Abstract
Phospholipase C (PLC)–β2 plays a major role in platelet activation. Previous studies have described a unique patient with impaired receptor-mediated platelet aggregation, secretion, calcium mobilization, and phospholipase C (PLC) activation associated with a selective decrease in platelet PLC-β2 isozyme. To identify the mechanisms leading to the defect, platelet RNA from the patient and healthy subjects was subjected to reverse transcription–polymerase chain reaction (RT-PCR) and the products sequenced. The PLC-β2 cDNA sequence in the patient showed no abnormalities. Platelet PLC-β2 and β-actin (internal control) mRNA levels were assessed by RT-PCR; the ratio of PLC-β2 to β-actin mRNA levels was 0.80 to 0.95 in 4 healthy subjects and 0.28 in the patient. PLC-β2 mRNA levels were similarly reduced compared with GPIIb and Gαq mRNA levels. PLC-γ2 and platelet factor 4 mRNA levels were normal. Calcium mobilization was studied in neutrophils upon activation with formyl-Met-Leu-Phe (fMLP), adenosine diphosphate (ADP), platelet-activating factor (PAF), interleukin-8 (IL-8), C5a, and leukotriene B4 (LTB4), and it was normal. Neutrophil elastase secretion upon activation with fMLP, ADP, PAF, IL-8, C5a, and LTB4 was normal, as were neutrophil PLC-β2 mRNA and PLC-β2 on immunoblotting. Thus, responses to activation, PLC-β2 protein, and PLC-β2 mRNA are decreased in patient platelets but not in neutrophils, providing evidence for a hitherto undescribed lineage (platelet)–specific defect in PLC-β2 gene expression. These studies provide a physiologically relevant model to delineate regulation of PLC-β2 gene and its tissue-specific expression.
Introduction
An early response to ligation of platelet G-protein–coupled receptors is activation of phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5 bisphosphate to yield 1,4,5 inositoltrisphosphate (IP3) and diacylglycerol (DG), 2 major mediators of platelet signal transduction. IP3mobilizes Ca++ from intracellular stores and DG activates protein kinase C, events that ultimately result in the end responses, such as aggregation and secretion. Phospholipase C exists in multiple isoforms,1,2 which are divided into 3 types: PLC-β, PLC-γ, and PLC-δ. Platelets contain at least 7 PLC-isozymes.3 PLC-β2 is the isozyme present in the largest amount in platelets next to PLC-γ2,3 and plays an important role in signal transduction mediated by G-protein–coupled receptors.
We have previously described a unique platelet function defect characterized by a deficiency in platelet PLC-β2 isozyme.3-5 The propositus and her son had impaired platelet aggregation, serotonin secretion, mobilization of cytoplasmic ionized calcium, and PLC activation in response to several G-protein–coupled receptor-mediated agonists, including adenosine diphosphate (ADP), thrombin, platelet-activating factor (PAF), and thromboxane A2.4 Platelet levels of PLC-β2 were decreased to approximately one third with normal levels of other PLC-isozymes.3 Described here are further studies to characterize the mechanisms leading to the PLC-β2 deficiency. We report that PLC-β2 mRNA and protein levels are decreased in platelets but normal in the neutrophils from the patient, which suggests a lineage-specific defect in PLC-β2 gene expression.
Patient, materials, and methods
Patient information
Details regarding the clinical presentation and the platelet responses have been previously described.3-5
Materials
Taq polymerase, dNTP, and peroxidase-conjugated goat anti–rabbit IgG were purchased from Promega Biotech (Madison, WI). All oligonucleotides were synthesized by Custom Primers, Gibco BRL (Grand Island, NY). α–32P-dCTP and Gene Screen-Plus hybridization transfer membrane were purchased from DuPont-NEN (Boston, MA). Rabbit antibodies against PLC-β2 and PLC-γ2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Carbacyclin was obtained from Biomol (Plymouth Meeting, PA). Fura-2/AM was purchased from Calbiochem (San Diego, CA). Betaine and other chemicals and reagents were from Sigma Chemical (St Louis, MO). LTB4 was obtained form Cayman (Ann Arbor, MI), C5a from Bachem (King of Prussia, PA), and PAF from Avanti Polar-Lipids (Alabaster, AL).
Preparation of platelet RNA
Two tubes of 50 mL blood were collected from the patient and healthy volunteer donors into 7.5 mL each of acid citrate dextrose (ACD) solution (71.4 mM citric acid, 85 mM sodium citrate-dihydrate, 11.1 mM dextrose). A stable prostacyclin analog, carbacyclin (30 nM), was added to the blood, and platelet-rich plasma (PRP) was obtained by centrifugation at 200g for 20 minutes at room temperature. Carbacyclin (30 nM) and ACD (one-tenth volume) were added to the PRP and the platelets pelleted by centrifugation at 650g for 15 minutes at room temperature. The platelet pellet was resuspended in Hepes buffer (pH 6.5, 20 mM Hepes, 5.5 mM dextrose, 0.376 mM NaH2PO4, 1 mM MgCl2, 2.7 mM KCl, 137 mM NaCl, and 1 mg/mL bovine serum albumin), and was washed twice using the same buffer by centrifugation at 650g for 10 minutes at room temperature. Platelet pellets were resuspended in 4 mL TRIzol Reagent (Gibco BRL, Gaithersburg, MD) and total RNA was isolated as recommended by the manufacturer. The final RNA preparations were stored in diethyl pyrocarbonate–treated water at −80°C.
Preparation of neutrophil RNA
Blood was drawn into one-tenth volume of 3.8% sodium citrate and overlaid on neutrophil isolation medium (NIM) essentially as described by the manufacturer (Cardinal Associates, Santa Fe, NM). Red blood cells were sedimented by centrifugation at 400g for 40 minutes at room temperature. The neutrophil layer was collected and washed by centrifugation with Hanks balanced salt solution (HBSS) twice. The red blood cells were lysed by incubating with 2.5 volumes of erythrocyte lysing buffer (E-lyse) (Cardinal Associates) for 10 minutes at room temperature. The neutrophils were recovered from the lysis solution by centrifugation at 250g for 5 minutes at room temperature. The preparation was assessed for viability by trypan blue dye exclusion. The RNA was extracted as described above for platelets.
PCR amplification of cDNA
First-strand cDNA was synthesized from total RNA (5 μg) using either random or oligo(dT) primers (Stratagene, La Jolla, CA). MMLV Reverse Transcriptase (Stratagene) was used under reaction conditions according to the manufacturer's protocol. Following incubation at 37°C for 60 minutes, the enzyme was inactivated by heating the samples for 5 minutes at 90°C. PCR amplification of cDNA was performed in a Perkin Elmer DNA thermal Cycler (Perkin Elmer Cetus, Norwalk, CT). Amplification primers were designed from a known PLC-β2 cDNA sequence6 and have been previously described.7 PCR conditions were optimized for each primer pair. In some experiments 1M betaine (N, N, N-trimethylglycine)8 was added to the PCR reaction. The PCR products were visualized by 0.8% to 2% of ethidium bromide–stained agarose gel electrophoresis and excised. The amplified product was purified using the Qiagen purification kit (Chatsworth, CA). PCR products were sequenced by automatic sequencing performed using the PRISM Ready Reaction DyeDeoxy Terminator cycle Sequencing Kit on the Applied Biosystems model 377 DNA Sequencing System.
Estimation of PLC-β2 mRNA levels
Platelet and neutrophil cDNA were amplified by PCR in the presence of 32P-dCTP using PLC-β2 primers as indicated, under conditions optimized for linearity of amplification. The reactions were assembled by making a master mix of all components for the PCR amplification reaction except the target cDNA. The PCR cycling conditions for β-actin, PLC-β2, PLC-γ2, Gαq, PF4, and GPIIb were initially optimized in order to compare the DNA fragments produced during a period of exponential application. The number of PCR cycles was chosen so that no component of the reaction, other then the amount of template present, was limiting. Initial tests of 20 to 60 cycles established that PCR amplification of the PLC-β2 and β-actin cDNA product were well below the point of PCR plateau at the end of 30 cycles. The amplified fragments were radiolabeled by including 1 μCi (0.037 MBq) α–32P-dCTP in the PCR. In the PCR amplification of PLC-β2, β-actin was coamplified as an internal control. In these reactions, 0.2 μM of PLC-β2 primers, 0.08 μM of β-actin primers, 2 mM MgCl2, 0.3 mM dNTPs, 1 M betaine, and 3.75 U of Taq polymerase were combined with 2 μL of the first-strand template to a final volume of 50 μL. The first-strand cDNA was quantitatively normalized using densitometric measurements from scanned phosphorimages of β-actin PCR amplification with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). After an initial incubation at 94°C for 45 seconds, 30 cycles of the PCR were performed at 94°C for 45 seconds, 62°C for 45 seconds, and 72°C for 2 minutes, followed by a 7-minute final extension step in a Perkin Elmer DNA thermal cycler (Perkin Elmer Cetus). When the PCR products from these reactions were visualized, the resulting fluorescent signals remained within the linear phase of absorption for ethidium bromide. A quantity of 14 μL of each PCR product was electrophoresed on a 1.2% agarose gel (Sigma Chemical) containing 0.5 μg/mL ethidium bromide in 0.5 X TBE buffer (44.5 mM Tris-base, 44.5 mM boric acid, 1 mM EDTA) at 100 V for 60 minutes. The gels were transferred by the alkaline transfer method to membrane GeneScreen Plus hybridization transfer membrane (DuPont-NEN, Boston, MA). The radioactivity in the bands was quantified using the Fujix Bas 2000TR Phosphor-Imager (Fuji Medical Systems, Stamford, CT). The relative amount of product in each band was quantified from the scanned plate with ImageQuant software (Molecular Dynamics). In some experiments, the bands of interest were excised from gels and the radioactivity was measured by liquid scintillation counting.
To assess expression of PLC-γ2 mRNA, platelet cDNA was PCR-amplified using primers coding for a 435-bp fragment (forward: 3208-3229 nt 5′–AGTACATGCAGATGAATCACGC-3′; reverse 3621-3642 nt 5′–ACCTGAATCCTGATTTGACTGC-3′). In the same reaction, β-actin was simultaneously amplified as an internal control as described above. The reaction mixture (50 μL) contained 0.2 μM of each PLC-γ2 primer, 0.1 μM of each β-actin primer, 2 μL of normalized first-strand cDNA, 1.5 mM MgCl2, 1 μCi (0.037 MBq)32P-dCTP, 2 mM dNTPs, and 2.5 U Taq polymerase. The reaction was started with an incubation at 94°C for 45 seconds followed by 28 cycles of 94°C for 45 seconds, 58°C for 30 seconds, and 72°C for 90 seconds. The final incubation was for 7 minutes at 72°C. The products were analyzed as described above. To analyze Gαq and glycoprotein (GP) IIb expression, platelet cDNA was PCR-amplified in separate reactions, under conditions optimized for linearity of amplification, using primers coding for the entire coding region (1080 bp) of Gαq, (forward, 1-22 nt: 5′-ATGACTCTGGAGTCCATCATGG-3′; reverse: 1063-1080 nt: 5′-TTAGACCAGATTGTACTC-3′) (GenBank Accession number: AF329 284) and a 1230-bp fragment of GPIIb (forward: 4-26 nt 5′-GGCCAGAGCTTTG TGTCCACTGC-3′; reverse: 1303-1323 nt 5′-GGGCTGTCCAGGACCTGGGAG-3′.9 The 50-μL PCR reaction volumes contained 2 μL normalized first-strand cDNA, 0.2 μM each of forward and reverse primers, 1 μCi (0.037 MBq)32P-dCTP, 2 mM dNTPs, 1.5 mM MgCl2, and 2.5 U Taq polymerase. The reaction was started with an incubation at 94°C for 45 seconds followed by 30 cycles in the reaction for Gαq and 28 cycles for GPIIb of 94°C for 45 seconds, 55°C for 45 seconds, and 72°C for 2 minutes. The final incubation was at 72°C for 7 minutes. The products were analyzed as described above.
To assess expression of PF4 mRNA, platelet cDNA was PCR amplified using primers coding for a 290 bp fragment (forward: 48-68 nt 5′-TGCTGTTCCTGGGGTTGCTGC-3′; reverse 313-337 nt 5′-TGCACACACGTAGGCAGCTAGTAGC-3′). In the same reaction β-actin was simultaneously amplified as an internal control as described above. The reaction mixture (50 μL) contained 0.2 μM of each PF4 primer, 0.2 μM of each β-actin primer, 2 μL of normalized first strand cDNA, 1.5 mM MgCl2, 1 μCi (0.037 MBq) 32P-dCTP, 2mM dNTPs, and 2.5 U of Taq polymerase. The reaction was started with an incubation at 94°C for 45 seconds followed by 22 cycles of 94°C for 45 seconds, 56°C for 30 seconds, and 72°C for 90 seconds. The final incubation was for 7 minutes at 72°C. The products were analyzed as described above.
Immunoblotting for neutrophil PLC-β2 and PLC-γ2
The neutrophils were washed 3 times with ice-cold extraction buffer (50 mM Tris-base, pH 8.3, 1 mM ethyleneglycotetraacetic acid [EGTA], 1 mM dithiothreiotol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 10 μg/mL aprotinin). Cells were sonicated on ice (twice for 10 seconds at 40 kHz). The homogenate was centrifuged for 10 minutes at 600g and supernatants were collected. The protein content in total homogenates was determined by the bicinchoninic acid method (BCA) using a commercial assay (Pierce Chemical, Rockford, IL) and the aliquots stored at −80°C. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (10% resolving gel) and electrophoretically transferred to polyvinylidene fluoride (PVDF) nitrocellulose membranes (Millipore, Bedford, MA). The membranes were washed in tris-buffered saline (TBS) with 0.1% Tween 20 and incubated for 1 hour at room temperature with specific polyclonal rabbit antibodies (0.1 μg/mL PLC-β2 or 0.2 μg/mL PLC-γ2) in TBS with 1% bovine serum albumin and 0.1% Tween 20. The antibodies bound to nitrocellulose were detected using peroxidase-conjugated goat anti–rabbit IgG for 1 hour at room temperature, developed by Western Blot Chemiluminecence Reagent Plus (NEN, Boston, MA) and analyzed on a Kodak X-OMAT Blue imaging film.
Studies on calcium mobilization and elastase release in neutrophils
Blood was drawn from the patient and healthy donors in ACD as anticoagulant (ratio 6:1) and neutrophils were isolated as described.10 Leukocyte-rich plasma (LRP) was prepared from the whole blood by sedimentation using 3% dextran T-500 (Amersham Pharmacia Biotech, Piscataway, NJ) gradient on ice for 20 minutes. The LRP was centrifuged for 10 minutes at 250g at 4°C. The pellet was suspended in HBSS (Gibco BRL) without added calcium chloride, magnesium chloride, magnesium sulfate, and sodium bicarbonate. The suspension was subjected to density centrifugation (400g, 45 minutes, room temperature) using Ficoll-hypaque (Amersham Pharmacia Biotech AB). The top layers were aspirated and erythrocytes removed by hypotonic lysis with ice-chilled 0.2% sodium chloride for 20 seconds. The neutrophil pellet was resuspended in HBSS. The neutrophil cell count was adjusted to 1 × 107cells/mL and viability judged by trypan blue dye exclusion.
For studies on Ca++ mobilization, the neutrophils were loaded with 3 μM fura-2 AM (Calbiochem) for 30 minutes at 37°C. After the incubation period, the neutrophils were washed twice in HBSS and kept at room temperature. Aliquots (2 mL) of neutrophils suspension (3.4 × 106/mL) were incubated in a quartz cuvette (1-cm light path) for 2 minutes at 37°C in the presence of 1 mM external calcium prior to activation. Fluorescence measurements were recorded in a Perkin-Elmer model LS-5 fluorimeter using a quartz cuvette continuously stirred and thermostatically controlled at 37°C. Fura-2 fluorescence signals were recorded using an excitation wavelength of 340 nm and an emission wavelength of 510 nm. Cytosolic free calcium concentrations were calculated as previously described.4 11 Autofluorescence was determined by the addition of 1 mM manganese chloride, and maximal fluorescence (Fmax) was determined by the addition of 0.5% Triton to lyse the cells. The agonists used include N-formyl-Met-Leu-Phe (fMLP) (0.1 μM-10 μM), ADP (50 μM-200 μM), IL-8 (1 nM-5 nM), LTB4 (0.1 μg/mL-0.25 μg/mL), PAF (0.1 μM-1 μM), and C5a (1 μM-5 μM). After recording the fluorescence changes, aliquots of the samples were utilized to assess elastase release. The sample was incubated at 37°C and 500 uL aliquots were removed at 2 minutes and 10 minutes. The suspension was centrifuged at 14 000g for one minute, and supernatant collected for elastase assay. A quantity of 10 μL normal plasma was added to each sample. Elastase levels were measured by enzyme immunoassay (Merck Immunoassay kit; E.M. Sciences, Gibbstown, NJ). The data are expressed as a percentage of total elastase, measured in a separate aliquot subjected to lysis with 0.5% Triton.
Results
To determine the mechanisms leading to the decreased platelet PLC-β2 levels, platelet RNA from the patient and healthy subjects was subjected to RT-PCR using 5 sets of overlapping primers (Figure1) and the products sequenced. The PLC-β2 coding sequence (165-3707 nt) in the patient was identical to that from healthy subjects and the published sequence12(GenBank accession number M95678). In addition, the 3′ UTR sequence extending from 3708 nt to 4516 nt was also identical to the human PLC-β2 sequence.12
A schematic representation of the PLC-β2 coding sequence and the primers used to amplify PLC-β2 cDNA.
The numbering is as per Park et al.12 The coding sequence is shown by the filled area (165 nt to 3707 nt). Sequence 2755-2799 represents alternative splicing of the PLC-β2 mRNA, resulting in 2 variants with and without this sequence.7
A schematic representation of the PLC-β2 coding sequence and the primers used to amplify PLC-β2 cDNA.
The numbering is as per Park et al.12 The coding sequence is shown by the filled area (165 nt to 3707 nt). Sequence 2755-2799 represents alternative splicing of the PLC-β2 mRNA, resulting in 2 variants with and without this sequence.7
Platelet PLC-β2 and PLC-γ2 mRNA levels
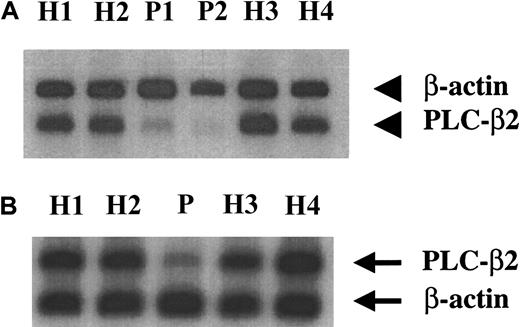
Because the coding sequence was intact, we assessed platelet PLC-β2 mRNA levels. Platelet RNA from 4 healthy subjects and the patient was subjected to RT-PCR using primers corresponding to 2864 nt-2885 nt (forward) and 3328 nt-3352 nt (reverse), and under conditions carefully optimized for linearity of amplification. These primers are downstream of the site of alternative splicing of PLC-β2 mRNA (2755-2799 nt) (Figure 1). A 758-bp fragment of β-actin was simultaneously amplified. The PLC-β2 mRNA levels were decreased in the patient compared with 4 healthy subjects (Figure2A). The ratio of PLC-β2 to actin was 0.7 to 0.9 in the 4 healthy subjects and 0.3 in the patient. To obtain further evidence of decreased PLC-β2 mRNA levels, platelet mRNA was amplified by using a second set of PLC-β2 primers corresponding to the regions 3188 nt-3212 nt (forward) and 4492 nt-4516 nt (reverse) with the reverse primer being in the 3′ untranslated region (Figure 1). The mRNA levels were decreased in the patient compared with healthy subjects (Figure 2B). The ratio of PLC-β2 to actin was 0.28 in the patient compared with 0.80 to 0.95 in the healthy subjects. Each of these analyses have been performed at least 2 to 3 times and using platelet mRNA obtained from the patient on 3 separate visits over a period of 2 to 3 years. Previous studies have shown that platelet PLC-γ2 activity and antigen levels were normal in our patient.3 Platelet PLC-γ2 mRNA level was comparable with that in healthy subjects (Figure 3). The ratio of PLC-γ2 mRNA level to actin ranged from 0.35 to 0.49 in 4 healthy subjects; it was 0.40 in the patient.
Platelet PLC-β2 mRNA levels in the patient and healthy subjects.
Platelet RNA from the patient (P) and healthy subjects (H) was subjected to RT-PCR in the presence of 32P-dCTP using 2 sets of PLC-β2 primers (A,B) and β-actin primers, under conditions of linearity of amplification, as described in “Patient, materials, and methods.” The products were subjected to gel electrophoresis, transferred to membrane, and analyzed by phosphorimaging. (A) The PLC-β2 primers used were forward: 2864 nt to 2885 nt; reverse: 3328 nt to 3352 nt. P1 and P2 represent patient samples from 2 separate occasions. (B) The PLC-β2 primers used were forward: 3188 nt to 3212 nt, reverse: 4492 nt to 4516 nt, with the latter being in the 3′ untranslated region (see Figure 1).
Platelet PLC-β2 mRNA levels in the patient and healthy subjects.
Platelet RNA from the patient (P) and healthy subjects (H) was subjected to RT-PCR in the presence of 32P-dCTP using 2 sets of PLC-β2 primers (A,B) and β-actin primers, under conditions of linearity of amplification, as described in “Patient, materials, and methods.” The products were subjected to gel electrophoresis, transferred to membrane, and analyzed by phosphorimaging. (A) The PLC-β2 primers used were forward: 2864 nt to 2885 nt; reverse: 3328 nt to 3352 nt. P1 and P2 represent patient samples from 2 separate occasions. (B) The PLC-β2 primers used were forward: 3188 nt to 3212 nt, reverse: 4492 nt to 4516 nt, with the latter being in the 3′ untranslated region (see Figure 1).
Platelet PLC-γ2 mRNA levels in the patient and healthy subjects.
Platelet RNA from the patient (P) and healthy subjects (H) was subjected to RT-PCR in the presence of 32P-dCTPs using primers for PLC-γ2 corresponding to 3208 nt to 3229 nt (forward) and 3621 nt to 3642 nt (reverse), and for β-actin as described in “Patient, materials, and methods.” The products were subjected to gel electrophoresis, transferred to membrane, and analyzed by phosphorimaging. Figure is representative of 2 separate experiments.
Platelet PLC-γ2 mRNA levels in the patient and healthy subjects.
Platelet RNA from the patient (P) and healthy subjects (H) was subjected to RT-PCR in the presence of 32P-dCTPs using primers for PLC-γ2 corresponding to 3208 nt to 3229 nt (forward) and 3621 nt to 3642 nt (reverse), and for β-actin as described in “Patient, materials, and methods.” The products were subjected to gel electrophoresis, transferred to membrane, and analyzed by phosphorimaging. Figure is representative of 2 separate experiments.
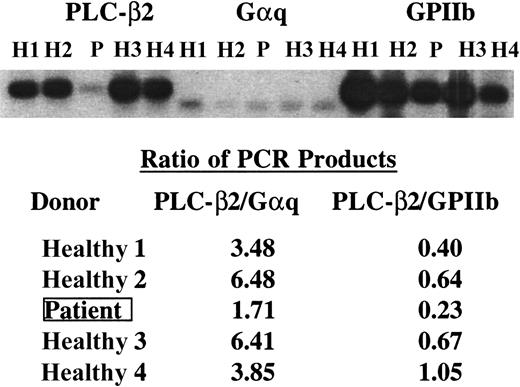
Comparison of PLC-β2, Gαq, and GPIIb mRNA levels in platelets
In additional experiments, the PLC-β2 mRNA levels were compared against those for 2 other platelet proteins, Gαq and GPIIb, the latter being specific for platelets. The entire coding sequence of Gαq was amplified using primers yielding a product of 1080 bp. GPIIb was amplified with primers corresponding to 4 nt-16 nt (forward) and 1303 nt-1323 nt (reverse) yielding a product of 1230 bp. PLC-β2 mRNA was decreased in the patient relative to Gαq and GPIIb mRNA (Figure4). The ratio of PLC-β2 to Gαq mRNA ranged from 3.5 to 6.5 in the 4 healthy subjects and was 1.7 in the patient (Figure 4). The corresponding values for GPIIb ranged from 0.40 to 1.05 in healthy subjects and 0.23 in the patient. These analyses have been performed 3 times with similar results. In some experiments the specific bands were excised from the gels and the radioactivity was quantified by scintillation counting (not shown). The results were similar to those from phosphorimaging.
PLC-β2, GPIIb, and Gαq mRNA levels in the patient and healthy subjects.
cDNA from the patient (P) and healthy subjects (H) was amplified under conditions of linearity of amplification by PCR using specific primers for PLC-β2 corresponding to 3188 nt to 3212 nt (forward) and 4492 nt to 4516 nt (reverse), Gαq (full coding sequence, 1080 nt), and a 1230-bp fragment of GPIIb. The products were transferred to membrane and analyzed by phosphorimaging. The ratios of the PCR products in PLC-β2 to those in Gαq and GPIIb are shown. The findings indicate a decrease in PLC-β2 mRNA in the patient relative to Gαq and GPIIb mRNA.
PLC-β2, GPIIb, and Gαq mRNA levels in the patient and healthy subjects.
cDNA from the patient (P) and healthy subjects (H) was amplified under conditions of linearity of amplification by PCR using specific primers for PLC-β2 corresponding to 3188 nt to 3212 nt (forward) and 4492 nt to 4516 nt (reverse), Gαq (full coding sequence, 1080 nt), and a 1230-bp fragment of GPIIb. The products were transferred to membrane and analyzed by phosphorimaging. The ratios of the PCR products in PLC-β2 to those in Gαq and GPIIb are shown. The findings indicate a decrease in PLC-β2 mRNA in the patient relative to Gαq and GPIIb mRNA.
PF4 mRNA levels in platelets
We assessed mRNA levels for PF4, which is specific to platelets. These levels were normal in the patient (Figure5). The ratio of PF4 to β-actin in the patient was 1.18 (4 healthy subjects ranged 1.13 to 1.28).
Platelet PF4 mRNA levels in patient and healthy subjects.
Platelet RNA from the patient (P) and healthy subjects (H) was subject to RT-PCR in the presence of 32P-dCTPs using primers for PF4 corresponding to 48-68 nt (forward) and 313-337 nt (reverse), and for β-actin, as described in “Patient, materials, and methods.” The products were subjected to gel electrophoresis, transferred to membrane, and analyzed. Results shown are representative of 2 separate experiments.
Platelet PF4 mRNA levels in patient and healthy subjects.
Platelet RNA from the patient (P) and healthy subjects (H) was subject to RT-PCR in the presence of 32P-dCTPs using primers for PF4 corresponding to 48-68 nt (forward) and 313-337 nt (reverse), and for β-actin, as described in “Patient, materials, and methods.” The products were subjected to gel electrophoresis, transferred to membrane, and analyzed. Results shown are representative of 2 separate experiments.
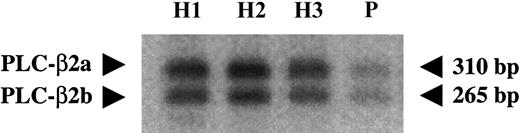
Relative levels of the alternatively spliced transcripts in platelets
Our previous studies with platelet RNA and genomic DNA from healthy subjects revealed evidence for alternative splicing of PLC-β2 mRNA, with and without a 45-bp sequence (2755 nt-2799 nt), and corresponding to deduced proteins of 1181 amino acids (PLC-β2a) and 1161 amino acids (PLC-β2b), respectively.7 Platelet RNA was subjected to RT-PCR in the presence of 32P-labeled dCTPs using primers flanking the alternative splicing site and corresponding to 2575 nt-2596 nt (forward) and 2864 nt-2885 nt (reverse) (Figure 1). The 2 splice variants with sizes of 310 bp and 265 bp were noted (Figure 6). In healthy subjects, PLC-β2a was slightly more than PLC-β2b (ratio 1.3). In the patient the relative amounts of the 2 splice variants were identical to those in the healthy subjects (ratio 1.2); however, the levels of both variants were decreased (Figure 6).
Relative amounts of alternatively spliced products of PLC-β2.
Normalized first-strand cDNA from the patient (P) and healthy subjects (H) was amplified using PLC-β2 primers corresponding to 2575 nt to 2596 nt (forward) and 2864 nt to 2885 nt (reverse), flanking the alternative splicing site (2755 nt to 2799 nt). The products were subjected to gel electrophoresis, transferred to membrane, and subjected to phosphorimaging. Both splice variants were expressed in the patient but at decreased levels.
Relative amounts of alternatively spliced products of PLC-β2.
Normalized first-strand cDNA from the patient (P) and healthy subjects (H) was amplified using PLC-β2 primers corresponding to 2575 nt to 2596 nt (forward) and 2864 nt to 2885 nt (reverse), flanking the alternative splicing site (2755 nt to 2799 nt). The products were subjected to gel electrophoresis, transferred to membrane, and subjected to phosphorimaging. Both splice variants were expressed in the patient but at decreased levels.
Agonist-induced calcium mobilization and elastase release in neutrophils
To determine if the defect observed in the platelets occurs in other hematopoietic cells, we studied neutrophil responses on activation with several agonists, chosen for the following reasons: (1) receptor activation with fMLP, C5a, or IL-8 is recognized to activate PLC-β213,14; (2) Ca++ mobilization in response to ADP and PAF is impaired in the patient's platelets4; and (3) in knock-out mice deficient in PLC-β2, neutrophil Ca++ mobilization in response to fMLP and IL-8 is impaired.15 The basal (resting) cytoplasmic Ca++ levels in the patient were comparable to those in normal platelets. The rise in cytoplasmic Ca++ levels on activation with fMLP (0.1, 1.0, and 10 μM), ADP (50, 200 μM), PAF (0.1, 1.0 μM), C5a (1, 5 μM), IL-8 (1, 5 nM), and LTB4 (0.1, 0.25 μg/mL) were normal in the patient (Table 1). In parallel with studies on neutrophils, we assessed the Ca++ responses in platelets and the rise in cytoplasmic Ca++ levels were impaired in response to ADP, U46619, and thrombin, as previously described.4
Agonist-induced calcium mobilization in neutrophils
| Agonist . | Healthy donors Mean; range (n) . | Patient (nM) . |
|---|---|---|
| fMLP (0.1 μM) | 171; 38-531 (24) | 121 |
| fMLP (1 μM) | 187; 49-663 (25) | 100 |
| fMLP (10 μM) | 226; 61-501 (25) | 138 |
| ADP (50 μM) | 41; 18-100 (21) | 40 |
| ADP (200 μM) | 127; 14-479 (24) | 165 |
| PAF (0.1 μM) | 146; 25-263 (23) | 37 |
| PAF (1 μM) | 191; 38-320 (22) | 126 |
| C5a (1 μM) | 76; 18-144 (18) | 40 |
| C5a (5 μM) | 146; 38-416 (19) | 100 |
| IL-8 (1 nM) | 126; 41-307 (20) | 51 |
| IL-8 (5 nM) | 132; 38-238 (18) | 79 |
| LTB4 (0.1 μg/mL) | 175; 139-234 (4) | 146 |
| LTB4 (0.25 μg/mL) | 211; 139-301 (4) | 99 |
| Agonist . | Healthy donors Mean; range (n) . | Patient (nM) . |
|---|---|---|
| fMLP (0.1 μM) | 171; 38-531 (24) | 121 |
| fMLP (1 μM) | 187; 49-663 (25) | 100 |
| fMLP (10 μM) | 226; 61-501 (25) | 138 |
| ADP (50 μM) | 41; 18-100 (21) | 40 |
| ADP (200 μM) | 127; 14-479 (24) | 165 |
| PAF (0.1 μM) | 146; 25-263 (23) | 37 |
| PAF (1 μM) | 191; 38-320 (22) | 126 |
| C5a (1 μM) | 76; 18-144 (18) | 40 |
| C5a (5 μM) | 146; 38-416 (19) | 100 |
| IL-8 (1 nM) | 126; 41-307 (20) | 51 |
| IL-8 (5 nM) | 132; 38-238 (18) | 79 |
| LTB4 (0.1 μg/mL) | 175; 139-234 (4) | 146 |
| LTB4 (0.25 μg/mL) | 211; 139-301 (4) | 99 |
Shown are the increase in cytoplasmic Ca++concentration over the basal level upon activation.
The patient results are representative of 2 separate studies.
A major abnormality documented in this patient is markedly decreased platelet secretion in response to several different G-protein–coupled agonists.4 We, therefore, assessed elastase release on neutrophil activation. Elastase release at 2 and 10 minutes in response to fMLP (0.1, 1.0, and 10 μM) was comparable with that in healthy subjects. For example, elastase release in the healthy subjects and patient at 10 minutes were as follows: 0.1 μM fMLP: patient 22%, healthy subjects 17% ± 11% (n = 4, mean ± SD); 1.0 μM fMLP: patient 38%, healthy subjects 25% ± 9% (n = 3); and 10 μM fMLP: patient 41%, healthy subjects 41% ± 11%. In addition, elastase release in response to 1 μM PAF (patient 25%, 4 healthy subjects 15% ± 10%), 1 μM C5a (patient 30%, 2 healthy subjects 15% ± 16%), 5 nM IL-8 (patient 35%, 2 healthy subjects 10% ± 14%) and 0.25 μg/mL LTB4 (patient 33%, 2 healthy subjects 13% ± 17%) were not decreased. Thus, in our patient neither Ca++ mobilization nor elastase release were impaired on activation of neutrophils with several agonists.
Neutrophil PLC-β2 and PLC-β2 mRNA
Neutrophil PLC-β2 mRNA levels, assessed on 2 separate occasions, were normal (Figure7A). The ratio of PLC-β2 to actin was 0.90 and 0.97 (2 visits) in the patient compared with 0.6 to 0.9 in 4 healthy subjects. In parallel studies, platelet PLC-β2 mRNA from the same occasion were decreased. On immunoblots, PLC-β2 and PLC-γ2 levels in neutrophils were comparable with those in healthy subjects (Figure 7B).
Neutrophil PLC-β2 and PLC-γ2 levels.
(A) PLC-β2 mRNA levels in neutrophils. First-strand cDNA from neutrophils from the patient (P) and healthy subjects (H) was amplified by PCR using primers for PLC-β2 corresponding to 2864 nt to 2882 nt (forward) and 3328 nt to 3352 nt (reverse) along with primers for β-actin (758-bp fragment). The products were analyzed as described in the Figure 2 legend. P1 and P2 represent patient samples from 2 separate visits. Results shown are representative of 3 separate experiments. (B) Immunoblots showing PLC-β2 and PLC-γ2 levels in neutrophil lysates from patient (P) and 3 healthy subjects. The neutrophil lysates were subjected to electrophoresis and transferred to membrane. The PLC-β2 and PLC-γ2 were detected using specific antibodies and by chemiluminescence. Samples from healthy subject H1 were analyzed at increasing protein concentration from 0.5 μg to 12.0 μg subjected to gel electrophoresis. Samples from patient (P) and healthy subjects H2 and H3 were analyzed at 6 μg protein. Results shown are representative of 3 separate experiments.
Neutrophil PLC-β2 and PLC-γ2 levels.
(A) PLC-β2 mRNA levels in neutrophils. First-strand cDNA from neutrophils from the patient (P) and healthy subjects (H) was amplified by PCR using primers for PLC-β2 corresponding to 2864 nt to 2882 nt (forward) and 3328 nt to 3352 nt (reverse) along with primers for β-actin (758-bp fragment). The products were analyzed as described in the Figure 2 legend. P1 and P2 represent patient samples from 2 separate visits. Results shown are representative of 3 separate experiments. (B) Immunoblots showing PLC-β2 and PLC-γ2 levels in neutrophil lysates from patient (P) and 3 healthy subjects. The neutrophil lysates were subjected to electrophoresis and transferred to membrane. The PLC-β2 and PLC-γ2 were detected using specific antibodies and by chemiluminescence. Samples from healthy subject H1 were analyzed at increasing protein concentration from 0.5 μg to 12.0 μg subjected to gel electrophoresis. Samples from patient (P) and healthy subjects H2 and H3 were analyzed at 6 μg protein. Results shown are representative of 3 separate experiments.
Discussion
The present studies demonstrate that our patient with platelet PLC-β2 deficiency (protein and activity)3 has a normal PLC-β2 coding sequence and this is associated with decreased PLC-β2 mRNA in platelets. PLC-β2 mRNA levels were reduced more than 50% in line with the reductions in the protein and activity.3 The reduction in platelet PLC-β2 mRNA with normal levels of Gαq, and platelet-specific GPIIb and PF4 mRNA indicates a specific rather than a generalized decrease in platelet mRNA. This is further supported by normal platelet mRNA levels for PLC-γ2, the most abundant PLC isozymes in human platelets.3 We have previously shown that platelet PLC-γ2 protein and activity are normal in the patient.3 The finding of a platelet protein deficiency associated with a normal coding sequence and decreased mRNA levels is a very uncommon mechanism among the various platelet function abnormalities studied to date, including thrombasthenia and the Bernard Soulier syndrome,16,17 the entities best studied from a molecular genetics point of view. In an overwhelming majority of these patients, the mutations have been in the coding sequence; in a few patients the mRNA levels have been decreased, but this has been secondary to mutations in the coding sequence leading to premature termination of protein translation. Our patient provides a striking example of a platelet protein deficiency due to abnormalities not in the coding sequence. A second major finding in this patient is in relation to the neutrophils. Neutrophil functions of Ca++mobilization and elastase secretion were preserved in response to several agonists, including fMLP and IL-8, the responses to which are abnormal in PLC-β2–deficient mice.15 Moreover, neutrophil PLC-β2 protein and mRNA levels were unaffected in the patient. Together, these studies suggest that the patient has a defect in PLC-β2 gene expression, and, equally important, that the defect is hematopoietic lineage-specific, restricted to platelet-megakaryocytes.
There are multiple mechanisms that regulate mRNA levels, including transcription initiation, transcription elongation, and mRNA stability.18 For most genes, mRNA abundance is regulated, at least in part, at the level of transcription initiation.18 In general, the abundance of mRNA is dependent on its rate of synthesis and rate of decay,19,20although mRNA stabilities can be regulated coordinately with transcription initiation. Alterations in mRNA stability may occur due to mutations in the 3′ and 5′ mRNA untranslated regions, which are implicated in mRNA stability.21-24 Thus, altered mRNA stability would provide a cogent explanation for our findings. However, tissue-specific expression of several megakaryocytic-specific genes is governed at the transcription level (discussed below), suggesting that the lineage-specific aberration in our patient may be in transcription regulation rather than in altered mRNA stability. In future studies, both mechanisms need to be pursued. A pivotal finding in our study, therefore, is the hematopoietic lineage-specificity of the defect, which was noted in platelets but not neutrophils.
The pluripotent hematopoietic stem cells differentiate into highly specialized circulating blood cell lines with lymphoid, myelomonocytic, erythroid, and megakaryocytic features. Erythroid cells and megakaryocytes share a common precursor as well as certain transcription regulatory mechanisms.25 For example, transcription factor GATA-1 is expressed in both erythroid and megakaryocytic cell lines and regulates expression of several lineage-specific genes.26,27 Studies in megakaryocytic-specific genes, especially GPIIb, have provided insights into the mechanisms regulating lineage-specific gene expression. Transcription factors GATA-1 and Ets, and their combinatorial association, play a major role in the megakaryocytic-specific expression of several genes including GPIIb,27-29 platelet factor-4,30 GPIX,31 GPV,32 and the thrombopoietin receptor.33 Another transcription factor that plays an important regulatory role is the ubiquitous factor Sp1, which interacts with Ets-like proteins in transcriptional activation of genes that lack the TATA box, such as the GPIIb gene.28 Defects in transcription factors have been associated with abnormalities of platelet number and function. Transcription factor NF-E2 deficiency in mice is associated with thrombocytopenia,26 and, interestingly, a specific enzyme deficiency in thromboxane synthase,34,35 platelet granule deficiency,36 and defective inside-out signaling governing GPIIb-IIIa activation.37,38 In addition, β1 tubulin protein and mRNA, which are exquisitely restricted to platelets and megakaryocytes, are virtually absent.39 Further, patients with haplodeficiency of the transcription factor CBFA2 have impaired aggregation in response to agonists40; we have shown that secretion of granule constituents is defective in some patients.41 Because of the possibility that a cluster of platelet proteins may be potentially regulated by an abnormal transcription factor, we assessed platelet mRNA levels for several proteins (PLC-γ2, PF4, GPIIb, and Gαq) in addition to PLC-β2 in our patient; these levels were normal.
DNA cis-acting elements in the gene promoters, the targets of the transcription factors, represent the other major participants in promoter activity regulation and in tissue-specific expression. A mutation in the GATA binding site of GPIβ promoter has been reported in a patient with Bernard Soulier syndrome.42 Interestingly, severe factor VII deficiency has been reported in association with a mutation disrupting an Sp1 binding site in factor VII promoter.43 In addition to the enhancer elements, other studies29,44 suggest that tissue-specific transcriptional regulation of the megakaryocytic GPIIb gene is controlled by interactions between positivecis-acting elements and a repressor sequence. The repressor sequence is also found in the promoters for other megakaryocytic genes, including for platelet factor 4, GPIbα, GPV, and GPIX.29GPIIIa is expressed in a number of tissues including platelets, osteoclasts, endothelial cells, monocyte-derived macrophages, and cultured human embryonic fibroblasts.45 Jin et al45 have identified a megakaryocytic cell-specificcis-acting element controlling GPIIIa gene expression. These observations and the evidence from transcription factor–deficient mice and patients constitute strong evidence for the role of transcriptional regulation in tissue-specific expression of megakaryocytic genes and provide a paradigm for a possible defect in the promoter andcis-acting DNA regulatory elements and/or in the interactions of transacting factors leading to decreased tissue-specific PLC-β2 levels in our patient.
PLC-β2 expression is restricted to hematopoietic cells,2and to our knowledge, there is little information available on PLC-β2 gene regulation. The transcriptional regulatory elements have been elucidated for human PLC-γ1,46 PLC-γ2,46and PLC-β3.47 The promoter regions of these PLC isozymes have not had a canonical TATA box and have been uniformly GC-rich with multiple binding sites for transcription factors, especially Sp1. The PLC-β3 gene has been shown to have an initiator element sequence but the Inr alone is insufficient to increase PLC-β3 promoter activity significantly, and an activator (such as Sp1) is additionally required.47 The precise mechanisms underlying the reduced PLC-β2 mRNA levels in our patient need to be delineated. Further studies in our patient will provide a physiologically relevant model to delineate not only the regulation of the human PLC-β2 gene but also important aspects of its tissue-specific expression.
We thank Dr S. G. Rhee (National Heart, Lung, Blood Institute, National Institutes of Health) for providing us with PLC-β2 cDNA (pTMI plasmid); Dr Danny Dhanasekaran for critical discussions and review of the manuscript; and Ms JoAnn Hamilton for assistance in preparation of the manuscript.
Supported by grant R01 HL 56724 from the National Heart, Lung, and Blood Institute (A.K.R.), and grant RR-349 (General Clinical Research Center).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. Koneti Rao, Temple University School of Medicine, Division of Hematology and Thromboembolic Diseases, 3400 N Broad St, Suite 300, Philadelphia, PA 19140; e-mail:koneti@astro.temple.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal