Abstract
The relationship between class switch recombination (CSR) and somatic hypermutation has been unclear. By using human CD27− naive B cells, we investigated the somatic hypermutation and producibility of immunoglobulins (Igs) that occur after CSR. Although neither adult CD27− nor cord blood B cells, which showed the unmutated Ig V-region genes, produced IgG, IgM, or IgA in response to conventional stimuli, they produced IgG and IgM but not IgA in the presence of Staphylococcus aureus Cowan strain (SAC) + interleukin-2 (IL-2) + IL-10 + anti-CD40 mAb + CD32 transfectants (CD40/CD32T). The naive B cells also produced IgE when combined with IL-4 + CD40/CD32T. In parallel with IgG production, the expression of mature γ1 and γ 2 transcripts was induced from naive B cells by the stimuli. The CD27 expression on human naive B cells was induced remarkably by CD40 signaling or B-cell receptor engagement, but somatic hypermutation could not be induced. The proliferation and differentiation into plasma cells were induced from naive B cells, whereas most of the plasma cells displayed very low levels of mutations in Ig V-region genes. CD27− naive B cells expressed activation-induced cytidine deaminase messenger RNA by the stimuli later than CD27+memory B cells. Our results demonstrate that CSR, but not noticeable somatic hypermutation, can be induced from CD27− naive B cells upon B-cell receptor engagement and CD40 signaling in cooperation with cytokines, suggesting that CSR and somatic hypermutation processes can occur independently, and the antibodies produced in this in vitro system are low-affinity antibodies.
Introduction
B-lineage cells undergo characteristic changes in their immunoglobulin (Ig) genes during differentiation. In bone marrow, B-cell precursors bring together heavy- and light-chain variable (VH and VL) region genes by means of V(D)J recombination between the V, diversity (D), and joining (J) regions. After they succeed in generating a functional antigen receptor, they are released into the B-cell pool as naive B cells. The differentiation of naive B cells into memory B cells occurs within the germinal centers (GCs) in secondary lymphoid organs, where activated naive B cells undergo vigorous proliferation, somatic hypermutation of Ig V-region genes, isotype switching, interaction with antigens, antigen-driven selection, and differentiation into memory B cells and plasma cells.1 2
The CD27 molecule belongs to the nerve growth factor receptor/tumor necrosis factor receptor family3 and plays an important role in Ig production.4 Adult peripheral blood (PB) B cells can be divided into at least 2 subtypes on the basis of the expression of CD27 antigen. CD27+ B cells have been recently identified as memory B cells by virtue of their Ig production, morphology, and increased CD27 expression with age5,6 as well as the fact that they carry somatically mutated V-region genes.6-8 The absence of switched IgD−CD27+ B cells in X-linked hyper-IgM syndrome,9,10 in which GCs are impaired, supports the view that IgD−CD27+ B cells are memory cells. In this respect, IgD+CD27+ B cells, a portion of memory B cells, are generated in this disease probably via a GC-independent pathway.10 CD27 signaling is also important for the generation of plasma cells. CD27+ memory B cells differentiate into plasma cells as a result of contact with CD27 ligand (CD70) transfectants in conjunction with interleukin-10 (IL-10),11Staphylococcus aureus Cowan strain (SAC) + IL-2,12 or IL-4 + CD40 signaling.13
However, the characteristics of naive B cells have remained unclear because of the absence of true naive B-cell markers. IgD+CD27− B cells in tonsils14 and PB5 as naive B cells do not produce IgA, IgM, or IgG in the presence of SAC + IL-2. It has also been reported that IL-10 and transforming growth factor (TGF)–β cooperate in inducing anti-CD40–activated IgD+ B cells to secrete IgA.15 However, IgA production is not investigated by true naive B cells.
Activation-induced cytidine deaminase (AID), a novel number of the RNA editing cytidine deaminase family, was identified by use of complementary DNA (cDNA) subtraction from murine lymphoma cells to understand the molecular mechanism of class switch recombination (CSR) in B cells. Because AID-deficient mice failed to undergo CSR and somatic hypermutation, AID may be involved in regulation or catalysis of the DNA modification step of both class switching and somatic hypermutation.16
In the study presented here, we investigated the features of IgD+CD27− naive B cells by analyzing their ability to induce CSR, somatic hypermutation, and plasma cell differentiation.
Materials and methods
Antibodies and reagents
Anti-CD27 monoclonal antibody (mAb) (8H5, IgG1), which does not block the ligation of CD27/CD70,4 17 was provided by Dr T. Morimoto (Dana-Farber Cancer Institute, Boston, MA). Fluorescein isothiocyanate (FITC)– conjugated anti-CD20 mAb and phycoerythrin (PE)-conjugated anti-CD20 mAb were purchased from DAKO Japan (Tokyo, Japan) and FITC-conjugated anti-CD38 mAb (T16, IgG1) and anti-CD40 mAb (MAB89, IgG1) from Immunotech (Marseille, France). Conjugation of biotin to anti-CD27 mAb (8H5) was performed with the standard technique using N-hydroxysuccinimido-biotin (Sigma, St Louis, MO) in our laboratory. SAC was purchased from Sigma, and human IL-2, IL-4, IL-10, and TGF-β1 from Genzyme (Cambridge, MA).
Cell preparation
Human adult PB samples were obtained from healthy volunteers. Heparinized cord blood (CB) was collected after obtaining informed consent from the parents. PB mononuclear cells (MNCs) and CB MNCs were isolated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density gradient centrifugation and separated with 5% sheep erythrocytes into erythrocyte rosette-positive (E+) and -negative (E−) populations.18 The adult E−and CB E− cells were depleted of monocytes with the aid of silica (IBL, Fujioka, Japan) or by adherence to the plastic surface. Adult CD20+CD27+ and CD20+CD27− B cells were isolated from the monocyte-depleted E− cells by sorting with a FACStar Plus (Becton Dickinson, Mountain View, CA) under sterile conditions. The purity of both populations was more than 98%. The monocyte-depleted CB E− cells were further purified into B cells by positive selection with anti-CD19 mAb-coated immunomagnetic beads (Dynal, Oslo, Norway), after which the anti-CD19 mAb was removed with the use of Detach-a-Bead (Dynal). More than 97% of the B cells in the CB B cell populations reacted with anti-CD20 mAbs. The B cells thus obtained did not show any signs of proliferation or activation.
Preparation and fixation of CD32 transfectants
CD32 (FcγRII) transfectants (CD32T) were prepared as described elsewhere.19 Briefly, total RNA was isolated with the single-step method from the CD32+ human monocytic cell line U937. After reverse transcriptase–polymerase chain reaction (RT-PCR), the amplified cDNA was digested and ligated with the mammalian expression vector BCMGSHyg.20 The resulting plasmid was transfected into the murine pre–B-cell line 300-19 21 by electroporation. CD32T was selected by growth in a culture medium with hygromycin B (Boehringer Mannheim, Mannheim, Germany) at 1 mg/mL. The cell lines highly expressing CD32 were picked up. The transfectant cells were then incubated with 1% paraformaldehyde in phosphate-buffered saline (PBS) for 5 minutes. After 3 washings with PBS, the cells were cultured in RPMI 1640 with 10% fetal calf serum for 30 minutes and then used for the analysis.
Flow cytometric analysis
Activated adult and CB-purified B cells were stained with anti-CD38–FITC and anti-CD20–PE for analyzing the differentiation into plasma cells or with anti-CD20–FITC and biotin-CD27 conjugates followed by streptavidin-PE for the CD27 expression. Two-color analysis of B-cell surface molecules was performed by a FACScan (Becton Dickinson). The antibody-coated cells were gated on living cells by cell size and granularity and then counted by means of flow cytometric analysis.
B-cell proliferation assay
Highly purified adult CD20+CD27+, CD20+CD27−, and CB B cells were cultured in the presence of medium alone, SAC + IL-2, anti-CD40 mAb cross-linked with CD32T (CD40/CD32T), SAC + IL-2 + IL-10 + CD40/CD32T, or IL-4 with or without CD40/CD32T at a final cell density of 2.5 × 105 to 5 × 105/mL in a volume of 200 μL per well. The cells were cultured in 96-well round-bottom plates (Nunc, Roskilde, Denmark) for 3 days at 37°C in a humidified atmosphere with 5% CO2. The cultures were then pulsed with 0.5 μCi (18.5 Bq) [3H]thymidine. After 18 hours of incubation, the cells were harvested by an automatic cell harvester (Packard, Meriden, CT), and [3H]thymidine incorporation was measured on a liquid scintillation analyzer (Packard).
Ig assay by ELISA
For the IgG, IgM, and IgA syntheses, highly purified adult CD20+CD27+, CD20+CD27−, and CB B cells were cultured with medium alone, SAC + IL-2, IL-10 + CD40/CD32T, or SAC + IL-2 + IL-10 + CD40/CD32T with or without various concentrations (0.1, 1 and 10 ng/mL) of TGF-β. The cells were cultured for 8 to 10 days at 37°C in a humidified atmosphere with 5% CO2. For the IgE synthesis, the cells were cultured with medium alone, IL-4, or IL-4 + CD40/CD32T for 14 days under the same conditions. The final cell density was 2.5 × 105 to 5 × 105/mL in a volume of 200 μL per well. The plates were coated with goat antihuman Igs (Southern Biotechnology, Birmingham, AL) for the detection of IgG, IgM, and IgA and with antihuman IgE (CIA-E− 7.12 and CIA-E− 4.15, provided by Dr A. Saxon, Division of Clinical Immunology/Allergy, UCLA School of Medicine, Los Angeles, CA) for the detection of IgE. The cultured supernatants were harvested and added to 96-well flat enzyme-linked immunosorbent assay (ELISA) plates (Nunc). The standard human IgG, IgM, IgA (Sigma), or IgE (Chemicon International, Temecula, CA) was also added to the plates. After an overnight culture at 4°C, the supernatants were discarded and the wells were washed with 0.05% Tween 20 in PBS. Alkaline phosphatase–labeled goat antihuman IgG, IgM, IgA, and IgE (Sigma) at a dilution of 1:2500 was added for the detection of IgG, IgM, IgA, and IgE, respectively. After 2 hours of incubation at room temperature, color detection was performed with 3-[cyclohexylamino]-1-propanesulfonic acid buffer containing p-Nitrophenyl phosphate (Sigma). Calibration was performed with PBS at standard zero levels. No cross-reaction among IgG, IgM, IgA, and IgE occurred in this ELISA system.
RT-PCR of mature γ1, γ2, and AID
Highly purified adult CD20+CD27+, CD20+CD27−, or CB B cells at the cell numbers of 0.5 × 106 to 1 × 106 cells were cultured with medium only or 0.01% SAC, 50 U/mL IL-2, 50 ng/mL IL-10, and 1 μg/mL anti-CD40 mAb cross-linked with CD32T (for indicating times). Total RNA was extracted by the acid-guanidine thiocyanate-phenol-chloroform method using an RNAzol rapid RNA purification kit (Biotex, Houston, TX). First-strand cDNA copies were synthesized by using Superscript II Reverse Transcriptase (Life Technologies, Grand Island, NY) with oligo (dT) (Life Technologies) as a primer in a total volume of 20 μL, and then PCR was performed. The following oligonucleotide primers were used for PCR: for germline Cγ1, sense primer, 5′-ACGAGGAACATGACTGGATGC-3′; antisense primer, 5′-TGTGAGTTTTGTCACAAGATTTGGG-3′; for germline Cγ2, 5′-TCTCAGCCAGGACCAAGGAC-3′ and 5′-ACTCGACACAACATTTGCG-3′; for mature Cγ1, 5′-CCTGGTCACCGTCTCCTCA-3′ and 5′-TGTGAGTTTTGTCACAAGATTTGGG-3′; for mature Cγ2, 5′-CCTGGTCACCGTCTCCTCA-3′ and 5′-ACTCGACACAACATTTGCG-3′; and for AID, 5′-AGCTGACAATGATGAATCTCA-3′ and 5′-CTTGGGGTAGTGAGCGTTGTA-3′. A total of 2 to 5 μL cDNA was amplified in PCR using each primer and Taq DNA polymerase (Life Technologies). The amplified products were analyzed on a 1.2% agarose gel containing ethidium bromide and visualized by UV light illumination. The β2-microglobulin sense primer 5′-GCTATGTGTCTGGGTTTCAT-3′ and antisense primer 5′-ATCTTCAAACCTCCATGATG-3′ were used as controls.
Sequence analysis of Ig V-region genes
Total RNA was isolated from the sorted B cells or plasma cells derived from naive B cells with the aid of the RNAzol rapid RNA purification kit (Biotex) and then reverse transcribed into cDNA using the oligo (dT) primer and Superscript II Reverse Transcriptase (Life Technologies). VH5 genes were amplified by using primers corresponding to the 5′ region of the VH5 leader sequence (ATGGGGTCAACCGCCATCCT) and to the 3′ Cμ constant region (GTCCTGTGCGAGGCAGCCAA). PCR products were ligated into the pCR2.1 vector (Invitrogen, Carlsbad, CA) and transformed into TOP10F′ bacteria (Invitrogen). Individual clones were selected and expressed, and then plasmid DNA was purified. DNA sequencing was performed with a dideoxy termination technique using an ABI 377 sequencer (Perkin-Elmer Applied Biosystems, Weiterstadt, Germany).
Results
Characteristics of CD27− B cells and their Ig production
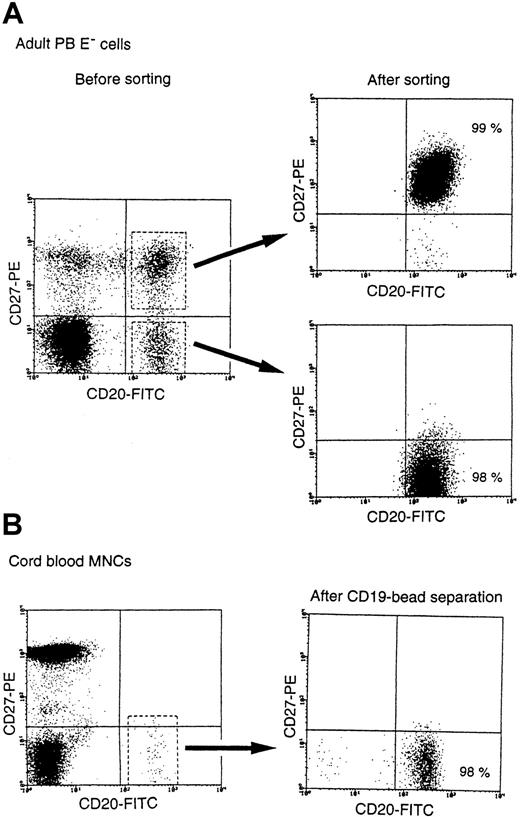
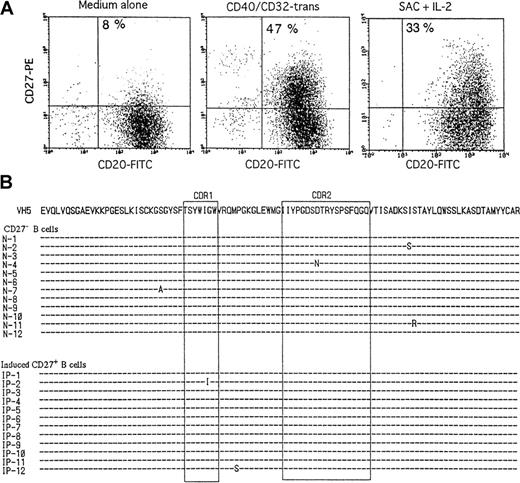
CD27+ B cells produced IgG, IgM, and IgA as a result of SAC + IL-2 stimulation, whereas CD27− B cells did not, as previously reported.5,14 22 To clarify the production of Igs by CD27− B cells, we first attempted to promote IgG, IgM, and IgA production by CD27− B cells with various stimuli. For this purpose, we obtained highly purified adult CD27− B cells by sorting and CB B cells, most of which were CD27− cells, by anti-CD19 mAb-coated immunomagnetic bead separation (Figure 1). Sequence analyses of the Ig VH5 region genes isolated from both the sorting-purified adult CD27− B cells (average mutation frequency 0.09%, n = 4, 48 clones) and the purified CB B cells (average mutation frequency 0.08%, n = 4, 48 clones) produced evidence of unmutated sequences, indicating that they did not carry somatic hypermutation and were naive B cells. Adult CD27−B cells did not produce IgG, IgM, and IgA in the presence of medium alone, of SAC + IL-2, or of IL-10 + CD40/CD32T. In contrast, adult CD27+ B cells produced large amounts of IgG, IgM, and IgA (Table 1). It was remarkable that adult CD27− B cells produced large amounts of IgG and IgM but not IgA in the presence of SAC + IL-10 + IL-2 + CD40/CD32T (Table 1). Similarly, highly purified CB B cells did not produce IgG or IgA, although they produced marginal levels of IgM, in the presence of SAC + IL-2 or IL-10 + CD40/CD32T and produced large amounts of IgG and IgM but not IgA when stimulated with SAC + IL-10 + IL-2 + CD40/CD32T (Table 1). In addition, both adult CD27− and CB B cells, CD27+ memory B cells, produced all subclasses of IgG with the same stimuli (data not shown). Detectable levels of IgG and IgM were also produced by both naive B cells with SAC + IL-2 + CD40/CD32T as the stimulus (data not shown). These findings indicate that adult CD27−naive B cells and CB B cells have the ability to produce IgG and IgM but not IgA in conjunction with SAC as reagents of B-cell receptor (BCR) engagement and CD40 signaling in the presence of IL-2 and IL-10.
Preparation of adult CD27− naive and CB B cells.
Highly purified adult CD27− B cells were separated by means of flow cytometry, and CB B cells were separated with the aid of CD19-coated immunomagnetic beads. The purity of both types of B cells was more than 97%.
Preparation of adult CD27− naive and CB B cells.
Highly purified adult CD27− B cells were separated by means of flow cytometry, and CB B cells were separated with the aid of CD19-coated immunomagnetic beads. The purity of both types of B cells was more than 97%.
IgG, IgM, and IgA production by purified adult PB CD27+, CD27−, and CB B cells
| . | Adult PB CD27+ B cells . | Adult PB CD27− B cells . | CB B cells . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IgG (ng/mL) . | IgM (ng/mL) . | IgA (ng/mL) . | IgG (ng/mL) . | IgM (ng/mL) . | IgA (ng/mL) . | IgG (ng/mL) . | IgM (ng/mL) . | IgA (ng/mL) . | |
| Medium | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 |
| SAC* + IL-2* | 8960 ± 3015‡ | 6938 ± 2509 | 7533 ± 2340 | < 62 | < 62 | < 62 | < 62 | 487 ± 123 | < 62 |
| IL-10* + CD40*/CD32T† | 1966 ± 492 | 1935 ± 604 | 2054 ± 612 | < 62 | < 62 | < 62 | < 62 | 185 ± 55 | < 62 |
| SAC + IL-2 + IL-10 + CD40/CD32T | 9980 ± 3436 | 9257 ± 3350 | 9918 ± 2685 | 4643 ± 1336 | 4569 ± 1453 | < 62 | 6430 ± 1908 | 6225 ± 1911 | < 62 |
| . | Adult PB CD27+ B cells . | Adult PB CD27− B cells . | CB B cells . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IgG (ng/mL) . | IgM (ng/mL) . | IgA (ng/mL) . | IgG (ng/mL) . | IgM (ng/mL) . | IgA (ng/mL) . | IgG (ng/mL) . | IgM (ng/mL) . | IgA (ng/mL) . | |
| Medium | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 | < 62 |
| SAC* + IL-2* | 8960 ± 3015‡ | 6938 ± 2509 | 7533 ± 2340 | < 62 | < 62 | < 62 | < 62 | 487 ± 123 | < 62 |
| IL-10* + CD40*/CD32T† | 1966 ± 492 | 1935 ± 604 | 2054 ± 612 | < 62 | < 62 | < 62 | < 62 | 185 ± 55 | < 62 |
| SAC + IL-2 + IL-10 + CD40/CD32T | 9980 ± 3436 | 9257 ± 3350 | 9918 ± 2685 | 4643 ± 1336 | 4569 ± 1453 | < 62 | 6430 ± 1908 | 6225 ± 1911 | < 62 |
IgG, IgM, and IgA production assays, using 2.5 × 104 to 105 per well of highly purified adult PB CD27+, CD27−, and CB B cells, were performed as described in “Materials and methods.”
The final concentrations of SAC, IL-2, IL-10, and anti-CD40 were 0.01%, 50 U/mL, 50 ng/mL, and 1 μg/mL, respectively.
The transfectants accounted for 40% of the number of B cells added.
Results are expressed as the mean ± SD of triplicate experiments.
IgE synthesis by CD27− naive B cells
Adult CD27+ B cells, CD27− B cells, and CB B cells did not produce IgE with medium or IL-4 alone, but these B cells produced substantial amounts of IgE in the presence of IL-4 + anti-CD40 mAb. The IgE production by CD27+, CD27−, and CB B cells was greatly enhanced with IL-4 + anti-CD40 mAb cross-linked with CD32 transfectants (Table2). These data demonstrate that CD27− naive B cells and CB B cells have the ability to produce IgE if they obtain substantial levels of CD40 signaling.
IgE production by purified adult PB CD27+, CD27−, and CB B cells
| . | Adult PB CD27+ B cells . | Adult PB CD27− B cells . | CB B cells . |
|---|---|---|---|
| Medium | < 8 | < 8 | < 8 |
| IL-4 | < 8 | < 8 | < 8 |
| IL-4* + CD40* | 26 ± 8‡ | 16 ± 4 | 18 ± 5 |
| IL-4 + CD40/CD32T† | 67 ± 14 | 49 ± 15 | 53 ± 12 |
| . | Adult PB CD27+ B cells . | Adult PB CD27− B cells . | CB B cells . |
|---|---|---|---|
| Medium | < 8 | < 8 | < 8 |
| IL-4 | < 8 | < 8 | < 8 |
| IL-4* + CD40* | 26 ± 8‡ | 16 ± 4 | 18 ± 5 |
| IL-4 + CD40/CD32T† | 67 ± 14 | 49 ± 15 | 53 ± 12 |
Immunoglobulin E concentration is given in ng/mL. IgE production assay, using 1 × 105 per well of highly purified adult PB CD27+, CD27−, and CB B cells, was performed as described in “Materials and methods.”
The final concentrations of IL-4 and anti-CD40 were 50 ng/mL and 1 μg/mL, respectively.
The transfectants accounted for 40% of the number of B cells added.
Results are expressed as the mean ± SD of triplicate experiments.
Naive B-cell proliferation
To define the characteristics of naive B cells, we next investigated the proliferation of adult CD27− naive B cells and CB B cells in comparison with that of CD27+memory B cells. CD40/CD32T alone, SAC + IL-2, or IL-4 + CD40/CD32T induced the proliferation of naive B cells as well as of CD27+ B cells, while SAC + IL-10 + IL-2 + CD40/CD32T induced much higher levels of proliferation. IL-4 could synergize with CD40 pathway for the naive B-cell proliferation (Table3). These findings indicate that the prominent proliferation of naive B cells can be induced by CD40 signaling or SAC + IL-2 and be augmented by a combination of such stimuli.
Proliferation by purified adult PB CD27+, CD27−, and CB B cells
| . | [3H]thymidine incorporation (cpm) . | ||
|---|---|---|---|
| Adult PB CD27+ B cells . | Adult PB CD27− B cells . | CB B cells . | |
| Medium | 136 ± 353-152 | 136 ± 33 | 175 ± 38 |
| CD403-150/CD32T3-151 | 1513 ± 178 | 1185 ± 289 | 1759 ± 359 |
| SAC3-150 + IL-23-150 | 5370 ± 1245 | 4728 ± 956 | 8083 ± 1747 |
| SAC + IL-2 + IL-103-150 + CD40/CD32T | 15 369 ± 3013 | 11 080 ± 2574 | 14 366 ± 2984 |
| IL-43-150 | 1338 ± 348 | 989 ± 261 | 1294 ± 335 |
| IL-4 + CD40/CD32T | 4810 ± 1328 | 4170 ± 1248 | 7007 ± 1512 |
| . | [3H]thymidine incorporation (cpm) . | ||
|---|---|---|---|
| Adult PB CD27+ B cells . | Adult PB CD27− B cells . | CB B cells . | |
| Medium | 136 ± 353-152 | 136 ± 33 | 175 ± 38 |
| CD403-150/CD32T3-151 | 1513 ± 178 | 1185 ± 289 | 1759 ± 359 |
| SAC3-150 + IL-23-150 | 5370 ± 1245 | 4728 ± 956 | 8083 ± 1747 |
| SAC + IL-2 + IL-103-150 + CD40/CD32T | 15 369 ± 3013 | 11 080 ± 2574 | 14 366 ± 2984 |
| IL-43-150 | 1338 ± 348 | 989 ± 261 | 1294 ± 335 |
| IL-4 + CD40/CD32T | 4810 ± 1328 | 4170 ± 1248 | 7007 ± 1512 |
Proliferation assay, using 1 × 105 per well of highly purified adult PB CD27+, CD27−, and CB B cells, was performed as described in “Materials and methods.”
The final concentrations of SAC, IL-2, IL-10, IL-4, and anti-CD40 were 0.01%, 50 U/mL, 50 ng/mL, 50 ng/mL, and 1 μg/mL, respectively.
The transfectants accounted for 40% of the number of B cells added.
Results are expressed as the mean ± SD of triplicate experiments.
The effect of TGF-β on B-cell IgA synthesis
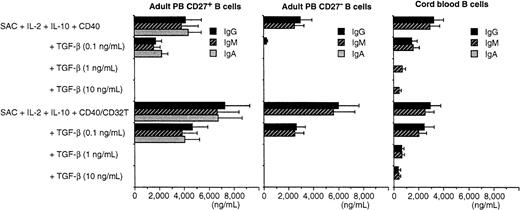
Only IgA was not induced by naive B cells in our system. TGF-β is a potent cytokine that regulates the IgA synthesis by B cells, as previously demonstrated.15 23-25 Therefore, we investigated whether TGF-β could induce IgA production in the presence of SAC + IL-10 + IL-2 + CD40/CD32T in highly purified adult CD27− naive B cells and CB B cells. Unexpectedly, neither adult CD27− nor CB B cells produced IgA when TGF-β was added in the presence of SAC + IL-10 + IL-2 + anti-CD40 mAb with or without cross-linking by CD32T. On the other hand, TGF-β inhibited the IgG and IgM synthesis induced by SAC + IL-10 + IL-2 + anti-CD40 mAb with or without CD32T in a dose-dependent manner (Figure 2). In addition, the production of IgA as well as of IgG and IgM from sorting-purified CD27+ memory B cells in the presence of SAC + IL-2 or of SAC + IL-10 + IL-2 + anti-CD40 mAb with or without CD32T (Figure 2) was reduced by the addition of TGF-β in a dose-dependent manner. These findings indicate that TGF-β cannot induce IgA production by naive B cells and has a suppressive effect on IgA, IgM, and IgG synthesis by memory B cells induced by the stimuli. TGF-β could inhibit the B-cell proliferation in a dose-dependent manner by various stimuli in both of memory and naive B cells (data not shown).
TGF-β does not induce IgA production from adult CD27− and CB B cells.
Highly purified adult CD27+, CD27−, and CB B cells were cultured with TGF-β (0.1, 1, and 10 ng/mL) in the presence of SAC (0.01%) + IL-2 (50 U/mL) + IL-10 (50 ng/mL) + anti-CD40 mAb (1 μg/mL) with or without cross-linking by CD32T (40%) at a final cell density of 0.5 × 105 per well for 8 to 10 days. ELISA was used to measure IgG, IgM, and IgA contents. Similar results were obtained in 3 independent experiments. Results are expressed as the mean ± SD of triplicate determinations.
TGF-β does not induce IgA production from adult CD27− and CB B cells.
Highly purified adult CD27+, CD27−, and CB B cells were cultured with TGF-β (0.1, 1, and 10 ng/mL) in the presence of SAC (0.01%) + IL-2 (50 U/mL) + IL-10 (50 ng/mL) + anti-CD40 mAb (1 μg/mL) with or without cross-linking by CD32T (40%) at a final cell density of 0.5 × 105 per well for 8 to 10 days. ELISA was used to measure IgG, IgM, and IgA contents. Similar results were obtained in 3 independent experiments. Results are expressed as the mean ± SD of triplicate determinations.
The expression of mature γ1 and γ2 transcripts by CD27− naive B cells
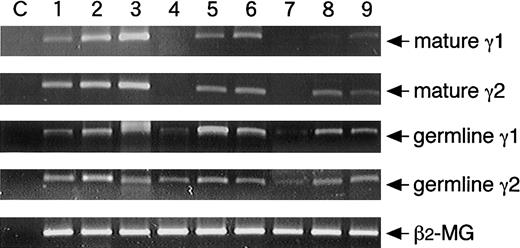
The induction of germline transcripts occurs before class switching, and their expression are apparently essential for CSR. Mature transcripts are induced after CSR, and then Ig synthesis is initiated. Inasmuch as adult CD27− and CB B cells produced IgG, IgM, and IgE, we ascertained whether CSR in transcriptional levels is also induced by the naive B cells. The germline γ1 and γ2 messenger RNA (mRNA) was expressed spontaneously from resting CD27+ and CD27− B cells. Spontaneous expression of mature γ1 and γ2 transcripts also be found in adult CD27+ memory B cells but not in naive B cells (Figure3). The enhancement of mature γ1 and γ2 transcripts was observed by CD27+ memory B cells in the presence of SAC + IL-2 + IL-10 + CD40/CD32T, and the induction of mature γ1 and γ2 transcripts was obtained by adult CD27− and CB B cells with the stimuli (Figure 3). These results clearly indicate that CSR can be induced by naive B cells.
Induction of mature γ1 and γ2 transcripts from naive B cells.
Highly purified adult CD20+CD27+, CD20+CD27−, or CB B cells at the cell numbers of 0.5 × 106 cells were cultured with medium alone (lanes 1, 4, 7), 0.01% SAC, 50 U/mL IL-2, 50 ng/mL IL-10, and 1 μg/mL anti-CD40 cross-linked with CD32T for 1 day (lanes 2, 5, 8) or 4 days (lanes 3, 6, 9). After extraction of total RNA, RT-PCR was performed as described in “Materials and methods.” PCR primers of germline Cγ transcripts were prepared in the initiation region (Iγ1 or Iγ2) and the hinge of constant region (Cγ1 or Cγ2). PCR primers of mature Cγ transcripts were prepared in the regions of JH of V region and of the hinge of constant region. Each template contained the same of cDNA from RNA extracted from highly purified B cells. The C lanes represent control of contamination-free reaction, in which all PCR reagents are present, but there is no cDNA. The β2-microglobulin (β2-MG) was used as a positive control. Data are representative of the results of 3 experiments using different donors.
Induction of mature γ1 and γ2 transcripts from naive B cells.
Highly purified adult CD20+CD27+, CD20+CD27−, or CB B cells at the cell numbers of 0.5 × 106 cells were cultured with medium alone (lanes 1, 4, 7), 0.01% SAC, 50 U/mL IL-2, 50 ng/mL IL-10, and 1 μg/mL anti-CD40 cross-linked with CD32T for 1 day (lanes 2, 5, 8) or 4 days (lanes 3, 6, 9). After extraction of total RNA, RT-PCR was performed as described in “Materials and methods.” PCR primers of germline Cγ transcripts were prepared in the initiation region (Iγ1 or Iγ2) and the hinge of constant region (Cγ1 or Cγ2). PCR primers of mature Cγ transcripts were prepared in the regions of JH of V region and of the hinge of constant region. Each template contained the same of cDNA from RNA extracted from highly purified B cells. The C lanes represent control of contamination-free reaction, in which all PCR reagents are present, but there is no cDNA. The β2-microglobulin (β2-MG) was used as a positive control. Data are representative of the results of 3 experiments using different donors.
AID expression in naive B cells
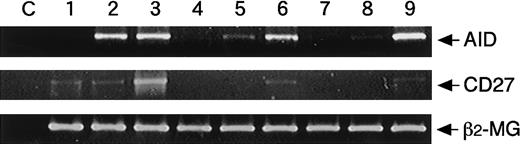
AID is the essential component for both CSR and somatic hypermutation.16 Therefore, we investigated whether AID is inducible by naive B cells. Experiments were conducted in which naive B cells were stimulated, and the expression of AID mRNA was investigated in comparison to that of CD27 mRNA. AID mRNA was not found in resting CD27+ and CD27− B cells. The expression of AID mRNA was induced by adult CD27− and CB B cells as well as CD27+ B cells with SAC + IL-2 + IL-10 + CD40/CD32T, whereas its expression by CD27+ B cells was earlier than that by naive B cells (Figure4). In parallel with AID mRNA expression, CD27 mRNA expression was also induced by naive B cells (Figure 4). These findings indicate that AID is induced by naive B cells by the stimuli probably before CSR.
Induction of AID from naive B cells.
Highly purified adult CD20+CD27+, CD20+CD27−, or CB B cells at the cell numbers of 0.5 × 106 cells were cultured with medium alone (lanes 1, 4, 7), 0.01% SAC, 50 U/mL IL-2, 50 ng/mL IL-10, and 1 μg/mL anti-CD40 cross-linked with CD32T for 1 day (lanes 2, 5, 8) or 4 days (lanes 3, 6, 9). After extraction of total RNA, RT-PCR was performed as described in “Materials and methods.” Each template contained the same of cDNA from RNA extracted from highly purified B cells. The C lanes represent control of contamination-free reaction, in which all PCR reagents are present, but there is no cDNA. The β2-microglobulin (β2-MG) was used as a positive control. Data are representative of the results of 3 experiments using different donors.
Induction of AID from naive B cells.
Highly purified adult CD20+CD27+, CD20+CD27−, or CB B cells at the cell numbers of 0.5 × 106 cells were cultured with medium alone (lanes 1, 4, 7), 0.01% SAC, 50 U/mL IL-2, 50 ng/mL IL-10, and 1 μg/mL anti-CD40 cross-linked with CD32T for 1 day (lanes 2, 5, 8) or 4 days (lanes 3, 6, 9). After extraction of total RNA, RT-PCR was performed as described in “Materials and methods.” Each template contained the same of cDNA from RNA extracted from highly purified B cells. The C lanes represent control of contamination-free reaction, in which all PCR reagents are present, but there is no cDNA. The β2-microglobulin (β2-MG) was used as a positive control. Data are representative of the results of 3 experiments using different donors.
Analysis of somatic hypermutation in CD27+ B cells induced from naive B cells
Because the CD40-CD154 interaction and BCR engagement can promote the B-cell activation, we attempted to induce the CD27 expression and somatic hypermutation from human naive B cells. Because CD40 stimulation by anti-CD40 mAb + CD32T enhanced the B-cell proliferation and IgE synthesis, we used this cross-linking system via CD40 for the B-cell stimulation. Upon CD40/CD32T or SAC + IL-2, CD27+ B cells were generated from CB B cells, most of which were CD27− B cells, and underwent unmutated sequences of VH5 genes before stimulation (Figure5A). However, CD27+ B cells generated from naive B cells by the stimuli underwent no to low levels of somatic hypermutation, like CD27− B cells (Figure 5B). Somatic hypermutation was not induced even by SAC + IL-2 + IL-10 + CD40/CD32T, although the CD27 expression was remarkably induced by the stimuli (data not shown). In regard to CD27 expression and somatic hypermutation after the activation of naive B cells, the same results were obtained when we used adult CD27− B cells (data not shown). The findings demonstrate that somatic hypermutation cannot be induced in vitro by the CD40 engagement or BCR engagement despite the remarkable induction of the CD27 expression.
Induction of CD27 and analysis of somatic hypermutations in CD27+ B cells derived from naive B cells.
(A) Highly purified CB B cells were stimulated with medium alone, 1 μg/mL anti-CD40 + CD32T, or 0.01% SAC + 50 U/mL IL-2. After 3 days of culture, the cells were stained with anti-CD20–FITC and anti-CD27− biotin followed by streptavidin-PE. (B) Highly purified CB B cells were stimulated with CD40/CD32T. After 3 days, the cells were incubated with anti-CD20–FITC and anti-CD27− biotin followed by streptavidin-PE, and CD27+ and CD27− B cells were sorted by FACStar Plus. The top sequence is the germline gene of VH5 with amino acid translation. Complementarity determining regions are boxed. These data are the representative of 2 different experiments. The same results were obtained when sort-pure adult CD27− B cells were stimulated with CD40/CD32T or SAC + IL-2.
Induction of CD27 and analysis of somatic hypermutations in CD27+ B cells derived from naive B cells.
(A) Highly purified CB B cells were stimulated with medium alone, 1 μg/mL anti-CD40 + CD32T, or 0.01% SAC + 50 U/mL IL-2. After 3 days of culture, the cells were stained with anti-CD20–FITC and anti-CD27− biotin followed by streptavidin-PE. (B) Highly purified CB B cells were stimulated with CD40/CD32T. After 3 days, the cells were incubated with anti-CD20–FITC and anti-CD27− biotin followed by streptavidin-PE, and CD27+ and CD27− B cells were sorted by FACStar Plus. The top sequence is the germline gene of VH5 with amino acid translation. Complementarity determining regions are boxed. These data are the representative of 2 different experiments. The same results were obtained when sort-pure adult CD27− B cells were stimulated with CD40/CD32T or SAC + IL-2.
Generation of plasma cells from CD27− naive B cells
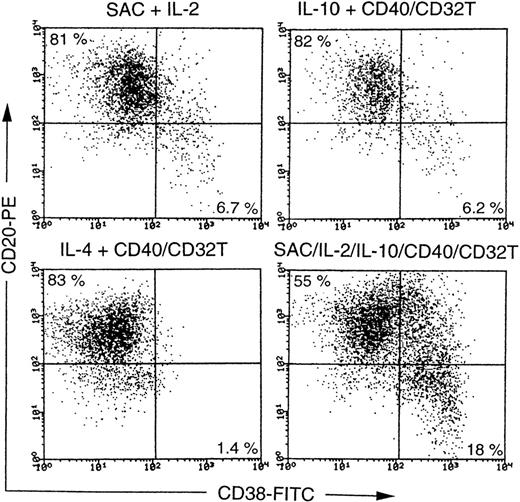
The production of antibodies requires the differentiation of B cells into plasma cells. In the present study, CD27−B cells produced large amounts of Igs in response to a combination of the stimuli, which prompted us to ascertain whether plasma cells can be generated from CD27− naive B cells. We therefore studied the effects of various stimuli on the generation of plasma cells from CD27− naive B cells. Flow cytometric analyses with anti-CD38 and anti-CD20 identified noticeable levels of the differentiation of adult CD27− B cells into plasma cells in the presence of SAC + IL-10 + IL-2 + CD40/CD32T and mild to moderate levels of differentiation in the presence of SAC + IL-2, IL-10 + CD40/CD32T, or IL-4 + CD40/CD32T (Figure6). Similar results were observed in CB B cells (data not shown). These results clearly demonstrate that the differentiation of naive B cells into plasma cells can be induced in parallel with Ig synthesis as a result of engagement with the B-cell Ig receptor and CD40 signaling in the presence of IL-2 and IL-10.
Induction of plasma cells from CD27− naive B cells.
Highly purified adult CD27− B cells were cultured with SAC (0.01%) + IL-2 (50 U/mL), or IL-10 (50 ng/mL) + anti-CD40 mAb (1 μg/mL), SAC + IL-2 + IL-10 + anti-CD40 mAb in conjunction with cross-linking by CD32T (40%) at a final cell density of 0.5 × 105 per well in 96-well round-bottom plates for 8 to 10 days. Alternatively, the cells were cultured with IL-4 (50 ng/mL) + anti-CD40 mAb in conjunction with cross-linking by CD32T (40%) at a final cell density of 1 × 105 per well for 12 to 14 days. The cells were then stained with anti-CD38–FITC and anti-CD20–PE. The antibody-coated cells were gated on living cells by cell size and granularity, and dead cells were removed by propidium iodide (PI) staining and then counted by means of flow cytometric analysis. Expression of CD38 and CD20 on B cells are shown with a log scale. Data are representative of the results of 2 experiments using different donors. The same results were obtained when CB B cells were cultured with above-mentioned stimuli.
Induction of plasma cells from CD27− naive B cells.
Highly purified adult CD27− B cells were cultured with SAC (0.01%) + IL-2 (50 U/mL), or IL-10 (50 ng/mL) + anti-CD40 mAb (1 μg/mL), SAC + IL-2 + IL-10 + anti-CD40 mAb in conjunction with cross-linking by CD32T (40%) at a final cell density of 0.5 × 105 per well in 96-well round-bottom plates for 8 to 10 days. Alternatively, the cells were cultured with IL-4 (50 ng/mL) + anti-CD40 mAb in conjunction with cross-linking by CD32T (40%) at a final cell density of 1 × 105 per well for 12 to 14 days. The cells were then stained with anti-CD38–FITC and anti-CD20–PE. The antibody-coated cells were gated on living cells by cell size and granularity, and dead cells were removed by propidium iodide (PI) staining and then counted by means of flow cytometric analysis. Expression of CD38 and CD20 on B cells are shown with a log scale. Data are representative of the results of 2 experiments using different donors. The same results were obtained when CB B cells were cultured with above-mentioned stimuli.
Somatic hypermutation in plasma cells generated from naive B cells
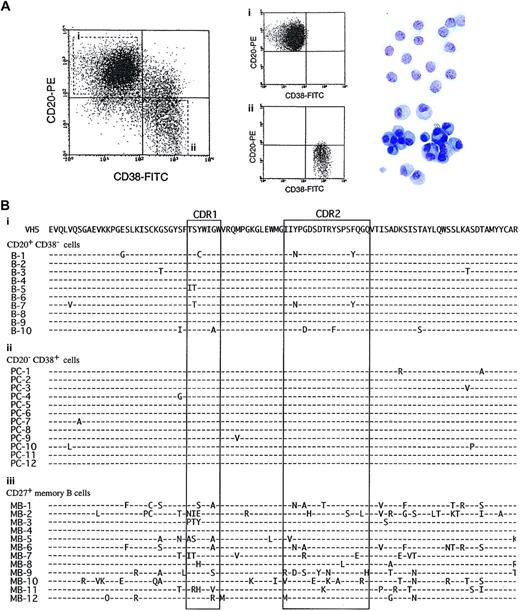
Because large amounts of IgM, IgG, and IgE were produced from naive B cells by the appropriate stimuli, we finally investigated whether plasma cells generated from naive B cells carried somatic hypermutation. We obtained highly pure plasma cells, generated from CB B cells with SAC + IL-10 + IL-2 + CD40/CD32T, with a basophilic cytoplasm with a pale Golgi zone and an eccentric nucleus (Figure 7A). Naive B cells before the stimulation displayed the germline in VH5 genes (data not shown). In contrast, adult CD27+ B cells carried mutated V-region genes with amino acid changes (frequency of nucleotide changes 4.8%). As shown in the amino acid sequence of the VH5 genes in plasma cells generated from CB B cells, no noticeable induction of somatic hypermutation was observed, and the same was true for CD20+CD38− B cells (Figure 7B). The frequency of amino acid changes of CD20+CD38−B cells and CD20−CD38+ cells in experiment 1 is shown in Figure 7B. This frequency was above the background PCR error. Table 4 shows the frequency of nucleotide changes and number of nucleotide changes inducing amino acid replacement and silent mutations. No statistical significance was obtained in the frequency of nucleotide changes in the Ig V region between resting CB B cells and CD20+CD38−/CD20−CD38+cells after the stimulation (Table 4). Similar results were obtained by using adult CD27− B cells (data not shown). These findings suggest that the antibodies produced from naive B cells in our system may be low-affinity antibodies.
The characteristics and amino acid sequences of VH5 genes in plasma cells generated from naive B cells.
(A) Highly purified CB B cells were cultured with SAC (0.01%), IL-2 (50 U/mL), IL-10 (50 ng/mL), and anti-CD40 mAb (1 μg/mL) with CD32T for 8 days. The cells were then stained with anti-CD38–FITC and anti-CD20–PE and the antibody-coated cells gated on living cells by cell size and granularity and finally counted by means of flow cytometric analysis. Expressions of CD38 and CD20 on B cells are shown on a log scale. The cells were separated into CD20+CD38− B cells (Ai) and CD20−CD38+ plasma cells (Aii) by sorting, cytospun, and stained with May-Giemsa staining. Original magnification, × 400. (B) Total RNA was isolated from the CD20+CD38− B cells (Bi), CD20−CD38+ plasma cells (Bii), and resting CD27+ memory B cells (Biii) and then reverse transcribed into cDNA. VH5 genes were amplified by using primers corresponding to the 5′ region of the VH5 leader sequence and to the 3′ Cμ constant region. PCR products were ligated and transformed. The DNA sequencing of individual clones was performed with a dideoxy termination technique. Each dash represents identity with the germline sequence; amino acid differences are indicated. Complementarity determining regions are boxed. These data represent each of 3 different experiments. The same results were obtained when sort-pure adult CD27− B cells were stimulated with SAC + IL-2 + IL-10 + CD40/CD32T.
The characteristics and amino acid sequences of VH5 genes in plasma cells generated from naive B cells.
(A) Highly purified CB B cells were cultured with SAC (0.01%), IL-2 (50 U/mL), IL-10 (50 ng/mL), and anti-CD40 mAb (1 μg/mL) with CD32T for 8 days. The cells were then stained with anti-CD38–FITC and anti-CD20–PE and the antibody-coated cells gated on living cells by cell size and granularity and finally counted by means of flow cytometric analysis. Expressions of CD38 and CD20 on B cells are shown on a log scale. The cells were separated into CD20+CD38− B cells (Ai) and CD20−CD38+ plasma cells (Aii) by sorting, cytospun, and stained with May-Giemsa staining. Original magnification, × 400. (B) Total RNA was isolated from the CD20+CD38− B cells (Bi), CD20−CD38+ plasma cells (Bii), and resting CD27+ memory B cells (Biii) and then reverse transcribed into cDNA. VH5 genes were amplified by using primers corresponding to the 5′ region of the VH5 leader sequence and to the 3′ Cμ constant region. PCR products were ligated and transformed. The DNA sequencing of individual clones was performed with a dideoxy termination technique. Each dash represents identity with the germline sequence; amino acid differences are indicated. Complementarity determining regions are boxed. These data represent each of 3 different experiments. The same results were obtained when sort-pure adult CD27− B cells were stimulated with SAC + IL-2 + IL-10 + CD40/CD32T.
Somatic hypermutation in rearranged VH5 sequences in unstimulated and stimulated CB B cells
| . | Number of sequences . | Mutations . | |||||
|---|---|---|---|---|---|---|---|
| Total . | Mutated . | Range . | Total no. . | R . | S . | Frequency/total sequences, % . | |
| Before stimulation | |||||||
| CB B cells | |||||||
| 1 | 12 | 4 (33%) | 0-2 | 7 | 5 | 2 | 0.20 |
| 2 | 12 | 3 (25%) | 0-1 | 3 | 3 | 0 | 0.08 |
| 3 | 12 | 1 (8%) | 0-1 | 1 | 1 | 0 | 0.03 |
| 4 | 12 | 0 (0%) | 0 | 0 | 0 | 0 | 0 |
| 5 | 10 | 3 (30%) | 0-3 | 7 | 5 | 2 | 0.24 |
| After stimulation | |||||||
| CD20+CD38−cells | |||||||
| 1 | 10 | 5 (50%) | 0-5 | 19 | 17 | 2 | 0.65 |
| 2 | 10 | 3 (30%) | 0-5 | 10 | 8 | 2 | 0.34 |
| 3 | 10 | 4 (40%) | 0-1 | 4 | 3 | 1 | 0.14 |
| 4 | 10 | 1 (10%) | 0-1 | 1 | 1 | 0 | 0.03 |
| 5 | 10 | 3 (30%) | 0-3 | 6 | 5 | 1 | 0.2 |
| CD20−CD38+ B cells | |||||||
| 1 | 12 | 6 (50%) | 0-3 | 13 | 10 | 3 | 0.37 |
| 2 | 12 | 5 (42%) | 0-4 | 11 | 10 | 1 | 0.31 |
| 3 | 12 | 4 (33%) | 0-1 | 4 | 4 | 0 | 0.11 |
| 4 | 10 | 5 (50%) | 0-6 | 12 | 10 | 2 | 0.41 |
| 5 | 10 | 1 (10%) | 0-1 | 1 | 1 | 0 | 0.03 |
| . | Number of sequences . | Mutations . | |||||
|---|---|---|---|---|---|---|---|
| Total . | Mutated . | Range . | Total no. . | R . | S . | Frequency/total sequences, % . | |
| Before stimulation | |||||||
| CB B cells | |||||||
| 1 | 12 | 4 (33%) | 0-2 | 7 | 5 | 2 | 0.20 |
| 2 | 12 | 3 (25%) | 0-1 | 3 | 3 | 0 | 0.08 |
| 3 | 12 | 1 (8%) | 0-1 | 1 | 1 | 0 | 0.03 |
| 4 | 12 | 0 (0%) | 0 | 0 | 0 | 0 | 0 |
| 5 | 10 | 3 (30%) | 0-3 | 7 | 5 | 2 | 0.24 |
| After stimulation | |||||||
| CD20+CD38−cells | |||||||
| 1 | 10 | 5 (50%) | 0-5 | 19 | 17 | 2 | 0.65 |
| 2 | 10 | 3 (30%) | 0-5 | 10 | 8 | 2 | 0.34 |
| 3 | 10 | 4 (40%) | 0-1 | 4 | 3 | 1 | 0.14 |
| 4 | 10 | 1 (10%) | 0-1 | 1 | 1 | 0 | 0.03 |
| 5 | 10 | 3 (30%) | 0-3 | 6 | 5 | 1 | 0.2 |
| CD20−CD38+ B cells | |||||||
| 1 | 12 | 6 (50%) | 0-3 | 13 | 10 | 3 | 0.37 |
| 2 | 12 | 5 (42%) | 0-4 | 11 | 10 | 1 | 0.31 |
| 3 | 12 | 4 (33%) | 0-1 | 4 | 4 | 0 | 0.11 |
| 4 | 10 | 5 (50%) | 0-6 | 12 | 10 | 2 | 0.41 |
| 5 | 10 | 1 (10%) | 0-1 | 1 | 1 | 0 | 0.03 |
Nucleotide exchange frequency in the VH5 segments of Cμ transcripts. The total number of nucleotides sequenced for the VH5 segments is 294 base pairs.
R and S indicate replacement and silent mutations, respectively.
Discussion
The characteristics of naive B cells have remained obscure. Our study demonstrates that naive B cells, which do not express CD27 and carry the unmutated sequences of the Ig V-region gene, can induce CSR, AID, and all Ig isotypes but IgA. The naive B cells, like CD27+ memory B cells, were found to proliferate and differentiate into plasma cells without noticeable somatic hypermutation, suggesting the produced antibodies are low affinity.
The production of IgG and IgM but not IgA from naive B cells could be induced in our experiment by the stimulation of Ig receptors by SAC and CD40 signaling in conjunction with such cytokines as IL-2 and IL-10. Several investigators15,26 have reported that IgD+ B cells used as naive B cells produced Igs, even though the population contains CD27+ B cells. Their results indicate that tonsillar surface IgD+ B cells produce IgG1, IgG2, and IgM in the presence of SAC + IL-10 + CD40/CD32T27 and possess the unique tendency to generate IgM in response to simultaneous cross-linking of surface Igs and CD40.15
Both IL-4 and CD40 signalings are necessary for IgE synthesis.27-29 We previously reported13 that CD27− naive B cells did not produce IgE with IL-4 + anti-CD40 mAb alone. However, in this experiment, not only CD27+ memory B cells but also CD27− naive B cells could produce IgE when the B cells were stimulated with IL-4 + CD40/CD32T. Differentiation of CD27− B cells into plasma cells was observed, although to a minor degree, in the presence of IL-4 + CD40/CD32T. In this regard, we9 and others30 demonstrated that IgE can be produced by naive B cells in X-linked hyper-IgM syndrome patients. Thus, it is apparent that both CD27+ memory and CD27− naive B cells have the ability to produce IgE in response to IL-4 and enough CD40 signaling. However, IL-4 and CD40 signaling induced only marginal levels of IgG and IgM production by adult CD27− and CB B cells (data not shown).
TGF-β was found to inhibit the growth of lymphocytes and the secretion of IgG and IgM from B cells.31,32 Subsequently, it was reported that TGF-β stimulated the IgA secretion from lipopolysaccharid-stimulated spleen or Peyer patch B cells in murine systems23,24 as well as the TGF-β–directed IgA class switching in human B cells in the presence of PWM + activated CD4+ T-cell clones.33 One report states that TGF-β and IL-10 cooperate in enhancing the IgA production from anti-CD40–activated IgD+ human B cells and in inhibiting the IgA production from IgD− B cells.15 In another study,34 the IgA production was induced from IgD+ B cells with dendritic cells in the presence of IL-10 + TGF-β + CD154 transfectants. The same study found that TGF-β unexpectedly reduced the production of IgA as well as of IgG and IgM from adult unseparated B cells or memory B cells in the presence of SAC + IL-10 + IL-2 + CD40/CD32T, while CD27− B cells did not produce any IgA in our study even after the addition of TGF-β in the presence of SAC + IL-10 + IL-2 + CD40/CD32T. The reasons for this discrepancy in IgA production findings between previously published data and ours are not clear. One possible explanation is that the naive B cells used in the various experiments are different. The other investigators15 34 used IgD+ B cells as naive B cells even though IgD+ B cells contain IgD+CD27+ memory B cells in the adult B cells.
B-cell Ig synthesis is preceded by transcription of the germline mRNA, and mature mRNA was expressed after CSR. CSR replaces the Ig heavy chain constant region genes to be expressed from Cμ to other CH genes. CSR take places between 2 broad areas surrounding S regions, resulting in looped-out deletion of an intervening DNA segment.16 Because naive B cells in our study produced IgG, IgM, and IgE, we further investigated the inducibility of CSR by naive B cells after stimulation. The findings that naive B cells expressed germline and mature γ transcripts demonstrated that the cells have an ability to occur CSR with the appropriate stimuli. In contrast to naive B cells, memory B cells, which have Ig receptors such as IgA, IgM, IgG, and IgE in their surface, constitutively expressed mature γ transcripts without stimulation.
AID−/− spleen cells failed to undergo CSR although they expressed germline transcripts.16 AID may be involved in regulation or catalysis of the DNA modification step of both class switching and somatic hypermutation.16 Probably, AID expression is required before CSR. To clarify this point, we investigated AID expression by naive B cells. Naive B cells remarkably expressed AID upon stimulation, probably involved in the accuracy of CSR as a consequence. Because CD27, which is memory B-cell marker, was also induced by naive B cells, AID may be involved in CD27 expression and may regulate the differentiation into memory B cells with somatic hypermutation.
Because somatic hypermutation may occur in GCs, in which B cells are activated and proliferating, and CD40 or BCR signaling significantly promotes the B-cell proliferation, the CD40-CD154 or BCR-antigen interaction still remains as a candidate for the inducer of somatic hypermutation. In addition, cross-linking of CD40 by anti-CD40 mAb + CD32T with or without IL-4 greatly enhanced the B-cell proliferation and IgE production as compared with anti-CD40 mAb alone. To clarify effects of the CD40-CD154 interaction and BCR engagement on the induction of somatic mutation, we used CB B cells and adult PB CD27− naive B cells, both of which carried unmutated sequences in Ig VH5-region genes. CD40 signaling induced no somatic mutations from CB and sort-pure adult IgD+CD27− naive B cells. In addition, somatic hypermutations were not induced by CB B cells in the presence of CD40/CD32T and CD3-activated adult T cells in our hand (data not shown). CD5+ B cells are reported to be mainly engaged in the production of low-affinity antibodies,35 and CD5 expression on CB and adult PB CD27− B cells is different. Hence, the function of CB and adult CD27− B cells may be different, and CB B cells may not represent an ideal target to study the somatic hypermutation. In this regard, of interest is our result that there was no difference in the induction of somatic hypermutation between adult CD27− naive B cells and CB B cells (data not shown).
During clinically relevant infections, neutralizing antibodies are soon generated, and such antibodies are devoid of somatic hypermutation in their Ig V-region genes.36 The analysis of genealogically related antigen-specific antibodies indicates that their binding avidities can be enhanced by as much as a factor of 30 through somatic mutation.37 The question thus arises whether the antibodies produced by naive B cells in our in vitro system have high binding avidities. We therefore analyzed somatic hypermutation in Ig V-region genes obtained from plasma cells generated from naive B cells with SAC + IL-10 + IL-2 + CD40/CD32T stimulation. The sequencing analysis of the Ig V-region genes disclosed that plasma cells generated from naive B cells in our system were mostly composed of unmutated compartments, which suggests that they produce low-affinity antibodies (Table 4). We also studied somatic hypermutation of induced plasma cells after 14 days of stimulation in culture. In this experiment, the frequency of mutations of plasma cells induced from activated CB B cells was not different between 8-day culture and 14-day culture (data not shown). Several reports demonstrated that somatic hypermutation of B cells and some B-lymphoma cells are inducible in vitro with T-cell help and upon the BCR engagement.38-40
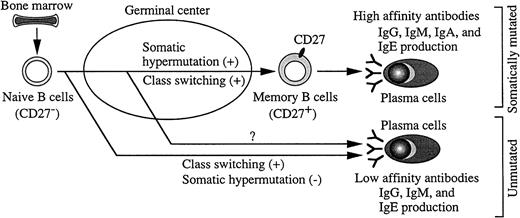
A hypothetical scheme of CSR, somatic hypermutation, and plasma cell generation from naive B cells is shown (Figure8). Naive B cells undergo CSR and somatic hypermutation in GCs in vivo. However, independent regulation of class switching and somatic hypermutation has been shown in various systems, by using such things as a Burkitt lymphoma cell line,41human GC B cells,42 or mouse in vitro system.36 43 In our present study, we highlighted the ability to induce class switching and somatic hypermutation by using human IgD+CD27− clean naive B cells. In our in vitro system CSR, the Ig production and plasma cell differentiation, but not noticeable somatic hypermutation were achieved by IgD+CD27− naive B cells, indicating the different process of class switching and somatic hypermutation in human naive B cells. This observation elucidates some mechanisms of the susceptibility to infections in neonates and certain primary immunodeficiencies.
The different process of class switching and somatic hypermutation and proposed generation of plasma cells in humans.
After B-cell precursors succeed in generating functional antigen receptors in bone marrow, they are released into the B-cell pool as naive B cells. Naive B cells without CD27 undergo oligoclonal expansion, class switching, and somatic hypermutation in GCs, and CD27 is expressed on their surface, which results in their differentiation into memory B cells. Plasma cells generated from memory B cells, which carry a high load of somatic mutation, may produce high-affinity antibodies (IgG, IgM, IgA, IgE). Plasma cells from unmutated B cells outside GCs or through GCs before carrying somatic hypermutation may produce low-affinity antibodies (IgG, IgM, IgE).
The different process of class switching and somatic hypermutation and proposed generation of plasma cells in humans.
After B-cell precursors succeed in generating functional antigen receptors in bone marrow, they are released into the B-cell pool as naive B cells. Naive B cells without CD27 undergo oligoclonal expansion, class switching, and somatic hypermutation in GCs, and CD27 is expressed on their surface, which results in their differentiation into memory B cells. Plasma cells generated from memory B cells, which carry a high load of somatic mutation, may produce high-affinity antibodies (IgG, IgM, IgA, IgE). Plasma cells from unmutated B cells outside GCs or through GCs before carrying somatic hypermutation may produce low-affinity antibodies (IgG, IgM, IgE).
The authors thank Drs S. Nonoyama, T. Kobata, S. Ito, and C. Morimoto for their support.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kazunaga Agematsu, Shinshu University, Graduate School of Medicine, Dept of Infectious Immunology and Pediatrics, Asahi 3-1-1, Matsumoto 390-8621, Japan; e-mail:agemats@gipac.shinshu-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal