Abstract
Ex vivo expanded Epstein-Barr virus (EBV)–specific T cells have been successfully applied clinically for adoptive immunotherapy. However, the role of CD4+ T cells in the therapeutic T-cell culture has not been established for the reconstitution of EBV-specific immunity. We isolated and characterized CD4+ T-cell lines from the ex vivo T-cell cultures. Monoclonal line PD-F4 and oligoclonal lines ND-R4 and TD-B4 were CD3+CD4+CD8−. Cytolytic tests with targets of mismatched major histocompatibility complex (MHC) and anti-MHC antibodies confirmed that the cytotoxicity of these CD4+ cells was restricted by MHC class II. Single cells of ND-R4 expressed interferon-γ (IFN-γ, or interleukin 4 (IL-4), but rarely coexpressed these 2 cytokines. In contrast, PD-F4 coexpressed IFN-γ, IL-2, and IL-4. Kinetic studies with PD-F4 showed that expression of the 3 cytokines plateaued 5 hours upon stimulation and was then drastically reduced, with a pattern consistent with independent modulation and differential off-cycle signal requirements. The cytotoxicity of these CD4+ cells was largely resistant to brefeldin A, an inhibitor for cytolytic pathways by Fas-ligand family molecules. Although sensitive to concanamycin A and ethyleneglycotetraacetic acid, which inhibit cytotoxicity by granule exocytosis, the CD4+ cytotoxic T lymphocytes (CTLs) did not express perforin, suggesting a cytotoxic mechanism independent of perforin although involving exocytosis. Flow cytometric analysis showed that the CD4+ CTLs expressed granulysin, a recently identified cytolytic molecule associated with exocytotic cytolytic granules. These data suggested that CD4+ T cells in the therapeutic B-lymphoblastoid cell lines–primed T-cell culture are diverse in producing TH1 and TH2 cytokines, and may exert specific cytotoxicity via exocytosis of granulysin.
Introduction
T-cell–mediated immunity is the major mechanism providing specific protection against microbial infections, including those by viruses.1 Virus-infected cells process viral polypeptides and present antigenic epitopes on the cell surface. T cells mount immune responses upon recognizing the antigen epitope in the context of the major histocompatibility complex (MHC) molecules and receiving various signals from the antigen-presenting cells. It has been well established that CD8+ T cells are mostly cytotoxic T lymphocytes (CTLs) that directly destroy virus-infected cells,2 while CD4+ T cells serve primarily as helpers by secreting various cytokines to regulate and coordinate functions of T cells, B cells, and other immune cells.3
Viruses have evolved different strategies to escape T-cell–mediated immunity.4 Some viruses maintain a state of latent infection in immunocompetent individuals. As exemplified by the Epstein-Barr virus (EBV) and cytomegalovirus (CMV), viruses may disrupt the host cell mechanisms of antigen presentation and/or adapt viral replication programs to minimize the expression of viral targets recognizable by immune surveillance. In individuals with compromised immunity, such as recipients of stem cell transplants (SCTs), latently infecting EBV and CMV may reactivate and cause morbidity and mortality.5,6 Guided by the increased understanding of mechanisms of cellular immunity against viral pathogens, strategies of adoptive immunotherapy have been developed7 and successfully applied to patients following SCT to prevent and treat these viral complications.8,9 Adoptive immunotherapy involves infusing ex vivo expanded, virus-specific T cells into susceptible patients. Therapeutic CMV- or EBV-specific CTLs have been prepared from ex vivo T-cell cultures stimulated with autologous CMV-infected fibroblasts,10 or B-lymphoblastoid cell lines (BLCLs),9 respectively.
In SCT patients, the infusion of polyclonal, BLCL-primed T-cell preparations reconstitutes long-term cellular immunity against EBV,11 but CMV-specific CD8+ clones were found to provide only short-term protection.12 It has been suggested that a deficiency in CD4+ helpers may be responsible for the failed long-term survival of the infused CMV-specific CD8+ CTL, as the persistence of the CD8+ CTL is correlated with CD4+ helper functions in recipients of T-cell infusons.12 Thus, the long-term efficacy of the BLCL-primed T cells may result from the presence of a minor component of EBV-specific CD4+ cells in the polyclonal T-cell culture. This is consistent with the findings that BLCLs express both HLA class I and HLA class II and have the capacity to present endogenously derived antigens to CD8+, as well as CD4+ T cells.13,14 Indeed, we were able to isolate specific CD4+ T cells that recognize autologous BLCLs from T-cell cultures primed with BLCLs, and we showed that they are cytolytic via a pathway independent of granzyme B.15 We are interested in further studying the CD4+ T cells derived from the BLCL-primed T-cell cultures, because the information obtained from these cells may provide insight into their functions and into the mechanism by which the CD4+ T cells contribute to adoptive immunotherapy. On the other hand, although CD4+ T cells may facilitate long-term reconstitution of specific immunity, they could also carry undesirable side effects. It has been documented that CD4+T cells possess nonspecific “bystander” cytotoxicity via Fas ligant (FasL) and other tumor necrosis factor (TNF)–α family molecules, which could result in toxicity in recipients.16 17
We report here that the CD4+ T cells in ex vivo expanded, BLCL-primed T-cell cultures (1) produced TH1 and TH2 cytokines in response to antigenic stimulation and (2) exerted MHC class II–restricted cytotoxicity through a mechanism that was dependent on exocytosis and possibly involved granulysin.
Materials and methods
Donors and cell lines
Healthy EBV-seropositive donors provided peripheral blood mononuclear cells (PBMCs) for this study under protocols approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB). Serostatus was determined by the UAB Core Immunology Laboratory with an immunoglobulin (Ig)–G enzyme-linked immunosorbent assay (Abbott Laboratories, Chicago, IL). MHC typing was performed by the UAB Histocompatibility Laboratory. Preparation of PBMCs and establishment of BLCLs have been described previously.13
Ex vivo T-cell culture
Ex vivo CTL cultures were established as described previously.13 Briefly, PBMCs were cocultivated with autologous BLCLs in 24-well plates (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) in RPMI 1640 supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah). BLCLs were exposed to 100 Gy gamma irradiation prior to use as stimulator cells. The CTL cultures were primed weekly following a regimen of decreasing responder-to-stimulator ratios from 40:1 at day 0 to 20:1 at day 7 and 5:1 on day 14 over a period of 3 weeks. Interleukin (IL)–2 (Collaborative Biomedical Products, Bedford, MA) was added 10 days after the initial stimulation to a final concentration of 2.5 IU/mL, and medium was changed every 3 days by replacing one-half of the supernatant with fresh medium containing IL-2.
Cloning of CTLs
CTLs were isolated by limiting dilution in 96-well, U-bottom tissue-culture plates. Cells were plated in serial dilutions, with 2.5 × 104 irradiated (30 Gy) allogeneic pooled PBMCs from healthy donors as feeders and 2.5 × 104 irradiated (100 Gy) autologous BLCLs as stimulators in a final volume of 200 μL. The cloning medium was supplemented with 200 U/mL IL-2 (Chiron, Emeryville, CA).
Cytotoxicity assay by chromium release assays and cytotoxicity blocking
Chromium release assays were performed as previously described.13 18 In brief, target cells were labeled with51Cr (New England Nuclear, Boston, MA) for 1 hour (300 μCi/106 cells [11.1 × 106Bq/106 cells]), harvested by centrifugation, washed in phosphate-buffered saline (PBS), and dispensed into 96-well V-bottom plates (ICN, Costa Mesa, CA) at 4 × 103 cells per well. Effector cells were added to indicated effector-to-target (E-to-T) ratios in equal volumes. After the cells were pelleted with centrifugation at 1000g and incubated for 4 hours at 37°C in 5% CO2, supernatant was harvested and counted in a gamma counter. Spontaneous release and total release for each target were used to calculate the percentage of specific release as:
Blocking of cytotoxicity was assayed by preincubating either the effector cells or the target cells with blocking reagents. Blocking reagents for the effector cells were as follows: concanamycin A (CMA) (Sigma, St Louis, MO); brefeldin A (BFA, Sigma); ethyleneglycotetraacetic acid (EGTA) (Sigma); antibodies against CD3 (clone OKT3) (Ortho Biotech, Raritan, NJ), FasL (clones NOK-1 and NOK-2) (BD PharMingen), or human TNF-related apoptosis-inducing ligand (hTRAIL) (clone 75 411.11) (R&D Systems, Minneapolis, MN); fusion proteins Fas-Ig and DR5-Ig (gifts from Dr T. Zhou, University of Alabama at Birmingham). For the target cells, blocking antibodies were against MHC class I (clone W6/32) (Leinco, St Louis, MO); HLA-DP (BD PharMingen), HLA-DQ (BD PharMingen), or HLA-DR (clone L243; Leinco). After a 30-minute incubation, equal volumes of effectors and targets were mixed and tested as above.
Reverse transcriptase–polymerase chain reaction for T-cell receptor Vβ and sequencing of Vβfragments
Total RNA was isolated from 3 to 5 × 106 T cells with 1 mL Tri Reagent (Molecular Research Center, Cincinnati, OH). First-strand complementary DNA (cDNA) was synthesized with the SuperScript Preamplification System (Life Technologies, Rockville, MD). The primers used for Vβ amplification were a common 3′ primer and one of the twenty-four 5′ primers according to Genevee et al.19 A pair of T-cell receptor (TCR) Cα primers were included in each reaction as an internal control. The reaction cycles were as follows: 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute, for a total 30 cycles, followed by 72°C for 5 minutes. The polymerase chain reaction (PCR) products were separated by agarose gel electrophoresis, stained with ethidium bromide, and evaluated visually. For sequencing of the PCR-amplified fragments, aliquots of reverse transcriptase (RT)–PCR reaction were used for sequencing from both directions with the PCR primers in an ABI Prism automatic sequencer (Applied Biosystems, Foster City, CA).
Flow cytometric determination of cell surface and intracellular markers
Flow cytometry was performed on a FACScan (BD PharMingen). Surface markers were determined as described previously15by staining with directly conjugated monoclonal antibodies specific for CD3, CD4, and CD8 (BD PharMingen). Multiple color staining of immunophenotypic markers, for both surface and intracellular antigens, was performed as described previously with modifications.15 In brief, T cells were incubated with stimulators at indicated ratios in a concentration of 1 × 106 cells/mL at 37°C with 5% CO2. For experiments in which stimulation lasted for up to 5 hours, BFA was added at 10 μg/mL at the beginning of the cocultivation, and fractions of the culture were harvested at indicated intervals. For experiments with stimulation longer than 5 hours, BFA was added 5 hours prior to cell harvest. After stimulation, EDTA was added to a final concentration of 2.5 mM, and the cells were incubated at room temperature for 10 minutes. Then, 10 vol Lysing Solution (BD PharMingen) was added and incubated for 10 minutes. The cells were either stained immediately or stored at −80°C.
For direct staining, the cells were washed with 3% fetal calf serum and 0.1% NaN3 in PBS, incubated with permeabilization buffer (BD PharMingen) for 10 minutes, aliquoted, and stained with the following labeled antibodies (BD PharMingen): CD3–peridinin chlorophyll protein (CD3-PerCP) or CD3-allophycocyanin (CD3-APC); CD4–fluorescein isothiocyanate (CD4-FITC) or CD4-PerCP; CD8-FITC or CD8-PerCP; CD69-FITC; perforin-phycoerythrin (perforin-PE); interferon-γ (IFN-γ)–APC; IL-4–PE; and IL-2–FITC. For granulysin staining, the cells were first incubated with monoclonal DH4, a gift from Dr A. Krensky (Stanford University, CA), at 1 μg/mL for 30 minutes and then stained with FITC-labeled antimouse IgG. Control antibodies were the respective isotype antibodies conjugated with relevant fluoresceins.
Results
CD4+ T-cell lines with MHC class II–restricted specific cytotoxicity
T-cell lines were cloned by limiting dilution from CTL cultures primed with autologous BLCLs. Several lines displayed an immunophenotype of CD3+CD4+CD8−(data not shown). PD-F4 showed specific cytotoxicity against autologous BLCLs (DQ0602/DQ0609), which was sensitive to a monoclonal antibody against HLA-DQ, but not to the antibodies against DR or DP (Figure 1A). This line did not exhibit cytotoxicity against allogeneic BLCLs with fully mismatched HLA alleles (Figure 1A; Allo C). While PD-F4 did not kill the allogeneic BLCLs sharing a single MHC allele DQ0609 (Allo B), it lysed the partially mismatched allogeneic BLCLs sharing the single MHC DQ0602 (Allo A), and this cytotoxicity was blocked again by the anti-DQ antibody. Two more CD3+CD4+CD8− lines, ND-R4 and TD-B4, displayed a similar pattern of specific cytotoxicity against autologous BLCLs, but not allogeneic BLCLs with fully mismatched HLA alleles (Figure 1B). While the specific cytotoxicity of TD-B4 was inhibited by the HLA-DR–specific antibody, ND-F4 was sensitive to antibodies against HLA-DR and HLA-DQ. The results from the above experiments of antibody blocking and HLA-mismatched targets suggested that the cytotoxicity of all 3 CD4 T-cell lines was restricted by MHC II alleles. A restriction of functions by MHC II is consistent with the characteristics of CD4+ T cells.
Specific cytotoxicity of CD4+ T-cell lines.
Specific cytotoxicity of CD4+ T-cell lines was restricted by MHC class II. CD4+ T-cell lines were tested in standard 4-hour chromium release assays. (A) PD-F4 killed autologous BLCLs and the allogeneic BLCLs sharing DQ0602 (Allo A), but not those sharing DQ0609 (Allo B) or with completely mismatched MHC (Allo C). The cytotoxicity of PD-F4 was inhibited by anti-DQ antibody. (B) ND-R4 was inhibited by anti-DQ, while TD-B4 by anti-DR and anti-DQ antibodies. E-to-T ratio was 3:1. A representative of 2 independent experiments is shown. Error bars represent ± SD.
Specific cytotoxicity of CD4+ T-cell lines.
Specific cytotoxicity of CD4+ T-cell lines was restricted by MHC class II. CD4+ T-cell lines were tested in standard 4-hour chromium release assays. (A) PD-F4 killed autologous BLCLs and the allogeneic BLCLs sharing DQ0602 (Allo A), but not those sharing DQ0609 (Allo B) or with completely mismatched MHC (Allo C). The cytotoxicity of PD-F4 was inhibited by anti-DQ antibody. (B) ND-R4 was inhibited by anti-DQ, while TD-B4 by anti-DR and anti-DQ antibodies. E-to-T ratio was 3:1. A representative of 2 independent experiments is shown. Error bars represent ± SD.
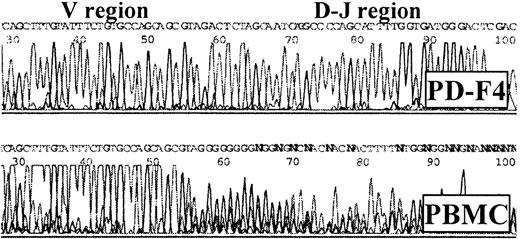
The clonality of the CD4+ lines was established by the expression patterns of TCR β chains (TCR-Vβ).19 RT-PCR detected only Vβ1 in PD-F4, suggesting a monoclonal line. In contrast, ND-R4 and TD-B4 expressed multiple Vβs, consistent with having oligoclonal origins. To exclude the possibility that PD-F4 was actually an oligoclonal line expressing multiple Vβ1, the Vβ1 cDNA fragment amplified by RT-PCR from PD-F4 was sequenced with the PCR primers. While the control Vβ1 fragment from PBMCs showed a unique sequence for the V region, the D-J sequence was ambiguous (Figure 2), indicating a polyclonal nature. In contrast, the equivalent PCR product from PD-F4 displayed no ambiguity in the V, as well as the D-J, region (Figure 2), confirming its monoclonality.20
Sequence of Vβ1 fragment amplified from PD-F4 by RT-PCR.
Note the unambiguous D-J region sequence for PD-F4, consistent with a monoclonal origin, in comparison with the ambiguous counterpart from PBMCs.
Sequence of Vβ1 fragment amplified from PD-F4 by RT-PCR.
Note the unambiguous D-J region sequence for PD-F4, consistent with a monoclonal origin, in comparison with the ambiguous counterpart from PBMCs.
Profile of cytokine production by CD4+ CTLs
CD4+ T cells regulate specific immunity mostly by producing cytokines in response to specific antigenic stimulation. To understand the profile and modulation of cytokine production by the CD4+ CTLs, we used flow cytometry to analyze cytokine production by simultaneous staining for intracellular antigens. We examined 3 cytokines in this study: IFN-γ, IL-2, and IL-4. The first 2 are TH1 cytokines that polarize the immune response to CTL generation, and the third is a TH2 factor promoting B-cell development and antibody production.21
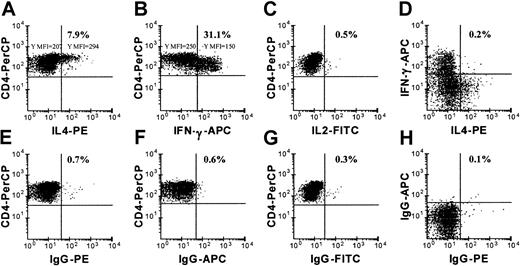
Figure 3 shows that among the oligoclonal ND-R4 cells, approximately 31.1% were positive for IFN-γ (Figure 3B) and 7.9% for IL-4 (Figure 3A) 5 hours after stimulation with autologous BLCLs. Few ND-R4 cells stained for IL-2 (Figure 3C). While the ND-R4 cells expressing IL-4 were mostly CD4high (Figure3A), with a mean fluorescence intensity (MFI) of 294 versus an MFI of 207 for IL-4–negative cells, the IFN-γ–expressing cells were CD4dim (Figure 3B), with an MFI of 150 versus an MFI of 250 for IFN-γ–negative cells. Few, if any, ND-R4 cells coexpressed IL-4 and IFN-γ (Figure 3D). Thus, ND-R4 appeared to consist of 2 distinct populations: one expressing IFN-γ with low CD4, and the other producing IL-4 with high CD4. The staining with control isotype antibodies was lower than 0.7% (Figure 3E-H).
Cytokine expression by ND-R4 cells.
ND-R4 cells expressed single cytokines. Expression of IFN-γ, IL-2, and IL-4 was examined by flow cytometric analysis in ND-R4, which was stimulated with autologous BLCLs for 5 hours in the presence of BFA. A gate was placed on CD4+ events. Panels E-H are for cells stimulated as in panels A-D, but stained with respective control isotype antibodies. Note that the CD4high cells expressed IL-4, while CD4dim cells were positive for IFN-γ only. A representative of 2 independent experiments is shown.
Cytokine expression by ND-R4 cells.
ND-R4 cells expressed single cytokines. Expression of IFN-γ, IL-2, and IL-4 was examined by flow cytometric analysis in ND-R4, which was stimulated with autologous BLCLs for 5 hours in the presence of BFA. A gate was placed on CD4+ events. Panels E-H are for cells stimulated as in panels A-D, but stained with respective control isotype antibodies. Note that the CD4high cells expressed IL-4, while CD4dim cells were positive for IFN-γ only. A representative of 2 independent experiments is shown.
Similarly to ND-R4, PD-F4 up-regulated the expression of cytokines in response to stimulation by autologous BLCLs (Figure4A-C; compare the staining with the corresponding isotype antibodies in Figure 4D-F). There was no detectable cytokine expression in PD-F4 7 days after priming without further stimulation (Figure 4G-I) or with stimulation by allogeneic BLCLs (Figure 4J-L). Unlike ND-R4, the monoclonal PD-F4 produced IL-2 (≈ 20%; Figure 4A,B), as well as IFN-γ (≈ 42%; Figure 4B,C) and IL-4 (≈ 5%; Figure 4A-C). More significantly, single PD-F4 cells were found to coexpress cytokines that have opposite polarizing functions. For example, a significant number of cells coexpressed not only the IL-2/IFN-γ pair (≈ 17%; Figure 4B), but also the IFN-γ/IL-4 pair (≈ 4%; Figure 4C) and IL-2/IL-4 (≈ 2.6%; Figure4A). While the coexpression of these 3 cytokine pairs was always observed in 5 separate experiments, the proportion of cells expressing a given cytokine varied in the different experiments (data not shown), possibly reflecting the plasticity of CD4+ T-cell function in response to subtle fluctuations in antigenic stimulation.22 These data suggest that CD4+ T cells in therapeutic T-cell cultures are diverse and produce cytokines promoting both TH1 and TH2 differentiation.
Cytokine expression by PD-F4 cells.
PD-F4 cells coexpressed cytokines. Expression of IFN-γ, IL-2, and IL-4 was examined by flow cytometric analysis in PD-F4, which was stimulated with autologous BLCLs (panels A-C), no stimulators (panels G-I), or allogeneic BLCLs (panels J-L) for 5 hours in the presence of BFA. A gate was placed on CD4+ events. Panels D-F are for PD-F4 stimulated as for panels A-C, but stained with respective control isotype antibodies. A representative of 5 independent experiments is shown.
Cytokine expression by PD-F4 cells.
PD-F4 cells coexpressed cytokines. Expression of IFN-γ, IL-2, and IL-4 was examined by flow cytometric analysis in PD-F4, which was stimulated with autologous BLCLs (panels A-C), no stimulators (panels G-I), or allogeneic BLCLs (panels J-L) for 5 hours in the presence of BFA. A gate was placed on CD4+ events. Panels D-F are for PD-F4 stimulated as for panels A-C, but stained with respective control isotype antibodies. A representative of 5 independent experiments is shown.
Kinetics of cytokine production by CD4+ CTLs
Recent evidence has shown that the on/off cycling of cytokine production in CD8+ T cells is tightly regulated in coordination with different phases of antigenic stimulation.23 24 The monoclonal PD-F4 cell line provided us an opportunity to study the on/off cycling for cytokine production in CD4+ T cells. We examined the expression of IFN-γ IL-2, and IL-4 in the PD-F4 cells over the course of 9 hours of postantigenic stimulation. Figure 5 shows the kinetics of cytokine expression by enumerating cells producing IFN-γ, IL-2, or IL-4 alone (Figure 5A) and coexpressing other cytokines (Figure 5B). Cells expressing cytokines became detectable at the first hour after stimulation. At the seventh hour, the number of cytokine-positive cells plateaued for all 3 cytokines. In this particular experiment, the numbers of cells expressing IFN-γ and IL-4 were comparable, while IL-2–expressing cells were significantly fewer (Figure 5A). During the seventh to ninth hours, the number of cytokine-positive cells significantly decreased. IL-2– and IL-4–expressing cells decreased most dramatically, almost to baseline levels. In contrast, more than half of the IFN-γ–expressing cells remained positive at the ninth hour after stimulation. Figure 5B shows the same PD-F4 sample analyzed for the kinetics of cells concurrently expressing any 2 cytokines. Consistent with the pattern constructed by enumerating cells positive for any one of the cytokines (Figure 5A), the numbers of cells coexpressing cytokines reached plateau levels at the seventh hour after stimulation. Similar percentages of cells coexpressed IFN-γ/IL-2 and IFN-γ/IL-4, while fewer cells coexpressed IL-2/IL-4. At the ninth hour after stimulation, all the cells concurrently expressing 2 cytokines, including those coexpressing IFN-γ (IFN-γ/IL-2 and IFN-γ/IL-4), declined to baseline levels (Figure 5B). These results indicated that, while the CD4+ T cells had on/off controls for cytokine expression similar to those seen in the CD8+ CTLs, the offswitching for IL-2 and IL-4 expression appeared more prompt than for IFN-γ, or the CD4+ T cells down-regulated the production of IFN-γ less synchronously than that of IL-2 and IL-4.
Time course of cytokine production in PD-F4 in response to antigenic stimulation.
PD-F4 was stimulated with autologous BLCLs at hour zero and harvested at different time points for flow cytometric analysis of cytokine expression as in Figure 4. (A) Enumeration of cells expressing IFN-γ, IL-2, or IL-4. (B) Enumeration for cells coexpressing 2 cytokines. A representative of 2 independent experiments is shown.
Time course of cytokine production in PD-F4 in response to antigenic stimulation.
PD-F4 was stimulated with autologous BLCLs at hour zero and harvested at different time points for flow cytometric analysis of cytokine expression as in Figure 4. (A) Enumeration of cells expressing IFN-γ, IL-2, or IL-4. (B) Enumeration for cells coexpressing 2 cytokines. A representative of 2 independent experiments is shown.
Mechanisms of specific cytotoxicity by CD4+CTLs
Our interest in investigating killing mechanisms of the BLCL-primed CD4+ CTLs arose from reports that CD4+ T cells exerting cytotoxicity through FasL-mediated apoptosis may contribute to pathological bystander cytotoxicity.16,17 We have previously shown that the CD4+ lines established in our laboratory from BLCL-primed T-cell cultures do not express granzyme B, a dominant apoptosis-inducing molecule in the perforin/granzyme–mediated cytotoxic pathway.15 Although a lack of granzyme B expression is consistent with killing mechanisms independent of perforin/granzyme pathways, it was possible that granzymes other than type B were involved in the killing. To address this question, we examined the expression of perforin in the CD4+ T-cell lines isolated from BLCL-primed cultures. While flow cytometric staining detected perforin in the control CD8+ CTL line PD-F8 in the activated state (5 hours after stimulation; data not shown) as well as in the resting state (7 days after stimulation, Figure 6), all 3 CD4+ lines were negative for this protein (Figure 6) under the same detection conditions. A lack of perforin expression in the CD4+ CTLs was consistent with the established observation that most CD4+ T cells exert their specific cytotoxicity predominantly via upregulating FasL and other TNF-α family molecules upon activation.25 To establish that these CD4+ lines indeed exerted specific cytotoxicity via FasL/TNF-α family molecules, we attempted to block the cytotoxicity with antibodies or receptor-immunoglobulin fusion proteins that are known to inhibit cytotoxicity by FasL (Nok1, Nok2, Fas-Ig) or TRAIL (anti-hTRAIL, DR5-Ig). To our surprise, none of the above specific biological reagents significantly inhibited the cytotoxicity of the CD4+ lines (data not shown).
Analysis of CD4+ T cells for perforin expression.
Perforin expression was examined by flow cytometric analysis in CD4+ and CD8+ CTLs. A gate was placed on CD3+ events. The gray shades indicate the background staining by the control isotype antibody. Note that the CD8+ line PD-F8, but not the CD4+ lines ND-R4, PD-F4, and TD-B4, stained positive for perforin. A representative of 2 independent experiments is shown.
Analysis of CD4+ T cells for perforin expression.
Perforin expression was examined by flow cytometric analysis in CD4+ and CD8+ CTLs. A gate was placed on CD3+ events. The gray shades indicate the background staining by the control isotype antibody. Note that the CD8+ line PD-F8, but not the CD4+ lines ND-R4, PD-F4, and TD-B4, stained positive for perforin. A representative of 2 independent experiments is shown.
A resistance to blocking antibodies and receptor-immunoglobulin fusion proteins suggested to us that FasL and TRAIL might not contribute significantly to the specific cytotoxicity of the CD4+CTLs. To confirm this possibility, we then examined the CD4+ CTL lines with chemical reagents that are known to block cytolytic pathways with a wider spectrum of specificity. CMA acidifies intracellular vacuolar granules and is thought to inhibit perforin/granzyme–mediated cytotoxicity by increasing degradation of the content in the exocytotic granules.26 In contrast, BFA selectively inhibits FasL and other TNF-α family molecule–mediated cytotoxicity by inhibiting surface upregulation of glycopolypeptide molecules.27 EGTA has been shown to efficiently inhibit perforin/granzyme–mediated cytotoxicity by chelating extracellular free calcium, which is required for exocytosis of cytolytic granules and pore formation by perforin.28 29
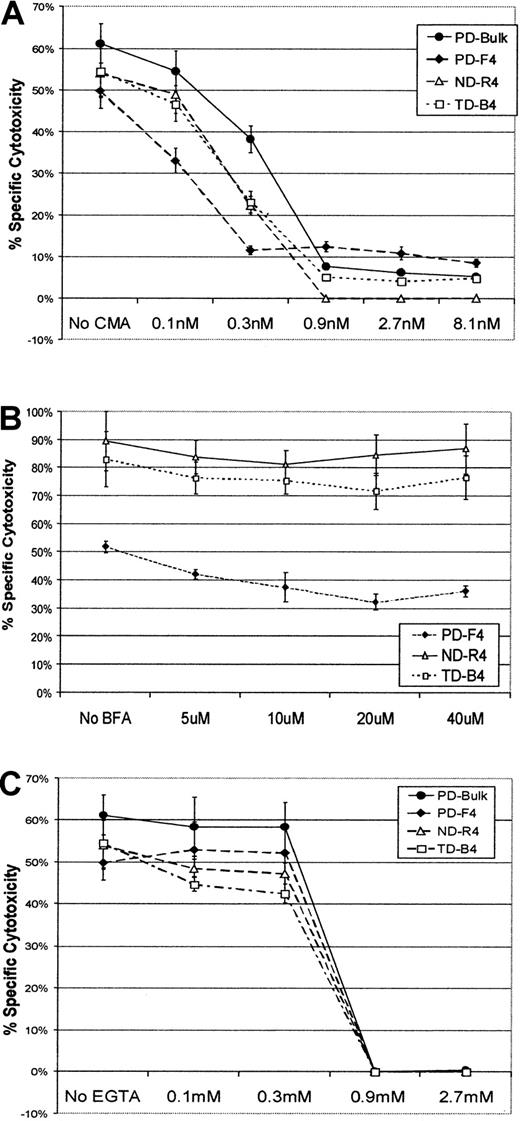
In the presence of 0.1 nM CMA, the specific cytotoxicity of the CD4 T cells showed a slight inhibition (Figure7A). Within the concentrations of 0.3 to 0.9 nM, CMA inhibition of cytotoxicity reached its fullest extent. In comparison with ND-R4 and TD-B4, PD-F4 appeared to have higher residual CMA-resistant cytotoxicity. Figure 7B shows that ND-R4 and TD-B4 displayed an almost complete resistance to BFA when tested with concentrations ranging from 5 to 40 μM. A small proportion of the cytotoxicity by PD-F4 was sensitive to BFA (Figure 7B). This inhibition was dose-dependent when BFA was within the concentration of 5 to 20 μM and appeared to reach the minimal level around 20 μM. Nevertheless, the major portion of the cytotoxicity by PD-F4 was resistant to BFA, in correlation with a residual cytotoxicity resistant to CMA (Figure 7A). EGTA completely blocked the cytotoxicity of all the 3 lines at a concentration of 0.9 mM (Figure 7C). It should be noted that the dose-dependent cytotoxicity inhibition to the CD4+ lines by CMA and EGTA was very similar to a bulk CTL culture (Figure 7A,C) that was composed mostly of BLCL-specific CD8+ T cells (data not shown). The bulk culture provided a control for cytotoxicity mediated by granule exocytosis, typically found in CD8+ CTLs. We have shown before that the cytotoxicity in the bulk T-cell culture primed with BLCL-based APC is detectable only in the CD8+fraction.13 These results suggest that the CD4+ lines may exert their specific cytotoxicity through granule exocytosis.
Inhibition of specific cytotoxicity of the CD4+ CTLs.
Specific cytotoxicity of the CD4+ CTLs was inhibited by CMA and EGTA. CD4+ T-cell lines were tested in chromium release assays for specific cytotoxicity in the presence of CMA (panel A), BFA (panel B), or EGTA (panel C). A representative of 2 independent experiments is shown. Error bars represent ± SD. E-to-T ratio was 3:1.
Inhibition of specific cytotoxicity of the CD4+ CTLs.
Specific cytotoxicity of the CD4+ CTLs was inhibited by CMA and EGTA. CD4+ T-cell lines were tested in chromium release assays for specific cytotoxicity in the presence of CMA (panel A), BFA (panel B), or EGTA (panel C). A representative of 2 independent experiments is shown. Error bars represent ± SD. E-to-T ratio was 3:1.
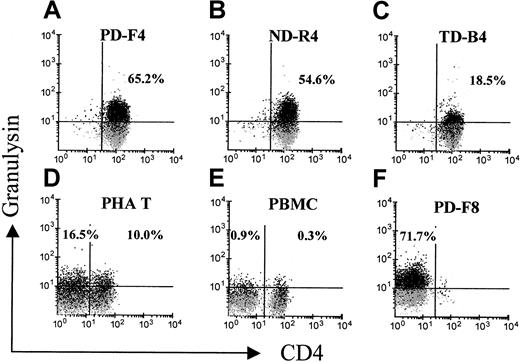
The above findings prompted us to examine the expression of cytolytic granule-associated effector molecules other than perforin and granzymes. Granulysin has recently been identified in the cytotoxic granules of CTL and natural killer (NK) cells and is cytolytic against tumor cells and microbes.30 With the monoclonal granulysin-specific antibody DH4, flow cytometric analysis revealed that granulysin was expressed in a low percentage of phytohemagglutinin-activated T blasts, approximately 17% for CD8+ and 10% for CD4+ cells (Figure8D). In contrast, few CD4+ or CD8+ cells in PBMCs expressed this protein (Figure 8E). Significantly, 65% of PD-F4, 55% of ND-R4, and 19% of TD-B4 expressed granulysin (Figure 8A-C). The CD8+ line PD-F8, which expressed perforin (Figure 6), was also positive for granulysin in 72% of the cells (Figure 8F). Granulysin expression was positive in the T cells of the activated state (5 hours after stimulation; data not shown), as well as of the resting state (7 days after priming; Figure 8A-C). This pattern of expression is similar to that of perforin, but different from the one for cytokines.23 The same cell preparation used for granulysin staining was also tested for indirect staining with the antibody against IFN-γ. The pattern from indirect staining for IFN-γ (data not shown) was similar to the one with directly conjugated anti–IFN-γ (Figures 3 and 4). Isotype antibodies did not show any significant staining (Figure 8 and not shown). These results suggested that the BLCL-specific CD4+CTLs might exert their major cytotoxicity by exocytosis of granulysin.
Analysis of CD4+ T cells for granulysin expression.
CD4+ T cells were stained with the granulysin-specific monoclonal antibody DH4 for flow cytometric analysis. A gate was placed on CD3+ events. The gray shadows represent overlaid background staining by the control isotype antibody (IgG1). A representative of 2 independent experiments is shown.
Analysis of CD4+ T cells for granulysin expression.
CD4+ T cells were stained with the granulysin-specific monoclonal antibody DH4 for flow cytometric analysis. A gate was placed on CD3+ events. The gray shadows represent overlaid background staining by the control isotype antibody (IgG1). A representative of 2 independent experiments is shown.
Discussion
CD4+ T cells have been extensively studied as helpers regulating the immune system via diffusable factors including cytokines.3 Although cytotoxic CD4+ T cells have been reported,17,31-35 little is known about their role in virus-specific immunity, especially the relationship between their specific cytotoxicity and helper functions. In this study, we characterized the antigen-specific CD4+ CTLs from T-cell cultures generated ex vivo with a protocol used in adoptive immunotherapy for EBV-related tumors.36 Although the specific cytotoxicity of these CD4+ T cells was clearly MHC class II restricted, as expected for classic CD4+ T cells, the CD4+ CTLs showed uncommon features in cytokine production and cytolytic mechanism in response to antigenic stimulation.
As expected for CD4+ T cells, ND-R4 and PD-F4 expressed immunoregulatory cytokines in response to antigenic stimulation. Single ND-R4 cells expressed either IL-4 or IFN-γ, but rarely expressed the 2 concomitantly. This is consistent with the results from several studies showing that the majority of CD4+ T cells express single cytokines.37,38 In contrast, the monoclonal PD-F4 was found to express IL-2, IFN-γ, and IL-4. Moreover, single PD-F4 cells coexpressed not only IFN-γ and IL-2, cytokines with the same polarizing effect, but also cytokines with opposite polarizing functions, IFN-γ/IL-4 and IL-2/IL-4. Coexpression of TH1 and TH2 cytokines has been reported recently for alloreactive T cells in a study with single-cell RT-PCR.39Our finding that most of the IL-2–and IL-4–positive cells in PD-F4 also expressed IFN-γ is consistent with the report showing a correlation between dosage of antigenic stimulation and quantitative modulation of cytokine expression in CD4+ T cells.22
Results from our study suggested that the CD4+ CTLs controlled the off cycling for IL-2 and IL-4 production in ways similar to those reported for IFN-γ and TNF-α in CD8+CTLs.23,24 Our data further revealed that the pattern of the off-cycling control for IL-2 and IL-4 production may be different from the one for IFN-γ Since cytolysis by the CD4+ T-cell lines had nearly plateaued after 4 hours of coincubation with target cells (data not shown), it would be reasonable to speculate that the drastic decrease in numbers of cytokine-expressing cells upon 7 hours of stimulation was due to diminished antigenic stimulation (Figure 5). Interestingly, while the PD-F4 cells almost completely lost their expression of IL-2 and IL-4 between the seventh and ninth hours, the reduction of cells expressing IFN-γ was not as drastic, suggesting a more resilient off-cycling control for IFN-γ production in response to the loss of antigenic stimulation. This result, while reinforcing the idea of an independently regulated expression of cytokines, also indicated that the requirement for signals to maintain cytokine expression would be more stringent for IL-2 and IL-4 than for IFN-γ. Thus, it is possible that the off-cycling regulation for cytokine production in activated CD4+ CTLs might be carried out with different sensitivity to diminishing antigenic stimulation. For example, while the early phase of target cell death alone would be sufficient for down-regulating IL-2 and IL-4 in the CD4+CTLs, a physical dissociation between the target and CTL might be required for the off-cycling switch of IFN-γ. A similar mode of differential off-switches for IFN-γ and TNF-α expression has been described in CD8+ CTLs.24 40 The cell lines described in this study would be useful tools to further delineate the mechanisms for the off-cycling regulation of cytokine expression.
We found that the CD4+ CTLs possessed specific cytotoxicity that could not be attributed to classic cytolytic pathways mediated by perforin/granzyme or FasL.25 For CTLs exerting cytotoxicity via the perforin/granzyme pathway, interaction between T cells and target cells results in release of cytolytic granules containing perforin and granzymes, which permeabilize the plasma membrane of the target cells and set off a chain of enzymatic reactions quickly leading to destruction of the plasma membrane and programmed cell death. In contrast, in response to specific antigenic stimulation, most CD4+ CTLs up-regulate the expression of FasL, which binds to its cognate receptor Fas/CD95 on the surface of target cells and induces apoptosis of the target cells. Other mechanisms of cytolysis, which mainly implicate members of the TNF-α family molecules, have also been appreciated recently.41
We showed in this study that the CD4+ CTL lines did not express the pore-forming protein perforin. This is in contrast to the CD8+ CTLs, which were found positive for perforin with the same experimental approach and detection technique (Figure 6). We have previously reported that granzyme B is not expressed in the CD4+ CTL lines isolated from BLCL-primed culture.15 Although this is in line with the mainstream concept that CD4+ T cells generally exert specific cytotoxicity by FasL, functional assays with specific blocking agents could not establish a confident link between the specific cytotoxicity and FasL/TRAIL. Further blocking experiments with chemical inhibitors confirmed that the CD4+ CTL-mediated killing was independent of FasL or TRAIL, as the major proportion of the cytotoxicity by the CD4+ CTL lines was resistant to BFA. Since BFA is inhibitory to surface transportation of polypeptides from the endoplasmic reticulum, a resistance to this substance would suggest that not only FasL and TRAIL but also other members of the TNF-α family molecules may not play a major role in the killing by these CD4+ CTLs. Consistent with this interpretation is our finding that the CD4+ CTL lines reached their maximum cytolysis in about 4 hours (data not shown). It has been reported that TNF-α– and TRAIL-mediated cell death often become obvious after 16 hours.41 42
The CD4+ CTL lines appeared to exert specific cytotoxicity via mechanism(s) involving exocytosis, possibly with granulysin. Supporting evidence includes our findings that the cytotoxicity mediated by the CD4+ CTLs was sensitive to CMA and EGTA. The detection of granulysin, but absence of other known cytolytic effectors in these CD4 CTLs, is consistent with the possibility that CD4+ CTLs might kill their targets by this protein. Granulysin is a newly discovered cytolytic molecule usually coexpressed with perforin in the cytolytic granules of CTL and NK cells.43 It has been found to be bactericidal against a broad range of microbes, including the intracellular parasiteMycobacterium tuberculosis.44 This particular study44 showed that granulysin, together with perforin, is expressed in antigen-specific CD8+CTLs. A very recent work further demonstrated that granulysin can be expressed in the CD4+ T cells specific toMycobacterium leprae, but did not determine whether theseM leprae–specific CD4+ T cells produce any cytokines.45 It is speculated that granulysin works synergistically with perforin, which allows granulysin to enter the cells and kill the intracellular microbes. Recently, it was also found that synthetic granulysin itself is cytolytic to tumor and virus-infected cells.46,47 Granulysin causes damage to cell membranes and disrupts the transmembrane potential in mitochondria, which leads to apoptosis.48 Results from our study further the significance of granulysin by directly showing that T cells expressing granulysin were cytolytic to EBV-infected B cells. These CTLs may have a CD8+ phenotype, as exemplified by PD-F8, which coexpressed perforin and granulysin (Figures 6 and 8). Of interest is our finding that CD4+ CTLs expressed granulysin without coexpressing detectable amounts of perforin, suggesting that granulysin alone could be sufficient as a physiologically relevant cytolytic effector. Since the same CD4+ lines coexpressed multiple immunoregulatory cytokines, the CD4+ CTLs described in our study have the potential to play important roles in specific immunity. It should be noted that although from our study the correlation between granulysin and CD4+ CTL-mediated cytotoxicity is strong, a definitive conclusion awaits technical means that would directly and specifically disrupt the function of granulysin. Moreover, a granulysin-mediated pathway does not necessarily exclude the use of other cytolytic pathways by CD4 T cells, especially under different physiological conditions or against specific pathogens. Although our data suggested that the major part of the CD4+ CTL-mediated cytolysis, which occurred during the initial 4 hours upon encountering antigen-bearing cells, was independent of FasL, one could not exclude the possibility that FasL might be induced and contribute cytolysis at a later phase of antigen stimulation. The coordination between the specific cytotoxicity and immunoregulation for the CD4+ T cells also needs further study.
The results reported in this study are relevant to clinical application of adoptive immunotherapy against EBV. Since the antigen-specific CD4+ T cells in the BLCL-primed T-cell culture possessed helper functions to regulate both TH1 and TH2 differentiation, potentially these cells would contribute to the efficacy of T-cell therapy by promoting a full spectrum of specific immunity against a given target. Furthermore, because the specific cytotoxicity of the CD4+ CTLs was largely independent of FasL and possibly other TNF-α family molecules, these cells would be unlikely to carry risks of bystander cytotoxicity.
The authors thank Dr R. Lopez (UAB) for the use of a flow cytometer, Dr T. Zhou for Fas-Ig and DR5-Ig, Dr W. Britt for discussion of results, and Drs A. Krensky and S. Okada (Stanford University) for the antibody DH4.
Supported by grants from the American Cancer Society (CRTG 97-043-EDT), the National Institutes of Health (R01 CA75566-01 and R21 CA84398-01), and the Amy Stretzer-Manasevit Award from the National Marrow Donor Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth G. Lucas, Bone Marrow Transplantation Program, University of Alabama at Birmingham, 1900 University Blvd, THT 513, Birmingham, AL 35294; e-mail klucas@uabmc.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal