Key Points
CV risk factors are common in older men with hemophilia.
Although older men with hemophilia have less CV disease than comparable unaffected men, CV events do occur and require treatment.
Abstract
Men with hemophilia were initially thought to be protected from cardiovascular disease (CVD), but it is now clear that atherothrombotic events occur. The primary objective of the CVD in Hemophilia study was to determine the prevalence of CVD and CVD risk factors in US older men with moderate and severe hemophilia and to compare findings with those reported in age-comparable men in the Atherosclerosis Risk in Communities (ARIC) cohort. We hypothesized if lower factor levels are protective from CVD, we would see a difference in CVD rates between more severely affected and unaffected men. Beginning in October 2012, 200 patients with moderate or severe hemophilia A or B (factor VIII or IX level ≤ 5%), aged 54 to 73 years, were enrolled at 19 US hemophilia treatment centers. Data were collected from patient interview and medical records. A fasting blood sample and electrocardiogram (ECG) were obtained and assayed and read centrally. CVD was defined as any angina, any myocardial infarction by ECG or physician diagnosis, any self-reported nonhemorrhagic stroke or transient ischemic attack verified by physicians, or any history of coronary bypass graft surgery or coronary artery angioplasty. CVD risk factors were common in the population. Compared with men of similar age in the ARIC cohort, patients with hemophilia had significantly less CVD (15% vs 25.8%; P < .001). However, on an individual patient level, CVD events occur and efforts to prevent cardiovascular events are warranted. Few men were receiving secondary prophylaxis with low-dose aspirin, despite published opinion that it can be used safely in this patient population.
Introduction
Hemophilia A and B are X-linked disorders that result in bleeding as the result of an absence or decrease in coagulation factor VIII or factor IX, respectively. Hemophilia is classified as severe (<1% clotting factor activity), moderate (1%-5%), and mild (6%-40%).1 Without treatment, patients with hemophilia, particularly those with severe disease, have recurrent disabling and life-threatening bleeding episodes. More recent use of prophylactic factor therapy to prevent bleeding has changed the landscape of hemophilia, and patients with hemophilia are living longer because of improvements in treatment and general medical care.2-4
Among 14 990 US men with hemophilia A or B reported in September 2017 in the American Thrombosis and Hemostasis Network data set, 14.5% were aged 50 years or older.5 As cardiovascular disease (CVD) is a disease that increases with age, these men are at risk for cardiovascular complications. Men with hemophilia were initially thought to be protected from CVD, but it is now clear that atherothrombotic events do occur.6-13 Limited data suggest that hemophilia is not protective against atherosclerosis,13-15 but this has not been well studied in men with severe hemophilia without other CVD risk factors, such as active HIV infection. CVD risk factors are prevalent in the hemophilia population, and for reasons not understood, hypertension appears to be more common, even in younger men.16,17 Overall, most, but not all, studies have shown some protection from CVD in the hemophilia population.8 In general, these studies have evaluated patients with all severities of hemophilia and have not focused on those with moderate and severe disease, who in theory may be most protected from CVD.
The primary objective of the CVD in Hemophilia study reported here was to determine the prevalence of CVD and CVD risk factors in older men with moderate and severe hemophilia in the United States. In addition, we compared the prevalence of CVD and CVD risk factors in our hemophilia cohort with age-comparable men in the US Atherosclerosis Risk in Communities (ARIC) and National Health and Nutrition Examination Survey (NHANES) cohorts. CVD was defined as any angina validated by the Rose questionnaire,18 any myocardial infarction (MI) by electrocardiogram (ECG) or physician diagnosis, any self-reported nonhemorrhagic stroke or transient ischemic attack that was verified by physicians, or any history of coronary bypass graft surgery or coronary artery angioplasty.
Methods
The CVD in Hemophilia study was a US national cross-sectional study, which began enrollment in October 2012. Patients were enrolled at 19 hemophilia treatment centers (HTCs) in the United States. The study included men with moderate or severe congenital hemophilia A or B (factor VIII [FVIII] or IX level ≤ 5%) aged 54 to 73 years. The age range was established to allow data to be compared with that in the ARIC study.19 The enrollment goal of 200 subjects was determined on the basis of 80% power to show a difference in the prevalence of CVD compared with the same-aged ARIC cohort, assuming the prevalence of CVD in the subjects with hemophilia was 14.9% (vs 23% in ARIC). Men with an additional bleeding disorder (besides liver dysfunction) were excluded. The study was approved by the local Institutional Review Board at each site.
Men with mild hemophilia (FVIII or FIX level 6%-40%) were excluded because, with our hypothesis that a low factor activity level is protective from CVD, we would expect to see a greater difference in CVD between men with normal factor levels and those with more severely decreased factor levels. Patients with mild disease would be at intermediate risk, and a larger number of patients would be needed to compare CVD events between those with mild and more severe disease or between those with mild disease and nonhemophilic control patients.
After obtaining written informed consent, data were collected from patient interview and medical records and included CVD risk factors, medications, and history of thrombotic events. A fasting blood sample was collected and assayed centrally (University of Washington, Seattle, WA) for glucose, lipids, creatinine, and high-sensitivity C-reactive protein (hsCRP). A 12-lead ECG was performed and read centrally and independently by 2 masked cardiologists. Evidence of an MI was defined by the presence of Minnesota Code (MC) classifications as a major Q/QS abnormality (MC 1.1 or 1.2) or minor Q/QS abnormality (MC 1.3) and major ST-T abnormality (MC 4.1, 4.2, 5.1, or 5.2).20 If the independent ECG readings differed, a final reading was given by consensus. CVD was defined as any angina validated by the Rose questionnaire,18 any MI by ECG or physician diagnosis, any self-reported nonhemorrhagic stroke or transient ischemic attack that was verified by physicians, or any history of coronary bypass graft surgery or coronary artery angioplasty. Additional definitions used were prophylaxis, regular infusions of FVIII (≥2 doses/week) or FIX (≥1 dose/week) to prevent bleeding (3 patients receiving prophylaxis were receiving extended half-life products); obesity, body mass index higher than 30 kg/m2; elevated waist circumference, higher than 102 cm; hypertension, sitting systolic blood pressure higher than 140 mm Hg, diastolic blood pressure higher than 90 mm Hg (elevated levels were confirmed with repeat testing ≥30 minutes later) or receiving antihypertensive medications; dyslipidemia, elevated fasting total cholesterol (>200 mg/dL), triglycerides (>150 mg/dL), low-density lipoprotein (LDL) (>130 mg/dL), decreased fasting HDL (<40 mg/dL), or receiving cholesterol lowering agents; diabetes mellitus, subject reported, elevated fasting glucose (>125 mg/dL), or receiving medication; and elevated hsCRP (>1.0 mg/dL). Angina was defined using the validated Rose questionnaire as the presence of chest pain on exertion that was relieved within 10 minutes after stopping or slowing down.18 A positive family history of CVD was defined as a sibling or parent with a heart attack or stroke.
Male participants in the ARIC study were used as the primary comparison population. This CVD in Hemophilia study was structured using the same CVD definition as was used in ARIC, except that hemorrhagic stroke was not specifically excluded. The ARIC study is an National Institutes of Health-funded prospective epidemiologic study to investigate the etiology and natural history of atherosclerosis and cardiovascular disease in 4 US communities.19 The cohort component of the study was begun in 1987, and in the fourth and last follow-up period in 1996 to 1998, 4826 men were aged 54 to 73 years, which is the age focus of this CVD in Hemophilia study. The ARIC cohort was chosen as a control because of the type and focus of its data collection and an age range comparable to that in which individuals frequently are diagnosed with CVD, and for which the hemophilia population size was sufficiently feasible to conduct this study. Peripheral vascular disease was not included in our definition of CVD because it was not part of the ARIC definition. Peripheral vascular disease includes pain with walking alleviated with rest, which can also occur in older men with hemophilia resulting from joint disease. We did not have resources for an objective measure of peripheral vascular disease, such as ankle brachial index.
To compare CVD prevalence in patients with hemophilia with a more contemporaneous population cohort, we used the NHANES 2013 to 2014 examination (https://wwwn.cdc.gov/nchs/nhanes). NHANES is a US population-based cross-sectional survey conducted by the National Center of Health Statistics at the Centers for Disease Control and Prevention. The study collects survey data on health and nutritional status of noninstitutionalized civilians of the US population through a series of interviews, examinations, and laboratory measurements. Participants are selected using a random sampling method, designed to oversample certain populations, including Hispanics, non-Hispanic African Americans, and non-Hispanic Asians. Of note, in NHANES, cardiac events are by history only, and ECGs are not obtained; transient ischemic attack (TIA) is also not recorded. A total of 862 men were chosen because their age at interview was 54 to 73 years, with a cohort mean age of 63 (standard deviation, 6) years. As per its design, NHANES had a higher percentage of Hispanic, African American, and Asian participants than our hemophilia cohort.
Statistical analysis
For categorical variables, χ2 or Fisher’s exact tests were used to compare clinical and laboratory characteristics in those with hemophilia vs nonhemophilic men in the ARIC and NHANES studies. A logistic regression model using multivariate analysis followed by bivariate analyses was used to identify CVD risk factors in male patients with hemophilia, as well as to compare the prevalence of CVD between hemophilia and ARIC cohorts. The bivariate analyses built a foundation of covariates to be considered in the multiple logistic regression analysis. The models presented in the Results have the smallest Akaine Information Criterion or Bayesian Information Criterion values that best explain the data.
Results
Patients meeting all the inclusion criteria and none of the exclusion criteria were approached at each HTC. Two hundred one subjects were enrolled from 2012 to 2015. One subject had a baseline factor activity level above 5% and was excluded from the analysis. Approximately 60% had hemophilia A, and just over half had severe disease; of the 40% with hemophilia B, 42% had severe disease. Patients with hemophilia A were more likely to have severe disease (P = .002) and to have hepatitis C (P = .009) and HIV (P < .001) infections than those with hemophilia B. African American subjects were more likely to have severe hemophilia than white subjects (76.2% vs 52.5%; P = .039). Almost one-third of patients were receiving routine prophylaxis with factor therapy. Patient demographics and baseline clinical characteristics are shown in Table 1. Approximately 60% of patients had been infected with hepatitis C and 27% with HIV. Forty-three (21.5%) subjects had been diagnosed with cirrhosis, of whom 11 (5.5%) had an elevated prothrombin time and 21 (10.5%) had thrombocytopenia. Fifty-two of the 54 HIV-positive patients had a recent CD4 count available, with a median count of 484/mm3 (mean, 514/mm3; range, 86-867/mm3). Only 4 patients had CD4 counts below 200/mm3.
Cardiovascular risk factors were common in the population (Table 2). More than half the individuals had dyslipidemia and hypertension, and nearly one-third were obese. Although the mean creatinine was 1.1 mg/dL (standard deviation, 0.4 mg/dL), 26.8% of the subjects had an elevated value. Of the 119 subjects with hypertension, 72 (56%) used antihypertensive medication. Of the 97 subjects who ever smoked, 32 were current smokers. African American patients were less likely to have dyslipidemia (38.1% vs 66.1%; P = .012), but other risk factors were similar between races.
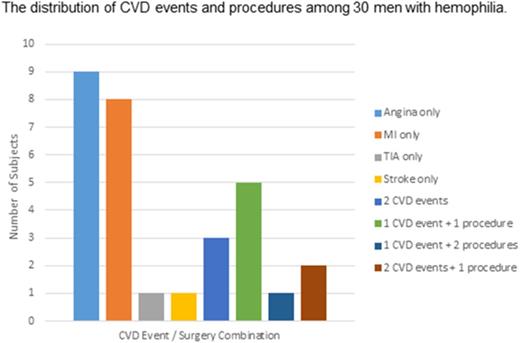
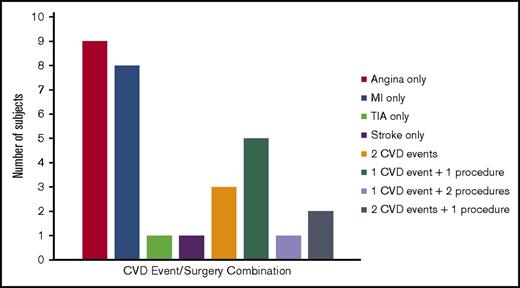
In total, 30 subjects met criteria for CVD (defined as angina, MI, TIA, or ischemic or embolic stroke), for a prevalence of 15%. Fifteen subjects (7.5%) had MI (8 diagnosed by ECG only), 16 (8%) had prior angina, 3 had a history of a TIA, and 1 had a history of an ischemic or embolic stroke. In addition, 15 subjects had atrial fibrillation/flutter, 6 had coronary stent placement, 3 had coronary bypass grafting, and 6 had venous thromboembolism (2 had both deep vein thrombosis and pulmonary embolism, 2 had deep vein thrombosis only, and 2 had pulmonary embolism only). As demonstrated in Figure 1, among the 30 subjects, 19 had a single CVD event, 3 had 2 CVD events, and 8 had at least 1 CVD event plus 1 CVD surgery.
The distribution of CVD events and procedures among 30 subjects with CVD. The number of subjects who experienced CVD events (angina, MI, TIA, or stroke) alone or in combination with other events or procedures (coronary artery bypass grafting or angioplasty).
The distribution of CVD events and procedures among 30 subjects with CVD. The number of subjects who experienced CVD events (angina, MI, TIA, or stroke) alone or in combination with other events or procedures (coronary artery bypass grafting or angioplasty).
On bivariate analysis, the development of CVD among the patients with hemophilia was significantly associated with hypertension (P = .002), dyslipidemia (P = .014), and ever smoking (P = .031). CVD risk factors for obesity, increased waist circumference, elevated hsCRP, and not engaging in physical activity were not associated with CVD in this population (P > .8 for all). Hemophilia type (A vs B), severity (severe vs moderate), and treatment regimen (prophylaxis vs on-demand) had no significant associations (P values = .160 and .291, respectively) in this analysis. A multiple logistic regression model was built to describe the relationship among the hemophilia cohort between the presence of CVD and a priori chosen explanatory variables including CVD risk factors (age, race, family history of CVD, smoking, hypertension, diabetes, dyslipidemia) and hemophilia characteristics (type, severity, inhibitor presence, hepatitis C virus [HCV] and HIV infections, and interaction between severity and HCV). Among generally known CVD risk factors, nonwhite race, dyslipidemia, hypertension, and ever smoking all were significantly associated with CVD (Table 3). Among hemophilia characteristics, the presence of an inhibitor was significantly associated with CVD in the total cohort. In the absence of hepatitis C, moderate hemophilia was significantly associated with CVD compared with severe hemophilia.
Compared with men of similar age in the ARIC cohort, patients with hemophilia had significantly less CVD (15% vs 25.8%; P < .001). Although the age ranges for the 2 cohorts were the same (53-74 years), the proportion of those in ARIC aged 65 years and older (43.5%) was significantly higher than in the hemophilia cohort (25%). There were other significant differences between the 2 cohorts, as shown in Table 4. The patients with hemophilia were more likely to be of white race, have hypertension, have a family history of CVD, and have an elevated hsCRP and creatinine. Participants in the ARIC cohort were more likely to have ever smoked, have an elevated waist circumference, and be receiving antihypertensive medications. In a logistic regression model, the odds of having CVD in men with hemophilia vs ARIC, after adjusting for the effects of age, race, ever smoking, family history of CVD, hypertension, diabetes mellitus, dyslipidemia, elevated hsCRP, and elevated creatinine, was 0.42 (95% confidence interval, 0.28-0.63). In other words, if the values of other predictors were equal, men without hemophilia had 2.4 times higher odds of developing CVD.
To compare CVD rates in patients with hemophilia with a more contemporaneous population cohort, we used the NHANES 2013 to 2014 examination (https://wwwn.cdc.gov/nchs/nhanes). About 8.2% (n = 71) of selected NHANES subjects had angina either through self-reporting or Rose questionnaire, 8.6% (n = 74) reported myocardial infarction, and 4.9% reported a stroke (n = 42). Without accounting for MI detected via ECG only, or the occurrence of TIA, 17.9% (n = 154) of NHANES patients had CVD, which was significantly higher than for the patients with hemophilia (P < .001), of whom only 10% (n = 20) reported angina (n = 16), MI (n = 6), or stroke (n = 1).
There were differences in CVD risk factors between the NHANES (ages 53-74 years) and hemophilia subjects (Table 4). Compared with the patients with hemophilia, NHANES subjects were older, had increased waist circumference, and had a history of ever smoking, diabetes, and elevated total cholesterol. Patients with hemophilia were more likely than NHANES subjects to have a low HDL and to have hypertension. However, NHANES estimated triglyceride and LDL values on the basis of data from fewer than 50% of subjects, and thus may not be a true reflection of the population. Because of different measures used between the cohorts, we could not apply the logistic regression model used for the ARIC comparison.
Discussion
In this study, we analyzed the prevalence of cardiovascular disease in older men with moderate and severe hemophilia. Although CVD risk factors were common in these men with hemophilia, cardiac events were less common than in similarly aged US male populations. This may not be explained by differences in atherosclerosis. Studies in men with hemophilia have shown similar atherosclerosis, as measured by carotid and femoral artery intima-media thickness and on autopsy, although these studies included many men with milder hemophilia or untreated HIV infections and may not reflect findings in our population.13-15,21,22 Given the role of FVIII and FIX in thrombin generation, there may be less thrombus formation with an acute event, such as plaque rupture, decreasing the likelihood of an acute event.
The demographics of our subjects were generally representative of the hemophilia population in the United States. In contrast to younger patients with hemophilia, there were proportionally more patients with hemophilia B. Our population characteristics are reflected in the American Thrombosis and Hemostasis Network data set, in which men aged 50 to 74 years represent 15.5% of those with moderate or severe hemophilia B, but only 9.2% with moderate or severe hemophilia A.5 This is likely a result of the higher rate of HIV infection and subsequent death in those being treated with contaminated FVIII-containing vs FIX-containing concentrates in the past.23 This could affect our analysis, although no difference in risk factors or CVD events by type of hemophilia was noted. The reason why there were proportionally more African American subjects with severe disease is not clear, but may have been influenced by the small number of African American subjects enrolled in the study.
We did not find a difference in the prevalence of CVD by treatment approach (prophylaxis vs on-demand therapy). One could hypothesize that patients using routine prophylactic factor infusions would have less protection from CVD, given higher overall factor activity levels. Alternatively, prophylactic therapy could prevent joint bleeding and associated inflammation, which might increase CVD risk. Although we do not have detailed information on the length of prophylactic therapy, given the ages of the men in our cohort and clinical practice patterns in the United States, the introduction of prophylaxis would likely have been as adults. Thus, our data cannot inform questions around CVD in patients receiving prophylaxis from early childhood.
The patient population we studied is of an age severely affected by factor replacement-related infections with HIV and hepatitis C and B that occurred before adoption of viral-inactivation methodology, beginning in the mid-1980s.24,25 Twenty-seven percent of our population was HIV infected. This represents a group that likely infused less at the infection risk time, and thus may have phenotypically less severe disease. In some ways, our cohort represents a “survivor” cohort, and our results could be influenced by factors that contributed to their ability to live long enough to participate in this study. Although HIV and medications for HIV are associated with accelerated CVD in other populations,26 we did not find a higher prevalence of CVD in our HIV-positive subjects. Our findings may provide insight into the pathogenesis of HIV and HIV medication-associated CVD, and particularly the role of coagulation in reported events.
Chronic HCV infection has also been associated with an increased risk for CVD, particularly in those with other CVD risk factors.27 This was not observed in our population. However, we did find an increased incidence of CVD in patients with moderate vs severe disease in patients who were not HCV infected. It is possible that a risk associated with HCV, such as chronic inflammation, masks a protective effect of a lower factor level.
As has been described in several other cohort studies,16 28-33 we also found an increased prevalence of hypertension compared with ARIC and NHANES subjects in our population. The reason why men with hemophilia have more hypertension, as well as higher blood pressures, compared with the general population is unknown, but may be linked to vascular abnormalities in arthropathic joints.34 We found a higher prevalence of abnormal serum creatinine in our cohort compared with ARIC subjects; this was not collected for the NHANES cohort. Given the link between hypertension and renal disease,35 it is possible that renal disease is an etiology. von Drygalski et al also found an association between hypertension and elevated creatinine in subjects with hemophilia, which was similar to that found in NHANES subjects.16 The large Advance study of men with hemophilia (all severities) in Europe also found an association between hypertension and renal dysfunction.36 However, such an association has not been found in all cohort studies.30 A follow-up study to our current study is underway to better define renal disease in hemophilia. One consideration may be the role of hematuria. Patients with hemophilia frequently have hematuria, which is treated conservatively unless there is significant blood loss.37 However, an association with hematuria and hypertension has not been found in 2 studies,32,36 so another etiology may be present. If an association between decreased renal function and hypertension is confirmed, further measures to control blood pressure and prevent renal disease would be warranted. If hematuria appears to increase the risk for subsequent renal disease, population characteristics should be explored further to explain differences in findings between studies.
Our patients reported a low usage of antihypertensive therapy. The Centers for Disease Control and Prevention has reported that approximately 50% of people with hypertension achieve blood pressure control with medication.38 In our study, only 36% of patients with hypertension were receiving antihypertensive medication. Hypertension is reported in younger men with hemophilia as well, with poor control.16 It is important that hemophilia treaters inform patients and their primary care providers of the increased prevalence of hypertension in hemophilia, screen for hypertension at hemophilia clinic visits, and facilitate appropriate care.
In our cohort, 30 subjects met the definition of CVD, but only 4 were receiving low-dose aspirin, 3 as secondary and 1 started before, as primary prevention. This was lower than those in the ARIC and NHANES cohorts. Data from the National Health Interview Survey in 2012 found that 70% of people with CVD were receiving low-dose aspirin.39 Patients with hemophilia of this era were strongly advised not to take aspirin when they were younger, and for this reason, many have aspirin in their medical record as an “allergy.” This advice was instituted when aspirin was used at much higher doses, and before the cardiovascular benefits of low-dose aspirin had been described. There are minimal data on the safety of low-dose aspirin in patients with hemophilia, although expert opinion has recommended that patients receiving low-dose aspirin with FVIII below 1% use prophylaxis, but not those with higher factor levels.40-42 We have no data to suggest that low-dose aspirin has less cardioprotective effect in men with hemophilia than in the general population. Given that 30% of our subjects were receiving regular prophylactic therapy, and approximately half of subjects had moderate disease, it is likely that many could tolerate low-dose aspirin without a change in treatment regimen. In addition, given that we found no difference in CVD events between those receiving prophylaxis vs on-demand therapy, underlying atherosclerotic vascular disease should not be a reason to withhold prophylaxis, and prophylaxis, or other regimens such as gene therapy, would allow use of more optimal cardiovascular medications and interventions.
Six of the 9 patients with neutralizing antibodies (inhibitors) to FVIII had CVD in our hemophilia cohort. The presence of an inhibitor was significantly associated with CVD in our analysis (odds ratio, 9.18; 95% confidence interval, 1.26-66.90; P = .029). This finding was likely influenced by the small number of inhibitor patients, which is reflected in the wide confidence interval, and this makes it unclear whether it is a true physiological finding. The patients with inhibitors treated bleeding episodes with bypassing agents, which contain activated clotting factors. However, no acute thrombotic event temporally associated with bypassing agent treatment was recorded in the study.
The strengths of our study are that it was multicenter and focused on men with more severe disease. If a low baseline factor level is protective against CVD, it would be more likely to be detected in a population with more severe disease, as the protective effect could be diluted or masked in cohorts that include patients with mildly decreased factor levels. This is particularly true of rare diseases such as hemophilia, for which subject numbers in studies tend to be low. Our study also used medical records to confirm CVD diagnoses, central laboratory testing for lipids, and hsCRP and central reading of ECGs.
A major limitation of our study was the absence of an active control group. We chose the ARIC cohort for comparison and used the same definition of CVD because of characteristics similar to those in our older hemophilia population and study measurements that we could obtain, although the cohort is noncontemporaneous. In the NHANES study of 21 472 adults aged 40 years and older, although the prevalence of coronary heart disease decreased during that time, the reduction was seen mainly in individuals without established CVD risk factors.43 However, there were differences in risk factors between cohorts, and prevalence of specific diseases has changed over time. The NHANES cohort is more contemporaneous, but fewer data are available to compare with the hemophilia cohort, and risk factors also differ between the populations.
Another limitation is potential bias in subject selection. Each HTC was to approach all patients who met inclusion criteria at their HTC. However, patients with more medical problems may visit the HTC more frequently and be available for study. In addition, our population was limited to those seen in HTCs, which is estimated to represent 70% to 80% of the overall hemophilia population in the United States. Both these points could potentially bias our cohort to those with more comorbidities that might result in overestimation of CVD in the population.
As noted earlier, a prospective study to further investigate the significantly increased rate of renal dysfunction noted in our hemophilia population, and to follow this cohort as they continue to age, is underway. In addition, more data are needed to determine the role of inhibitors as a potential risk factor for CVD. Given the prevalence of CVD risk factors in men with hemophilia and low rates of hypertension control and aspirin use, efforts to optimize care are needed. Patients with hemophilia who present with acute coronary syndromes often receive nonstandard care, which may negatively affect outcomes.41,44
In summary, although patients with moderate and severe hemophilia appear to have some protection from cardiovascular thrombotic events compared with the general population, cardiovascular risk factors are common, including an increased prevalence of hypertension. Thus, on an individual-patient level, optimal regimens for cardioprevention are warranted. For example, only 28% of our patients with dyslipidemia were receiving statins, medications that are not associated with increased bleeding. A meta-analysis of 27 randomized trials of pravastatin therapy in patients at low risk for vascular disease found that for every 40 mg/dL decrease in LDL cholesterol, there was a 20% to 25% decrease in major cardiovascular events. Thus, given the occurrence of CVD in men with hemophilia, and the challenges in its treatment in this population, measures directed at screening for and managing cardiovascular risk factors and optimizing management of CVD in this population are needed.
Acknowledgments
The authors thank the research coordinators at each site: Natasia Weiss and Jacqueline Buckley (Indiana Hemophilia and Thrombosis Center), Helena Jacobs (Georgetown University), Rebecca Hauke (University of Michigan), Gail Long (Hemophilia Center of Central Pennsylvania/Penn State Milton S. Hershey Medical Center), Stephanie Whitten (Emory University Comprehensive Hemophilia Program), Manny Mangilit (Hemophilia Treatment Center, Orthopaedic Hospital), Mary Kelty (Penn Comprehensive Hemophilia and Thrombosis Program/Hospital of the University of Pennsylvania), Jacqueline Washington and Judith Kadosh (Hemophilia Center of Western Pennsylvania), Deborah Bennett (Mary M. Gooley Hemophilia Center, Inc./Rochester General Hospital), Carol deKernion (Louisiana Comprehensive Hemophilia Care Center/Tulane University Health Science Center), Robin Kellerman (Hemophilia and Thrombosis Center/University of North Carolina), Karen Stephany (Blood Center of Wisconsin), Molly Brown and Kristi Norton (Hemophilia and Thrombosis Center/University of Colorado), Madeline Cantini and Krishna Cannon (Gulf States Hemophilia and Thrombophilia Center), Ilana Levin (Hemophilia Treatment Center, University of California, San Diego), Mary Jones (The Vanderbilt Hemostasis/Hemophilia Clinic), Melanie Heinlein (The Ohio State University Medical Center Hemophilia Treatment Center), and Linda Mueller and Nancy Sullivan (Henry Ford Hemophilia and Thrombosis Treatment Center)
The authors acknowledge research funding from the Centers for Disease Control (B.A.K. and the American Thrombosis and Hemostasis Network) and from the US Department of Health and Human Services (H30MC24049) for use of Washington Center for Bleeding Disorders 340B program income for research (B.A.K.).
Authorship
Contribution: S.L.S. and B.A.K. devised the study and received funding; D.C. and C.W. further modified the study and data collection; D.C. provided statistical analysis; M.R., C.M.K., D.Q., A.D.S., N.S.K., M.J.M.-J., A.C., C.K., T.-F.W., M.E.E., P.K., A.v.D., J.C.G., A.W., P.K., M.A.E., C.L., and S.G. gave input into study design, collected data, and gave input into the manuscript; and M.C. analyzed ECGs and gave input into the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara A. Konkle, Bloodworks Northwest, 921 Terry Ave, Seattle WA 98104; e-mail: barbarak@bloodworksnw.org.