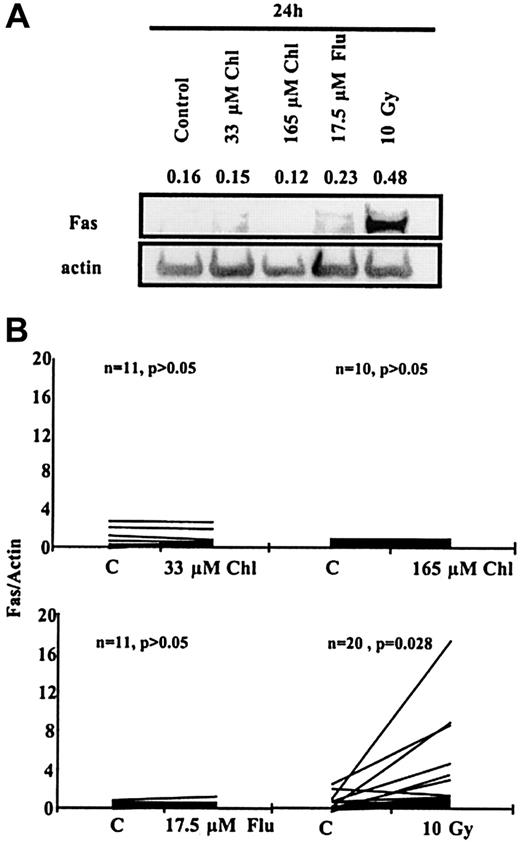
Western blot analysis of cell lysates from a representative B-CLL sample also showed the induction of Fas protein 24 hours after exposure to 10 Gy γ-radiation but not after treatment with fludarabine or chlorambucil (Figure 3A). Analysis of multiple samples showed a mean 5-fold increase in Fas expression (P = .028) after irradiation, with 15 of 20 samples showing increases greater than 1.5-fold (Figure 3B). In contrast, no significant increase in Fas expression was observed after 24-hour incubation with 33 μM chlorambucil, 165 μM chlorambucil, or 17.5 μM fludarabine (Figure 3B). Fas-L protein was not detected in any untreated or treated B-CLL sample by either Western blot or FACScan analysis (data not shown).
Western blot analysis of B-CLL cells.
(A) Fas protein expression in a B-CLL sample after 24-hour incubation with Chl or Flu or after γ irradiation. Numbers above bands are the intensities of individual bands normalized with respect to actin band intensities in the same lane. (B) Fas Western blot data for multiple B-CLL samples normalized with respect to actin.
Western blot analysis of B-CLL cells.
(A) Fas protein expression in a B-CLL sample after 24-hour incubation with Chl or Flu or after γ irradiation. Numbers above bands are the intensities of individual bands normalized with respect to actin band intensities in the same lane. (B) Fas Western blot data for multiple B-CLL samples normalized with respect to actin.
Fas ligation does not augment killing of B-CLL cells by cytotoxic agents
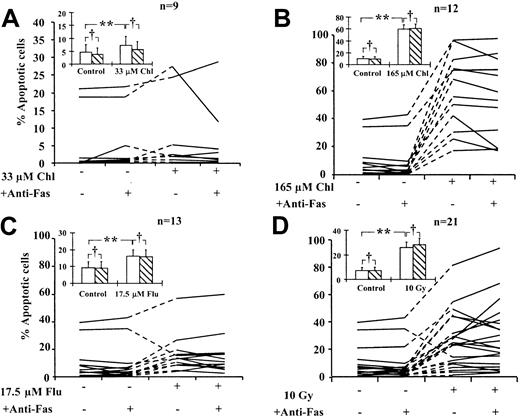
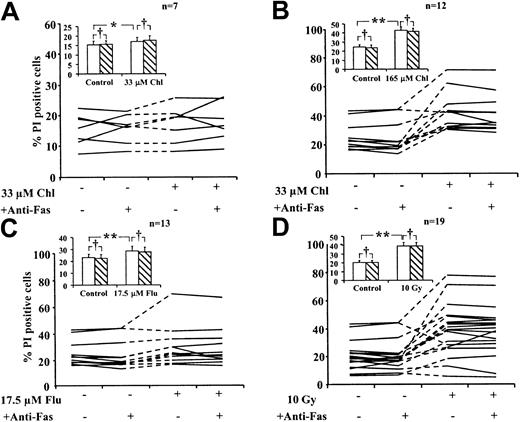
To determine whether treatment with cytotoxic drugs or exposure to γ radiation enhanced the sensitivity of B-CLL cells to Fas-induced killing, we incubated treated cells for 24 hours in the presence or absence of an agonistic anti-Fas IgM antibody. Apoptosis of B-CLL cells was quantified by morphologic criteria (Figure4). Spontaneous apoptosis at 24 hours ranged from less than 0.5% to 39%. Different isolates showed varying sensitivity to different cytotoxic agents. The increase over spontaneous apoptosis ranged from less than 0.5% to 8.6% (33 μM chlorambucil), 17% to 74% (165 μM chlorambucil), 1% to 26% (17.5 μM fludarabine), and 1.5% to 44% (10 Gy radiation). Apoptosis was increased by a mean of 1.5-fold (P = .031) after treatment with 33 μM chlorambucil, 6-fold (P < .001) with 165 μM chlorambucil, and 1.7-fold (P = .031) with 17.5 μM fludarabine. A 3.6-fold (P < .001) increase in mean apoptosis was observed in cells exposed to 10 Gy γ radiation. All these increases were statistically significant (Figure 4). The inclusion of anti-Fas IgM in the incubations did not result in significant additional increases in mean apoptosis under any of the incubation conditions (P > .05). However, inspection of data pertaining to individual isolates showed that incubation with anti-Fas IgM resulted in small increases in the percentage of apoptotic cells induced by fludarabine in 2 of 13 isolates and by γ radiation in 5 of 21 isolates (Figure 4C-D). We also observed that fludarabine treatment or γ irradiation of the cells from one patient resulted in a decrease in apoptosis (Figure 4C-D). This was consistently observed in 3 separate experiments and may reflect the ability of these agents to induce nonapoptotic death in the isolates from this patient.
Action of anti-Fas IgM on apoptosis induction in B-CLL cells.
B-CLL cells were treated with cytotoxic agents in the presence or absence of 50 ng/mL anti-Fas IgM as indicated. Percentage of apoptotic cells was determined by morphologic criteria. Lines connect data points obtained using individual B-CLL isolates. Standard errors of individual determinations were less than 10% of the mean values reported. Mean data for all isolates (inset). No anti-Fas IgM (open bars). Plus anti-Fas IgM (hatched bars). Bars represent standard errors of the means. Data were analyzed by Student t test. Percentages of apoptotic cells in drug-treated or irradiated samples were compared with those of corresponding controls (**P < .05). For each incubation condition, percentage apoptotic cells in the presence of anti-Fas were also compared with the corresponding percentages in the absence of anti-Fas (†P > .05).
Action of anti-Fas IgM on apoptosis induction in B-CLL cells.
B-CLL cells were treated with cytotoxic agents in the presence or absence of 50 ng/mL anti-Fas IgM as indicated. Percentage of apoptotic cells was determined by morphologic criteria. Lines connect data points obtained using individual B-CLL isolates. Standard errors of individual determinations were less than 10% of the mean values reported. Mean data for all isolates (inset). No anti-Fas IgM (open bars). Plus anti-Fas IgM (hatched bars). Bars represent standard errors of the means. Data were analyzed by Student t test. Percentages of apoptotic cells in drug-treated or irradiated samples were compared with those of corresponding controls (**P < .05). For each incubation condition, percentage apoptotic cells in the presence of anti-Fas were also compared with the corresponding percentages in the absence of anti-Fas (†P > .05).
Quantitation of overall spontaneous cell death by flow cytometric analysis of PI-stained cells showed that the percentage of dead cells after 24-hour incubation varied between 7.5% and 41% (Figure5). Additional killing by cytotoxic agents ranged between less than 0.5% and 4% (33 μM chlorambucil), 6% and 39% (165 μM) chlorambucil, 2% and 29% (17.5 μM fludarabine), and 2% and 37% (10 Gy radiation). No increases in mean cell killing were induced by coincubation with anti-Fas IgM under any of the treatment conditions. Examination of data obtained for the individual isolates also failed to reveal a synergistic cytotoxic action between anti-Fas IgM and cytotoxic drugs or radiation. These conclusions were verified by manual counting of trypan blue–stained cells (not shown).
Action of anti-Fas IgM on viability of B-CLL cells.
B-CLL cells were treated with cytotoxic agents in the presence or absence of 50 ng/mL anti-Fas IgM as indicated. Percentage of nonviable cells was determined by flow cytometric analysis of PI-stained cells. Mean data for all isolates (inset). Bars represent standard errors of the means. No anti-Fas IgM (open bars). Plus anti-Fas IgM (hatched bars). Data were analyzed by Student t test. Percentages of apoptotic cells in drug-treated or irradiated samples were compared with those of the corresponding controls (*P > .05; **P < .05). For each incubation condition, percentage nonviable cells in the presence of anti-Fas were also compared with the corresponding percentages in the absence of anti-Fas (†P > .05)
Action of anti-Fas IgM on viability of B-CLL cells.
B-CLL cells were treated with cytotoxic agents in the presence or absence of 50 ng/mL anti-Fas IgM as indicated. Percentage of nonviable cells was determined by flow cytometric analysis of PI-stained cells. Mean data for all isolates (inset). Bars represent standard errors of the means. No anti-Fas IgM (open bars). Plus anti-Fas IgM (hatched bars). Data were analyzed by Student t test. Percentages of apoptotic cells in drug-treated or irradiated samples were compared with those of the corresponding controls (*P > .05; **P < .05). For each incubation condition, percentage nonviable cells in the presence of anti-Fas were also compared with the corresponding percentages in the absence of anti-Fas (†P > .05)
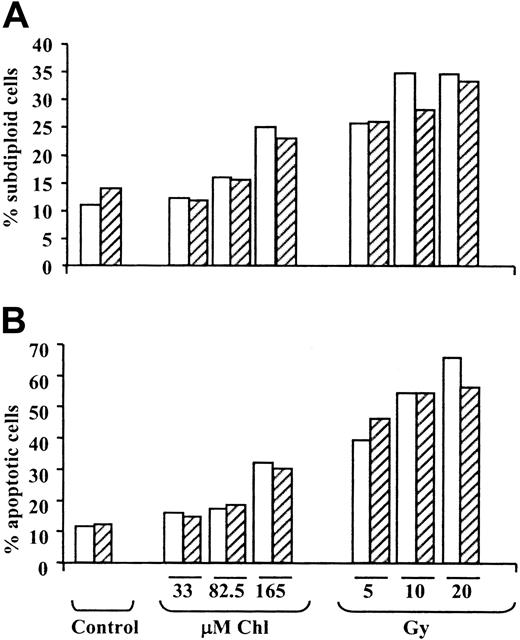
We also quantified apoptosis induction by determining the proportion of cells with subdiploid DNA content36 (Figure6A) and by assessment of light-scattering properties37 (Figure 6B). Both criteria clearly showed the dose-dependent induction of apoptosis by chlorambucil and γ radiation. Anti-Fas IgM did not substantially augment the induction of apoptosis at any of the concentrations of chlorambucil or doses of radiation tested. Similar results were obtained using 2 additional B-CLL isolates.
Quantitation of B-CLL apoptosis by flow cytometric analysis.
Percentage of apoptotic B-CLL cells was determined by flow cytometric quantitation of the proportion of cells with a subdiploid DNA content (A) or changes in light-scattering properties (B). Incubations were carried out in the absence (open bars) or presence (hatched bars) of 50 ng/mL anti-Fas IgM.
Quantitation of B-CLL apoptosis by flow cytometric analysis.
Percentage of apoptotic B-CLL cells was determined by flow cytometric quantitation of the proportion of cells with a subdiploid DNA content (A) or changes in light-scattering properties (B). Incubations were carried out in the absence (open bars) or presence (hatched bars) of 50 ng/mL anti-Fas IgM.
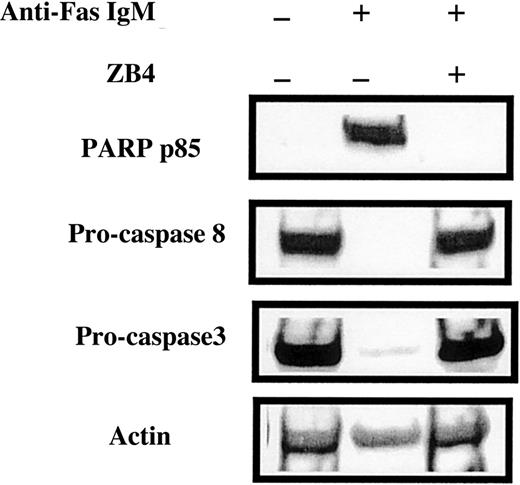
We used the Jurkat cell line, which is sensitive to Fas ligation, to verify that the anti-Fas IgM antibody induced apoptosis at the concentration routinely used in the experiments shown in Figures 4 to6. Western blot analysis showed that 24-hour incubation with 50 ng/mL anti-Fas IgM induced extensive processing of procaspases 3 and 8 and generation of the p85 PARP fragment (Figure7). The anti-p85 PARP antibody used here and in subsequent figures is highly specific for the neo-epitope generated after caspase 3 cleavage of PARP and, therefore, serves as a sensitive molecular criterion for caspase 3 activation.6All these anti-Fas IgM–induced apoptotic events were abrogated by the Fas-blocking antibody ZB4 (Figure 7). Anti-Fas IgM also increased the percentage of morphologically detectable apoptotic cells from 2% to 85% (not shown).
Effect of anti-Fas antibodies on caspase processing in Jurkat cells.
Jurkat cells were incubated with 50 ng/mL agonistic anti-Fas IgM or 250 ng/mL Fas-blocking antibody ZB4, as indicated above the lanes. After 24-hour incubation, cells were processed for Western blot analysis.
Effect of anti-Fas antibodies on caspase processing in Jurkat cells.
Jurkat cells were incubated with 50 ng/mL agonistic anti-Fas IgM or 250 ng/mL Fas-blocking antibody ZB4, as indicated above the lanes. After 24-hour incubation, cells were processed for Western blot analysis.
Processing of procaspases and PARP
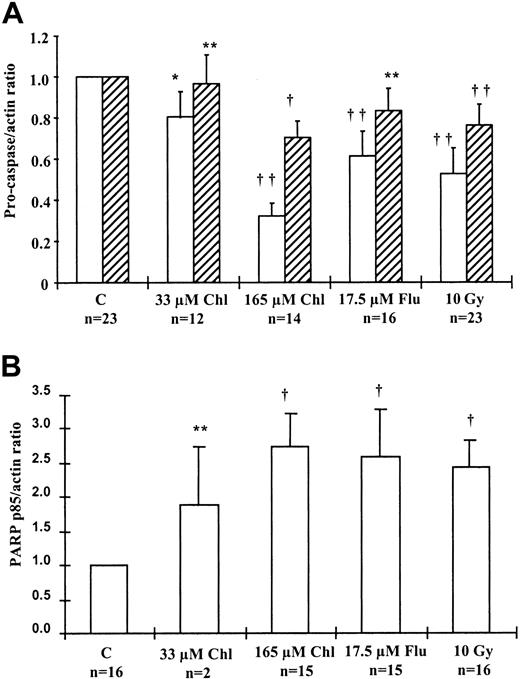
We analyzed the processing of procaspases 3 and 8 and the generation of the p85 PARP fragment after treatment of B-CLL cells with apoptosis-inducing agents. Data from studies on multiple isolates were normalized to procaspase or p85 PARP levels in the 24-hour control sample and are summarized in Figure 8. Mean procaspase 8 levels in B-CLL samples decreased by 20% (P = .051) after treatment with 33 μM chlorambucil, 62% (P = .003) with 165 μM chlorambucil, 39% (P = .002) with 17.5 μM fludarabine, and 45% (P < .001) after γ irradiation compared to the 24-hour control. Procaspase 3 levels also decreased by a mean of 3.5% (P > .05) after treatment with 33 μM chlorambucil, 30% (P = .016) with 165 μM chlorambucil, 19% (P > .05) with 17.5 μM fludarabine, and 23% (P = .009) after γ irradiation (Figure 8A). The p85 PARP fragment in B-CLL samples (Figure 8B) increased by a mean of 1.9-fold (P > .05) after treatment with 33 μM chlorambucil, 2.7-fold (P = .01) with 165 μM chlorambucil, 2.6 fold (P = .03) with 17.5 μM fludarabine, and 2.4-fold (P = .042) after γ irradiation compared to the 24-hour control.
Caspase processing in B-CLL cells.
(A) Processing of procaspase 8 (open bars) and procaspase 3 (hatched bars) in B-CLL samples after 24-hour treatment, estimated by Western blotting. Procaspase band intensities were normalized to actin, and the ratio was set to 1.0 for the control for each sample. (B) Processing of PARP to its p85 fragment in B-CLL samples after 24-hour treatment. p85 PARP was compared with actin levels, and the ratio was set to 1.0 for the control in each sample. Student t test was carried out using procaspase-actin or p85 PARP–actin ratios (*P = .05; **P > .05; †.01 <P < .05; ††P < .01).
Caspase processing in B-CLL cells.
(A) Processing of procaspase 8 (open bars) and procaspase 3 (hatched bars) in B-CLL samples after 24-hour treatment, estimated by Western blotting. Procaspase band intensities were normalized to actin, and the ratio was set to 1.0 for the control for each sample. (B) Processing of PARP to its p85 fragment in B-CLL samples after 24-hour treatment. p85 PARP was compared with actin levels, and the ratio was set to 1.0 for the control in each sample. Student t test was carried out using procaspase-actin or p85 PARP–actin ratios (*P = .05; **P > .05; †.01 <P < .05; ††P < .01).
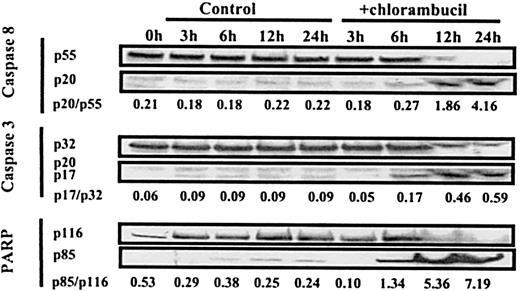
The time-course study of caspase activation and PARP cleavage shown in Figure 9 confirms that loss of immunoreactive procaspase 3 and 8 is accompanied by the generation of active subunits. After treatment with 165 μM chlorambucil, an increase in generation of the subunits of caspases 3 and 8 and of the p85 PARP fragment was first evident at 6 hours but was more pronounced by 12 hours. In this experiment, we observed the complete processing of procaspase 8 by 24 hours, whereas residual levels of procaspase 3 were still present at this time. We have consistently observed that a greater proportion of procaspase 8 than of procaspase 3 was cleaved in response to each of the cytotoxic stimuli studied here (Figure 8A).
Time-course of processing of procaspases and PARP in a B-CLL isolate.
B-CLL cells were incubated with no additions or with 165 μM chlorambucil. Samples were processed for Western blot analysis at the times indicated. Procaspase 8 was detected using the Pharmingen antibody. The processed form of caspase 8 was detected using goat polyclonal anti-caspase 8 p20 antibody (sc-6136).
Time-course of processing of procaspases and PARP in a B-CLL isolate.
B-CLL cells were incubated with no additions or with 165 μM chlorambucil. Samples were processed for Western blot analysis at the times indicated. Procaspase 8 was detected using the Pharmingen antibody. The processed form of caspase 8 was detected using goat polyclonal anti-caspase 8 p20 antibody (sc-6136).
Fas-blocking antibody ZB4 does not abrogate apoptosis of B-CLL cells
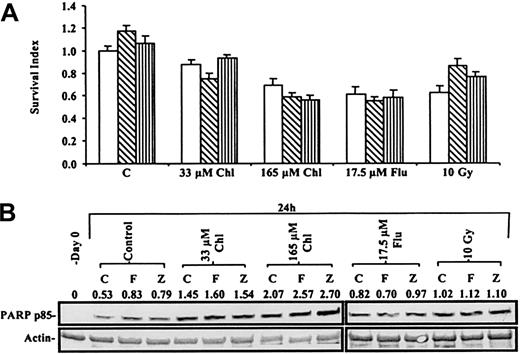
Augmented interaction of pre-existing cell-surface Fas and Fas-L may contribute to the induction of apoptosis in some cellular contexts.31 Although we failed to detect expression of Fas-L on B-CLL cells by either Western blot or flow cytometric analysis, we wanted to eliminate the possibility that interaction of Fas with undetectable levels of Fas-L might have played a role in the killing of B-CLL cells by cytotoxic agents. Therefore, we used the ZB4 monoclonal antibody, whose ability to completely eliminate anti-Fas IgM–induced PARP cleavage in Jurkat cells is documented in Figure 7. Figure 10A shows that the viability of a B-CLL isolate, quantified by the MTT dye reduction assay, was decreased by chlorambucil, fludarabine, or γ radiation. Neither the agonistic anti-Fas IgM nor the ZB4 blocking antibody significantly altered the cytotoxic action of any of the agents.
Actions of anti-Fas antibodies on B-CLL killing.
(A) MTT assay of a B-CLL sample incubated with no antibody (open bars), 50 ng/mL anti-Fas IgM (hatched bars), or 250 ng/mL ZB4 (striped bars) in addition to cytotoxic agents. The absorbance of MTT reduced by the 24-hour control was set at 1, and the viability of treated cells was compared to this value. (B) Western blot of the processing of PARP to p85 PARP in B-CLL cells from same patient after treatment with Chl, Flu, or γ irradiation with or without 50 ng/mL anti-Fas IgM (F) or 250 ng/mL ZB4 (Z).
Actions of anti-Fas antibodies on B-CLL killing.
(A) MTT assay of a B-CLL sample incubated with no antibody (open bars), 50 ng/mL anti-Fas IgM (hatched bars), or 250 ng/mL ZB4 (striped bars) in addition to cytotoxic agents. The absorbance of MTT reduced by the 24-hour control was set at 1, and the viability of treated cells was compared to this value. (B) Western blot of the processing of PARP to p85 PARP in B-CLL cells from same patient after treatment with Chl, Flu, or γ irradiation with or without 50 ng/mL anti-Fas IgM (F) or 250 ng/mL ZB4 (Z).
The inability of the anti-Fas IgM to augment generation of the p85 PARP fragment by any of the agents used is shown in Figure 10B. This figure also confirms that the ZB4 blocking antibody did not protect B-CLL cells from apoptosis induction by cytotoxic drugs or by radiation. The observations shown in Figure 10 were repeated in 2 additional experiments.
Discussion
The potential role of Fas signaling in cytotoxic killing of cancer cells remains controversial. Most studies on the role of the Fas pathway in mediating apoptosis induction by cytotoxic drugs have been carried out using leukemic and solid tumor cell lines. We addressed this issue by studying the potential roles of Fas and Fas-L in the killing of freshly isolated B-CLL cells by cytotoxic drugs and γ radiation. Treatment of B-CLL cells with chlorambucil, fludarabine, or radiation resulted in augmented expression of Fas transcripts. A substantial increase in cell-surface Fas expression, however, was only observed after irradiation. This observation suggests that Fas expression may be regulated by a complex mechanism that permits translation of Fas transcripts after irradiation, but not in cells treated with chlorambucil or fludarabine. We were unable to detect Fas-L protein expression in untreated B-CLL cells or after treatment with drugs or radiation.
We used morphologic criteria, PI and trypan blue exclusion, quantitation of cells with subdiploid DNA content, FACScan analysis of light-scattering, the MTT test, and generation of the p85 PARP fragment to assess the putative roles of Fas and Fas-L in the killing of B-CLL cells by cytotoxic agents. Analysis of data obtained using multiple B-CLL isolates showed that cell killing by chlorambucil, fludarabine, or γ radiation was not significantly augmented by an agonistic anti-Fas antibody. Inspection of individual experiments suggested that Fas ligation resulted in the augmentation of morphologically detectable apoptosis in a minority of samples treated with fludarabine or γ radiation. These effects were modest and were not evident when other cell death criteria were used. Killing by each of the agents was also refractory to inhibition by the ZB4 Fas-blocking antibody, ruling out the possibility that interactions between Fas and pre-existing Fas-L may contribute to the killing mechanism. Taken together, our data show that signaling by the Fas/Fas-L system does not play a major role in the apoptotic killing of B-CLL cells by cytotoxic agents.
Cytotoxic drugs induce apoptosis by triggering cytochrome-crelease with the consequent APAF-1–dependent activation of caspase 9.3,5 Western blot experiments have shown that procaspase 9 processing was only observed when B-CLL cells were treated with high-dose chlorambucil (D.T.J., unpublished observations, 2000). Activation of procaspase 9 after binding to APAF-1 does not necessitate its processing.39 Hence, the role of caspase 9 in killing of B-CLL cells by cytotoxic treatments other than high-dose chlorambucil is unclear at present.
Here we observed that procaspase 8 processing accompanied the activation of procaspase 3 in response to all the death stimuli studied here. In contrast to an earlier report,40 we found that a substantial proportion of the procaspase 8 of B-CLL cells was processed in response to cytotoxic treatments, suggestive of an important role in apoptosis induction. In some cellular contexts, cytotoxic drugs can activate caspase 8 in a Fas-independent manner.41-43Because neither an agonistic nor a blocking anti-Fas antibody significantly affected apoptosis induction or procaspase 8 processing (data not shown), we conclude that the cytotoxic agents used here also activated caspase 8 by a mechanism independent of Fas signaling. The death receptors DR4 and DR5 also activate caspase 8 as a consequence of binding their cognate ligand, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL).4 We observed that B-CLL cells were completely refractory to killing by TRAIL, either when added alone or in conjunction with chlorambucil, fludarabine, or γ radiation (D.T.J., unpublished observations, 2000).
Although APAF-1 itself does not activate caspase 8,44procaspase 8 may be processed by a purely intracellular mechanism analogous to the activation of caspase 9 within the apoptosome. For example, recent studies on the Jurkat45 and NCI-H46046 lung cancer cell lines have shown that caspase 8 activation in response to cytotoxic drugs is mediated by mitochondria rather than by death receptors. In Jurkat and MCF-7 cells, caspase 8 may function as a terminal executioner caspase rather than as an initiator of caspase-processing pathways.47 The similarity in the time courses of activation of caspases 3 and 8 in chlorambucil-treated B-CLL cells is consistent with the possibility that both proteases function as executioners in the apoptotic pathway. The low specificity of peptide-aldehyde caspase 8 inhibitors and the refractory nature of B-CLL cells to transfection with dominant-negative inhibitory constructs or with the selective caspase 8 inhibitor crmA have precluded a definitive assessment of the mechanism of caspase 8 activation and of the relation between the activation of caspases 3 and 8 in B-CLL cells.
Sensitivity of B-CLL cells to apoptosis induction consequent to Fas ligation remains controversial. Some reports suggest that these cells are refractory to Fas killing,32,48 whereas others suggest that they are sensitive.49,50 Our data here suggest that B-CLL cells, even when induced to express substantial levels of cell-surface Fas by γ radiation, cannot activate the apoptotic pathway in response to Fas ligation. The reasons for this defect are unclear. B-CLL cells express functional procaspase 8 and substantial levels of FADD (data not shown), the signaling components required for downstream signaling by Fas.4 The existence of inactivating mutations in the Fas molecule have also been ruled out.32 c-FLIP, an inhibitor of death signaling by Fas,51 is expressed in B-CLL cells. However, the ratio of Fas to c-FLIP expression in Fas-sensitive Jurkat cells is similar to that in B-CLL cells (not shown), suggesting that c-FLIP expression may not account for Fas-resistance. Recent studies have suggested that molecules other than c-FLIP may block Fas signaling.52 It is plausible that the resistance of B-CLL cells to killing through death receptor ligation may result from elevated expression of an unidentified inhibitor. Elucidation of the basis of Fas resistance in B-CLL may be of importance because this may contribute to the expansion of malignant cells in this disease.
In conclusion, the observations here argue against a major role for the Fas–Fas-L signaling pathway in drug- or γ irradiation–induced apoptosis in B-CLL cells. Nevertheless, our data suggest that both caspase 3 and caspase 8 play important roles in the killing of these cells by these agents. The caspase 8 and caspase 9 pathways may overlap or synergize in securing the apoptotic demise of leukemia cells. Elucidation of these mechanisms in primary malignant cells may be of importance in developing novel and effective strategies for cancer therapy.
Supported by the Leukemia Research Fund, United Kingdom; the Trustees of the Royal Free Hospital; and a bequest from William Price.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. Gitendra Wickremasinghe, Department of Hematology, Royal Free and University College Medical School, Royal Free Campus, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail:r.wickremasinghe@rfc.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal