Abstract
Hematopoietic stem cells (HSCs) represent an important target for the treatment of various blood disorders. As the source of critical cells within the immune system, genetic modification of HSCs can also be used to modulate immune responses. The effectiveness of HSC-mediated gene therapy largely depends on efficient gene delivery into long-term repopulating progenitors and targeted transgene expression in an appropriate progeny of the transduced pluripotent HSCs. Self-inactivating (SIN) lentiviral vectors have been demonstrated to be capable of transducing mitotically inactive cells, including HSCs, and accommodating a nonviral promoter to control the transgene expression in transduced cells. In this study, we constructed 2 SIN lentiviral vectors, EF.GFP and DR.GFP, to express the green fluorescent protein (GFP) gene controlled solely by the promoter of either a housekeeping gene EF-1α or the human HLA-DRα gene, which is selectively expressed in antigen-presenting cells (APCs). We demonstrated that both vectors efficiently transduced human pluripotent CD34+cells capable of engrafting nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice. When the EF.GFP vector was used, constitutive high-level GFP expression was obtained in all the human HSC progeny detectable in NOD/SCID mice and in subsequent in vitro differentiation assays, indicating that engrafting human HSCs have been transduced. In contrast, the DR.GFP vector mediated transgene expression specifically in human HLA-DR+ cells and highly in differentiated dendritic cells (DCs), which are critical in regulating immunity. Furthermore, human DCs derived from transduced and engrafted human cells potently stimulated allogeneic T-cell proliferation. This study demonstrated successful targeting of transgene expression to APCs/DCs after stable gene transduction of pluripotent HSCs.
Introduction
Hematopoietic stem cells have the unique capability of repopulating the entire hematopoietic system, including the immune system, due to their self-renewal and pluripotent differentiation potentials. Therefore, stable gene transfer to these stem cells has great potential to achieve both long-term and short-term therapeutic effects for the treatment of inherited and acquired hematopoietic disorders.1-6 Currently, retroviral (including oncoretroviral and lentiviral) vectors remain the only choice to stably transduce hematopoietic stem/progenitor cells (HSPCs) efficiently, resulting in permanent integration of the transgene into the host genome.7-9 However, transgene expression in all progenies of the transduced HSPCs is often unnecessary and may even be detrimental in many circumstances.9,10 Thus, targeted and/or restricted expression of transgenes efficiently and specifically in one relevant differentiation lineage derived from the transduced HSPCs may be crucial to achieve maximal therapeutic effectiveness and to limit potential adverse effects.
Until recently, oncoretroviral vectors (RVs) have been the only delivery vehicles for HSPCs.1-10 However, RV transduction requires division of the target cells while most of primitive HSPCs are mitotically quiescent. Although significant progress has been made in improving conditions for ex vivo HSPC culture and transduction, RV design and production, and RV-mediated transgene expression,11-16 RVs still possess intrinsic limitations. For example, a preactivation lasting for 2 to 3 days is required for efficient RV transduction of HSPCs. Because HSPC engraftment activities can be only maintained in culture for several days currently and extended culture often results in HSPC reduction, a subtle balance of HSPC maintenance and high-level transduction during ex vivo culture is critical. Second, the RV long terminal repeat (LTR) is used in almost all the high-titer RVs as the promoter to transcribe the transgene as well as the viral genomic RNA.9,10 However, the RV LTR has been found attributing to the inactive or attenuated transgene expression in transduced cells after differentiation and/or long-term culture and engraftment.17-20 Insertion of an additional promoter into RVs often resulted in “promoter interference” that reduced promoter activity of the internal promoter and/or the LTR.21-23 Deletion of the promoter element within the RV LTR to avoid the promoter interference often resulted in a severe (100-fold) reduction of viral titers.9,10 Except in a few cases such as the GATA-1 transcriptional factor binding and self-activation system,24 it has been generally difficult to construct high-titer RVs in which a transgene is solely controlled by a non-LTR promoter.
Recently, attention has focused on vectors derived from lentiviruses (LVs) such as human immunodeficiency virus-1 (HIV-1), which have been shown to transduce a variety of mitotically inactive cells, including human HSPCs.25-31 This is consistent with the notion that LVs can efficiently transduce cells that are in the G1, S, and G2 phases before cell division. In contrast, RV integration can occur only after breakdown of the nuclear envelope, concomitant with progression through the M phase of the cell cycle. We and others have found the pseudotyped LVs can transduce human HSPCs efficiently as assayed in a surrogate transplantation model using nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.26,29,32-34 Recent improvements, such as minimizing HIV sequences and eliminating viral accessory proteins for improved transduction efficiency and safety,35,36 further enhanced favorability of LVs as delivery vehicles for HSPCs. Moreover, the self-inactivating (SIN) modification of LV,37-39 which permanently disables the viral promoter after integration, enables transgene expression to be controlled solely by an internal promoter. Importantly, the SIN modification of LVs does not reduce viral titers, which is in sharp contrast to what was observed previously with RV.9,10Thus, using SIN LVs with a specific promoter, we may achieve targeted transgene expression in a specific lineage of transduced pluripotent HSPCs.
In the present study, we evaluated the ability of SIN LVs to target genes into antigen-presenting cells (APCs) differentiated in vitro and in vivo from HSPCs. It is now appreciated that processing and presentation of antigens by different APC types are the initial events in determining immune responses. Indeed, major endeavors in engineering antigen-specific immunotherapy such as vaccines are focused upon the targeting of antigens to various APC subsets such as dendritic cells (DCs), which are critical mediators of T-cell immunity.40-43 We postulated that large numbers of transduced APCs/DCs could be generated from engrafted HSPCs after transduction with specific genes to modulate immunity. To achieve APC-specific transgene expression, we took advantage of the fact that major histocompatibility complex class II (MHC II) genes are expressed selectively in APCs and highly in DCs after differentiation and maturation. We constructed and evaluated 2 SIN LVs expressing the green fluorescent protein (GFP) gene controlled by 2 different internal promoters: DR.GFP, which employs a human MHC II–specific HLA-DRα promoter, and EF.GFP, which employs a strong constitutive promoter from the human translation elongation factor 1α (EF-1α) gene. Using the NOD/SCID mouse engraftment model, we demonstrated selective expression of transgene in MHC II+ human cells derived from the DR.GFP-transduced HSPCs before and after engraftment of NOD/SCID mice. Furthermore, GFP-transduced human DCs obtained from transduced and engrafted HSPCs are functional in stimulating allogeneic T-cell proliferation.
Materials and methods
Animals, cell lines, and cytokines
Immunodeficient NOD/LtSz-scid/scid (NOD/SCID) mice, originally obtained from Jackson Laboratory (Bar Harbor, ME), were bred and housed in the animal facility at Johns Hopkins Oncology Center. They were kept in a pathogen-free environment following a protocol approved by the Johns Hopkins Animal Care and Use Committee. Human cell lines HeLa and 293T were maintained in Dulbecco modified Eagle medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS, GIBO BRL). Epstein-Barr virus–transformed human B cells (HLA-DR+) and a HLA-DR+ human leukemia cell line (TF1) and HLA-DR− (U937, K562, HL60, and EOL) cell lines were cultured in RPMI 1640 medium (GIBCO BRL) supplemented with 10% FBS. For the TF1 cell line, which was derived from bone marrow (BM) blastic cells in a patient with erythroleukemia and is growth factor–dependent,11 44 granulocyte-macrophage colony-stimulating factor (GM-CSF) (Immunex, Seattle, WA) at 1 ng/mL was added. All the other cytokines were purchased from PeproTech (Rocky Hill, NJ), except G-CSF and erythropoietin (Amgen, Thousand Oaks, CA).
LV construction and virus production
A SIN LV pRLLhPGK.GFP Sin-18 (abbreviated as PGK.GFP) was kindly provided by Dr Didier Trono, University of Geneva Medical School.38 We constructed EF.GFP by replacing the human PGK promoter (EcoRV to BamHI sites in the PGK.GFP vector) with human EF-1α promoter from plasmid pEF1/Myc-His-A (Invitrogen, Carlsbad, CA). The EF-1α promoter (nucleotide 373 to 1560 as numbered in GenBank accession J04616) contains the first exon, first intron, and part of the second exon before the coding sequence (starting at nucleotide 1582). Similarly, we constructed DR.GFP by using the human (MHC II) HLA-DRα promoter (nucleotide 182 to 480, as numbered in GenBank accession X00274) to replace the PGK promoter (XhoI to BamHI sites in the PGK.GFP vector). This 300–base pair promoter sequence has been shown to confer selective expression of a transgene in MHC II+ cells.45Detailed sequences are available upon request.
Recombinant LVs were generated using the 3-plasmid system by cotransfection of 293T cells through calcium phosphate precipitation.35,38,46 In addition to a transducing vector (PGK.GFP, EF.GFP or DR.GFP), 2 other vectors were used in viral production. One, pMD.G, expresses the vesicular stomatitis virus G envelope protein.46 The other, pCMVΔR8.91, expresses the HIV-1 gag/pol, tat, and rev genes required for efficient LV production.35 The latter 2 plasmids were also provided by Dr Trono. The ratio of a transducing vector, pMD.G, and pCMVΔR8.91 was fixed at 1.5:0.5:2 μg for 106 293T cells plated in a 35-mm dish or scaled up proportionally for larger dishes. Viruses were harvested at 48 and 72 hours after transfection and titered based on percentages of GFP+ cells after transduction with serially diluted viral supernatants. TF1 cells (MHC II+) were used for DR.GFP as well as EF.GFP and PGK.GFP vectors, whereas 293T cells were used for titering the latter 2 vectors (with similar results). The titers, calculated as transducing units (TUs) per milliliter of supernatants, were usually in the range of 1 × 106 to 6 × 106 TUs per milliliter for all 3 vectors, while EF.GFP consistently had a higher titer than PGK.GFP or DR.GFP. To obtain more concentrated viruses, viral supernatants were transferred to a filtration column (Centricon Plus-20, MWCO 100 000; Millipore, Bedford, MA) and centrifuged at 2000g for 30 to 90 minutes at 4°C. The method was also developed independently by Dr J. Reiser and described in detail.47 The concentrated viruses were tittered as above (usually in the range of 0.8 × 108-3 × 108 TUs/mL) and stored at −80°C.
Detection of replication-competent retroviruses
To rule out the possibility that replication-competent retroviruses (RCRs) could be generated from the DR.GFP or EF.GFP LV, we randomly tested the presence of viral proteins in culture media of stably transduced cells (after continuous culture after transduction) and in sera of NOD/SCID mice engrafted with transduced human cells. The amount of the HIV-1 p24 viral protein, which would exist in sera or culture media and gradually amplify if RCRs arose, was determined using a p24 enzyme-linked immunosorbent assay kit (ZeptoMetrix, Buffalo, NY). Within the detection limit (5 pg per sample) of the assay, all the samples we examined were negative for RCRs in human cells and mouse sera.
Antibodies and flow cytometric analysis
R-phycoerythrin (PE)–conjugated antihuman HLA-DR (MHC II), CD1a, CD14, CD40, CD80, CD86, biotin-conjugated antimouse CD45 and Ter119, and CyChrome-conjugated antihuman CD45 monoclonal antibodies (mAbs) were purchased from PharMingen (San Diego, CA). PE-conjugated antihuman CD13, CD19, and CD83 were obtained from Caltag Laboratories (Burlingame, CA). PE-conjugated antihuman CD34 was purchased from Becton Dickinson (San Jose, CA). PE-conjugated antihuman glycophorin A was obtained from Immunotech (Marseille, France). Fluorescence-activated cell sorter (FACS) analysis was carried out using a FACScan or FACSort (Becton Dickinson).
Macrophage and DC differentiation after LV transduction of human blood monocytes
Human peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia, Sweden) from healthy donors. Monocytes were enriched by plastic adherence after culturing peripheral blood mononuclear cells (8 × 106 cells/well [9.4 cm2]) with RPMI 1640 medium plus 10% FBS for 2 hours at 37°C. After washing, adherent cells were cultured in the same medium supplemented with M-CSF (25 ng/mL) to induce macrophage differentiation for 7 to 10 days. At days 2 and 3, adherent cells were transduced with EF.GFP or DR.GFP LV twice with 8 μg/mL polybrene. The multiplicity of infection (MOI) was 30 for each round of transduction. Cells were harvested on day 10 and analyzed for expression of GFP and macrophage markers (CD14 and HLA-DR). For DC differentiation, adherent monocytes were cultured in the same medium supplemented with GM-CSF (800 U/mL or 150 ng/mL) and interleukin-4 (IL-4) (1000 U/mL or 20 ng/mL). At days 1 and 2, adherent cells were transduced with DR.GFP or EF.GFP LV (2 mL/well, MOI = 10) with 8 μg/mL polybrene. After 4 to 6 hours of incubation with LV, 2 mL fresh medium containing 2 × GM-CSF and IL-4 was added, and transduction continued overnight. At day 2, transduction was repeated. At day 3, transduced cells were harvested and further cultured in 1 mL fresh medium containing GM-CSF and IL-4. When IL-4 was added, no sign of cell proliferation was observed, in contrast to the case when GM-CSF alone or M-CSF was added to monocyte cultures. At day 6, 1 mL fresh medium containing tumor necrosis factor (TNF)–α (final concentration 50 ng/mL) was added to promote DC maturation. At day 8, total cells were harvested and analyzed for expression of GFP and DC markers (HLA-DR and CD83) with PE-conjugated mAbs.
To quantitatively determine the specific transgene expression in MHC II+ cells, we defined the term “specificity factor” (SF) as the ratio of percentages of GFP+ cells in all the MHC II+ cells versus that in all the MHC II−cells:
For example, the SF of EF.GFP in transduced macrophages (Figure1A) is [56/(56 + 36)]/[5/(5 + 3)] = 0.95. SF is independent of exact levels of transduction efficiencies and is 1.0 if there are equal percentages of GFP+ cells in MHC II+ and MHC II− cell populations. SF works best if both MHC II+ and MHC II− cells exist in fair percentages (Figures 2-4).
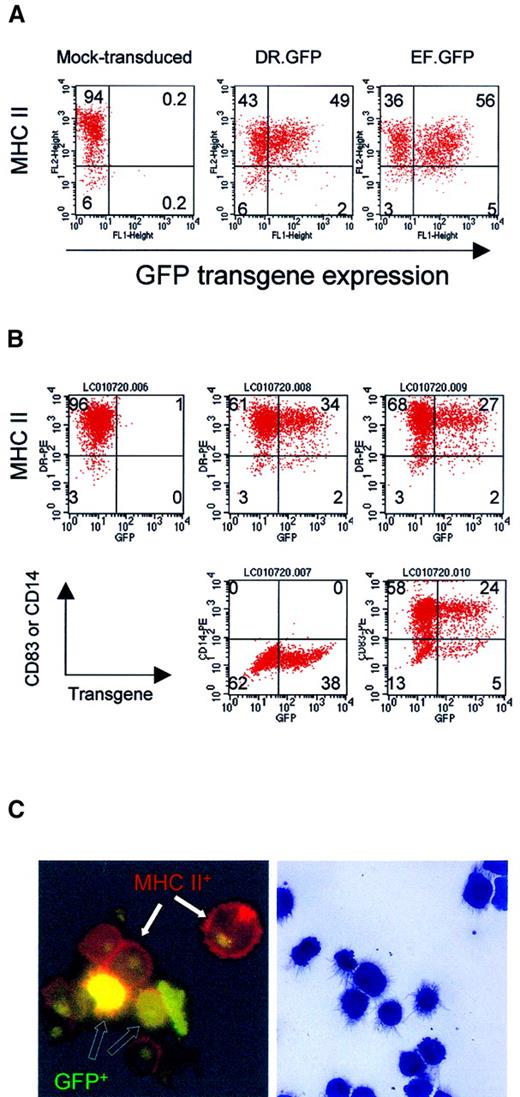
GFP expression in differentiated macrophages and DCs from human blood monocytes after LV transduction.
(A) Adherent monocytes enriched from PBL were induced in culture to differentiate into macrophage by M-CSF. Adherent cells were transduced with DR.GFP or EF.GFP twice in 2 days. For mock transduction, the medium used to collect LVs was added. Transduced cells were continuously cultured with M-CSF, and adherent cells were harvested 7 days after transduction to analyze GFP and MHC II (HLA-DR) expression (A). (B) For DC differentiation and maturation, adherent monocytes were first cultured with GM-CSF and IL-4. Cultured monocytes were transduced twice as before. Four days after transduction, motile cells were collected and cultured for 2 more days with TNF-α in addition to GM-CSF and IL-4. At day 8 of culture, transduced cells were harvested and stained by PE-conjugated antibodies to characterize GFP-transduced cells (B). In addition to MHC II staining (top panels), the DC phenotypes were further confirmed by the expression of a DC-specific marker CD83 (bottom right panels) and lacking of a monocyte/macrophage marker CD14 (middle panels). Quadrant setting was based on background staining by isotype-matched control antibodies. Percentages of various cell subsets in each quadrant are indicated. (C) Photomicrographs of DR.GFP-transduced DCs. After MHC II staining (B), DCs were spun onto glass slides and examined by fluorescence microscopy (left, × 1000 magnification) of DC morphology. Cells with GFP transgene expression (green to yellow, open arrows) within transduced cells and MHC II on cell surface (red, closed arrows) are illustrated. Background green fluorescence was seen in nuclei of all the cells. The remaining cytospin slides were stained with the Wright-Giemsa solution (right, × 400 magnification). Note that the staining diminished GFP fluorescence signals but it better revealed DC morphology.
GFP expression in differentiated macrophages and DCs from human blood monocytes after LV transduction.
(A) Adherent monocytes enriched from PBL were induced in culture to differentiate into macrophage by M-CSF. Adherent cells were transduced with DR.GFP or EF.GFP twice in 2 days. For mock transduction, the medium used to collect LVs was added. Transduced cells were continuously cultured with M-CSF, and adherent cells were harvested 7 days after transduction to analyze GFP and MHC II (HLA-DR) expression (A). (B) For DC differentiation and maturation, adherent monocytes were first cultured with GM-CSF and IL-4. Cultured monocytes were transduced twice as before. Four days after transduction, motile cells were collected and cultured for 2 more days with TNF-α in addition to GM-CSF and IL-4. At day 8 of culture, transduced cells were harvested and stained by PE-conjugated antibodies to characterize GFP-transduced cells (B). In addition to MHC II staining (top panels), the DC phenotypes were further confirmed by the expression of a DC-specific marker CD83 (bottom right panels) and lacking of a monocyte/macrophage marker CD14 (middle panels). Quadrant setting was based on background staining by isotype-matched control antibodies. Percentages of various cell subsets in each quadrant are indicated. (C) Photomicrographs of DR.GFP-transduced DCs. After MHC II staining (B), DCs were spun onto glass slides and examined by fluorescence microscopy (left, × 1000 magnification) of DC morphology. Cells with GFP transgene expression (green to yellow, open arrows) within transduced cells and MHC II on cell surface (red, closed arrows) are illustrated. Background green fluorescence was seen in nuclei of all the cells. The remaining cytospin slides were stained with the Wright-Giemsa solution (right, × 400 magnification). Note that the staining diminished GFP fluorescence signals but it better revealed DC morphology.
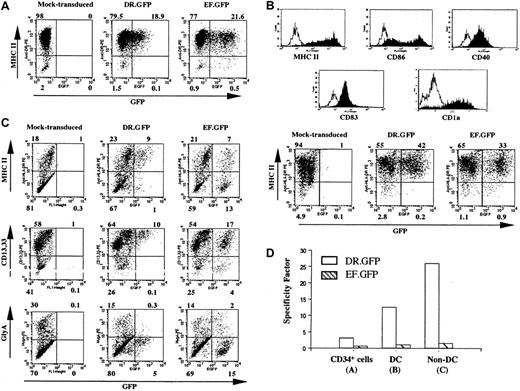
Specific GFP expression in DR.GFP-transduced blood CD34+ cells and their progeny differentiated in vitro.
(A) Human CD34+ PBSCs were transduced with DR.GFP or EF.GFP LV at an MOI of 10. GFP and MHC II (HLA-DR) expression of these transduced CD34+ cells was analyzed 3 days after transduction. (B) Human DCs were differentiated from the above-transduced CD34+ cells for 16 days in the presence of human GM-CSF, IL-4, and TNF-α. Expression of the DC cell surface markers and the correlation of GFP with MHC II expression in these human DCs were examined by FACS analysis. The histogram of staining with each specific antibody (filled lines) overlays on the background staining (open lines) using the corresponding isotype-matched control antibody. (C) Transduced cells were differentiated in methylcellulose for erythroid/myeloid CFC assays. CFC-derived differentiated cells (non-DCs) were harvested on day 14 and examined for GFP, MHC II, myeloid (CD13 and CD33), and erythroid (glycophorin A, GlyA) cell lineage marker expression. (D) The SF of each vector to direct MHC II−-specific transgene expression (see “Materials and methods”) in transduced CD34+ cells, DCs, and non-DCs derived from the transduced CD34+ cells.
Specific GFP expression in DR.GFP-transduced blood CD34+ cells and their progeny differentiated in vitro.
(A) Human CD34+ PBSCs were transduced with DR.GFP or EF.GFP LV at an MOI of 10. GFP and MHC II (HLA-DR) expression of these transduced CD34+ cells was analyzed 3 days after transduction. (B) Human DCs were differentiated from the above-transduced CD34+ cells for 16 days in the presence of human GM-CSF, IL-4, and TNF-α. Expression of the DC cell surface markers and the correlation of GFP with MHC II expression in these human DCs were examined by FACS analysis. The histogram of staining with each specific antibody (filled lines) overlays on the background staining (open lines) using the corresponding isotype-matched control antibody. (C) Transduced cells were differentiated in methylcellulose for erythroid/myeloid CFC assays. CFC-derived differentiated cells (non-DCs) were harvested on day 14 and examined for GFP, MHC II, myeloid (CD13 and CD33), and erythroid (glycophorin A, GlyA) cell lineage marker expression. (D) The SF of each vector to direct MHC II−-specific transgene expression (see “Materials and methods”) in transduced CD34+ cells, DCs, and non-DCs derived from the transduced CD34+ cells.
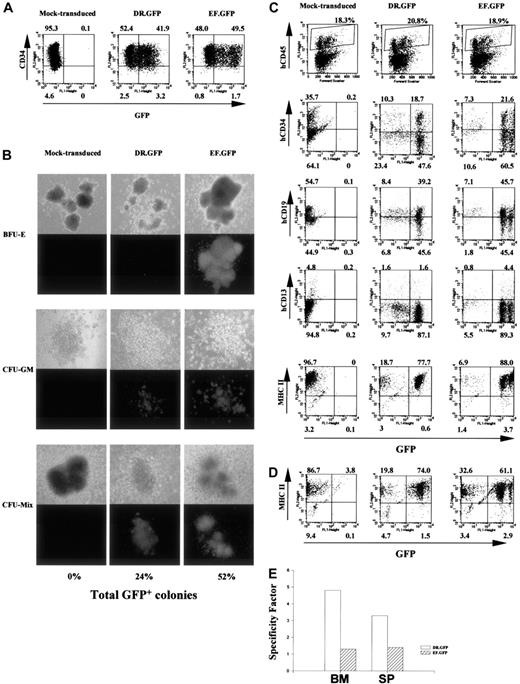
Targeted GFP expression in MHC II+ human cells before and after NOD/SCID mouse engraftment with DR.GFP-transduced CD34+ cells.
(A) Analysis of GFP and CD34 expression in human CB CD34+cells 3 days after DR.GFP, EF.GFP, or mock transduction. (B) Differentiation of the transduced CB CD34+ cells in methylcellulose for CFC assays. Representative photographs of BFU-E, CFU-GM, and CFU-mix under light (top panels) and fluorescence (bottom panels) microscopy after 14 days in culture by an inverted microscope (magnification, × 50). The percentages of GFP bright (GFP+) colonies in each group were calculated, and the averages of triplicates are shown at the bottom. (C) FACS analysis of engrafted human cells in the NOD/SCID BM 10 weeks after transplantation with DR.GFP- or EF.GFP-transduced CB CD34+cells (mice no. 15 and 20, respectively, in Table 1). Engrafted human cells were first identified in dot plots (top panels, human CD45 versus forward scatter) and gated for further analysis. The percentages of human (CD45+, shown in boxes) cells are indicated at the top of dot plots. Further examinations of GFP expression versus human CD34, CD19, CD13, and MHC II markers in gated human CD45+ populations are illustrated (bottom panels). (D) FACS analysis of GFP and MHC II expression in gated human populations in the spleen of engrafted NOD/SCID mice. (E) Quantitative analysis of transgene expression specificity in human MHC II+ populations found in the NOD/SCID BM and spleen (SP) after engraftment of DR.GFP- or EF.GFP- transduced CD34+ cells.
Targeted GFP expression in MHC II+ human cells before and after NOD/SCID mouse engraftment with DR.GFP-transduced CD34+ cells.
(A) Analysis of GFP and CD34 expression in human CB CD34+cells 3 days after DR.GFP, EF.GFP, or mock transduction. (B) Differentiation of the transduced CB CD34+ cells in methylcellulose for CFC assays. Representative photographs of BFU-E, CFU-GM, and CFU-mix under light (top panels) and fluorescence (bottom panels) microscopy after 14 days in culture by an inverted microscope (magnification, × 50). The percentages of GFP bright (GFP+) colonies in each group were calculated, and the averages of triplicates are shown at the bottom. (C) FACS analysis of engrafted human cells in the NOD/SCID BM 10 weeks after transplantation with DR.GFP- or EF.GFP-transduced CB CD34+cells (mice no. 15 and 20, respectively, in Table 1). Engrafted human cells were first identified in dot plots (top panels, human CD45 versus forward scatter) and gated for further analysis. The percentages of human (CD45+, shown in boxes) cells are indicated at the top of dot plots. Further examinations of GFP expression versus human CD34, CD19, CD13, and MHC II markers in gated human CD45+ populations are illustrated (bottom panels). (D) FACS analysis of GFP and MHC II expression in gated human populations in the spleen of engrafted NOD/SCID mice. (E) Quantitative analysis of transgene expression specificity in human MHC II+ populations found in the NOD/SCID BM and spleen (SP) after engraftment of DR.GFP- or EF.GFP- transduced CD34+ cells.
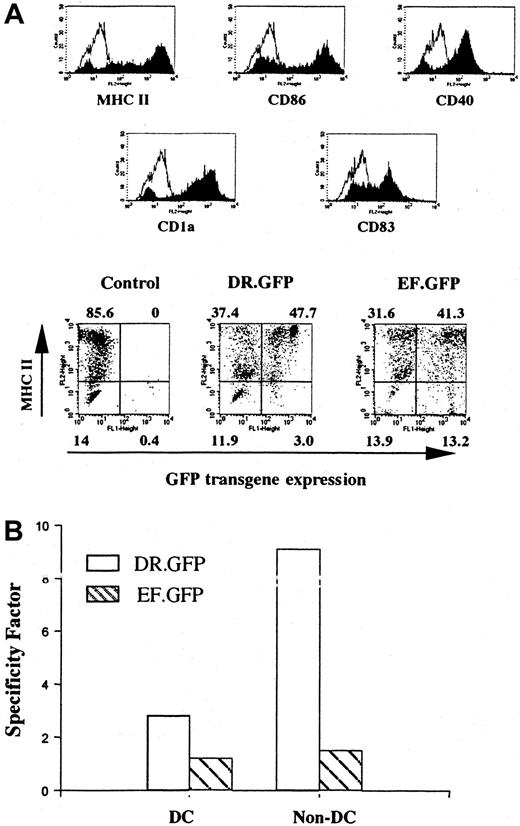
Differentiated DCs from transduced human cells that had engrafted in the NOD/SCID mice.
Human cells derived from transduced CB CD34+ cells were isolated from the BM of engrafted NOD/SCID mice (Figure 3C). Enriched human cells were cultured with human GM-CSF, IL-4, and TNF-α for 14 days to allow DC differentiation and maturation as in Figure 1. (A) FACS analysis of GFP and other DC surface marker (including MHC II) expression in differentiated human DCs after transplantation. The control group shows the differentiated DCs without GFP transduction. (B) Quantification of the transgene expression specificity of either vector in MHC II+ cells among DC- and CFC-derived progeny (non-DCs, as in Figure 2C) differentiated in vitro subsequent to the engraftment of transduced CD34+ cells.
Differentiated DCs from transduced human cells that had engrafted in the NOD/SCID mice.
Human cells derived from transduced CB CD34+ cells were isolated from the BM of engrafted NOD/SCID mice (Figure 3C). Enriched human cells were cultured with human GM-CSF, IL-4, and TNF-α for 14 days to allow DC differentiation and maturation as in Figure 1. (A) FACS analysis of GFP and other DC surface marker (including MHC II) expression in differentiated human DCs after transplantation. The control group shows the differentiated DCs without GFP transduction. (B) Quantification of the transgene expression specificity of either vector in MHC II+ cells among DC- and CFC-derived progeny (non-DCs, as in Figure 2C) differentiated in vitro subsequent to the engraftment of transduced CD34+ cells.
LV transduction of human CD34+ cells and DC differentiation
Human G-CSF–mobilized peripheral blood or cord blood CD34+ cells were obtained from AllCells (San Mateo, CA). CD34+ cells were purified by immunomagnetic selection (Miltenyi Biotec, Auburn, CA) and then cryopreserved. The purity of CD34+ cells was usually more than 95%. One day before transduction, cryopreserved CB CD34+ cells were thawed and cultured (106 cells/mL) overnight in QBSF-60 medium (Quality Biological, Gaithersburg, MD). Human thrombopoietin (10 ng/mL), stem cell factor/KIT ligand (SCF/KL) (100 ng/mL), and FLT3 ligand (FL) (100 ng/mL) were supplemented (theTpo/SCF/FL [TSF] medium). In the following days, these CD34+ cells were transduced with EF.GFP or DR.GFP LV (MOI = 10 to 60) once or twice by centrifugation at 2000g for 4 hours at room temperature. The cells with viruses were then diluted with one volume of the TSF medium, cultured overnight, and replaced with fresh TSF medium next morning. Three days after the first LV transduction, transduced CD34+ cells were harvested for FACS analysis and for subsequent in vitro and in vivo studies described below. DC in vitro differentiation was carried out by culturing the transduced CD34+ cells in RPMI 1640 supplemented with 10% FBS, GM-CSF (800 U/mL), IL-4 (200 U/mL), and TNF-α (20 ng/mL). The culture medium was replenished every 3 to 4 days. After 14 to 16 days of culture, mature human DCs in suspension were harvested and examined for GFP and MHC II expression.
CFC assays resulting in myeloid and erythroid lineage differentiation in methylcellulose
Transduced human CD34+ cells were seeded onto 35-mm dishes in triplicate, each containing 450 cells in 1.2 mL methylcellulose media (Marrow-Gro, Quality Biological) supplemented with human stem cell factor (100 ng/mL), IL-3 (10 ng/mL), IL-6 (10 ng/mL), G-CSF (10 ng/mL), GM-CSF (10 ng/mL), and erythropoietin (5 U/mL). After 14 days of culture, total numbers of granculocyte-monocyte colony-forming units (CFU-GMs), mixed colony-forming units (CFU-mix), and erythroid burst-forming units (BFU-Es), as well as GFP+colonies, were counted under a Nikon light/fluorescence microscope. Additionally, colony-forming cell (CFC)–derived cells were harvested by diluting and washing off methylcellulose with phosphate-buffered saline and then examined for GFP and MHC II expression.
Human CB CD34+ stem cell engraftment of NOD/SCID mice
The DR.GFP, EF.GFP, or mock-transduced CB CD34+cells were transplanted into 8- to 10-week-old sublethally irradiated (300 cGy) NOD/SCID mice via tail vein injection (2 × 105input cells per mouse). Six to 10 weeks after transplantation, the BM and spleens of these engrafted NOD/SCID mice were harvested and analyzed for the presence of human CD45+ cells. GFP expression in MHC II+ and MHC II− human cell populations was examined. Additionally, for further culture and in vitro differentiation assays of engrafted human cells, murine (CD45+ and Ter119+) cells were first removed. Briefly, harvested cells from NOD/SCID BM and spleens were resuspended in phosphate-buffered saline with 6% FBS at 2 × 107/mL and incubated with biotinylated antimurine CD45 and Ter119 antibodies (1 μg/106 cells) at 4°C for 30 minutes. After washing, the antibody-labeled cells were then incubated with streptavidin-conjugated magnetic beads (Dynal, Oslo, Norway) for 30 minutes at 4°C (4 beads per cell). The cell mixture was then placed in the magnetic rack (Dynal) for 5 minutes, and the unbound human cells were collected.
DC differentiation from the enriched human cells engrafted in NOD/SCID mouse BM was carried out similarly to peripheral blood stem cell (PBSC)–derived DC differentiation described above with the following modification. At day 6 of DC differentiation, the suspension cells were completely removed to further eliminate murine cells in suspension. For CFC assays after engraftment, enriched BM cells were added to methylcellulose media supplemented with human GM-CSF, IL-3, and erythropoietin in each of duplicate 35-mm dishes. After 19 days of culture, numbers of the total and GFP+ colonies were counted, and the cells were harvested for FACS analysis.
Alloantigen presentation of differentiated human DCs in mixed leukocyte reactions
In vitro–differentiated human DCs either directly from transduced CB CD34+ cells or from reconstituted NOD/SCID BM were irradiated at 3000 cGy and used as APCs in the mixed leukocyte reaction (MLR) T-cell proliferation assay. Briefly, cryopreserved human peripheral lymphocytes (effectors, 2 × 105/well) were seeded in 96-well plates with serially diluted human DCs (stimulators, stimulator:effector ratios 1:10, 1:80, and 1:640, respectively) in triplicate. After 3 days of culture, the cells were pulsed with 1 μCi/well (3.7 × 104 Bq) of [3H]thymidine and harvested 18 to 20 hours later with a Packard Micromate cell harvester (Packard BioScience, Meriden, CT). The [3H]thymidine incorporation was determined through a Packard Matrix 96 direct β-counter.
Results
Preferential transgene expression in MHC II+ cells mediated by the DR.GFP vector
To achieve restricted transgene expression in APCs that selectively express MHC II molecules such as HLA-DR, we constructed a SIN LV containing the HLA-DRα promoter as the sole internal promoter. Then we compared titers and transgene expression of DR.GFP with LVs in which transgene expression is controlled by either a human PGK promoter (PGK.GFP) or EF-1α promoter (EF.GFP). When tested in a variety of human MHC II+ (such as TF1 and Epstein-Barr virus–transformed B cells) and MHC II− (U937, K562, HL-60, and EOL) hematopoietic cell lines, the DR.GFP vector directed GFP expression preferentially (20- to 100-fold) in the MHC II+ cells with intensities higher than PGK.GFP but slightly weaker than EF.GFP (data not shown). In contrast, PGK.GFP or EF.GFP showed equivalent levels of GFP expression in both MHC II+or MHC II− cell lines (the GFP signal by EF.GFP was 3- to 20-fold brighter than that by PGK.GFP as reported recently30). To assess whether the induction of MHC II (HLA-DR) expression will result in transgene up-regulation, we treated the transduced HeLa cells with IFN-γ after DR.GFP transduction. Corresponding to the up-regulation of MHC II expression by IFN-γ, GFP transgene expression was also up-regulated by more than 10-fold in the transduced HeLa cells (data not shown). Subsequently, we used DR.GFP and EF.GFP vectors to evaluate gene transfer efficiency and promoter specificity of these SIN LVs in primary human hematopoietic cells.
Transgene expression in human blood monocyte–derived macrophages and DCs by the DR.GFP SIN vector
Adherent monocytes were cultured with M-CSF or GM-CSF plus IL-4 to induce macrophage or DC differentiation, respectively. After 2 rounds of transduction by DR.GFP or EF.GFP SIN LV, transduced cells were cultured in complete media for an additional 6 to 7 days before being analyzed by FACS for GFP and lineage marker expression. For macrophage differentiation, adherent cells were harvested for analysis (Figure1A). Nearly 100% of cells expressed CD14 (not shown), and most cells expressed moderate levels of MHC II (HLA-DR). After DR.GFP transduction, moderate levels of GFP expression were observed in these MHC II+ (and CD14+) macrophages, particularly in those expressing highest levels of MHC II (Figure 1A). GFP expression by EF.GFP appeared more uniform regardless of the MHC II expression levels.
To quantitatively determine the relative specificity of the transgene expression in the MHC II+ population, we employed the SF calculation (see “Materials and methods”). The dimensionless SF computes the percentage of GFP+ cells in MHC II+ population versus that in the MHC II−population. Equal percentages of GFP+ cells in MHC II+ and MHC II− cell populations result in SF = 1. If GFP transgene is preferentially expressed in MHC II+ cells, the SF of the vector will be more than 1. Based on this formula, the SF of DR.GFP in transduced macrophages was determined to be 2.13, whereas that of EF.GFP was 0.95 (Figure 1A).
For DC differentiation, motile cells were collected at day 6 and further cultured with TNF-α to promote DC maturation. Two days after, more than 94% of the cells were found to express high levels of MHC II. Most of these cells (> 80%) expressed a DC marker (CD83) and lacked the monocyte/macrophage marker CD14 (Figure 1B, lower panels). The morphology of DR.GFP-transduced DCs was further examined in slides by histology staining and fluorescence microscopy (Figure 1C). Very few cells showed the macrophage morphology, and GFP+ mature DCs were readily seen. By both methods, about 30% of mature DCs expressed GFP transgene after transduction by both vectors, and GFP signals were slightly lower in DR.GFP-transduced DCs. The lower transduction efficiency of DCs may be due to lower titers of the unconcentrated LVs used (MOI = 10) than in the transduction of macrophages (MOI = 30). For blood B cells that are also MHC II+, low levels of transduction were repeatedly observed by both vectors (with an MOI up to 50), although GFP+ cells were observed in MHC II+ cells (data not shown). It is unclear why peripheral blood–derived B cells were difficult to transduce even after activation.
Efficient transduction of human CD34+ HSPCs and selective transgene expression in MHC II+ cells by the DR.GFP vector
We next examined transgene expression in MHC II+ cells derived from transduced HSPCs by DR.GFP. Cryopreserved CD34+ PBSCs were cultured for 24 hours and transduced once (MOI 10-20). Three days after transduction by DR.GFP or EF.GFP, about 20% of transduced cells in both groups expressed GFP (Figure2A). More than 95% of the cells expressed MHC II (Figure 2A) as the starting CD34+ cell population. The MHC II expression in most CD34+ HSPCs has been reported previously.48,49 A closer examination of the MHC II (HLA-DR) expression in the transduced CD34+ cells revealed that GFP expression was almost exclusively restricted in the MHC II+ cell population in the DR.GFP transduction group. In contrast, similar percentages of GFP+ cells were detected in both MHC II+ and MHC II−populations in the EF.GFP-transduced group (Figure 2A). The SF of DR.GFP and EF.GFP in transduced PBSCs was determined to be 3.1 and 0.6, respectively (Figure 2A,D).
To further examine DR.GFP-mediated gene delivery into progenitor cells and the transgene expression in differentiated progeny with various levels of MHC II expression, the transduced human PBSCs were differentiated in culture into either DCs or erythroid/myeloid lineages. After 16 days of DC differentiation, more than 95% of the suspension cells exhibited the DC phenotype (ie, morphologically rich in surface dendrites, high levels of surface expression of MHC II, CD86, CD40, CD83, and CD1a) (Figure 2B). Further examination of GFP expression in DCs derived from the DR.GFP-transduced PBSCs demonstrated substantial specificity in MHC II+ cells (SF = 12.6, Figure 2B,D). In contrast, GFP-expressing cells derived from EF.GFP-transduced PBSCs were equally allocated between the MHC II+ and MHC II− populations (SF = 1.0, Figure2B,D).
DR.GFP- and EF.GFP-transduced PBSCs were also assayed in methylcellulose for CFCs, resulting in erythroid/myeloid differentiation. Mock-, DR.GFP-, and EF.GFP-transduced PBSCs all formed BFU-E, CFC-GM, and CFU-mix colonies, and colony numbers in 3 groups were similar, ranging from 185 to 207 (total). We found that 42% of the total colonies in the EF.GFP group displayed strong GFP signal by fluorescence microscopy. However, 25% of the colonies in the DR.GFP-transduced group were GFP+ and their GFP fluorescence intensities were much weaker, indicative of transgene down-regulation in differentiated erythorid/myeloid progeny. To further examine the correlation of GFP and MHC II expression in CFC-derived progeny, the cells were harvested from the methylcellulose assay (called non-DCs) and analyzed via FACS. In these mixed populations consisting of monocytes, granulocytes, and erythroid cells, about 20% of the cells expressed cell surface MHC II (Figure 2C). GFP+ cells were found only in MHC II+ cells in the DR.GFP transduction group (non-DC, SF = 26, Figure 2C). GFP+ cells in the DR.GFP group were restricted to cells expressing the highest level of CD13/CD33 differentiation marker (Figure 2C), and the intensity of GFP signals was lower than in differentiated DCs. These GFP+/MHC II+ cells are likely to be monocytes and macrophages present in the CFC assay. The other major cell type present in the CFC assay is erythroid cells (glycophorin A+), which are MHC II− (data not shown), contributing to 20% to 30% of the non-DCs (Figure 2C). Notably, none of these erythroid cells expressed GFP derived from DR.GFP-transduced PBSCs (Figure 2C). In contrast, in the EF.GFP transduction group, GFP was expressed in both MHC II+ and MHC II− non-DCs with no preference (SF = 1.1, Figure 2C,D).
Preferential transgene expression in MHC II+ human cells engrafted in NOD/SCID mice with DR.GFP-transduced CD34+ cells
We next tested the specificity of the HLA-DRα promoter in the DR.GFP vector to direct transgene expression in human cells in vivo using the NOD/SCID mouse model. We first used human cord blood stem cells (CBSCs) because they have higher engraftment capacity in this mouse model than PBSCs or BM CD34+cells.12,13,16 50-52 Cyropreserved CB CD34+cells were cultured overnight and transduced twice with DR.GFP or EF.GFP at the MOI of 60 in the next 2 days. Two days after the last transduction, GFP and CD34 expression of the transduced cells was examined by FACS analysis. Under our culture and transduction conditions, more than 90% of cells still maintained CD34 (Figure3A) and MHC II expression (data not shown), and about 50% of the transduced CBSCs expressed GFP 2 to 3 days after transduction (Figure 3A). These cells were then used for NOD/SCID transplantation as well as for in vitro differentiation assays.
After 14 days of in vitro differentiation in methylcellulose, numbers of total colonies and GFP+ colonies were determined. Figure3B is a representative illustration of colonies derived from mock-, DR.GFP-, and EF.GFP-transduced CBSCs and shows percentages of GFP+ colonies. Subsequent FACS analysis showed similar patterns of preferential transgene expression in MHC II+cells by DR.GFP (data not shown), as we observed with PBSCs (Figure2C). The reduction of GFP expression in MHC II− cells particularly in BFU-E colonies explains the reduced number of GFP+ colonies from DR.GFP-transduced CBSCs (Figure 3B).
To examine transgene expression in engrafted human cells from CBSCs after transduction, NOD/SCID mice were terminated 6 to 10 weeks after transplantation. Human (CD45+) cell engraftment and GFP expression in mouse BM and spleens were analyzed. Among the 15 animals that underwent transplantation, up to 20% human cells were detected in the NOD/SCID BM and about 1% to 2% in spleens (Table1), consistent with previous results published by us and others.12,13,15,16,29,32-34 Figure 3C illustrates a representative FACS analysis of NOD/SCID BM at 10 weeks after transplantation. These animals had about 20% human CD45+ cells in the BM (Figure 3C, top panels), and the engrafted human cells were further analyzed for the expression of GFP and human-specific surface markers such as CD34, CD19, and CD13 as well as MHC II (Figure 3C). In the animals engrafted with DR.GFP- or EF.GFP-transduced CBSCs, 41% to 88% of the human cells expressed GFP at high levels (Table 1 and Figure 3C). Ten weeks after transplantation, about 20% to 30% engrafted human cells in mouse BM retained the CD34+ phenotypes, about 60% expressed a human B cell marker (CD19), and about 5% expressed a marker for mature myeloid cells (CD13). Consistent with previous reports,51 52 more than 90% of the human cells in the BM of NOD/SCID mice of both groups were MHC II+ at 10 weeks after transplantation, including CD19+ B lymphocytes (about 60%), CD34+ cells (about 30%), and fewer CD13+ cells (Figure 3C). Cells expressing high-level GFP were found in all the expected human cell subsets in the NOD/SCID engraftment model, particularly in mice engrafted with EF.GFP-transduced CBSCs (Figure 3C and Table 1). Notably, these GFP+ cells found in vivo in the DR.GFP transduction group were exclusively in the MHC II+ population (SF = 4.8) but equally distributed between CD34+ and CD34− cells (Figure 3C). In contrast, GFP+cells in the EF.GFP transduction group were distributed between MHC II+ and MHC II− populations (SF = 1.3, Figure3C,E). Similar patterns and percentages of GFP+ human cells were observed in the spleen of engrafted NOD/SCID mice (Figure 3D and Table 1). The SF of either vector in human cells residing in mouse BM and spleen is shown in Figure 3E.
Percentages of human (h) CD45- and GFP-expressing cells in NOD/SCID mice after engraftment of lentiviral-transduced human CB CD34+ cells
| Mouse ID no. . | Weeks . | Bone marrow . | Spleen . | ||
|---|---|---|---|---|---|
| hCD45+ . | GFP+ in hCD45+ . | hCD45+ . | GFP+ in hCD45+ . | ||
| Mock-transduced | (Background) | (Background) | |||
| 12 | 6 | 8.4 | 1.0* | 2.6 | 0.3 |
| 14 | 6 | 0.8 | 1.0 | 0.8 | 2.6 |
| 11 | 10 | 18.3 | 0.2* | 1.7 | 0.8* |
| 13 | 10 | 4.4 | 0.8 | 0.7 | 1.0 |
| Mean ± SE | (n = 4) | 8.0 ± 3.3 | 0.8 ± 0.2 | 1.5 ± 0.4 | 1.2 ± 0.4 |
| DR.GFP-transduced | |||||
| 10 | 6 | 4.0 | 43.1* | 1.4 | 0.6 |
| 16 | 6 | 11.2 | 62.1* | 3.3 | 16.6 |
| 17 | 6 | 10.8 | 70.0 | 1.4 | 55.8* |
| 19 | 6 | 1.8 | 11.8 | 0.7 | 4.6 |
| 15 | 10 | 20.8 | 77.7* | 2.1 | 66.1* |
| 18 | 10 | 7.2 | 70.5* | 2.6 | 49.0 |
| Mean ± SE | (n = 6) | 9.3 ± 2.5 | 55.9 ± 9.2 | 1.9 ± 0.4 | 32.1 ± 10.5 |
| EF.GFP-transduced | |||||
| 21 | 6 | 4.7 | 33.3* | 1.8 | 41.6* |
| 23 | 6 | 3.4 | 17.0 | 1.3 | 4.3 |
| 24 | 6 | 0.7 | 10.5 | 0.7 | 4.7 |
| 20 | 10 | 18.9 | 88.0* | 1.8 | 56.6* |
| 22 | 10 | 13.8 | 41.0* | 0.7 | 50.1 |
| Mean ± SE | (n = 5) | 8.3 ± 3.1 | 38.0 ± 12.2 | 1.3 ± 0.2 | 31.5 ± 10.1 |
| Mouse ID no. . | Weeks . | Bone marrow . | Spleen . | ||
|---|---|---|---|---|---|
| hCD45+ . | GFP+ in hCD45+ . | hCD45+ . | GFP+ in hCD45+ . | ||
| Mock-transduced | (Background) | (Background) | |||
| 12 | 6 | 8.4 | 1.0* | 2.6 | 0.3 |
| 14 | 6 | 0.8 | 1.0 | 0.8 | 2.6 |
| 11 | 10 | 18.3 | 0.2* | 1.7 | 0.8* |
| 13 | 10 | 4.4 | 0.8 | 0.7 | 1.0 |
| Mean ± SE | (n = 4) | 8.0 ± 3.3 | 0.8 ± 0.2 | 1.5 ± 0.4 | 1.2 ± 0.4 |
| DR.GFP-transduced | |||||
| 10 | 6 | 4.0 | 43.1* | 1.4 | 0.6 |
| 16 | 6 | 11.2 | 62.1* | 3.3 | 16.6 |
| 17 | 6 | 10.8 | 70.0 | 1.4 | 55.8* |
| 19 | 6 | 1.8 | 11.8 | 0.7 | 4.6 |
| 15 | 10 | 20.8 | 77.7* | 2.1 | 66.1* |
| 18 | 10 | 7.2 | 70.5* | 2.6 | 49.0 |
| Mean ± SE | (n = 6) | 9.3 ± 2.5 | 55.9 ± 9.2 | 1.9 ± 0.4 | 32.1 ± 10.5 |
| EF.GFP-transduced | |||||
| 21 | 6 | 4.7 | 33.3* | 1.8 | 41.6* |
| 23 | 6 | 3.4 | 17.0 | 1.3 | 4.3 |
| 24 | 6 | 0.7 | 10.5 | 0.7 | 4.7 |
| 20 | 10 | 18.9 | 88.0* | 1.8 | 56.6* |
| 22 | 10 | 13.8 | 41.0* | 0.7 | 50.1 |
| Mean ± SE | (n = 5) | 8.3 ± 3.1 | 38.0 ± 12.2 | 1.3 ± 0.2 | 31.5 ± 10.1 |
Percentages of GFP+ in hCD45+cells were determined from human cell-enriched samples via murine CD45 and Ter119 depletion.
To further examine the gene transduction into pluripotent human HSPCs engrafted in the BM of NOD/SCID mice, human cells were further cultured under different conditions favoring either DC or myeloid/erythroid lineage differentiation. We found that human cells isolated from BM or spleens after engraftment were capable of differentiation into mature DCs. Most (> 80%) of the differentiated cells displayed a DC phenotype after being cultured with human GM-CSF, IL-4, and TNF-α (Figure 4A). In the DR.GFP transduction group, GFP+ cells were almost exclusively found in MHC II+ cells, particularly in those expressing the highest level of MHC II (Figure 4A). Both transgene and MHC II expression were up-regulated after DC differentiation. In contrast, GFP+cells in the EF.GFP group were more uniformly distributed between existent MHC II+ and MHC II− populations (Figure 4A).
Similarly, in the CFC assay resulting in myeloid/erythroid differentiation, we found that about 50% of colonies in the EF.GFP group were GFP+, whereas less than 10% of the colonies in the DR.GFP group were GFP+. We also examined the correlation of GFP and MHC II expression in CFC-derived erythroid/myeloid (non-DC) cells after engraftment, as in the CFC progeny before engraftment (Figures 2C and 3B). Once again, GFP expression was limited only to the MHC II+ populations in the DR.GFP transduction group (SF = 9.5). In contrast, in the EF.GFP group, GFP was expressed in both MHC II+ and MHC II− populations (SF = 1.5, Figure 4B).
Based on our in vivo and in vitro data, we conclude that engrafting and pluripotent human HSPCs have been transduced by both DR.GFP and EF.GFP vectors. Under the conditions employed (transduction at days 1 and 2), 50% SCID repopulating cells, DCs, as well as CFC progenitors were transduced, as evident in the EF.GFP transduction group. In contrast to the EF-1α promoter, which expressed more ubiquitously, the HLA-DRα promoter in the DR.GFP vector directed transgene expression preferentially in MHC II+ cells in vivo and in vitro and highly in mature DCs.
Human DCs derived from the BM and spleen of engrafted NOD/SCID mice were potent APCs in stimulating T-cell proliferation
To further confirm the proper antigen presentation function of the transduced DCs after engraftment, the immune stimulatory potency of these cells was examined in the MLR assay with allogeneic human T lymphocytes subsequent to transplantation and ex vivo differentiation/maturation. As shown in Figure5, these human DCs stimulated strong allogeneic-MLR responses even at stimulator:effector (T cells) ratios as low as 1:640. The stimulatory capacity of in vivo–derived DCs after transplantation was compatible to that of transduced DCs derived from untransplanted human CBSCs (Figure 5). Furthermore, there were no obvious differences in the stimulatory effects of DCs derived from mock- and LV-transduced cells.
Stimulation of human T cells by in vitro–differentiated DCs after engraftment of transduced CD34+ cells.
In this MLR T-cell proliferation assay, human PBLs (as effectors) were harvested from an unrelated (allogeneic) healthy donor and seeded (2 × 105/well) in 96-well plates in triplicate. Differentiated human DCs from BM (Figure 4) or spleen (SP) of the NOD/SCID mice that received transplants of DR.GFP-, EF.GFP-, or mock-transduced CD34+ cells were added in a serial dilution as APC stimulators. DC and T cells were cultured together for 3 days at stimulator-effector ratios of 1:10, 1:80, and 1:640. The stimulation of lymphocyte proliferation was determined by [3H]thymidine incorporation after the 3-day culture and 18-hour pulsing. The mean incorporations (counts per minute, cpm) from 3 triplicates were calculated and plotted, in comparison to the DCs differentiated from CB CD34+ cells without the transduction and engraftment steps (CB CD34-DC).
Stimulation of human T cells by in vitro–differentiated DCs after engraftment of transduced CD34+ cells.
In this MLR T-cell proliferation assay, human PBLs (as effectors) were harvested from an unrelated (allogeneic) healthy donor and seeded (2 × 105/well) in 96-well plates in triplicate. Differentiated human DCs from BM (Figure 4) or spleen (SP) of the NOD/SCID mice that received transplants of DR.GFP-, EF.GFP-, or mock-transduced CD34+ cells were added in a serial dilution as APC stimulators. DC and T cells were cultured together for 3 days at stimulator-effector ratios of 1:10, 1:80, and 1:640. The stimulation of lymphocyte proliferation was determined by [3H]thymidine incorporation after the 3-day culture and 18-hour pulsing. The mean incorporations (counts per minute, cpm) from 3 triplicates were calculated and plotted, in comparison to the DCs differentiated from CB CD34+ cells without the transduction and engraftment steps (CB CD34-DC).
Discussion
This study addressed 2 important aspects of stem cell gene therapy: efficient transgene delivery into pluripotent HSPCs and restricted transgene expression in a selected differentiation lineage of transduced HSPCs. We demonstrated efficient gene delivery into NOD/SCID repopulating human HSPCs by a short transduction protocol, as measured by GFP transgene expression in multiple lineages derived from transduced HSPCs. The high-level gene delivery and persistent expression was evident by the fact that 41% to 88% of human cells (including lymphoid and myeloid lineages) engrafted in NOD/SCID mice were GFP+ (Table 1). Similar transduction efficiencies were observed in human progenitor cells capable of generating erythroid/myeloid colonies and DCs, before and after transplantation. As evident with the transduction group by the EF.GFP vector, the transduction efficiency of pluripotent human HSPCs engrafted in NOD/SCID mice—evaluated by persistent transgene expression in multiple lineages—achieved in this study was comparable to or higher than other reported studies.26,29 32-34 These observations collectively confirmed the potential of LV in gene delivery into engrafting human HSPCs at high efficiencies.
The more unique and significant aspect of this study is the demonstration of restricted transgene expression in a specific lineage derived from transduced pluripotent HSPCs. We specifically focused on APCs/DCs in this study because of our interest in expressing genes selectively and highly in APCs/DCs. We constructed a SIN LV (DR.GFP) containing the MHC II–specific promoter from the human HLA-DRα gene, which is expressed selectively in APCs and highly in mature DCs. We found that almost all of the GFP+ cells derived from DR.GFP-transduced HSPCs were MHC II+ (such as DCs, B cells, and other APCs), whereas those derived from EF.GFP-transduced HSPCs were found in both MHC II+ and MHC II− (such as erythroid) populations. The restricted transgene expression by the DR.GFP vector in MHC II+ cells was observed in cells derived from freshly transduced PBSCs and CBSCs (Figures 2-4) as well as engrafted human cells in NOD/SCID mice (Figure 3 and 4). DR.GFP-mediated transgene expression was increased in progeny that acquired high-level expression of MHC II (such as DCs) after engraftment and/or in vitro differentiation, in parallel with the endogenous HLA-DR expression. Quantitatively, the SF of the DR.GFP vector was 3.5 to 26 in various progenies derived from transduced HSPCs. In contrast, the SF of the EF.GFP vector was about 1 (0.6 to 1.5), indicating no preference of transgene expression in MHC II+ cells. In addition to the SF measurement, which calculates the ratios of GFP+ cell numbers, we also found that the intensity of transgene expression after DR.GFP transduction largely correlated with that of MHC II. Notably, in mature DCs after in vitro differentiation/maturation, the intensity of transgene expression mediated by the HLA-DRα promoter was very high, close to those mediated by the EF-1α promoter. Therefore, we demonstrated targeting transgene expression to MHC II+cells such as APCs/DCs after transduction of pluripotent HSPCs. We conclude that selective expression of the transgene mediated by DR.GFP in MHC II+ cells results from the inactivation of HLA-DRα promoter in MHC II− cells and its activation in differentiated DCs as the endogenous gene. We also believe that similar strategies by the SIN LV can be used for targeting transgene expression to other specific hematopoietic lineages after HSPC transduction and differentiation if an autonomous and specific promoter is available. The SIN LV we used can accommodate both intronless and intron-containing promoters (such as EF-1α) up to 5 kilobases long (plus DNA sequences of gene and other genetic elements). Therefore, the SIN LV is more flexible in selecting the promoter of choice to achieve either sustained or restricted transgene expression after stable transduction of pluripotent HSPCs. For this reason, we may be able to achieve more DC-specific transgene expression using promoters from recently cloned DC-specific genes after the promoter sequences are delineated.53
By transducing pluripotent HSPCs, we can obtain genetically modified DC progenitors that are capable of engrafting into NOD/SCID mouse BM, differentiating into mature DCs, and potently stimulating human T cells (Figure 5). Our study has provided new insights on human APCs/DCs differentiated from engrafting HSPCs. For example, we found that DC progenitors can engraft and survive in mouse BM and spleens, because functional human DCs can be generated subsequently from engrafted human cells. However, we did not find human MHC II+ cells with the mature DC phenotype (CD83+, CD40+, and CD86high) either in mouse BM or spleens. Our results are consistent with a recent report that human DC differentiation is blocked prior to the CD83+ stage in NOD/SCID mice that underwent transplantation.54 It is unclear, however, whether there was true (partial) DC differentiation from human HSPCs in NOD/SCID mice or whether simply a fraction of engrafted human HSPCs maintained their pluripotency, including the ability to differentiate in subsequent DC cultures. The latter hypothesis is supported by the fact that about 30% of engrafted human cells retained the CD34+ phenotype (Figure 3C) 10 weeks after engraftment. Future experiments with the NOD/SCID model with the addition of human fetal thymus and/or human cytokines may allow us to examine the function, as well as transgene expression, in the human APC/DC progeny matured in vivo after HSPC transduction and transplantation. In parallel, we have also started to test the DR.GFP LV to transduce mouse HSPCs using immunocompetent mice. We observed similarly preferential and high-level transgene expression in mouse APCs/DCs in reconstituted mice after HSPC transplantation by the DR.GFP LV and another SIN LV containing a model antigen driven by the HLA-DRα promoter (unpublished data, March 2001). We hope to use this selective transgene expression system to functionally modify various subsets of APCs/DCs that are differentiated naturally in vivo from transduced HSPCs following transplantation and to examine immune responses mediated by transduced APCs/DCs expressing a specific antigen or regulatory molecule.
In summary, this study demonstrated the feasibility of targeted gene expression in specific differentiation lineage derived in vivo from transduced pluripotent stem cells. The methodology provides a basis for future targeted gene therapy applications to reduce potential adverse effects of broad transgene expression and increase therapeutic effectiveness mediated by gene transduction of HSPCs and other types of stem cells.
We thank Dr Didier Trono for providing the pRLLhPGK.GFP Sin-18 lentiviral vector and 2 packaging and envelope-expressing vectors, Dr Curt Civin and Ms Rene Smith for providing NOD/SCID mice, Dr Dae-Chul Joeng for Wright-Giemsa staining, Ms Leslie Meszler for technical assistance of microscopic photographing, and gifts from the Topercer family and Mrs Doris Needle to D.P. We gratefully appreciate the intellectual discussion and suggestions by Drs Peter Gao and Xianzheng Zhou. We also thank Drs X. Zhou, Katherine Whartenby, and Enrico Novelli for critical reading of the manuscript.
Supported in part by a National Institutes of Health grant (P30-CA06973 to L.C. and D.P.). Y.C. is supported by a Cancer Research Institute/Libby Bartnick Memorial Fellowship. L.C. is supported by the Alexander and Margaret Stewart Trust Scholarship. D.P. is a Janney Foundation Scholar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Linzhao Cheng, Div of Immunology and Hematopoiesis, Johns Hopkins Oncology Center, Bunting-Blaustein Cancer Research Bldg, Rm 208, 1650 Orleans St, Baltimore, MD 21231; e-mail:lcheng@welch.jhu.edu.

![Fig. 5. Stimulation of human T cells by in vitro–differentiated DCs after engraftment of transduced CD34+ cells. / In this MLR T-cell proliferation assay, human PBLs (as effectors) were harvested from an unrelated (allogeneic) healthy donor and seeded (2 × 105/well) in 96-well plates in triplicate. Differentiated human DCs from BM (Figure 4) or spleen (SP) of the NOD/SCID mice that received transplants of DR.GFP-, EF.GFP-, or mock-transduced CD34+ cells were added in a serial dilution as APC stimulators. DC and T cells were cultured together for 3 days at stimulator-effector ratios of 1:10, 1:80, and 1:640. The stimulation of lymphocyte proliferation was determined by [3H]thymidine incorporation after the 3-day culture and 18-hour pulsing. The mean incorporations (counts per minute, cpm) from 3 triplicates were calculated and plotted, in comparison to the DCs differentiated from CB CD34+ cells without the transduction and engraftment steps (CB CD34-DC).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.399/6/m_h80222017005.jpeg?Expires=1769952517&Signature=UntVzo3sUA1cjieitf-SFxU2lPfAi4Yvxp5oxvphKj994LjE29NLWP3-hgbcn7yZ0ijZNtyrEuP4RUoDY8sWmjDxYpyHbEIu72luwc2CrJNESeZ~t1bcGys4j31T4QFjo8-NCHH6CHiZyGNTT09VAz6M9n0A9C2dRd13lU2lo7KrEE7sTr2syden~yk~ZJIV3ZhnBtCFb-BONgTwbaY4zfIE23hi~ty1q2FZyWn01vy21zydV6-EaxsZA9NnrkQI5wjAQCvNiZfk9EjULOAIRSNxiIkdKElEYz~OWgHsBabjtUo-3eR0Ph98Z5tE7Ph5PltfcuMpMBCtebmu7hIfMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal