High-dose melphalan with autologous blood stem cell transplantation (SCT) can reverse the disease process in selected patients with primary systemic amyloidosis (AL); however, SCT for AL remains controversial because of the treatment-related mortality in patients with cardiac and multisystem organ involvement. In this review, we briefly discuss recent advances in AL, such as the free light-chain assay and the role of immunoglobulin light-chain variable region germline genes in the disease, and then we discuss the current status of SCT for AL with emphases on patient selection, approaches to stem cell mobilization, and peri-SCT management. It is clear that patients with AL who have advanced amyloid cardiomyopathy or more than 2 major viscera involved with disease are poor candidates for SCT. Therefore, the importance of patient selection cannot be overemphasized, and patients with 1 or 2 involved organs or with early cardiac involvement are usually appropriate candidates for SCT. Because the toxicity of melphalan is dose-related and survival with AL may be age-related, patient age and the extent of organ involvement can provide a basis for patient stratification. We discuss such a risk-adapted approach to melphalan dosing in detail and conclude with a brief overview of current research using SCT to treat patients with AL.
Introduction
Primary systemic amyloidosis (AL) is a protein conformation disorder and a clonal plasma cell dyscrasia.1Systemic disease results from amorphous extracellular deposits of material, composed in part of immunoglobulin light- or heavy-chain fragments, in key viscera such as the kidneys, heart, and liver and in the peripheral nervous system.2 Various presentations are observed at diagnosis, the most common of which is nephrotic-range proteinuria with or without renal insufficiency, congestive cardiomyopathy, unexplained hepatomegaly, and sensorimotor and autonomic peripheral neuropathy.2,3 As systemic deposits of amyloid accumulate, they disrupt organ function and ultimately lead to the death of the patient.3,4 The disorder has an incidence of 8 per million persons per year, and it is one fifth as common as multiple myeloma but more devastating because the median survival of patients seen within 1 month of diagnosis is 13.2 months.5 Moreover, for those with congestive heart failure the median survival is 4 months, and less than 5% of all AL patients survive 10 years or more from the time of diagnosis.6 7
Limited progress had been made in reversing the pathology of AL until the mid-1990s, when patients underwent dose-intensive intravenous melphalan therapy and autologous hematopoietic stem cell transplantation (SCT).8-10 As the production of deposits is halted and amyloid resorbed, the performance status and the quality of life of AL patients can improve.11-13 However, transplantation-related mortality was high in the early studies because the viscera of AL patients were compromised by deposits of amyloid. AL patients commonly have renal, cardiac, hepatic, gastrointestinal, or neuropathic problems that make them distinct from other SCT candidates.11,12 That is, most patients who undergo autologous transplantation have hematologic malignancies but no visceral organ dysfunction, whereas most patients with AL who undergo SCT have precisely the opposite findings. Therefore, refinement of patient selection and improvement of peri-transplantation clinical management have become priorities.12 14 In this review we describe relevant recent advances in AL and summarize the data on SCT for AL to improve current practice and to stimulate further clinical research. We also suggest a risk-adapted approach to patient selection and melphalan dosing with the expectation of reducing transplantation-related mortality, and we offer it as a basis for future research efforts.
Recent advances in primary systemic amyloidosis
AL is not a neoplasm per se. The monoclonal protein does not increase over time, as it often does in multiple myeloma, and the percentage of bone marrow plasma cells is comparatively low—60% of AL patients have 10% or fewer clonal marrow plasma cells, a percentage that remains stable over time.1 Because of extravascular monoclonal protein deposition, measurement of the monoclonal protein produced by these plasma cells is complex. Immunofixation of serum and urine specimens and the newly available quantitative light-chain assay are the most useful methods for measuring the low-level monoclonal proteins in AL.2,15 The enhanced sensitivity of the free light-chain assay is an important step forward in AL. One tenth of AL patients lack monoclonal proteins by immunofixation and, therefore, are difficult to distinguish from patients with nonimmunoglobulin forms of amyloidosis, such as familial (AF) or secondary (AA). The reliable detection of free light chains clearly allows such patients to be identified rapidly.16 Moreover, following SCT, it is important to ensure that free light-chain production has been eliminated. The ability to quantitate small amounts of monoclonal light chain is of use in monitoring the response of patients after SCT because it is likely that near complete elimination of precursor protein production is required for a response to occur. If light-chain production fails to completely stop, the patient is a candidate for investigational treatment or possibly second transplantation.
Recent diagnostic advances have also included the development of specific antisera that can identify the type of amyloid deposited in tissues and the development of new methods for sequencing amyloid proteins.17,18 Given the limited amount of sample available from diagnostic needle biopsies, it is often difficult to isolate and purify amyloid for direct sequencing. A new micro-method to isolate and purify the amyloid fibrils exists.18 The technique involves extraction of the amyloid with purification by polyacrylamide gel electrophoresis followed by electroblotting and elution of amyloid protein-related bands and then reverse-phase high-performance liquid chromatography. It is possible with this technique to obtain sufficient material to establish the chemical and molecular composition of fibrillar deposits.18
With respect to the immunoglobulin genetics that underlie AL, clonal plasma cells are more frequently λ than κ (3:1), and the immunoglobulin light-chain variable region genes expressed by AL clones include several that are less frequently expressed in the normal repertoire, indicating that germline-encoded features may contribute to the propensity of certain subtypes of light chains to form amyloid.19-21 In addition, associations between immunoglobulin light-chain variable region germline gene use and the pattern of amyloid-related organ involvement have recently been described.20-22 Patients with clones derived from the6a Vλ germline gene were significantly more likely to have dominant renal involvement, whereas those with clones derived from the 1c, 2a2, and 3r Vλ genes were more likely to have dominant cardiac and multisystem disease.21 These associations have been confirmed in several laboratories, indicating that light-chain variable region germline gene usage may determine a bias for amyloid deposition in specific organs and that this tropism may be related to the antigenic affinities of clonal light chains.23
Patient selection for stem cell transplantation
All patients with AL carry a poor prognosis. Therefore, all patients who have a systemic clinical amyloidosis syndrome (with the exceptions of purpura, focal soft tissue amyloid, or carpal tunnel syndrome only) should be considered candidates for SCT. Patients who have incidental amyloid deposits detected in a bone marrow performed for multiple myeloma should not be considered as having a systemic amyloid syndrome unless they have symptoms referable to viscera involved with amyloid. Because the monoclonal protein in AL does not increase over time as it does in multiple myeloma, it is not appropriate to observe patients for a change in the level of the M protein before considering SCT.24 Moreover, the distinction between AL with and without myeloma is usually based on a cut off of bone marrow plasma cells. Nearly 40% of patients with AL have more than 10% plasma cells in their bone marrow. However, if these patients are monitored over time, few if any will go on to acquire lytic bone disease, light-chain nephropathy, or soft tissue plasmacytomas.24 It is appropriate to treat AL patients with a high percentage of bone marrow plasma cells (eg, more than 20%) the same as patients with AL and a lower percentage of plasma cells in the bone marrow. There are no data to support a benefit from cytoreduction before SCT. Indeed, evidence from a recently reported randomized prospective clinical trial indicates that the delay associated with pre-SCT cytoreduction is likely to allow disease progression.25 Therefore, we usually elect to go directly to SCT in AL patients.
The toxicities of SCT for AL have been appreciated at all centers that have attempted to treat AL patients with SCT. The average 100-day mortality of SCT in 4 single-center clinical trials was 21%, and in 2 multicenter trials it was 39%, which is unacceptably high and requires attention (Table 1).10,26-30In addition, deaths have been reported during stem cell mobilization (with growth factor alone) and during stem cell component infusion, highlighting AL SCT patients as unusually prone to adverse events.10,28,29 Traditional selection criteria for autologous transplantation were designed to exclude patients with significant visceral dysfunction to minimize the morbidity and mortality of SCT and to allow standardized patient screening to conduct clinical research.31 Nevertheless, in most clinical trials in Table 1, standard criteria for autologous SCT were used to determine eligibility for transplantation. (One notable exception is the intermediate-dose melphalan trial reported in Comenzo et al28). Despite the use of such screening criteria, the transplantation-related mortality of patients with AL was 4 to 8 times higher than that currently reported for patients with multiple myeloma (which in our centers is much less than 5%).
Stem cell transplantation results in AL amyloidosis
| Reference . | Patients enrolled . | Deaths at SC collection . | Treated . | Deaths at SC infusion . | TRM . | Evaluable . | Responders . |
|---|---|---|---|---|---|---|---|
| Single-center clinical trials | |||||||
| 10 | 25 | 1 | 23 | 0 | 3 | 21 | 12 |
| 27 | 23 | 0 | 20 | 0 | 4 | 15 | 12 |
| 28 | 30 | 1 | 28 | 1 | 5 | 15 | 7 |
| 29 | 9 | 3 | 6 | 2 | 4 | 2 | 2 |
| Total | 87 | 5 | 77 | 3 | 16 (21%) | 53 | 33 (62%) |
| Multicenter clinical trials | |||||||
| 26 | 40 | 0 | 40 | 0 | 15 | 22 | 11 |
| 30 | 21 | 0 | 21 | 0 | 9 | 12 | 10 |
| Total | 61 | 0 | 61 | 0 | 24 (39%) | 34 | 21 (62%) |
| Reference . | Patients enrolled . | Deaths at SC collection . | Treated . | Deaths at SC infusion . | TRM . | Evaluable . | Responders . |
|---|---|---|---|---|---|---|---|
| Single-center clinical trials | |||||||
| 10 | 25 | 1 | 23 | 0 | 3 | 21 | 12 |
| 27 | 23 | 0 | 20 | 0 | 4 | 15 | 12 |
| 28 | 30 | 1 | 28 | 1 | 5 | 15 | 7 |
| 29 | 9 | 3 | 6 | 2 | 4 | 2 | 2 |
| Total | 87 | 5 | 77 | 3 | 16 (21%) | 53 | 33 (62%) |
| Multicenter clinical trials | |||||||
| 26 | 40 | 0 | 40 | 0 | 15 | 22 | 11 |
| 30 | 21 | 0 | 21 | 0 | 9 | 12 | 10 |
| Total | 61 | 0 | 61 | 0 | 24 (39%) | 34 | 21 (62%) |
TRM indicates transplantation-related mortality.
The extent of amyloid organ involvement before SCT clearly accounts for much transplantation-related mortality, as demonstrated by 2 early trials that used similar patient assessment and selection criteria and similar transplantation conditioning regimens.10,27 Of 43 patients who underwent transplantation, those with 2 or fewer organ systems involved had significantly superior 100-day survival rates (81%; 25 of 31) compared with those who had more than 2 systems involved (33%; 4 of 12; P < .01, Fisher exact test). Similar outcomes have been reported in several multicenter studies.26,30 Causes of death included cardiac arrhythmias, intractable hypotension, multiorgan failure, and gastrointestinal bleeding. Gastrointestinal bleeding, particularly unusual after autologous transplantation for hematologic malignancies, is frequently seen in patients with AL.10,26,30,32 33
In 66 patients who underwent transplantation at the Mayo Clinic for AL, the treatment-related mortality observed was 14%.34 In a multivariate analysis of survival, the 2 key predictors were the serum creatinine level at the time of transplantation and the number of visceral organs involved. The 30-month actuarial survival of patients transplanted is 72%; however, if more than 2 organs are involved at the time of transplantation, the actuarial survival is less than 20%. Not only does serum creatinine predict for an adverse survival with typical high-dose chemotherapy (ie, 140 mg/m2 melphalan and total body irradiation, or 200 mg/m2 melphalan), it also predicts for the development of renal failure during the transplantation procedure itself. The median creatinine level for 9 patients who required dialysis during transplantation was 1.7 mg/dL. Seven of the 9 patients subsequently died. Patients whose serum creatinine levels exceed 1.5 mg/dL or whose creatinine clearance is less than 51 mL/min are candidates for reduced-dose therapy in the risk-adapted model we propose (intermediate-risk patients).34
The presence of cardiac amyloid also contributes significantly to peri-transplantation mortality.29 Noninvasive criteria used to evaluate patients for cardiac involvement with AL may underestimate the incidence of cardiac deposition disease.35,36 In our experience, the peri-transplantation mortality rate in patients with cardiac amyloid and clinical congestive heart failure or a history of arrhythmias, syncope, or recurrent pleural effusions approaches 100%. In the British multicenter series, 3 of 7 cardiac patients underwent heart transplantation before SCT and survived SCT without complication.26 This therapeutic approach remains in search of a North American clinical trial.37 In a retrospective overview of 6 years' experience with SCT for AL, Sanchorawala et al10,28,38,39at Boston Medical Center aggregate the outcomes of a series of clinical trials using a range of melphalan doses. They do not evaluate post-SCT survival as a function of number of major viscera involved and, though they allude to the use of a range of melphalan doses, they do not describe their approach to melphalan dosing in detail. Nevertheless, they succinctly describe the association between dominant cardiac amyloid, peri-transplantation mortality, and reduced overall survival. Seventy percent of AL patients who experienced early mortality had amyloid cardiomyopathy; the median survival of cardiac patients was 2 years, whereas that of patients with other dominant organ involvement was more than 4 years. Of the 205 patients who began SCT during the 6-year period, 115 (56%) survived for at least 1 year after SCT, nearly half of whom had complete hematologic responses (undetectability of M proteins by immunofixation and absence of clonal plasma cells).39 Furthermore, it is clear from their data and analysis, and from that of others, that no other modality of therapy is as effective in achieving complete hematologic responses and reversal of amyloid-related organ dysfunction in two thirds of surviving patients (Table 1).10,14,26,27,30,39 Indeed, amyloid P component radionuclide scans have demonstrated resorption of AL deposits subsequent to the reduction or elimination of the clonal plasma cell disorder that is their root cause.26
Given the strict selection criteria for SCT, however, it is reasonable to think that the survival of a group of SCT-eligible patients with AL would exceed the 13 months reported for all AL patients. To address this issue, the amyloid database of the Mayo Clinic was queried by Gertz et al11 for patients who would in theory be eligible for SCT. Selection criteria included symptomatic disease, absence of multiple myeloma, age younger than 70 years, ventricular septal thickness less than 15 mm, cardiac ejection fraction greater than 55%, creatinine concentration less than 2 mg/dL, alkaline phosphatase value less than 3 times normal, and direct bilirubin value less than 2 mg/dL. Of the 1288 patients seen from 1983 to 1997, 234 (18%) met these criteria—131 men and 103 women with a median age of 57 years and a median follow-up time of 44.5 months. Nephrotic-range proteinuria and cardiac, hepatic, and nervous system involvement were present in 121 (52%), 98 (42%), 13 (5.5%), and 36 (16%) of them, respectively. Patients older than 60 years had a median survival of 30 months, whereas those younger than 50 years lived twice as long. The entire cohort had a median survival of 46 months. Thus, because it appears that patients eligible for SCT may be an inherently good risk population, it is likely important to stratify patients receiving SCT for risk factors known to impact on survival in this disease.
With an eye on such risk-adapted stratification, it is useful to compare this archival analysis with the results of a phase 2 trial in which 30 patients with AL (17 men and 13 women; median age, 62 years; range, 43-71 years) who were ineligible by standard criteria for SCT were treated with an intermediate dose of intravenous melphalan and stem cells.28 Although the incidence of significant morbidity with stem cell mobilization and collection was 17% and the peri-transplantation mortality rate was 20%, 17% of patients achieved complete hematologic responses and 40% of patients had stabilized or improved amyloid-related organ involvement, including 3 of 9 symptomatic cardiac patients who lived for more than 2 years after SCT. Median survival had not been reached at a median follow-up of 2 years. The lesson to be drawn from the comparison of this trial with the Mayo Clinic archival data is that though increasing age may be associated with shorter survival in good risk patients, a risk-adapted approach to the treatment of AL patients with intravenous melphalan might usefully accommodate the biology of the disease and improve tolerance to therapy. This could allow a much greater number of patients to receive SCT as the primary management of AL, a point borne out to some degree by the aggregate data from Boston Medical Center.39
Blood stem cell mobilization and collection
Given the impaired visceral reserve, vasculopathy, and coagulopathies associated with AL, it was predictable that regimen-related toxicity would be more severe in patients with AL who underwent SCT. It was not expected, however, that there would be significant toxicity associated with stem cell mobilization and collection.10 Deaths have been reported during the mobilization of patients with symptomatic cardiac amyloid or multisystem disease, at centers using moderate doses of cyclophosphamide (eg, 2.5 g/m2) or hematopoietic growth factors alone.10,29 During stem cell mobilization with granulocyte–colony-stimulating factor (G-CSF; 16 μg/kg per day for 5 days), we and others on rare occasions observed a sometimes fatal though unexplained syndrome associated with progressive hypoxia and hypotension unresponsive to supportive measures; it can occur in patients without cardiac involvement and may be caused by a combination of the effects of G-CSF, activated platelets returned during leukapheresis, pulmonary shunting, cytokines, or mediators of septic hemodynamics such as HMG-1.41 42
To minimize the risk for such toxicities, we recommend that G-CSF dosing for mobilization be given twice a day in lower doses (eg, 6 μg/kg every 12 hours) with collection beginning on the fifth day 2 to 4 hours after the morning dose of G-CSF.43 During mobilization and leukapheresis, patients with severe nephrotic syndrome who have hypoalbuminemia and are salt avid may become edematous and require diuresis and albumin infusions, whereas patients with renal and cardiac involvement may rarely experience complications such as rapidly accumulating pleural effusions and flash pulmonary edema. In addition, to minimize hypocalcemia and citrate toxicity in neuropathic and cardiac patients, it may help to use heparin anticoagulation during leukapheresis. The complication rate is approximately 15% in AL patients during mobilization and leukapheresis, and collections may have to be interrupted because of worsening edema or hypoxia.9 28
In the early SCT trials, the ability to mobilize CD34+ cells in patients who had previously received more than 200 mg oral melphalan was significantly lower than in patients who had previously received less or no melphalan.10,27 There were no significant differences with respect to the number of CD34+ cells collected on individual days or in total in patients receiving G-CSF alone for mobilization.10 In patients randomized to receive G-CSF (10 μg/kg per day) or serial GM-CSF then G-CSF, similar numbers of CD34+ cells were collected in both groups.28 In all these trials, two thirds of patients had amyloid identified in the bone marrow, and AL deposits did not obviously impair stem cell mobilization. Currently, G-CSF mobilization can be considered the standard approach in this population. Because of the clinical advantages associated with prompt myeloid and thrombopoietic recovery, we recommend that the optimal dose of CD34+ cells in AL patients who undergo SCT be at least 5 × 106 CD34+ cells/kg.
In all reported trials, complete engraftment following infusion of unmanipulated stem cell components was typical for the CD34 doses used, with median neutrophil and platelet recoveries occurring within 10 and 13 days, respectively, demonstrating that AL deposits did not interfere with engraftment. Contamination with clonotypic immunoglobulin-positive plasma cells has been demonstrated in the collected apheresis products from patients with amyloidosis undergoing leukapheresis after growth factor priming.38,40 CD34-selected cells were used in a clinical trial in which selection was performed from G-CSF–mobilized blood stem cells.38,44 Using the Isolex device (Nexell, Irvine, CA), median yield and purity were 42% and 85%, respectively, and median CD34+ cell dose per kilogram was 4.1 × 106. Four of 15 patients (27%) did not achieve the required dose of CD34-selected cells per kilogram (more than 2 × 106) following 2 column purification procedures; these patients (median age, 62 years; range, 56-70 years) required either additional stem cell collections or a bone marrow harvest to support high-dose therapy. Of note, in patients receiving CD34-selected stem cells, myeloid recovery was as rapid as that seen with unselected cells, but lymphoid recovery was significantly delayed and opportunistic infections were observed in several patients.38,44 In a phase 3 trial, CD34 selection, though capable of reducing clonotypic cells in the apheresis product, has not resulted in improved disease-free or overall survival in multiple myeloma. It is therefore unlikely to provide benefit in patients with amyloidosis.45
Peri-transplantation management
The frequency and grade of regimen-related toxicities are to some degree a function of the dose of intravenous melphalan, as indicated by a comparison of toxicities from cohorts treated at either 200 or 100 mg/m2 (Table 2). Of particular note, the gastrointestinal toxicity with 200 mg/m2 melphalan is striking, as are the higher rates of edema and bleeding. Gastrointestinal bleeding has been a significant cause of early mortality with SCT. Involvement of the gastrointestinal tract with AL may be focal or diffuse.8 Macroglossia occurs in approximately 10% of patients and can be massive, producing an inability to breathe, eat, or drink normally. Achalasia, hematemesis, gastroparesis, and pseudo-obstruction are among the many other manifestations of gastrointestinal amyloid. If amyloid extensively infiltrates the submucosa of the stomach or lower intestinal tract, the potential for severe mucositis with hemorrhage must be anticipated, whereas neuropathic compromise of the enteric plexus often results in atony, persistent posttransplantation nausea, and need for prolonged nutritional support. The potential for airway compromise exists in patients with macroglossia and dysphagia, particularly when mucositis develops and the risk for thrombocytopenic bleeding exists.
Toxicities (grade 2 or higher)
| Toxicity . | 200 mg/m2 (n = 23) Frequency, % (n) . | 100 mg/m2 (n = 27) Frequency, % (n) . |
|---|---|---|
| Nausea/vomiting | 83 (19) | 52 (14) |
| Diarrhea | 65 (15) | 48 (13) |
| Mucositis | 91 (21) | 37 (10) |
| Pulmonary edema | 35 (8) | 26 (7) |
| Peripheral edema | 48 (11) | 15 (4) |
| Non-GI bleeding | 71 (4) | 0 (0) |
| GI bleeding | 22 (5) | 7 (2) |
| Hepatic | 13 (3) | 22 (6) |
| Renal | 35 (8) | 19 (5) |
| Metabolic | 35 (8) | 7 (2) |
| Sepsis | 26 (6) | 11 (3) |
| Toxicity . | 200 mg/m2 (n = 23) Frequency, % (n) . | 100 mg/m2 (n = 27) Frequency, % (n) . |
|---|---|---|
| Nausea/vomiting | 83 (19) | 52 (14) |
| Diarrhea | 65 (15) | 48 (13) |
| Mucositis | 91 (21) | 37 (10) |
| Pulmonary edema | 35 (8) | 26 (7) |
| Peripheral edema | 48 (11) | 15 (4) |
| Non-GI bleeding | 71 (4) | 0 (0) |
| GI bleeding | 22 (5) | 7 (2) |
| Hepatic | 13 (3) | 22 (6) |
| Renal | 35 (8) | 19 (5) |
| Metabolic | 35 (8) | 7 (2) |
| Sepsis | 26 (6) | 11 (3) |
For these reasons, pretransplantation planning becomes essential. Patient evaluation should include a detailed review of gastrointestinal signs and symptoms, serial stool guaiacs, endoscopic studies to define disease when indicated by symptoms or other findings, and a complete assessment of coagulation status. In general, proton-pump inhibitors such as omeprazole should be used for prophylaxis, and, because dose-intensive intravenous melphalan can cause delayed emesis, an antiemetic regimen may be particularly useful beginning the day after stem cell infusion and consisting of 2 to 4 mg dexamethasone twice a day, 0.5 to 1.0 mg lorazepam 2 or 3 times a day, and 5 mg prochlorperazine 2 or 3 times a day. If breakthrough nausea and vomiting occur, daily granisetron may be used in place of prochlorperazine. This regimen is usually continued from days 1 through 7. Major gastrointestinal bleeds can present atypically as new-onset atrial fibrillation or supraventricular tachycardia or as hemodynamic instability. In SCT patients with known GI amyloid, the hematocrit should be maintained at 30% or greater and platelets at 50 000/μL or greater if stool guaiacs are positive.
Because splenic rupture can also occur acutely in SCT patients with AL during stem cell mobilization or the early transplantation period, vague or atypical left-sided abdominal or shoulder pain should raise a concern about splenic hemorrhage and lead to consideration of imaging the abdomen. Splenic rupture occurring during this period has been successfully managed surgically. Other viscera, such as the esophagus or small bowel, can also perforate and present life-threatening challenges.32 Of note, we have used corticosteroids at the time of stem cell infusion in patients with renal amyloidosis to reduce the risk for dimethyl sulfoxide–induced compromise of renal function and, because we have observed capillary leak syndrome in patients with amyloidosis, we have also used steroids to prevent alveolar hemorrhage at the time of engraftment.42
The management of intravascular volume and hypotension is a critical aspect of the care of AL patients who undergo SCT. Nephrotic syndrome causes salt avidity and hypoalbuminemia, often leading to significant edema. The risk for over-diuresis, however, may be greater than the risk for allowing some peripheral edema in patients with clinical euvolemia. Nevertheless, intravenous fluids administered should be sodium-free whenever possible, and maintaining a diuresis concurrently with melphalan administration and stem cell infusion is reasonable. Even mild intravascular volume depletion may exacerbate nausea and emesis; therefore, limited hydration and a limited period of diuresis are recommended. Because a major factor causing pulmonary and peripheral edema is hypoalbuminemia, albumin infusions should be used throughout the treatment period to maintain a serum level greater than 2.0 g/dL.
Volume depletion, bleeding, sepsis, and hypoadrenalism, followed by worsening autonomic neuropathy, are the most likely causes of hypotension after SCT. Rapid intravenous infusion of magnesium supplements can also cause vasodilation and hypotension. Use of morphine or fentanyl to treat mucositis can affect blood pressure and urine output and can complicate acyclovir prophylaxis. Acyclovir toxicity will increase (particularly central nervous system toxicity) in patients with reduced renal perfusion and urine output. Midodrine and fludrocortisone are useful agents to treat orthostasis, but they do not work reliably in transplantation.39 It is reasonable to omit post-SCT G-CSF administration in patients with severe nephrotic syndrome because of the fluid retention associated with its use. At the time of neutrophil recovery or myeloid engraftment, it is not uncommon for patients to experience orthostasis requiring more aggressive hydration.
In patients with dominant cardiac amyloid with minimal symptoms, preserved left ventricular function usually assures diuretic responsiveness. Maintenance of normal electrolyte levels in cardiac patients under diuresis is an obvious requirement. The mortality associated with cardiac amyloid in SCT is attributed to sudden cardiac death and to cardiopulmonary failure resulting in hypotension and hypoxia. Patients rarely, if ever, survive after ventricular arrhythmias or symptomatic bradycardia episodes begin to occur peri-SCT despite the addition of appropriate medications and the use of advanced life-support measures. Hemodynamically stable tachycardias, on the other hand, occur with some frequency and are usually well tolerated; use of β blockade simply for control of sinus tachycardia should not be routine. Whether prophylactic antiarrhythmic agents or devices can decrease mortality remains to be investigated.
Risk-adapted approach
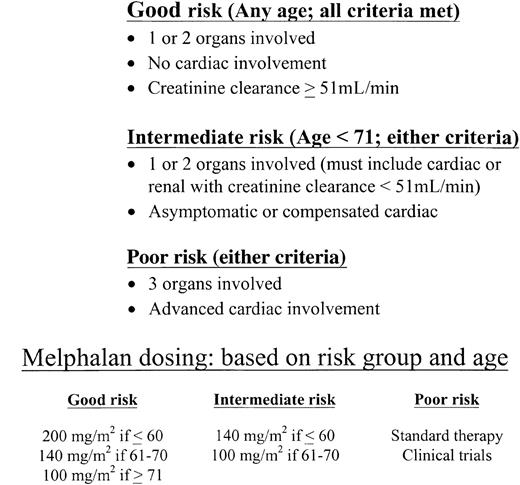
Patients with AL who have more than 2 major involved organs or who have advanced cardiomyopathy are at high risk for dying within the peri-transplantation period and, therefore, are poor risk candidates for SCT using high-dose regimens. At the same time, patients with involvement of 1 or 2 organs and those with uncomplicated cardiac disease are good candidates for SCT on clinical trials. We recommend that the dose of intravenous melphalan be attenuated based on age and organ involvement. We call this a risk-adapted approach, based on the dose-related differences in toxicity observed in clinical trials (at 100 and 200 mg/m2 of intravenous melphalan) and on the age-related differences in survival observed in the retrospective data from the Mayo Clinic amyloid database.
A suggested risk-adapted schema is described in Figure1. Patients with 1 or 2 involved organs without dominant cardiac amyloid are considered good risks and would receive either 200 or 140 mg/m2 intravenous melphalan based on age. Patients younger than 71 years with 1 or 2 involved organs and uncomplicated cardiac amyloid are considered intermediate risks and would receive either 140 or 100 mg/m2 based on age. It may be reasonable to provide more intensive therapy for patients in this category, as some have done, in a monitored setting. Good-risk patients should meet standard criteria for autologous SCT (see above). Patients with involvement by more than 2 organs or with advanced cardiomyopathy are considered poor risk (ie, not SCT candidates) and would be treated with investigational therapies or oral melphalan and prednisone. There will be patients whose disease does not fit these simple categories; for example, patients with only renal amyloid and a creatinine clearance less than 51 mL/min. Such patients are at higher than average risk for renal failure with SCT.34 Therefore, good risk AL patients who have 1 or 2 involved organs and no cardiac involvement may occasionally have impaired renal function and be at risk for becoming dialysis dependent after SCT at 200 mg/m2 melphalan. Dose-reductions to 140 or 100 mg/m2 (depending on age) are reasonable in such patients. In addition, patients with dominant hepatic disease, massive hepatomegaly, waning hepatic synthetic function, and elevated bilirubin require special consideration before commitment to SCT because of the risk for acute hepatic failure and peri-SCT death.46
A risk-adapted approach to assessing suitability of amyloidosis patients for stem cell transplantation.
A risk-adapted approach to assessing suitability of amyloidosis patients for stem cell transplantation.
Clinical research in amyloidosis
It is apparent that continued efforts to treat AL with SCT will depend on clinical trials designed to make SCT result in less morbidity or to answer specific questions of interest. The risk-adapted approach we suggest may expand the pool of AL patients as SCT candidates but would also, we hope, reduce the morbidity associated with SCT for AL patients. A multicenter phase 3 trial by a French myeloma intergroup is under way. AL patients are randomized to receive high-dose melphalan with SCT or oral melphalan and dexamethasone. Phase 2 tandem transplantation trials are also under way at several American centers, and a phase 2 ECOG trial has been completed but is yet to be reported. Although these trials did not use a risk-adapted approach, further insight may be gained from them with respect to the application of SCT to AL.
Of particular interest is the stratified randomized phase 2 trial recently reported in which AL patients received either SCT as initial therapy (arm 1) or 2 cycles of oral melphalan and prednisone and then SCT (arm 2).25 Overall survival was the primary endpoint. Although patients were stratified by dominant organ involvement for randomization, patients were not stratified based on number of organs involved or age. One hundred patients within 1 year of diagnosis (median age, 56 years; range, 37-80 years; male–female ratio, 1.8:1) were enrolled. Fifty-two patients were randomized to arm 1 and 48 to arm 2, and there were no differences in age or sex between the arms. The dominant symptomatic organ involved was renal in 56% of patients in both arms. A substantial proportion of patients also had evidence of cardiac involvement: 34 patients in arm 1 (65%) and 23 patients in arm 2 (48%). Of the 52 patients in arm 1, 9 (17%) did not proceed to SCT because of disease progression/death (n = 1), complications or death during stem cell mobilization (n = 4) or patient withdrawal (n = 4). Of the 48 patients in arm 2, 16 (33%) did not proceed to SCT because of progression of disease/death before SCT (n = 8), complications or death during stem cell mobilization (n = 6) or patient withdrawal (n = 2). With 12 to 58 months of follow-up, median survival has not been reached for either treatment arm by Kaplan-Meier. However, survival of patients 1 year after randomization was significantly higher for arm 1 (70%) than for arm 2 (58%) (P = .04). For patients with cardiac involvement, median survival was significantly longer for arm 1 (19.6 months) than for arm 2 (5.3 months) (P = .02). Overall, 35% of patients undergoing SCT in both arms had complete hematologic responses. Responses of the amyloid-related organ disease are still under evaluation. However, the overall incidence of disease progression, death, or severe complications with mobilization was 19%—that is, the sickest patients enrolled did not make it to SCT. This trial demonstrates that newly diagnosed patients with AL, eligible by minimal criteria for SCT, did not appear to benefit from initial treatment with oral melphalan and prednisone and that, for patients with cardiac involvement, there was a survival advantage to receiving intravenous melphalan. Yet the overall mortality attributed to progression of disease and TRM was nearly 40% in the first year after enrollment.
New approaches to AL may emerge from current efforts. For example, a transgenic mouse model has been developed carrying the human interleukin-6 gene with increased concentrations of the precursor secondary amyloid protein SAA.46 These mice develop renal and hepatosplenic amyloidosis at 3 months of age and have a clinical course remarkably similar to that of human AA. The availability of in vivo experimental models of AA provides a means to assess the therapeutic efficacy of new agents developed to prevent fibrillogenesis in amyloid-associated disease.47 In addition, a murine model of AL has been developed, clinically manifest as subcutaneous amyloidomas.48 When these AL-bearing animals receive injections of anti–light-chain monoclonal antibodies with specificity for an amyloid-related epitope, regression has been documented with the resolution of amyloid tumoral masses. This in vivo demonstration that amyloid deposits of immunoglobulin origin can be lysed by passive administration of an amyloid-reactive antibody has the potential for important clinical benefit to patients with AL.48 It is hoped that the antifibrillar and the serotherapeutic approaches to AL will be in clinical trials within this decade. We are also encouraged by the testing of novel biologic agents, such as amifostine and keratinocyte growth factor, that may reduce gastrointestinal toxicity in SCT.
Hypotheses of a translational nature that are worth examining in future trials include asking whether immunoglobulin VLgermline gene use has prognostic significance because germline gene use contributes to the organ tropism of AL and whether hematopoietic stem cells trans-differentiate to contribute to post-SCT tissue-specific recovery in patients with cardiac amyloid. We anticipate that the willingness of patients with AL to participate in clinical research will increase the likelihood of acquiring a better understanding of the mechanisms of AL disease and of amyloid resorption and organ recovery.
Conclusions
Stem cell transplantation for primary systemic amyloidosis is applicable to a minority of patients, such as those with limited organ disease and no significant cardiac involvement. Response rates with SCT appear to be higher than those seen in patients treated with traditional melphalan and prednisone. Morbidity and mortality are clearly higher than in patients with multiple myeloma or other hematologic malignancies undergoing autologous SCT. There is an unusually high rate of significant gastrointestinal tract hemorrhage, and cardiac complications including arrhythmias are prevalent. SCT for AL will remain controversial until there is either a multicenter phase 3 trial comparing it to standard therapy in newly diagnosed patients or a multicenter phase 2 trial using a risk-adapted approach showing reduced treatment-related mortality. We anticipate that improved patient selection and peri-transplantation management and adoption of a risk-adapted approach to melphalan dosing based on organ involvement and age will accelerate the acquisition of the expertise needed for the conduct of multicenter SCT trials and will enhance the impetus for early diagnosis and timely treatment of AL.
We thank Stephen Nimer for a critical reading of this manuscript and our colleagues at Memorial Sloan-Kettering, Mayo Clinic and in the amyloidosis community for many helpful discussions.
References
Author notes
Raymond L. Comenzo, Hematology Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY 10021; e-mail: comenzor@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal