Gab2, a newly identified pleckstrin homology domain-containing docking protein, is a major binding protein of SHP-2 tyrosine phosphatase in interleukin (IL)-3–stimulated hematopoietic cells. Its signaling mechanism remains largely unknown. We report here an important regulatory role for Gab2 in β1 integrin signaling pathway that mediates hematopoietic cell adhesion and migration. Cross-linking of the β1 integrin on Ba/F3 cells induced rapid tyrosine phosphorylation of Gab2 and its association with Syk kinase, SHP-2 phosphatase, and the p85 subunit of phosphatidylinositol (PI)-3 kinase. In addition, Gab2 was also constitutively associated with SHP-1 phosphatase via its C-terminal Src homology 2 domain. Overexpression of the pleckstrin homology domain or a mutant Gab2 molecule lacking SHP-2 binding sites resulted in significant reductions in Ba/F3 cell adhesion and migration. Biochemical analyses revealed that enforced expression of Gab2 mutant molecules dramatically reduced β1-integrin ligation-triggered PI3 kinase activation, whereas Erk kinase activation remained unaltered. Furthermore, transduction of primary hematopoietic progenitor cells from viable motheaten mice with these mutant Gab2 molecules also significantly ameliorated their enhanced migration capacity associated with theSHP1 gene mutation. Taken together, these results suggest an important signaling role for Gab2 in regulating hematopoietic cell adhesion and migration.
Introduction
Hematopoietic cell adhesion and migration are tightly regulated and mediated by cell surface adhesion molecules such as integrins. The β1 integrin is widely expressed on hematopoietic cells and is responsible for hematopoietic stem cell homing to the bone marrow, retention of progenitors in the bone marrow microenvironment, and leukocyte inflammatory responses.1-3However, little is known about the intracellular signaling mechanisms involved. A large body of evidence has demonstrated that after ligand engagement by fibronectin, fibrinogen, or anti-integrin antibodies, the cytoplasmic domains of integrins associate with multiple signaling proteins and together trigger intracellular signaling cascades that regulate cytoskeletal arrangement and the expression of target genes in the nucleus. The best characterized signaling pathway initiated by integrins involves focal adhesion kinase (Fak). Upon attachment of cells to extracellular matrix components such as fibronectin, Fak localizes to focal adhesion sites and binds to a variety of cytoskeletal and signaling proteins including paxillin, Src kinase, phosphatidylinositol (PI)-3 kinase, Cas, and Grb2, and then activates downstream signaling cascades.4,5 More recently, another distinct signaling pathway independent of Fak has emerged, which is mediated by a nonreceptor tyrosine kinase, Syk. Syk is a hematopoietic cell-specific kinase and has been demonstrated to be activated by the engagement of the β1, β2, α4, and αIIbβ3 integrins.6-9 Upon activation, Syk interacts with the guanine nucleotide exchange factor Vav and then signals to the Erk, Jnk, and Akt pathways. However, detailed mechanisms involved in the activation of these signaling pathways remain unclear.
SHP-2 and SHP-1 are Src homology 2 (SH2) domain-containing tyrosine phosphatases sharing high homology in their SH2 and catalytic domains.10-12 In contrast to the negative regulatory role for SHP-1 phosphatase in hematopoietic cell regulation,13-16 our previous work17-20suggested that SHP-2 phosphatase played a positive role in regulating hematopoietic cell development. We and others21-23 have also demonstrated that SHP-2 positively regulates integrin-mediated cell adhesion and migration, as fibroblasts lacking functional SHP-2 phosphatase were impaired in cell motility.21 Conversely, SHP-1 phosphatase was shown to have a negative regulatory role in hematopoietic cell adhesion and migration.22,23 The adhesion and migration capacities of hematopoietic cells fromviable motheaten mice that contain a point mutation in the coding region for the catalytic domain of SHP-124-26 were dramatically enhanced. Although both SHP-2 and SHP-1 phosphatases are highly expressed in hematopoietic cells, the role of SHP-2 in regulating hematopoietic cell adhesion and migration has not yet been documented, and we still lack an in-depth understanding of how these 2 phosphatases cooperate to properly control hematopoietic cell motility.
Recently, a potential downstream target of SHP-2 phosphatase called Gab2 has been identified.27,28 It is a widely expressed pleckstrin homology (PH) domain-containing docking protein that shares high homology with DOS,29,30 the putative downstream substrate of the Drosophila homologue of SHP-2,csw, and another mammalian PH domain-containing docking protein, Gab1.31 Gab2 was initially identified as a major binding protein of SHP-2 phosphatase in interleukin (IL)-3–stimulated hematopoietic cells. Subsequently, this docking molecule was found to be widely involved in a variety of other signaling processes including the erythropoietin, thrombopoietin, stem cell factor (SCF), Flt-3 ligand, B-cell receptor, and T-cell receptor (TCR) signaling pathways.28,32,33 An essential role for this docking protein in the allergic response has been recently reported.34 The close interaction between Gab2 and SHP-2 phosphatase prompted us to investigate the signaling mechanism for this docking protein. Because Gab2 contains a PH domain that is believed to be responsible for localizing the PH-containing protein-protein complex to phospholipids,35 we speculated that it might play a role in the regulation of cell motility. To test this hypothesis, we examined the involvement, the signaling function, and the biologic role of Gab2 in the β 1-integrin signal transduction through overexpressing dominant negative Gab2 molecules in a hematopoietic cell line and primary hematopoietic progenitor cells.
Materials and methods
Mice, cell line, and reagents
Heterozygous viable motheaten mice (C57BL/6J-Hcphme-v) were obtained from the Jackson Laboratory (Bar Harbor, ME). Wild-type and homozygous viable motheaten (mev/mev) mice were produced by intercrosses between heterozygous mutant mice. Ba/F3, a murine pro-B lymphoma cell line, was obtained from Dr Hal Broxmeyer (Indiana University, Indianapolis, IN). It is routinely maintained in RPMI 1640 medium with 0.2 ng/mL recombinant mouse IL-3 plus 10% fetal bovine serum (FBS). Anti-SHP-2, antiphospho Erk, and anti-Syk antibodies (Abs) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Gab2 Ab was obtained from Dr Gen-Sheng Feng (Burnham Institute, La Jolla, CA). Antiphosphotyrosine 4G10 and anti-p85 subunit of PI3 kinase Abs were purchased from Upstate Biotechnology (Lake Placid, NY). Piceatannol and cytochalasin D were obtained from Calbiochem (San Diego, CA). Human fibronectin was purchased from Sigma (St Louis, MO). GST-fusion protein expression plasmids containing the N-terminal and C-terminal SH2 domains of SHP-1 were provided by Dr Taolin Yi (The Cleveland Clinic Foundation Research Institute, Cleveland, OH). Rat insulin receptor substrate 1 (IRS-1) complementary DNA (cDNA) was obtained from Dr Achsah Keegan (Holland Laboratory, American Red Cross, Rockville, MD).
Generation of mutant Gab2 molecules and retroviral-mediated gene transfer
Murine full-length Gab2 cDNA was cloned from an embryonic day 15 mouse embryo cDNA library (Clontech, San Diego, CA) by using polymerase chain reaction according to the reported sequence.27 Gab2 wild-type cDNA, the PH domain-coding region, a deletion mutant cDNA (amino acids 1-603) without SHP-2 binding sites (Tyr604 and Tyr633), and rat IRS-1 PH domain-coding region (identical to the murine version) were subcloned into a retroviral expression vector, MSCV-IR-GFP36 (provided by Dr Kevin Bunting, Holland Laboratory, American Red Cross) that also contains an internal ribosomal entry sequence driving expression of a downstream green fluorescent protein (GFP) gene that facilitates tracking of the transduced cells. The ecotropic GP+E86-based retroviral producer cell lines were generated by transduction with retroviruses produced by 293T cells that were transiently cotransfected with pQEPAM3 (Minus E) packaging plasmid, pSrαG (VSV-G) envelope plasmid, and the recombinant retroviral plasmids. For Ba/F3 cell transfection, exponentially growing cells were washed in serum-free RPMI medium, resuspended at 5 × 106 cells/mL in phosphate-buffered saline (PBS), and incubated with 20 μg retroviral plasmids on ice for 10 minutes. Cells were then electroporated at 300 V/960 μF, and transferred to standard IL-3–containing culture medium. Forty-eight hours after electroporation, GFP+ cells were sorted by flow activated cell sorting (FACS). To transduce primary hematopoietic progenitor cells with wild-type and mutant Gab2 cDNAs, bone marrow cells harvested from femurs of 4-week-old wild-type and mev/mev mice were prestimulated with RPMI-1640 medium containing SCF (50 ng/mL), IL-3 (20 ng/mL), and IL-6 (50 ng/mL) for 2 days, and then cocultured with irradiated (1500 rads) retroviral producer cells for 48 hours. Transduced bone marrow cells were sorted for GFP expression by using FACS, or directly used for the progenitor migration assay.
Adhesion and migration assay
For the adhesion assay,37 24-well plates were coated with 20 μg/mL fibronectin at 4°C overnight and washed twice with PBS. Plates were then incubated with PBS plus 1% bovine serum albumin (BSA) at 37°C for 1 hour to block nonspecific binding. Wells coated with PBS/1% BSA alone were used as controls. Gene-transduced GFP+ Ba/F3 cells (5 × 105) in 200 μL RPMI-1640 with 1% BSA were seeded into each well and centrifuged at 600 rpm for 1 minute to allow attachment of cells to the bottom of the wells. After 30 minutes of incubation at 37°C in a 5% CO2 incubator, unattached cells were removed by washing twice with prewarmed RPMI 1640 medium containing 1% BSA. Adherent cells were quantitated using the One Solution Proliferation Kit from Promega (Madison, WI), and the percentage of cells attached was determined. Migration assays were performed using fibronectin-coated transwells (8 μm pore size, 6.5 mm diameter; Corning Costa, Cambridge, MA). The lower chambers contained 600 μL RPMI 1640 with 1% BSA. Transfected and sorted Ba/F3 cells (2 × 105cells/100 μL) were seeded into the upper chambers and allowed to migrate into the lower chamber for 7 hours in a 37°C, 5% CO2 incubator. Cells randomly migrating to the lower chambers were collected and counted on a hemacytometer.
Integrin cross-linking, immunoblotting, and immunoprecipitation
To induce integrin signaling, exponentially growing Ba/F3 cells were starved in RPMI 1640 medium with 1% BSA for 5 hours. Cells were then suspended in PBS (1 × 107 cells/mL) and incubated on ice for 15 minutes with monoclonal antibody against β1integrin (anti-CD29, Ha/5, Pharmingen, San Diego, CA) or hamster IgM as a control. Cells were washed once in PBS and then stimulated by cross-linking using antihamster IgM monoclonal Ab at 37°C for 5 and 15 minutes as reported.38-40 Stimulated cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 2 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride). Whole cell lysates (500 μg) were immunoprecipitated with 1 μg purified Abs or 4 μL antiserum as indicated. Immunoprecipitates were washed 3 times with HNTG buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 1% glycerol, 0.1% Triton X-100, and 1 mM Na3VO4), and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with indicated Abs.
PI3 kinase assay
Activity of PI3 kinase was determined by measuring the formation of PI 3 phosphate from PI as reported.31,41-43Cells were lysed after stimulation by β1-integrin cross-linking for 5 minutes. Cell lysates (500 μg) were immunoprecipitated with anti-p85 Ab. Immunoprecipitates were washed twice with washing buffer (100 mM Tris-HCl, pH 7.5, 5 mM LiCl, and 0.1 mM Na3VO4), once with TNE buffer (10 mM Tris-HCl, pH 7.5, 10 mM NaCl, 1 mM EDTA, and 0.1 mM Na3VO4), and resuspended in 50 μL TNE buffer. Samples were incubated at room temperature for 30 minutes after the addition of 10 μg PI (Avanti Polar-Lipids, Alabaster, AL). Adenosine triphosphate (ATP) solution (5 μL) containing 0.4 mM ATP, 5 μCi (0.185 MBq) 32P-ATP, and 20 mM MgCl2was then added and the reaction was conducted for 30 minutes at room temperature. To terminate the reaction, 20 μL 6 N HCl was added to the system and 160 μL CHCl3/MeOH (1:1) was added to extract phospholipids. Aqueous and organic phases were separated by centrifugation for 10 minutes; 50 μL of the organic phase was then spotted onto silicon gel 60 thin-layer chromatography (TLC) plates (Merck, Darmstadt, Germany) pretreated with 1% potassium oxidate. TLC plates were developed with chloroform-methanol-water-NH4OH (60:47:11.3:2). Unlabeled PI monophosphate was run in an adjacent lane to determine the migration position of PI3 phosphate. After chromatography, the plate was dried, and 32P-labeled PI3 phosphate was visualized by autoradiography; the PI monophosphate standard was stained by using iodine vapor staining.44
Primary hematopoietic progenitor cell migration assay
Both wild-type and mev/mev bone marrow cells were harvested and transduced through retroviral-mediated gene transfer as described above. GFP-expressing cells were sorted by FACS, and the sorted cells were examined for their migration potential toward a chemokine, stromal cell-derived factor (SDF)–1α.22Aliquots of sorted cells (2 × 104) in 100 μL RPMI 1640 with 1% BSA were seeded into fibronectin-coated transwells with 5 μm pore size and 100 ng/mL SDF-1α (Pharmingen, R&D Systems) was added to the medium in the lower chambers. Input cells and cells migrating to the lower chambers collected after 5 hours were assayed for colony-forming units in 0.9% methylcellulose α-MEM culture containing cocktails of hematopoietic growth factors (30% fetal calf serum [FCS], 5% pokeweed mitogen-stimulated mouse spleen cell conditioned medium, erythropoietin [2 U/mL], SCF [50 ng/mL], glutamine [10−4 M], β-mercaptoethanol [3.3 × 10−5 M], and hemin [100 μM]) as previously reported.18 19 Cells migrating from 2 transwells were combined to obtain enough hematopoietic progenitor cells for triplicate colony assays. After 7 days of incubation, colonies were counted under an inverted microscope. In some cases, whole bone marrow cells (2 × 105 cells/100 μL) transduced with the indicated retroviruses were used directly for migration assay without sorting. Hematopoietic progenitor assays were performed for the bone marrow cells before migration and for the cells migrating to the lower chambers. GFP+ hematopoietic colonies were scored under a fluorescence microscope after 7 days of incubation, and the migration percentage of hematopoietic progenitors was then determined.
Generation of bone marrow–derived macrophages
Bone marrow-derived macrophages were prepared from 4-week-old mev/mev mice and the wild-type littermates as previously described.15 45 Briefly, bone marrow cells harvested from femurs were incubated in Dulbecco modified Eagle medium (DMEM) supplemented with 15% FBS and 20 ng/mL recombinant mouse colony-stimulating factor 1. On the second day, nonadherent cells were collected and seeded into new tissue culture plates at the concentration of 2 × 105 cells/mL. After 4 to 5 days of culture, nonadherent cells were then collected and starved in 1% BSA containing RPMI 1640 medium for 5 hours before subjected to β1-integrin cross-linking as described above.
Results
Overexpression of mutant Gab2 molecules significantly suppressed hematopoietic cell adhesion and migration
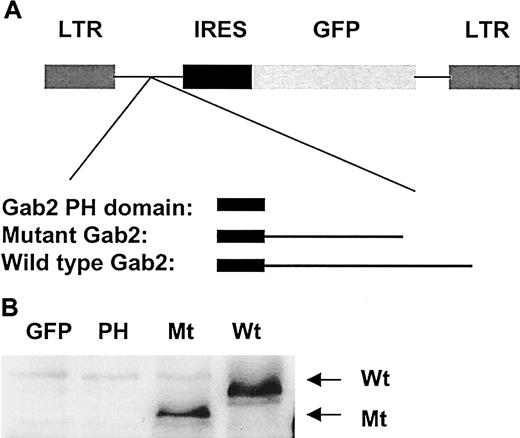
The Gab2 docking protein contains a PH domain, which is believed to localize the PH domain-containing protein-protein complex to phospholipids.35 Thus it may have a regulatory role in cell motility. To test this hypothesis, we transfected wild-type, the Gab2 PH domain-coding region, and a deletion mutant Gab2 cDNA without SHP-2 tyrosine phosphatase binding sites into a murine pro-B lymphoma cell line, Ba/F3, by electroporation. Transfected cells were sorted for GFP expression by FACS. As shown in Figure1, Western blotting clearly demonstrated that both wild-type and the deletion mutant Gab2 were highly expressed. Because anti-Gab2 antibody used does not recognize the N-terminal part of Gab2, the PH domain expressed in Ba/F3 cells was not detectable.
Overexpression of wild-type, the PH domain, and a deletion mutant Gab2 without SHP-2 binding sites in Ba/F3 cells.
(A) Wild-type (Wt), the PH domain encoding region (PH), and Gab2 deletion mutant (Mt) cDNAs were cloned into MSCV-IR-GFP vector and transfected into Ba/F3 cells by electroporation. (B) GFP-expressing cells were sorted by FACS after 48 hours. Sorted cells were expanded and lysed in RIPA buffer, 50 μg cell lysates was resolved by SDS-PAGE and blotted with anti-Gab2 antibody.
Overexpression of wild-type, the PH domain, and a deletion mutant Gab2 without SHP-2 binding sites in Ba/F3 cells.
(A) Wild-type (Wt), the PH domain encoding region (PH), and Gab2 deletion mutant (Mt) cDNAs were cloned into MSCV-IR-GFP vector and transfected into Ba/F3 cells by electroporation. (B) GFP-expressing cells were sorted by FACS after 48 hours. Sorted cells were expanded and lysed in RIPA buffer, 50 μg cell lysates was resolved by SDS-PAGE and blotted with anti-Gab2 antibody.
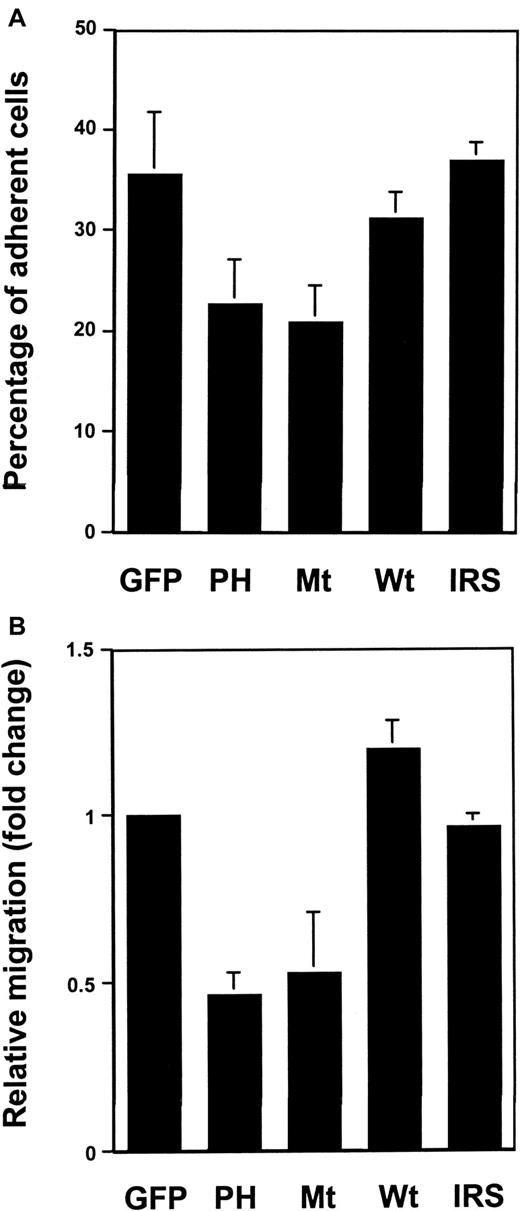
After the transfected cells were sorted by FACS, cell adhesion and migration assays were performed. As shown in Figure2A, overexpression of wild-type Gab2, like that of Grb2,38 did not have an obvious effect on cell adhesion. However, cell adhesion of Ba/F3 cells overexpressing either its PH domain or the deletion mutant was reduced by about 40% to 50%, compared to GFP vector-transfected cells. To further determine the role of Gab2 in regulating hematopoietic cell motility, migration analysis was performed using transwells coated with fibronectin on both surfaces. Similarly, the migration capacity of its PH domain-expressing or deletion mutant Gab2-expressing cells was also significantly decreased compared to the GFP control or the wild-type Gab2-expressing cells (Figure 2B). It appears that the phenotypes associated with the transfection of the Gab2 PH domain or the mutant Gab2 without SHP-2 binding sites resulted specifically from the reduced Gab2-mediated signal transduction, because overexpression of the rat IRS-1 PH domain (identical to the murine version) affected neither Ba/F3 cell adhesion nor migration (Figure 2A,B), but overexpression of a mutant Gab2 lacking PH domain also significantly decreased Ba/F3 cell adhesion and migration (data not shown). The effects of mutant Gab2s on the migration of Ba/F3 cells are likely to be mediated by β1integrin because there was no significant migration in transwells that were not coated with fibronectin. Thus, both fibronectin-related adhesion and migration data indicate that Gab2 might function in hematopoietic cell motility through β1-integrin signaling pathways.
The β1-integrin–mediated adhesion and migration of Ba/F3 cells transfected with dominant negative Gab2 molecules were reduced.
(A) Wild-type, mutant Gab2 cDNAs, the rat IRS-1 PH domain-coding region (IRS) as well as control vector transfected Ba/F3 cells were examined for their capacity to adhere to fibronectin-coated 24-well plates as described in “Materials and methods.” The percentage of adherent cells was determined using the One Solution Proliferation Kit. (B) Migration assays were performed using fibronectin-coated transwells as described in the text. The relative migration (fold change) was expressed as the ratio of the number of wild-type, mutant Gab2 cDNA, and IRS-1 PH domain-transfected cells migrating to the number of GFP vector-transfected cells migrating over the same period of time. Shown in both panels is the mean ± SD of 3 independent experiments; each experiment was performed in triplicate.
The β1-integrin–mediated adhesion and migration of Ba/F3 cells transfected with dominant negative Gab2 molecules were reduced.
(A) Wild-type, mutant Gab2 cDNAs, the rat IRS-1 PH domain-coding region (IRS) as well as control vector transfected Ba/F3 cells were examined for their capacity to adhere to fibronectin-coated 24-well plates as described in “Materials and methods.” The percentage of adherent cells was determined using the One Solution Proliferation Kit. (B) Migration assays were performed using fibronectin-coated transwells as described in the text. The relative migration (fold change) was expressed as the ratio of the number of wild-type, mutant Gab2 cDNA, and IRS-1 PH domain-transfected cells migrating to the number of GFP vector-transfected cells migrating over the same period of time. Shown in both panels is the mean ± SD of 3 independent experiments; each experiment was performed in triplicate.
The Gab2 molecule couples both SHP-2 and SHP-1 phosphatases to the β1-integrin signaling pathway
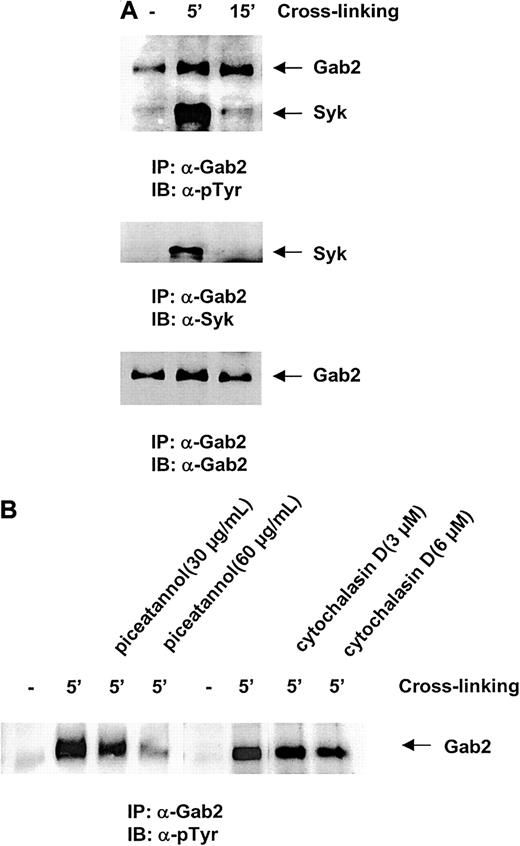
To test the involvement of the Gab2 molecule in β1-integrin signaling pathways, we next examined the tyrosine phosphorylation and protein-protein interactions of Gab2 in response to integrin cross-linking. The β1 integrins were cross-linked using an anti-integrin Ab, which is widely used to activate the “outside-in” integrin signaling pathway.38-40 As shown in Figure3A, upon β1-integrin cross-linking, Gab2 was rapidly tyrosine phosphorylated, indicating an involvement of this docking protein in the β1-integrin signaling pathway. Interestingly, another highly phosphorylated 70-kd protein was frequently observed in the anti-Gab2 immunocomplex. The phosphorylation response of this protein was rapid and transient; its phosphorylation decreased to the basal level in 10 to 15 minutes after β1-integrin engagement, suggesting that this protein is involved in the proximal events triggered by integrin cross-linking. To determine the identity of this Gab2-associated protein, we tested a number of Abs against signaling proteins that are potentially involved in β1-integrin signaling pathways. Finally, the Gab2-associated signaling protein turned out to be Syk, a hematopoietic cell-specific nonreceptor tyrosine kinase. This observation strongly suggested an interaction between the Gab2 docking protein and Syk kinase in response to integrin cross-linking. To determine the significance of the interaction of these 2 molecules, we pretreated the cells with piceatannol, a reportedly selective Syk kinase inhibitor46-48 before integrin cross-linking, and then examined the phosphorylation response of Gab2. As shown in Figure 3B, tyrosine phosphorylation of Gab2 was dramatically decreased by pretreatment with this compound at a concentration of 60 μg/mL. This result suggests that Gab2 acts downstream of Syk and that Syk kinase activity might be required for the phosphorylation of the Gab2 protein in the β1-integrin signaling pathway. Interestingly, preincubation of cells with cytochalasin D (3 and 6 μM for 45 minutes), which prevents actin polymerization and Fak kinase activation,49-51 did not obviously affect the phosphorylation response of Gab2 (Figure 3B), indicating that Gab2 appears to not be involved in the Fak-mediated pathway in hematopoietic cells.
Gab2 is phosphorylated in response to integrin cross-linking.
Ba/F3 cells were stimulated by β1-integrin cross-linking as described in “Materials and methods.” (A) Cell lysates were prepared and anti-Gab2 immunoprecipitation was performed. Immunoprecipitates were resolved by SDS-PAGE and blotted with antiphosphorylated tyrosine, anti-Syk, and anti-Gab2 Abs. (B) Ba/F3 cells were preincubated with 30 and 60 μg/mL piceatannol for 10 minutes or with 3 and 6 μM cytochalasin D for 45 minutes before β1-integrin cross-linking was conducted. Cell lysates were prepared and the phosphorylation status of Gab2 was then examined as above. Shown in both panels are representatives of 2 independent experiments.
Gab2 is phosphorylated in response to integrin cross-linking.
Ba/F3 cells were stimulated by β1-integrin cross-linking as described in “Materials and methods.” (A) Cell lysates were prepared and anti-Gab2 immunoprecipitation was performed. Immunoprecipitates were resolved by SDS-PAGE and blotted with antiphosphorylated tyrosine, anti-Syk, and anti-Gab2 Abs. (B) Ba/F3 cells were preincubated with 30 and 60 μg/mL piceatannol for 10 minutes or with 3 and 6 μM cytochalasin D for 45 minutes before β1-integrin cross-linking was conducted. Cell lysates were prepared and the phosphorylation status of Gab2 was then examined as above. Shown in both panels are representatives of 2 independent experiments.
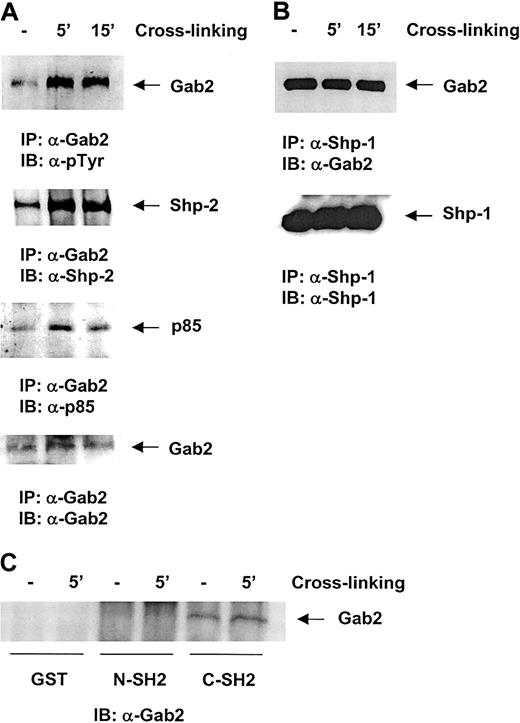
Because the Gab2 docking protein is a major binding partner for SHP-2 in hematopoietic cells stimulated by IL-3, we were interested in testing the association between Gab2 and SHP-2 in the β1-integrin signaling pathway. As shown in Figure4A, SHP-2 was indeed detected in the anti-Gab2 immunocomplex after β1- integrin cross-linking. In addition, the p85 subunit of PI3 kinase was also found to be associated with Gab2, suggesting that Gab2 may serve as a docking protein for Syk kinase, SHP-2 phosphatase, and also PI3 kinase. Because SHP-1, a hematopoietic-specific protein tyrosine phosphatase, shares high homology with SHP-2, the close relationship between SHP-2 and Gab2 led us to also test for a possible association between Gab2 and SHP-1. As shown in Figure 4B, Gab2 was detected in the anti-SHP-1 immunocomplex. Interestingly, Gab2 and SHP-1 were found to be coimmunoprecipitated independent of β 1-integrin cross-linking, suggesting that their association is constitutive. To define which domain within the SHP-1 protein is responsible for the interaction with Gab2, in vitro binding tests were performed. We found that only the C-terminal SH2 domain GST fusion protein was able to pull down Gab2 protein, indicating that SHP-1 binds to Gab2 via its C-terminal SH2 domain (Figure 4C). Taken together, these biochemical analyses suggest that both SHP-1 and SHP-2 phosphatases are linked to the β1-integrin signaling pathway through the common docking protein, Gab2.
Gab2 associates with the SHP-2 and SHP-1 phosphatases and the p85 subunit of PI3 kinase in the β1-integrin signaling pathway.
(A) Ba/F3 cells were stimulated by β1-integrin cross-linking for 5 and 15 minutes as indicated in the text. Cell lysates were prepared and anti-Gab2 immunoprecipitation was performed. Immunoprecipitates were resolved by SDS-PAGE and then blotted with anti-SHP-2, anti-p85 subunit of PI3 kinase, and anti-Gab2 Abs. (B) Anti-SHP-1 immunoprecipitation was conducted followed by anti-Gab2 Western blotting. (C) Cell lysates (500 μg) of Ba/F3 cells stimulated by β1-integrin cross-linking were incubated with 10 μg of the SHP-1 N- or C-terminal SH2 domain GST fusion proteins that were immobilized on glutathione beads for 4 hours. Bound proteins were resolved by SDS-PAGE and then immunoblotted with anti-Gab2 Ab. Shown are representatives of 2 independent experiments.
Gab2 associates with the SHP-2 and SHP-1 phosphatases and the p85 subunit of PI3 kinase in the β1-integrin signaling pathway.
(A) Ba/F3 cells were stimulated by β1-integrin cross-linking for 5 and 15 minutes as indicated in the text. Cell lysates were prepared and anti-Gab2 immunoprecipitation was performed. Immunoprecipitates were resolved by SDS-PAGE and then blotted with anti-SHP-2, anti-p85 subunit of PI3 kinase, and anti-Gab2 Abs. (B) Anti-SHP-1 immunoprecipitation was conducted followed by anti-Gab2 Western blotting. (C) Cell lysates (500 μg) of Ba/F3 cells stimulated by β1-integrin cross-linking were incubated with 10 μg of the SHP-1 N- or C-terminal SH2 domain GST fusion proteins that were immobilized on glutathione beads for 4 hours. Bound proteins were resolved by SDS-PAGE and then immunoblotted with anti-Gab2 Ab. Shown are representatives of 2 independent experiments.
Impaired activation of PI3 kinase, but not Erk kinase, contributes to the defective motility of the mutant Gab2-transfected hematopoietic cells
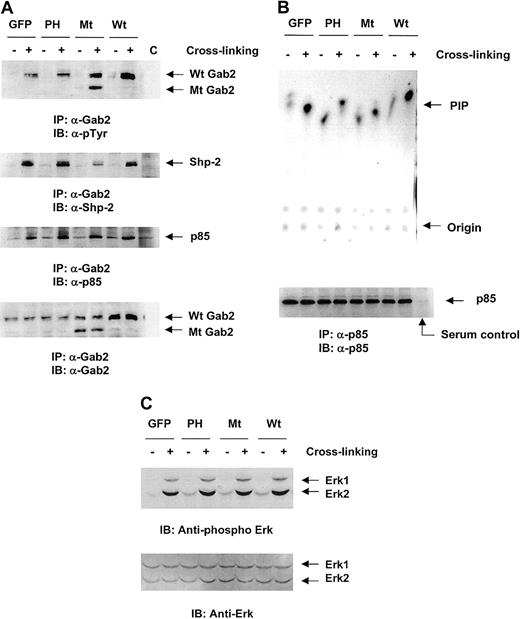
To define the molecular mechanisms underlying the defective adhesion and migration of hematopoietic cells transfected with dominant negative Gab2 molecules, we next examined the signaling pathways triggered by β1-integrin cross-linking in the mutant Gab2-transfected Ba/F3 cells. Anti-Gab2 immunoprecipitation followed by anti-phospho-tyrosine, anti-SHP-2, and anti-p85 immunoblottings were conducted. Because the Gab2 Ab used binds with both wild-type and the mutant Gab2 lacking SHP-2 binding sites (but not the PH domain), both endogenous and exogenous Gab2s (but not the PH domain) and their associated proteins could be precipitated. As shown in Figure5A, compared to the vector transfected control cells, tyrosine phosphorylation of Gab2 after β1-integrin cross-linking was enhanced in the wild-type Gab2-transfected cells due to the increased amount of Gab2. In the deletion mutant Gab2-expressing cells, both wild-type and mutant Gab2 were phosphorylated after integrin cross-linking. The phosphorylation level of mutant Gab2 was not found significantly higher than endogenous Gab2; this might be because the deletion mutant lacks the C-terminal 2 tyrosine sites. Phosphorylation of endogenous wild-type Gab2 in the PH domain transfected cells was not obviously changed. As expected, the amount of SHP-2 protein in the anti-Gab2 immunocomplex was significantly decreased in the cells overexpressing the deletion mutant Gab2 but not in the cells overexpressing PH domain, because anti-Gab2 antibody could only pull down endogenous wild-type Gab2 from the latter cells. In contrast, as the deletion mutant Gab2 still contains p85 binding sites, the levels of p85 subunit of PI3 kinase detected in the anti-Gab2 immunocomplexes after integrin cross-linking were not altered in all transfected cells.
Transfection of the PH domain or the deletion mutant Gab2 molecules resulted in a compromised activation of PI3 but not Erk kinases.
Wild-type, mutant Gab2, and control vector-transfected Ba/F3 cells were starved and stimulated by β1-integrin cross-linking for 5 minutes. (A) Cell lysates were prepared and immunoprecipitated using anti-Gab2 Ab was performed. Immunoprecipitates were resolved by SDS-PAGE and blotted with antiphosphorylated tyrosine, anti-SHP-2, anti-p85, and anti-Gab2 Abs. Serum control immunoprecipitation (c) was performed using the cell lysates of stimulated wild-type Gab2-transfected cells. (B) Cell lysates were immunoprecipitated using anti-p85 subunit of PI3 kinase Ab. Immunoprecipitates were washed and subjected to PI3 kinase assay as described in detail in “Materials and methods.” 32P-labeled phospholipids were visualized by autoradiography. Shown in the lower panel is the loading control of PI3 kinase precipitated by anti-p85 Ab. Serum control immunoprecipitation was performed using the cell lysates of stimulated wild-type Gab2 transfected cells. (C) Whole cell lysates (20 μg) were resolved by SDS-PAGE and blotted with antiphospho Erk Ab. For the loading control, membranes were stripped and reblotted with anti-Erk Ab. The pictures shown are representative of 2 independent experiments.
Transfection of the PH domain or the deletion mutant Gab2 molecules resulted in a compromised activation of PI3 but not Erk kinases.
Wild-type, mutant Gab2, and control vector-transfected Ba/F3 cells were starved and stimulated by β1-integrin cross-linking for 5 minutes. (A) Cell lysates were prepared and immunoprecipitated using anti-Gab2 Ab was performed. Immunoprecipitates were resolved by SDS-PAGE and blotted with antiphosphorylated tyrosine, anti-SHP-2, anti-p85, and anti-Gab2 Abs. Serum control immunoprecipitation (c) was performed using the cell lysates of stimulated wild-type Gab2-transfected cells. (B) Cell lysates were immunoprecipitated using anti-p85 subunit of PI3 kinase Ab. Immunoprecipitates were washed and subjected to PI3 kinase assay as described in detail in “Materials and methods.” 32P-labeled phospholipids were visualized by autoradiography. Shown in the lower panel is the loading control of PI3 kinase precipitated by anti-p85 Ab. Serum control immunoprecipitation was performed using the cell lysates of stimulated wild-type Gab2 transfected cells. (C) Whole cell lysates (20 μg) were resolved by SDS-PAGE and blotted with antiphospho Erk Ab. For the loading control, membranes were stripped and reblotted with anti-Erk Ab. The pictures shown are representative of 2 independent experiments.
As both Erk kinase and PI3 kinase pathways have been demonstrated to be involved in the signal transduction processes initiated by β1 integrin,4 52-54 the activation of downstream targets of these pathways, the Erk and PI3 kinases, was next examined. As shown in Figure 5C, both Erk1 and Erk2 were activated by integrin cross-linking and their activation was not altered by the transfection of mutant Gab2 molecules. By contrast, PI3 kinase activation in response to β1-integrin cross-linking was significantly reduced in the PH domain or mutant Gab2-transfected cells (Figure 5B). These data together strongly suggest that the cellular localization of the Gab2 complex to phospholipids and the association between Gab2 and SHP-2 are both required for the PI3 kinase but not Erk kinase activation. Impaired PI3 kinase activation may thus account for the decreased adhesion and migration of mutant Gab2-transfected hematopoietic cells.
The PH domain of Gab2 and the SHP-2/Gab2 complex are required for primary hematopoietic progenitor cell migration
The dynamic interaction between the SHP-2 and SHP-1 phosphatases and Gab2 prompted us to further define the cellular significance of their interactions. Previous reports demonstrated that SHP-1 phosphatase played a negative role in regulating hematopoietic cell migration.22 23 Although no direct evidence shows a regulation of SHP-2 in hematopoietic cell migration, the above data indicate a positive role for this phosphatase, because reduction of this enzyme in the Gab2 protein complex (Figure 5A) in the β1-integrin signaling pathway significantly reduced hematopoietic adhesion and migration. Thus, Gab2 might serve as a link for the functional interaction between SHP-2 and SHP-1 phosphatases in controlling hematopoietic cell motility.
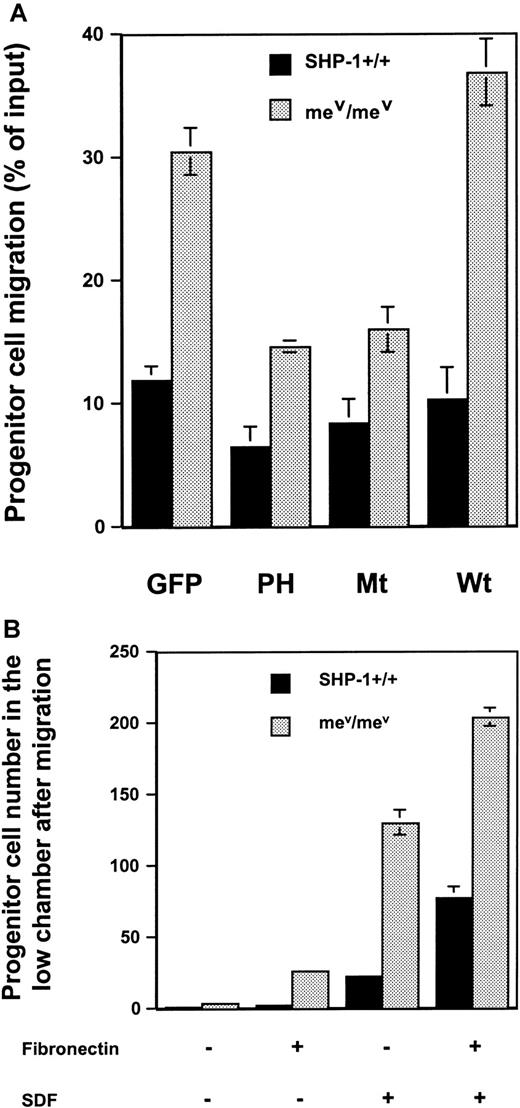
To test this hypothesis, the role of Gab2 in regulating primary hematopoietic progenitor cell migration was next assessed. Wild-type and SHP-1 mutant (viable motheaten) bone marrow cells were harvested and transduced with wild-type, the PH domain, and the deletion mutant Gab2 cDNAs as described in “Materials and methods.” Transduced bone marrow cells were sorted for progenitor migration assay. As shown in Figure 6A, interference of the endogenous Gab2 function by the PH domain or a mutant Gab2 molecule without SHP-2 binding sites significantly reduced progenitor cell migration, confirming that SHP-2 phosphatase has a positive role in regulating hematopoietic cell motility and that Gab2/SHP-2 association is required for this function. Moreover, interference of the Gab2 function or the association between SHP-2 and Gab2 in the context of the SHP1 gene mutation also significantly reduced the migration of hematopoietic progenitor cells from viable motheaten mice. These data further indicate opposing roles for SHP-2 and SHP-1 phosphatases in regulating hematopoietic cell motility mediated by β1 integrin.
Hematopoietic progenitor cell migration was decreased by the transduction of the PH domain or a Gab2 deletion mutant lacking SHP-2 binding sites.
(A) Primary bone marrow cells were transduced with the PH domain and the deletion version of Gab2 cDNAs by retroviral-mediated gene transfer. Transduced hematopoietic cells were sorted for GFP expression by FACS followed by migration assay as described in “Materials and methods.” (B) Primary bone marrow cells from wild-type andviable motheaten mice were directly analyzed for their migration (6 × 105 cells/well) on fibronectin-coated or uncoated transwells, with or without SDF in the lower chambers. Progenitor assays were conducted for the cells before migration and the cells migrating to the lower chambers.
Hematopoietic progenitor cell migration was decreased by the transduction of the PH domain or a Gab2 deletion mutant lacking SHP-2 binding sites.
(A) Primary bone marrow cells were transduced with the PH domain and the deletion version of Gab2 cDNAs by retroviral-mediated gene transfer. Transduced hematopoietic cells were sorted for GFP expression by FACS followed by migration assay as described in “Materials and methods.” (B) Primary bone marrow cells from wild-type andviable motheaten mice were directly analyzed for their migration (6 × 105 cells/well) on fibronectin-coated or uncoated transwells, with or without SDF in the lower chambers. Progenitor assays were conducted for the cells before migration and the cells migrating to the lower chambers.
Because chemokine SDF was used in the progenitor cell migration assay described above, to confirm that the increase in SHP-1 mutant hematopoietic cell migration is mediated through β1-integrin signaling pathway, the effect of fibronectin coating of transwells was tested. As shown in Figure 6B, without SDF, wild-type and SHP-1 mutant progenitor cells had minimal migration if transwells were not coated with fibronectin. However, the migration efficiency of the mutant cells on the fibronectin-coated transwells was dramatically increased compared to that of the wild-type cells, even though no SDF was used in the lower chambers. Because equal amounts of progenitor cells were used for the migration assay, the data shown in Figure 6B suggest that SHP-1 indeed plays a negative role in β1-integrin–mediated cell adhesion and therefore migration. Additionally, these results also indicate that SHP-1 may negatively regulate SDF signaling, because the SHP-1 mutant hematopoietic cell migration responding to SDF was dramatically enhanced compared to the wild-type cells.
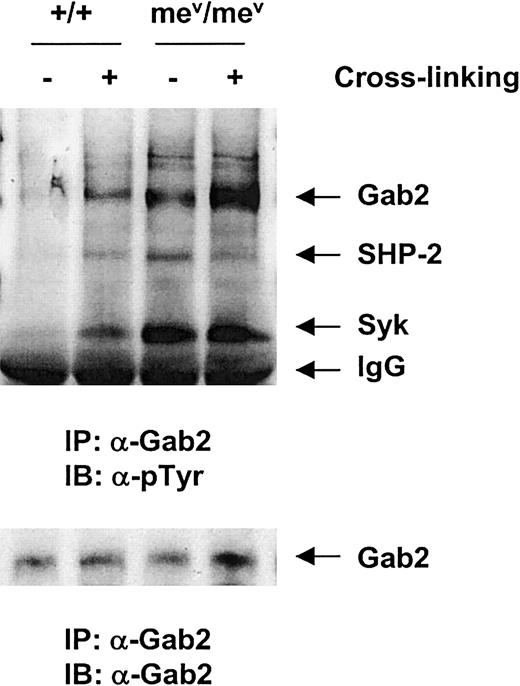
To define the mechanism by which SHP-1 phosphatase negatively regulates hematopoietic cell migration, we examined the β1-integrin signaling in the bone marrow–derived macrophages from viable motheaten mice and their littermates. As shown in Figure7, compared to the wild-type control, the basal level of Gab2 tyrosine phosphorylation was significantly higher in the SHP-1 mutant macrophages. After integrin cross-linking, its phosphorylation was further enhanced. Interestingly, phosphorylation of the Syk kinase associated with Gab2 was also dramatically increased in the macrophages from viable motheaten mice, suggesting a potential direct or indirect negative regulation of SHP-1 phosphatase on the Syk kinase.
The
SHP1 gene mutation led to elevated tyrosine phosphorylation of the Syk kinase and the Gab2 docking protein.Bone marrow–derived macrophages from mev/mevand wild-type (+/+) mice were generated, starved, and stimulated by β1- integrin cross-linking as detailed in “Materials and methods.” Anti-Gab2 immunoprecipitation was conducted followed by antiphosphotyrosine Western blotting. The membrane was stripped and reprobed with anti-SHP-2, Syk, and Gab2 Abs. Shown in the figure is a representative of 2 independent experiments.
The
SHP1 gene mutation led to elevated tyrosine phosphorylation of the Syk kinase and the Gab2 docking protein.Bone marrow–derived macrophages from mev/mevand wild-type (+/+) mice were generated, starved, and stimulated by β1- integrin cross-linking as detailed in “Materials and methods.” Anti-Gab2 immunoprecipitation was conducted followed by antiphosphotyrosine Western blotting. The membrane was stripped and reprobed with anti-SHP-2, Syk, and Gab2 Abs. Shown in the figure is a representative of 2 independent experiments.
Discussion
In this report, we defined an important regulatory role for the docking protein Gab2 in the β1-integrin signaling pathway–mediated hematopoietic cell adhesion and migration. Upon β1-integrin ligation on the cell surface, Gab2 is tyrosine phosphorylated, and then provides docking sites for SHP-2 phosphatase and PI3 kinase. Inhibition of the function of endogenous Gab2 by transducing the PH domain of Gab2 or a deletion mutant of Gab2 cDNA without SHP-2 binding sites significantly reduced hematopoietic cell adhesion and migration. Biochemical analyses revealed that PI3 kinase but not Erk kinase activation in response to integrin cross-linking was compromised in these mutant molecule transfected hematopoietic cells.
Gab2 is a docking or adaptor protein containing a PH domain and multiple tyrosine sites. Adaptor proteins do not possess catalytic activity. Rather, they usually create binding sites for other signaling intermediates on activation and thereby function as linkers to bring those effector molecules together. A number of adaptor or docking proteins are involved in integrin signaling pathways.54-56For instance, Crkl, an SH2-SH3-SH3 adaptor protein, has been implicated in β1-integrin signaling through a complex with Cbl and C3G.38,57 Further studies suggested that the SH2 and N-terminal SH3 domains of Crkl are essential for its function. Gab2 adaptor protein is also clearly involved in β1-integrin signaling pathway; its PH domain appears to be important, because enforced expression of the PH domain significantly reduced fibronectin-related hematopoietic adhesion and migration. This effect may be caused by the interference of the overexpressed PH domain on cellular localization of the Gab2 protein complex, as PH domain has been believed to bind to the phospholipids.35 In addition, our data suggest that the Gab2/SHP-2 association is also required for activating β1-integrin signaling pathway; inhibition of this association through overexpression of a mutant Gab2 molecule lacking SHP-2 binding sites (Tyr604 and Tyr633)27 also significantly decreased hematopoietic adhesion and migration mediated via fibronectin. Yet, it remains to be defined how SHP-2, a tyrosine phosphatase, promotes integrin signaling.
To date, 2 independent signaling pathways triggered by integrins have been characterized, which are mediated by the Fak and Syk kinases, respectively. It seems that Gab2 is involved in the Syk but not Fak-mediated β1-integrin signaling cascade, because Gab2 is closely associated with Syk kinase, and its tyrosine phosphorylation response to β1-integrin cross-linking was abolished after the cells were pretreated with piceatannol, a reportedly selective Syk kinase inhibitor.46-48 However, pretreatment with cytochalasin D, which prevents actin polymerization and Fak activation,49-51 did not show a significant effect in this respect. Additionally, the consistency of elevated activation (tyrosine phosphorylation) of Syk with increased phosphorylation of Gab2 in the SHP-1 mutant macrophages (Figure 7) also indicates that Syk may contribute to the phosphorylation of Gab2. Nevertheless, as piceatannol has recently been found not to be specific to Syk kinase,58 it remains a possibility that the abolished phosphorylation response of Gab2 might be caused by the nonspecific effects of piceatannol. To further confirm this point, it would be helpful to examine the Gab2 phosphorylation response to β1-integrin cross-linking in the Syk null mutant hematopoietic cells.
Gab2 associates with both SHP-2 and SHP-1 phosphatases. Compared to the inducible interaction between Gab2 and SHP-2, Gab2 associates with SHP-1 phosphatase via its C-terminal SH2 domain in a constitutive manner. Normally, SH2 domains mediate protein-protein interactions through phosphorylated tyrosine sites; however, the Gab2/SHP-1 interaction seems to be independent of the tyrosine phosphorylation of Gab2. It is worthwhile to mention that SHP-1 phosphatase has also been found to be associated with the Jak2 and Tyk2 kinases, and SLP-76 and p62DOK adaptor proteins independently of the phosphorylation of these partners.59-62 Their constitutive interactions with SHP-1 phosphatase are possibly good mechanisms for controlling the basal activities of these signaling molecules. Although SHP-1 constitutively associates with Gab2, and the basal phosphorylation of its associated proteins (Gab2 and Syk) is significantly elevated in the SHP-1 mutant cells (Figure 7), the negative regulation of SHP-1 on hematopoietic cell adhesion and thus migration appears to be mediated at least partially through the β1- integrin signaling pathways because migration efficiency of the SHP-1 mutant primary hematopoietic progenitor cells via fibronectin was dramatically enhanced even though no chemoattractant SDF was used (Figure 6B).
Phosphatidylinositol 3 kinase plays a critical role in mediating hematopoietic cell adhesion and migration.53,63 The data presented in this report have demonstrated that Gab2 plays an important role in PI3 kinase activation triggered by integrin cross-linking, consistent with the finding that Gab2-mediated PI3 kinase activation in TCR signaling.64 Gab2 may contribute to the activation of PI3 kinase through several mechanisms. First, Gab2 translocates PI3 kinase to the membrane and provides this enzyme with access to its phospholipid substrates. Membrane localization of PI3 kinase has been shown to be required for its enzymatic activity.65 Second, the interaction of Gab2 with the p85 regulatory subunit of PI3 kinase may also lead to a conformational activation of the p110 catalytic subunit of PI3 kinase. Finally, Gab2 may also function as a link for the functional interaction between SHP-2 and SHP-1 in regulating PI3 kinase activation in response to integrin cross-linking. SHP-1 has been previously reported to be involved in the regulation of PI3 kinase activity. Roach et al23 showed that mev/mev bone marrow cell–derived macrophages had 2- to 5-fold increases in the PI3 kinase activity associated with membranes. Our present observation that tyrosine phosphorylation of Syk was dramatically enhanced in the SHP-1 mutant macrophages suggests that SHP-1 phosphatase may control PI3 kinase activation through negatively regulating the upstream Syk kinase. To date, no available direct evidence shows SHP-2 phosphatase involvement in regulating PI3 kinase activity in the β1-integrin signaling process; the data presented in this report indicate that SHP-2 appears to positively regulate β1-integrin signaling in hematopoietic cells, because dissociation of SHP-2 from the Gab2 complex significantly reduced PI3 kinase activation. In contrast to the PI3 kinase activation, Erk kinase activation in response to integrin cross-linking was not changed by the transfection of mutant Gab2 molecules, suggesting that the PH domain of Gab2 and the association between Gab2 and SHP-2 are not absolutely required for signaling to Erk kinases. This is consistent with the finding of Gu et al27 that enforced expression of Gab2 mutant unable to bind to SHP-2 did not block IL-3–induced Erk activation, although a functional requirement of SHP-2 phosphatase in the Erk pathway was established.
In summary, we have identified the docking protein Gab2 as an important regulator in the β1-integrin signaling pathway-mediated hematopoietic cell adhesion and migration. Gab2 functions through interacting with Syk kinase, SHP-2 and SHP-1 phosphatases, and PI3 kinase. The findings will help to elucidate the cytoplasmic signaling mechanisms controlling hematopoietic cell adhesion and migration.
The authors are grateful to Dr Taolin Yi for the GST fusion protein expression plasmids containing N- and C-terminal SH2 domains of SHP-1; Dr Gen-Sheng Feng for anti-Gab2 antibody; Dr Kevin Bunting for the MSCV-IR-GFP retroviral vector; Dr Achsah Keegan for IRS-1 cDNA; and Dr Michael Chase for technical assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cheng-Kui Qu, Department of Hematopoiesis, Holland Laboratory, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855; e-mail: quc@usa.redcross.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal