Abstract
It has recently been shown that CD4+CD25+ T cells are immunoregulatory T cells that prevent CD4+ T-cell–mediated organ-specific autoimmune diseases. In this study, the regulatory mechanism of CD4+CD25+ T-cell development were investigated using T-cell receptor (TCR) transgenic mice. It was found that CD4+CD25+ T cells preferentially expressed the endogenous TCRα chain in DO10+ TCR transgenic mice compared with CD4+CD25− T cells. Moreover, it was found that CD4+CD25+ thymocytes were severely decreased in DO10+ TCR-α−/− mice in positively selecting and negatively selecting backgrounds, whereas CD4+CD25− thymocytes efficiently developed by transgenic TCR in DO10+ TCR-α−/− mice in positively selecting backgrounds, indicating that the appropriate affinity of TCR to major histocompatibility complex (MHC) for the development of CD4+CD25+ thymocytes is different from that of CD4+CD25− thymocytes and that a certain TCR–MHC affinity is required for the development of CD4+CD25+ thymocytes. Finally, it was found that, in contrast to thymus, CD4+CD25+ T cells were readily detected in spleen of DO10+TCR-α−/− mice in positively selecting backgrounds and that splenic CD4+CD25+ T cells, but not CD4+CD25+ thymocytes, were significantly decreased in B-cell–deficient mice, suggesting that B cells may control the peripheral pool of CD4+CD25+ T cells. Together, these results indicate that the development of CD4+CD25+ T cells in thymus and the homeostasis of CD4+CD25+ T cells in periphery are regulated by distinct mechanisms.
Introduction
Immunologic tolerance is a feature of the immune system essential for discrimination between self and nonself. Recent data suggest that in addition to clonal deletion and anergy, regulatory T cells play a significant role in the generation and maintenance of tolerance.1-4 The term regulatory T cells is used for a variety of immunoregulatory cells that can be subdivided into a number of subsets based on expression of cell surface proteins and pattern of cytokine production.1-4 Because these subsets of regulatory T cells have been characterized in experimental models using different assays, the interrelationship between the subsets is difficult to understand.1-4
One of the best-characterized subsets of CD4+ regulatory T cells is defined by its constitutive expression of interleukin-2 receptor (IL-2R)–α chain (CD25) (CD4+CD25+ T cells).3,4 CD4+CD25+ T cells constitute approximately 10% of peripheral CD4+ T cells in nonimmunized naive mice and exhibit a broad spectrum of autoimmunity-preventive activity.5-8CD4+CD25+ T cells are naturally anergic and, on T-cell receptor (TCR)–mediated activation, potently suppress the proliferation of CD4+CD25− T cells by an antigen-nonspecific mechanism.9-11 Molecular mechanisms by which CD4+CD25+ T cells mediate suppression are unclear but seem to be independent of cytokine production9,10 and dependent on cell contact9,10 and to require constitutive expression of CTLA-4.12 13
CD4+CD25+ T cells can be subdivided by the expression pattern of CD45RBhigh or CD45RBlow, CD38+ or CD38−, CD69+ or CD69−, or CD62Lhigh or CD62Llow.5,9,11,14 Although these findings suggest that CD4+CD25+ T cells might be heterogeneous and composed of a mixture of regulatory T cells and activated conventional T cells, it has recently been shown that the anergic and suppressive properties of CD4+CD25+T cells cannot be subdivided to a smaller subpopulation defined by the expression levels of CD45RB, CD62L, CD38, or CD69.11 14Therefore, CD4+CD25+ T cells may represent a relatively homogeneous population of regulatory T cells.
Precise signals that promote the development of CD4+CD25+ T cells remain elusive, but considerable evidence suggests that costimulatory molecules and cytokines play important roles. It has been shown that CD4+CD25+ T cell levels are severely decreased in mice lacking CD288 or CD40L,15 indicating that signaling by CD28 and by CD40L regulates the number of CD4+CD25+ T cells in periphery. In addition, CD4+CD25+ T-cell levels are decreased in mice lacking IL-216 or IL-2R component,17indicating that IL-2 signaling also regulates the number of CD4+CD25+ T cells.
The CD25+ population constitutes approximately 5% of CD4+CD8−CD3high mature thymocytes in normal mice (CD4+CD25+ thymocytes), and the CD4+CD25+ thymocytes exhibit a similar functional property to that found in peripheral CD4+CD25+ T cells.18 Moreover, thymectomy on day 3 of life decreases the number of CD4+CD25+ T cells from the peripheral lymphoid organ.6 Furthermore, CD4+CD25+ T cells cannot be produced by in vitro culture of CD4+CD25− T cells.7,9 14 Taken together, these findings suggest that CD4+CD25+T cells arise in the thymus and migrate to the periphery.
Recently, Itoh et al18 have shown that CD4+CD25+ T cells can develop in TCR transgenic mice of wild-type background but not of RAG-2–deficient background. Thus, it is possible that B cells, γδ T cells, or natural killer (NK) T cells, all of which are absent in TCR transgenic RAG-2–deficient mice but are present in TCR transgenic mice, are required for the development of CD4+CD25+ T cells. It is also possible that the expression of endogenous TCR and the subsequent interaction with self-major histocompatibility complex (MHC) at a certain affinity is required for the development of these cells. However, a great deal of uncertainty remains about differentiation factors and antigen specificity of CD4+CD25+ T cells.
In the current study, to determine whether positive or negative selection in thymus regulates the development of CD4+CD25+ T cells, we investigated CD4+CD25+ T-cell development in mice expressing TCR transgene in positively selecting and negatively selecting MHC backgrounds. We also investigated the requirement of B cells and NK T cells for the development of CD4+CD25+ T cells. Our results indicate that the size of the CD4+CD25+ T-cell pool in periphery is regulated at least 2 distinct levels. First, in the thymus, where CD4+CD25+ T cells are produced, a certain range of TCR–MHC affinity is required for their development. In addition, B cells, but not NK T cells, regulate the expansion and/or survival of CD4+CD25+ T cells in periphery.
Materials and methods
Mice
BALB/c and C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). Immunoglobulin μ-chain–deficient mice19 and NK T-cell–deficient mice20 of C57BL/6 background were previously described. Ovalbumin-specific DO11.10 (DO10+) TCR transgenic mice21 were backcrossed to BALB/c mice for more than 10 generations. BALB/c RAG-2−/−mice22 were a kind gift from Dr T. Saito (Chiba University). TCR-α−/− mice23(H-2b/b) were crossed with DO10+ mice (H-2d/d), and then offspring DO10+TCR-α+/− H-2d/b mice were crossed with TCR-α+/− H-2d/b mice to develop DO10+ TCR-α−/− mice in H-2d/d, H-2d/b, or H-2b/b background. Expressions of DO10+ transgene and H-2 haplotypes were determined by fluorescence-activated cell sorter analysis (FACS), as described below. The genotype of TCR-α allele was determined by polymerase chain reaction using the following primer pairs: to detect TCR-α wild-type allele, 5′-aagatcctcggtctcaggacagc-3′ and 5′-ggtaggtggcgttggtctctttg-3′; to detect TCR-α mutant allele, 5′-attcgcagcgcatcgccttctatcg-3′ and 5′-ggtaggtggcgttggtctctttg-3′. Homozygosity for the TCR-α mutant allele was confirmed, using FACS, by the absence of T cells that express endogenous TCR V-α2. Mice were housed in micro-isolator cages under pathogen-free conditions; 8- to 10-week-old mice were used in all experiments.
Flow cytometric analysis
Cells from thymus and spleen were stained and analyzed on a FACScalibur (Becton Dickinson, San Jose, CA) using CELLQuest software. For direct staining, the following conjugated antibodies were purchased from PharMingen (San Diego, CA): anti-CD3 fluorescein isothiocyanate (FITC) (145-2C11), anti-CD4 FITC, phycoerythrin (PE), PerCP, allophycocyanin (APC) (H129.19), anti-B220 FITC (RA3-6B2), anti-CD8 FITC, APC (53.6.7), anti-CD25 PE (PC61), anti-CD25 FITC (7D4), anti-CD45RB FITC (16A), anti–NK-1.1 PE (PK136), anti-TCR V-α2 FITC (B20.1), anti-TCR V-α11 FITC (RR8-1), anti-TCR Vβ8 FITC (F23.1), anti–H-2Kb PE (AF6-88.5), anti–H-2Kd FITC (SF1-1.1), anti–I-Ab PE (AF6-120.1), and anti–I-Ad FITC (AMS-32.1). KJ1-26 monoclonal antibody, anti-idiotype for DO10 TCR,24 was purified from supernatants of hybridoma cells using protein G columns (Pharmacia, Uppsala, Sweden) and conjugated to FITC or biotin. Before staining, Fc receptors were blocked with anti-CD16/32 antibody (2.4G2; PharMingen).
Annexin V staining
After cells were stained with anti-CD4 APC and anti-CD25 PE and washed twice with phosphate-buffered saline–1% bovine serum albumin, cells were stained with annexin V–FITC (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Cells were then analyzed on a FACScalibur.
Data analysis
Data are summarized as mean ± SD. Statistical analysis of the results was performed by the unpaired t test.P < .05 was considered significant.
Results
CD4+CD25+ T cells preferentially express the second TCR-α chain in TCR transgenic mice
It is well established that allelic exclusion of the TCR-β chain is almost perfect, whereas that of the TCR-α chain is incomplete.25 Thus, a considerable fraction of T cells express 2 different TCR-αβ pairs.26 Because it has been shown that CD4+CD25+ T cells can develop in TCR transgenic mice of wild-type background but not of RAG-2–deficient background,18 it is possible that CD4+CD25+ T cells preferentially express the endogenous TCR-α chain and that signaling through the endogenous TCR-α chain coupled with the transgenic TCR-β chain is involved in the development of CD4+CD25+ T cells in TCR transgenic mice. To examine this possibility, we first investigated the frequency of CD4+ T cells that express both transgenic TCR (recognized by anti-idiotypic monoclonal antibody, KJ1-26) and one of the endogenous TCR V-α chains, V-α2, in the CD25+ or CD25− population in ovalbumin-specific TCR transgenic (DO10+) mice. As shown in Figure1A, splenic CD4+ T cells that express transgenic TCR and TCR V-α2 chain were found at a higher frequency in CD4+CD25+ T cells than in CD4+CD25− T cells (CD4+CD25+ T cells 12.0% ± 1.6% versus CD4+CD25− T cells 2.5% ± 1.0%, mean ± SD; n = 5; P < .001). Moreover, CD4+ T cells that express both transgenic TCR and TCR V-α11 were also found at a higher frequency in CD4+CD25+ T cells (data not shown). These results suggest that CD4+CD25+ T cells preferentially express the second TCR-α chain in DO10+ mice. For controls, we performed the same analysis for splenocytes in DO10+RAG-2–deficient mice. We found that, consistent with the previous report by Itoh et al,18 CD4+CD25+T cells were almost absent in these mice (Figure 1B) and that no CD4+CD25− T cell expressed TCR V-α2 (Figure1B) or TCR V-α11 (data not shown) in these mice.
CD4+CD25+ T cells preferentially express the second TCRα chain in DO10+ TCR transgenic mice.
Single-cell suspension of splenocytes from DO10+ mice (A) and DO10+ RAG-2−/− mice (B) were stained with anti-TCR Vα2 FITC, anti-CD25 PE, anti-CD4 PerCP, and KJ1-26 biotin. After biotinylated antibody was visualized with streptavidin APC, cells were analyzed on FACScalibur. Shown are representative FACS profiles of CD4 versus CD25 of CD4+ splenocytes (upper panels) and KJ1-26 versus Vα2 (lower panels) on either CD4+CD25+ T cells or CD4+CD25− T cells from 5 mice in each group.
CD4+CD25+ T cells preferentially express the second TCRα chain in DO10+ TCR transgenic mice.
Single-cell suspension of splenocytes from DO10+ mice (A) and DO10+ RAG-2−/− mice (B) were stained with anti-TCR Vα2 FITC, anti-CD25 PE, anti-CD4 PerCP, and KJ1-26 biotin. After biotinylated antibody was visualized with streptavidin APC, cells were analyzed on FACScalibur. Shown are representative FACS profiles of CD4 versus CD25 of CD4+ splenocytes (upper panels) and KJ1-26 versus Vα2 (lower panels) on either CD4+CD25+ T cells or CD4+CD25− T cells from 5 mice in each group.
Dual TCR-α expression is not essential for the development of CD4+CD25+ T cells
CD4+CD25+ T cells preferentially expressed dual TCR-α chains in DO10+ mice (Figure 1A). To determine whether dual TCR-α expression is required for the development of CD4+CD25+ T cells, we investigated the development of CD4+CD25+ T cells in TCR-α heterozygous mice, in which only one allele of TCR-α was available for the expression. As shown in Figure 2, the number of CD4+CD25+ T cells in thymus and in spleen was normal in TCR-α heterozygous mice, suggesting that though CD4+CD25+ T cells preferentially expressed the second TCR-α chain in TCR transgenic mice, dual TCR-α expression was not essential for the development of CD4+CD25+ T cells.
Dual TCRα expression is not essential for the development of CD4+CD25+ T cells.
(A) Thymocytes from TCRα+/− mice and TCRα+/+ mice were stained with anti-CD3 FITC, anti-CD25 PE, anti-CD8 APC, and anti-CD4 PerCP. Shown are representative FACS profiles of CD4 versus CD25 on a CD4+CD8−CD3high population. FACS profile of CD4 versus CD8 and cell number of thymocytes was indistinguishable between TCRα+/− mice and TCRα+/+ mice (data not shown). (B) Splenocytes from TCRα+/− mice and TCRα+/+ mice were stained with anti-CD25 PE and anti-CD4 FITC. Shown are representative FACS profiles of CD4 versus CD25 on the CD4+ population from 5 mice in each group.
Dual TCRα expression is not essential for the development of CD4+CD25+ T cells.
(A) Thymocytes from TCRα+/− mice and TCRα+/+ mice were stained with anti-CD3 FITC, anti-CD25 PE, anti-CD8 APC, and anti-CD4 PerCP. Shown are representative FACS profiles of CD4 versus CD25 on a CD4+CD8−CD3high population. FACS profile of CD4 versus CD8 and cell number of thymocytes was indistinguishable between TCRα+/− mice and TCRα+/+ mice (data not shown). (B) Splenocytes from TCRα+/− mice and TCRα+/+ mice were stained with anti-CD25 PE and anti-CD4 FITC. Shown are representative FACS profiles of CD4 versus CD25 on the CD4+ population from 5 mice in each group.
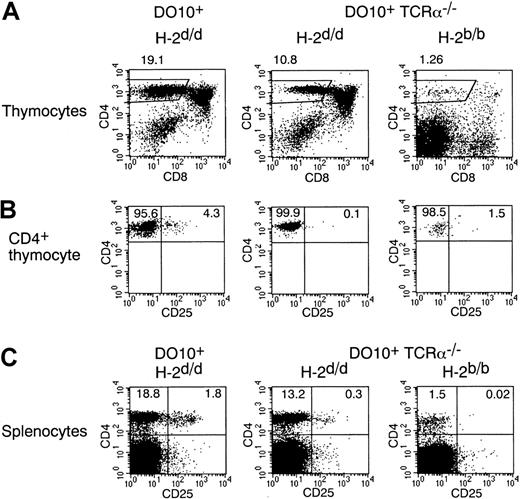
CD4+CD25+ thymocytes are severely decreased in DO10+ TCR-α−/− mice in positively selecting and negatively selecting backgrounds
Because CD4+CD25+ T cells are absent in DO10+ RAG-2−/− mice (Figure 1B) and because CD4+CD25+ T cells preferentially express the second TCR-α chain in DO10+ mice (Figure 1A), the expression of endogenous TCR-α chain and subsequent interaction with self-MHC at an appropriate affinity may be required for the development of CD4+CD25+ T cells. To test this possibility, we investigated the development of CD4+CD25+ T cells in DO10+ TCR-α–deficient (TCR-α−/−) mice (Figure 3). Indeed, the frequency of the CD25+ population in CD4+CD8− mature thymocytes was severely decreased in DO10+ TCR-α−/− mice with positively selecting H-2d background compared with that in DO10+ TCR-α+/+ mice (Figure 3B). In contrast, no significant difference was observed in the number of conventional CD4+CD25− mature thymocytes between DO10+ TCR-α−/− mice and DO10+ TCR-α+/+ mice (Figure 3B). These results indicate that expression of the endogenous TCR-α chain is required for the development of CD4+CD25+ T cells in DO10+ mice. In addition, they indicate that the appropriate affinity of TCR to self-MHC for the development of CD4+CD25+ thymocytes is different from that of CD4+CD25− thymocytes and that the interaction of TCR with self-MHC at a certain affinity is essential for the development of CD4+CD25+ thymocytes.
CD4+CD25+ thymocytes are severely decreased in DO10+TCRα−/− mice in positively selecting and negatively selecting backgrounds.
(A, B) Thymocytes from DO10+ mice (H-2d/d), DO10+ TCRα−/− mice (H-2d/d), and DO10+ TCRα−/−mice (H-2b/b) were stained with KJ1-26 FITC, anti-CD25 PE, anti-CD8 APC, and anti-CD4 PerCP. Shown are representative FACS profiles of CD4 versus CD8 on total thymocytes (A) and CD4 versus CD25 on CD4+ CD8− KJ1-26high thymocytes (B) from 5 mice in each group. The numbers of thymocytes are as follows: DO10+ TCRα+/+H-2d/d mice (10.8 ± 2.6 × 107), DO10+ TCRα−/− H-2d/d mice (13.2 ± 3.0 × 107), and DO10+TCRα−/− H-2b/b mice (0.6 ± 0.2 × 107) (n = 5 mice each;P < .01). (C) Splenocytes from DO10+H-2d/d mice, DO10+ TCRα−/−H-2d/d mice, and DO10+ TCRα−/−H-2b/b mice were stained with anti-CD25 PE and anti-CD4 FITC. Shown are representative FACS profiles of CD4 versus CD25 on splenocytes from 5 mice in each group.
CD4+CD25+ thymocytes are severely decreased in DO10+TCRα−/− mice in positively selecting and negatively selecting backgrounds.
(A, B) Thymocytes from DO10+ mice (H-2d/d), DO10+ TCRα−/− mice (H-2d/d), and DO10+ TCRα−/−mice (H-2b/b) were stained with KJ1-26 FITC, anti-CD25 PE, anti-CD8 APC, and anti-CD4 PerCP. Shown are representative FACS profiles of CD4 versus CD8 on total thymocytes (A) and CD4 versus CD25 on CD4+ CD8− KJ1-26high thymocytes (B) from 5 mice in each group. The numbers of thymocytes are as follows: DO10+ TCRα+/+H-2d/d mice (10.8 ± 2.6 × 107), DO10+ TCRα−/− H-2d/d mice (13.2 ± 3.0 × 107), and DO10+TCRα−/− H-2b/b mice (0.6 ± 0.2 × 107) (n = 5 mice each;P < .01). (C) Splenocytes from DO10+H-2d/d mice, DO10+ TCRα−/−H-2d/d mice, and DO10+ TCRα−/−H-2b/b mice were stained with anti-CD25 PE and anti-CD4 FITC. Shown are representative FACS profiles of CD4 versus CD25 on splenocytes from 5 mice in each group.
We next analyzed CD4+CD25+ T-cell development by transgenic TCR in DO10+ mice in a negatively selecting H-2b background.27 28 For this purpose, we investigated DO10+ TCR-α−/− mice to exclude the effect of endogenous TCR-α expression. Interestingly, the number of total thymocytes in DO10+ TCR-α−/− mice was severely decreased in H-2b background (DO10+ TCR-α−/− H-2b mice compared with DO10+ TCR-α−/−H-2d mice; 0.6 ± 0.2 × 107 versus 13.2 ± 3.0 × 107; n = 5 each;P < .001). Most thymocytes were within the CD4−CD8− stage (Figure 3A); consequently, the number of CD4+CD8− thymocytes was severely decreased in these mice. When gated on the CD4+CD8− thymocytes that could escape from negative selection in DO10+ TCR-α−/− mice in H-2b background, the frequency of the CD25+population was still severely decreased (Figure 3B). These results suggest that CD4+CD25+ T cells could not develop when the affinity between TCR and MHC was so high.
Interestingly, though the number of CD4+CD25+thymocytes was severely decreased in DO10+TCR-α−/− mice, CD4+CD25+ T cells were readily observed in the spleens of DO10+TCR-α−/− mice in H-2d background (Figure3C). The population of CD4+CD25+ T cells was also approximately 2-fold in the spleens of DO10+TCR-α+/+ mice compared with that of CD4+CD25+ thymocytes (Figure 3B-C). Most splenic CD4+CD25+ T cells in DO10+TCR-α−/− mice and DO10+TCR-α+/+ mice were of the CD45RBlowCD69− phenotype, similar to that in wild-type mice (data not shown). These results suggest that there existed some homeostatic machinery that regulated the size of the CD4+CD25+ T-cell pool in periphery.
B cells but not natural killer T cells regulate the peripheral pool of CD4+CD25+ T cells
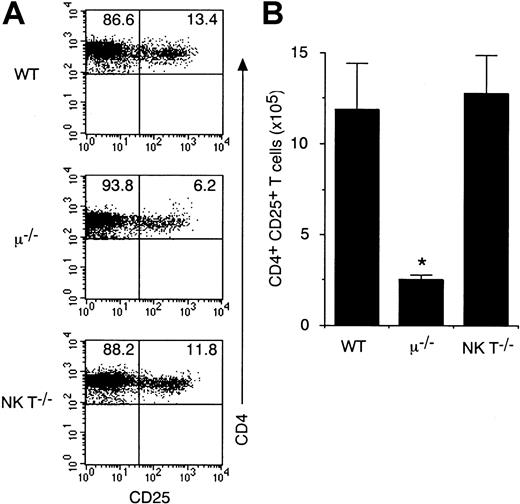
CD4+CD25+ T cells exist in the spleens of DO10+ TCR-α−/− H-2d mice (Figure 3C) but not in spleens of DO10+RAG-2−/− mice (Figure 1B). Thus, it is possible that a certain population of cells absent in RAG-2–deficient mice regulates the size of the CD4+CD25+ T-cell pool in periphery. The possibility that B cells might affect CD4+CD25+ T-cell development was examined by flow cytometric analyses of CD4+CD25+ T cells in μ-chain–deficient mice, which lack mature B cells.19Interestingly, a profound decrease of splenic CD4+CD25+ T cells was observed in μ-chain–deficient mice (Figure 4A). Less than 7% of CD4+ T cells in μ-chain–deficient mice expressed CD25 compared with 13% in wild-type mice (n = 5 each) (Figure 4A). Because the absolute number of splenic T cells was decreased in μ-chain–deficient mice, the number of splenic CD4+CD25+ T cells was significantly decreased in μ-chain–deficient mice (approximately 20% of wild-type levels;P < .001; Figure 4B). Interestingly, in contrast to splenic CD4+CD25+ T cells, the number of CD25+ population in CD4+ CD8−mature thymocytes was normal in μ-chain–deficient mice (Figure5). Taken together, these results indicate that though the development of CD4+CD25+ T cells in thymus does not require help from B cells, the size of CD4+CD25+ T-cell pool in periphery is regulated by B cells. In contrast, the number of splenic CD4+CD25+ T cells (Figure 4) and CD4+CD25+ thymocytes (Figure 5) was normal in NK T cell-deficient mice, indicating that NK T cells are not essential for the development of CD4+CD25+ T cells.
Splenic CD4+CD25+ T cells are decreased in μ-chain–deficient mice but not in NK T-cell–deficient mice.
Splenocytes from μ-chain–deficient mice, NK T-cell–deficient mice, and wild-type (WT) mice were stained with anti-CD25 PE and anti-CD4 FITC. Shown are representative FACS profiles of CD4 versus CD25 on the CD4+ population (A) and the absolute number of CD4+CD25+ T cells in spleen (B) from 4 to 5 mice in each group. *Significantly different from the mean value of wild-type mice; *P < .001.
Splenic CD4+CD25+ T cells are decreased in μ-chain–deficient mice but not in NK T-cell–deficient mice.
Splenocytes from μ-chain–deficient mice, NK T-cell–deficient mice, and wild-type (WT) mice were stained with anti-CD25 PE and anti-CD4 FITC. Shown are representative FACS profiles of CD4 versus CD25 on the CD4+ population (A) and the absolute number of CD4+CD25+ T cells in spleen (B) from 4 to 5 mice in each group. *Significantly different from the mean value of wild-type mice; *P < .001.
CD4+CD25+ thymocytes can develop normally in μ-chain–deficient mice and NK T-cell–deficient mice.
Thymocytes from μ-chain–deficient mice, NK T-cell–deficient mice, and wild-type (WT) mice were stained with anti-CD3 FITC, anti-CD25 PE, anti-CD8 APC, and anti-CD4 PerCP. Shown are representative FACS profiles of CD4 versus CD25 on CD4+CD8−CD3high thymocytes from 4 to 5 mice in each group. FACS profile of CD4 versus CD8 and cell number of thymocytes was indistinguishable among these mice (data not shown).
CD4+CD25+ thymocytes can develop normally in μ-chain–deficient mice and NK T-cell–deficient mice.
Thymocytes from μ-chain–deficient mice, NK T-cell–deficient mice, and wild-type (WT) mice were stained with anti-CD3 FITC, anti-CD25 PE, anti-CD8 APC, and anti-CD4 PerCP. Shown are representative FACS profiles of CD4 versus CD25 on CD4+CD8−CD3high thymocytes from 4 to 5 mice in each group. FACS profile of CD4 versus CD8 and cell number of thymocytes was indistinguishable among these mice (data not shown).
Discussion
In this study, we show that the size of the peripheral CD4+CD25+ T-cell pool is regulated by at least 2 distinct mechanisms. First, in the thymus, where CD4+CD25+ T cells are produced, a certain TCR–MHC affinity is required for the development of CD4+CD25+ T cells. We found that CD4+CD25+ T cells preferentially expressed the second TCR-α chain in DO10+ TCR transgenic mice (Figure1A) and that the development of CD4+CD25+thymocytes was severely impaired in DO10+TCR-α−/− mice (Figure 3B), indicating that the expression of the endogenous TCR-α chain is required for the development of CD4+CD25+ T cells in TCR transgenic mice. Moreover, we found that CD4+CD25+ thymocytes were severely decreased in DO10+ TCR-α−/− mice in the positively selecting background and the negatively selecting background, whereas CD4+CD25− thymocytes effectively developed by transgenic TCR in DO10+ TCR-α−/− mice in the positively selecting background (Figure 3B). Taken together, these results indicate that the appropriate affinity of TCR to self-MHC for the development of CD4+CD25+ thymocytes is different from that of CD4+CD25− thymocytes and that the interaction of TCR with self-MHC at a certain affinity is essential for the development of CD4+CD25+thymocytes. Second, in the periphery, B cells regulate the expansion and/or survival of the developed CD4+CD25+ T cells. We found that, in contrast to the thymus, CD4+CD25+ T cells were readily detected in the spleens of DO10+ TCR-α−/− mice in positively selecting background (Figure 3C), suggesting that a small number of CD4+CD25+ T cells that developed in the thymus might have expanded in the periphery. In addition, we found that the number of splenic CD4+CD25+ T cells, but not of CD4+CD25+ thymocytes, was decreased in B-cell–deficient mice (Figures 4, 5). Therefore, B cells may control the peripheral pool of CD4+CD25+ T cells, possibly by inducing the expansion or prolonging the survival of these cells.
Although it is accepted that CD4+CD25+ T cells need activation by TCR for regulatory function, the antigen specificity of CD4+CD25+ T cells is unknown. We found that CD4+CD25+ thymocytes were severely decreased in DO10+ TCR-α−/− mice in negatively selecting H-2b background (Figure 3). Moreover, we found that Mtv-9–induced clonal deletion of TCR V-β5 cells proceeded normally in CD4+CD25+ T cells in BALB/c mice (data not shown). Furthermore, Papiernik et al29 have shown that Mls-1a–induced clonal deletion of TCR V-β6 cells is also normal in CD4+CD25+ T cells. These findings indicate that CD4+CD25+ T cells are not resistant to clonal deletion in thymus. Recently, an altered negative-selection model of CD4+CD25+ T-cell development was proposed.3 In this model, the suboptimal stimulation of thymocytes with low-affinity self-antigens would result not in deletion but in a permanent change in TCR signaling. Although we could not identify the TCR–MHC affinity that specifically induced the development of CD4+CD25+ T cells, identification will extend the understanding of the nature of CD4+CD25+ T cells.
Although the expression of dual TCR-α chain is not essential for the development of CD4+CD25+ T cells (Figure 2), CD4+CD25+ T cells preferentially express the second TCR-α chain in DO10+ mice (Figure 1). In addition, though transgenic TCR-α expression is not sufficient for the development of CD4+CD25+ T cells in DO10+ mice (Figure 3), the ligation of transgenic TCR has been shown to be sufficient for the regulatory function of CD4+CD25+ T cells.10 11 These findings suggest that both TCRs on dual TCR-α–expressing cells are functional for their regulatory activity of CD4+CD25+ T cells. Therefore, dual TCR-α chain expression may increase the chance for TCR signaling of CD4+CD25+ T cells.
We found that B cells played a significant role in the regulation of the CD4+CD25+ T-cell pool in the spleen (Figure4) but not in the development of CD4+CD25+ T cells in the thymus (Figure 5). Because it has been shown that B7/CD28 interaction8 and CD40/CD40L interaction15regulate the CD4+CD25+ T-cell pool in periphery, B cells may regulate the CD4+CD25+T-cell pool through B7/CD28 interaction or CD40/CD40L interaction. Recently, it has been shown that the number of dividing cells is modestly increased in CD4+CD25+ T cells compared with that in CD4+CD25− T cells in periphery.29 Moreover, we found that apoptotic cells, which were assessed as annexin V binding cells, were not increased in CD4+CD25+ T cells (data not shown). Thus, it is likely that B cells regulate the cell expansion of CD4+CD25+ T cells in periphery.
Another subset of CD4+ regulatory T cells, isolated after T cells were activated with alloantigens in the presence of IL-10, was termed type 1 T regulatory (Tr1) cells.30 Tr1 cells are distinct from classical Th1 or Th2 cells in that they produce large amounts of IL-10 and moderate amounts of transforming growth factor-β.30 Significantly, Tr1 cells suppress immune responses in vitro and in vivo through a mechanism dependent on the production of the immunoregulatory cytokine IL-10.31 In contrast, the regulatory function of CD4+CD25+T cells is independent of IL-109. Although it is still possible that they are in fact the same subset of regulatory T cells in different stages of differentiation, the observation that CD4+CD25+ T cells cannot be differentiated from naive CD4+CD25− T cells in vitro,7,9,14 whereas Tr1 cells can be differentiated from naive cells,30 favors the hypothesis that CD4+CD25+ T cells and Tr1 cells are 2 distinct regulatory T cells with similar functions. Moreover, though NK T cells have been shown to be involved in the development of IL-10–dependent regulatory T cells,32 NK T cells were not required for the development of CD4+CD25+ T cells (Figures 4,5). These observations also support the above hypothesis.
In the past 10 years, a great deal has been learned about the regulation of the CD25 gene. It is known that the CD25 gene has at least 3 important elements for regulation, denoted positively regulatory regions (PRR) I, II, and III.33 NF-κB and SRF bind PRRI, Elf-1 and HMG-I (Y) bind PRRII, and Stat5 and Elf-1 bind PRRIII.33 Although PRRI is essential for mitogen–antigen-mediated induction of the gene and PRRIII is essential for IL-2–induced gene expression, PRRII is essential for transcription in response to either mitogen or IL-2. Thus, the CD25 gene can be controlled not only by activation-dependent signals (NF-κB and Stat5) but also by lineage-specific signals (Elf-1). Consistent with these in vitro findings, we found that the CD4+CD25+ T cells were decreased in both DO10+ TCR-α−/−mice (Figure 3) and DO10+ Stat5a−/−mice,34 suggesting that both TCR-mediated signaling and Stat5-mediated signaling are required for the development of CD4+CD25+ T cells in vivo. More detailed mechanisms will be uncovered by promoter analyses of the CD25 gene in CD4+CD25+ T cells.
In summary, we have shown that the peripheral pool of CD4+CD25+ T cells is regulated at least 2 distinct levels. First, a certain TCR–MHC affinity is required for the development of CD4+CD25+ T cells in thymus. Second, B cells are required for the expansion of CD4+CD25+ T cells in periphery. The identification of TCR–MHC affinity that preferentially induces the development of CD4+CD25+ T cells will extend our understanding of the nature of CD4+CD25+ T cells.
We thank Dr T. Saito for BALB/c RAG-2−/− mice, Dr D. Y. Loh for DO10+ mice, and Drs. K. M. Murphy, K. Suzuki, and S.-I. Kagami for valuable discussions.
Supported in part by grants from the Ministry of Education, Science and Culture, Japan and from Uehara Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
After our submission of this paper to Blood, Jordan et al reported that selection of CD4+CD25+ thymocytes required a TCR with high affinity for a self-peptide.35
Author notes
Hiroshi Nakajima, Dept of Internal Medicine II, Chiba University School of Medicine, 1-8-1 Inohana, Chiba 260-8670, Japan; e-mail: nakajimh@intmed02.m.chiba-u.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal