Key Points
We found no significant impact of any ΔIKZF1 on survival outcomes among patients aged 23 to 65 years with ALL in the UKALL14 trial.
The prognostic impact of ΔIKZF1 on adult ALL remains to be determined in large harmonized data sets.
Abstract
IKZF1 deletions (ΔIKZF1) are commonly detected in B-precursor acute lymphoblastic leukemia (ALL; B-ALL) and are widely assumed to have a significant impact on outcome. We compared the ability of multiplex ligand-dependent probe amplification (MLPA) and polymerase chain reaction (PCR) to detect ΔIKZF1 and to determine the impact on event-free survival of patients with precursor B-ALL aged 23 to 65 years recruited to the completed trial UKALL14 (ISRCTN 66541317). From 655 recruits with BCR-ABL1+ and BCR-ABL1− B-ALL, all available diagnostic DNA samples (76% of the recruited population) were screened by multiplex end point PCR covering 4 deletions: dominant-negative (DN) Δ4-7 or the loss of function Δ2-7, Δ4-8, and Δ2-8 (n = 498), MLPA (n = 436), or by both (n = 420). Although patients with BCR-ABL1− ΔIKZF1 were more likely to have minimal residual disease at the end of induction, we did not find any impact of ΔIKZF1 (including subgroup analysis for DN or loss-of-function lesions) or the IKZF1plus genotype on event-free, overall survival, or relapse risk by univariable or multivariable analyses. Consistent with the technical approach, MLPA not only detected a wider range of deletions than PCR but also failed to detect some PCR-detected lesions. The main difference between our study and others reporting an association between ΔIKZF1 and outcome is the older age of participants in our population. The impact of ΔIKZF1 in ALL may be less marked in an older population of patients. Our study underscores the need for analyses in large, harmonized data sets. This trial was registered at www.clinicaltrials.gov as #NCT01085617.
Introduction
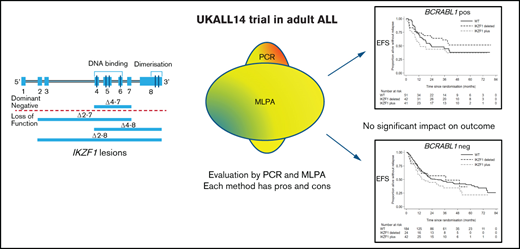
In B-precursor acute lymphoblastic leukemia (ALL; B-ALL), deletions of the transcription factor Ikaros, encoded by IKZF1, oc cur in up to 70% of BCR-ABL1+ and 30% of BCR-ABL1− cases and have been associated with a higher risk of primary treatment failure in both pediatric and adult disease.1-3 Despite its potential clinical utility, the optimal approach for defining IKZF1 status in a clinical setting also remains uncertain. In normal B-cell ontogeny, IKZF1 is a crucial protein for development and differentiation. Alternative splicing generates normal Ikaros isoforms, which differ in their subcellular localization and functional properties, depending on which part of the protein is expressed. IKZF1 deletion (ΔIKZF1) lesions, mediated by RAG enzyme complex–mediated cleavage at the heptamer sequence,1 result in several different types of deletions. Those which delete exons 4 to 7 (ΔIKZF1 4-7) lead to short, nonfunctional, cytoplasmically retained isoforms that do not bind DNA. However, in retaining the C-terminal zinc finger domains, they can still dimerize with residual normal isoforms, suppressing function of the normal Ikaros protein expressed from the nondeleted allele, the so-called “dominant-negative” (DN) effect.4-6 Other deletions lead to loss of function (LOF). There is some evidence of differential impact of LOF vs DN lesions on prognosis in ALL.7,8
In the present study, we evaluated diagnostic specimens from all patients with available DNA who were enrolled in the UK National Cancer Research Institute trial for adults with de novo B-ALL (Standard Chemotherapy With or Without Nelarabine or Rituximab in Treating Patients With Newly Diagnosed Acute Lymphoblastic Leukemia [UKALL14 (ISRCTN 66541317)]). We sought to determine (1) the relationship between IKZF1 lesions and survival outcomes and (2) the optimal method to ascertain IKZF1 status in a clinical context by comparing a multiplex polymerase chain reaction (PCR) assay with standard multiplex ligand-dependent probe amplification (MLPA).
Methods
Patients
Between December 2010 and July 2018, all patients were enrolled in the UKALL14 study, a multicenter phase 3 clinical trial that recruited patients aged 25 to 65 years with de novo BCR-ABL1− ALL and patients aged 23 to 65 years with BCR-ABL1+ ALL. All patients gave written informed consent to trial treatment and correlative science studies. The study was conducted in accordance with the Declaration of Helsinki. Approval was given by the UK Research Ethics Committee (16/LO/2055). Wherever possible, cytogenetic subgroup was determined locally by conventional cytogenetics and fluorescence in situ hybridization, and results were collated and centrally reviewed by A.V.M. at the Leukaemia Research Cytogenetics Group (Newcastle University). In the case of BCR-ABL1, the breakpoint was confirmed by PCR.
UKALL14 therapy
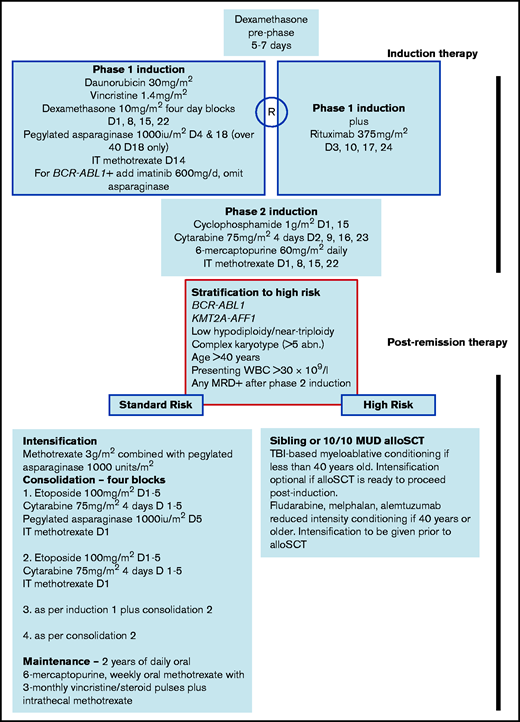
Summary details of UKALL14 treatment are provided in Figure 1. In summary, all patients underwent a 2-phase induction, followed by stratification to continuing chemotherapy or allogeneic stem cell transplant (alloSCT) based on a risk assessment. “High risk” was defined by the presence of BCR-ABL1, KMT2A-AFF1, low hypodiploidy/near triploidy, or complex karyotype; presenting white blood cell count (WBC) >30 × 109/L; any level of minimal residual disease (MRD) at the end of the second phase of induction; and age ≥41 years. Patients with any of these features were assigned to alloSCT if they were fit and had a 10 of 10 antigen-matched sibling or unrelated donor. Conditioning was myeloablative for patients aged up to 41 years. The safety and efficacy of nonmyeloablative alloSCT was evaluated as a trial question for patients 41 years of age and older. Patients with B-ALL were randomized between standard of care or standard or care plus 4 doses of rituximab given during the first phase of induction.
Diagnostic sample processing
All diagnostic samples were processed by the UK Adult ALL MRD laboratory at the UCL Cancer Institute. Bone marrow samples were layered onto Lymphosep separation (MP Biochemicals Europe) and spun at 1600 rpm for 30 minutes at room temperature to isolate ALL blasts. Where possible, DNA extraction was performed on at least 1 × 107 cells using the DNeasy mini kit (Qiagen) and diluted to a concentration between 50 and 150 ng/μL using AE buffer (Qiagen). Concentration and purity were checked by measuring absorbance at 260 nm and 280 nm using a Nanodrop spectrophotometer (Thermo Scientific). Final DNA concentration and quality were assessed by TaqMan (Thermo Scientific) real-time quantitative PCR amplification of the albumin housekeeping gene, and compared with a standard curve derived from human genomic DNA (Bioline).
MLPA and IKZF1plus definition
MLPA assays were prepared and analyzed at the Leukaemia Research Cytogenetics Group, Newcastle University, and were performed on DNA extracted from the diagnostic sample using the SALSA MLPA P335 kit (ALL-IKZF1, versions B1, B2, and C1) and the P327 kit (iAMP21-ERG, version B2) (MRC Holland, The Netherlands), as previously described.9 Analysis was carried out with GeneMarker V1.85 analysis software (SoftGenetics) and Coffalyser (MRC Holland). IKZF1plus was defined as per standard: ΔIKZF1 with concomitant CDKN2A/B, PAX5, or PAR1 region deletion, in the absence of an ERG deletion. Among 170 cases with ΔIKZF1, 93 had concomitant deletions of the CDKN2A/B, PAX5, or PAR1 region. ERG deletions are synonymous with IGH-DUX4 fusion and are extremely rare in other genetic subgroups. Among these 93 cases, 68 had an established primary genetic abnormality (BCR-ABL1, n = 49; JAK-STAT, n = 14; ZNF384 fusion, n = 1; KMT2A fusion, n = 1; TCF3-PBX1, n = 1; ABL class, n = 1; low hypodiploid, n = 1) and were assumed not to harbor an ERG deletion. Among the remaining 25 cases, 18 were tested by MLPA (none had an ERG deletion) and 7 were not tested for ERG due to lack of material. Thus, the final cohort of IKZF1plus cases comprised 93 cases including 7 cases not specifically tested for ERG.
PCR
Diagnostic DNA specimens were screened for the 4 most common IKZF1 intergenic deletions: Δ4-7, Δ2-7, Δ4-8, and Δ2-8 by end point multiplex PCR as described by Caye et al10 using a DYAD thermocycler. Albumin amplification served as internal control (details available in supplemental Methods). DNA from the IKZF1-deleted cell line SUP-B15 served as positive control. All PCR products were analyzed on a 10% polyacrylamide gel, purified using Illustra ExoProStar (GE Healthcare), and subjected to Sanger sequencing by Eurofins Genomics to determine the breakpoints. Sequence was analyzed using Seqman Lasergene software (DNASTAR). The lower limit of detection of the PCR assay was 10−4 as determined by serial 10-fold dilutions of the Δ4-7 positive cell line SUP-B15.
MRD analysis
MRD analysis done in a single, central EuroMRD-accredited laboratory detected and quantified patient-specific immunoglobulin and T-cell receptor (TCR) gene rearrangements by real-time quantitative PCR for all those with BCR-ABL1− ALL or BCR-ABL1 transcripts for those with BCR-ABL1+ ALL. MRD analysis was performed and interpreted strictly according to EuroMRD guidelines.11 Within the trial protocol, all of those with missing MRD data or results that were classified as “positive outside of the quantitative range” (POQR) or “indeterminate” were assumed as negative for the purposes of treatment assignment.
Statistical analysis
Event-free survival (EFS) and overall survival (OS) times were calculated from the date of randomization until the date of the first event (relapse and death for EFS or death for OS) with patients not experiencing an event censored at the date last seen. Time to relapse was calculated, including only those who achieved remission after induction, as the time from first remission until relapse. Competing risks analysis by the method of Fine and Gray was used, with death in first remission treated as a competing risk. Comparisons between groups were made using χ2 or Fisher exact tests (discrete variables), Student t tests or Wilcoxon Mann-Whitney tests (continuous variables), or Cox regression and the log-rank test (time-to-event variables).
The assumption of proportional hazards was tested using the Schoenfeld residuals. Analyses were performed using STATA version 15.1 (STATACorp).
Results
Frequency and subclassification of ΔIKZF1 lesions
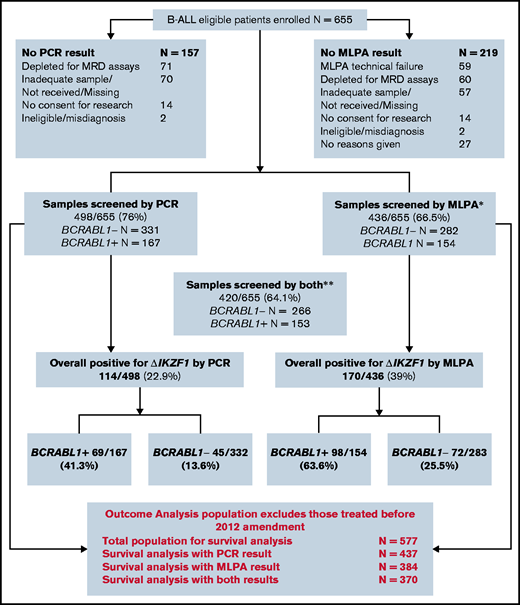
Of 655 eligible patients enrolled into the B-cell arm of UKALL14, 498 had sufficient diagnostic DNA for IKZF1 screening by PCR (N = 498), MLPA (N = 436), or both (N = 420). The baseline characteristics of the patients screened by each technique compared with those who could not be screened are shown in Table 1. The baseline characteristics of the overall population are also given. The P values show a comparison between those who could and could not be screened for each technique. There was a modest but statistically significantly higher presenting WBC in the screened population compared with the nonscreened population for both MLPA and PCR, reflecting the greater availability of stored material for experimental analyses. There were also more likely to be samples available in BCR-ABL1+ ALL. A consort diagram representing the ΔIKZF1 status of all enrolled patients is shown in Figure 2.
Consort diagram showing derivation of specimens from the total patient population. *N = 59 additional samples that were subjected to MLPA and failed quality control. **A small number of samples underwent MLPA but not PCR.
Consort diagram showing derivation of specimens from the total patient population. *N = 59 additional samples that were subjected to MLPA and failed quality control. **A small number of samples underwent MLPA but not PCR.
The large number of samples screened by both PCR and MLPA allowed a robust comparison between methods. Table 2 summarizes the specific lesions detected and shows the concordance between PCR and MLPA. Overall, there was disagreement between the methods in 87 of 420 cases. PCR identified ΔIKZF1 in 14 patients in whom MLPA did not detect a deletion and MLPA identified ΔIKZF1 in 73 patients in whom PCR did not detect a deletion. Fifty-three ΔIKZF1 lesions (in 52 patients) were identified by MLPA but not PCR: 1-2 (N = 8), 1-3 (N = 3), 1-7 (N = 3), 1-8 (N = 34), 2-3 (N = 4) and 6-8 (N = 1); these lesions were “expectedly discordant” as the PCR assay was not designed to identify them. PCR detected 21 lesions not seen by MLPA, likely due to the higher sensitivity of the technique.
The relationship between baseline characteristics and the various ΔIKZF1 lesions as determined by both PCR and MLPA is shown in Table 3. Patients with ΔIKZF1 lesions were slightly but significantly older than those without and had significantly higher presenting WBCs. As expected, ΔIKZF1 lesions were much more common in BCR-ABL1+ ALL than BCR-ABL1− ALL, whereas there was only 1 case detected in each of KMT2A-AFF1 and low hypodiploid/near-triploid ALL.
Population analyzed for survival outcomes
Survival analyses were confined to those patients treated after an April 2012 protocol amendment that was made because of excess toxicity in the BCR-ABL1+ cohort.12 Hence, the relationship between ΔIKZF1 and MRD and survival outcomes was analyzed for those recruited after that amendment. The outcome analysis population with available ΔIKZF1 screens differed from the from the non-ΔIKZF1 screened population in exactly the same way as demonstrated for the overall population, as shown in supplemental Table 1. Outcomes for the PCR cohort are based on 437 patients; for the MLPA cohort, 384 patients; and for those tested by both methods, 370 patients (as per Figure 2). The 3-year OS of this overall population, at a median follow-up of 50 months, is 53.1% (49.1% to 57.1%), and is 54.3% (49.3% to 59.0%) for the PCR-screened group, and 55.3% (50.0% to 60.3%) for the MLPA-screened group. The corresponding EFS was 46.1% (42.1% to 50.0%), 46.4% (41.4% to 51.2%), and 47.0% (41.7% to 52.1%). Cumulative incidence of relapse (CIR) was 29.0% (25.4% to 32.9%), 31.3% (26.8% to 36.2%) for the PCR-screened group, and 30.2% (25.6% to 35.4%) for the MLPA-screened group (Kaplan-Meier survival curves illustrating these data are shown in supplemental Figure 1).
Relationship between IKZF1 status and MRD
The relationship between IKZF1 status and MRD levels is shown in Table 4. Patients with MRD values POQR or indeterminate were grouped with “negative” in order to follow the treatment stratification used in the trial. An end-of-induction MRD result was a prerequisite for inclusion in this analysis. Overall, those with ΔIKZF1 were significantly more likely to have a positive MRD result. When the outcomes for BCR-ABL1− and BCR-ABL1+ ALL were analyzed separately, a significant difference in the rate of MRD positivity in the presence of ΔIKZF1 was only evident for patients with BCR-ABL1− ALL. By PCR, only 23% of IKZF1 wild type (WT) had residual MRD vs 48.6% of those with any ΔIKZF1 lesion (P = .002). One-half of patients with LOF lesions (50%) and 47.4% of patients with DN lesions were MRD+: both types of lesion were significantly more likely to be associated with residual MRD than IKZF1 WT, P = .019 and P = .023, respectively. By MLPA, similar differences were seen in all ΔIKZF1 lesions: 23.8% of IKZF1 WT patients had residual MRD vs 47.6% with ΔIKZF1 (P = .021). The IKZF1plus genotype had higher MRD positivity rates than WT (33.3%) although this was not significant (P = .26). As assessed by MLPA, greater numbers of patients with either DN or LOF lesions were MRD+ but the differences did not reach statistical significance.
Impact of IKZF1 status on EFS, OS, and relapse risk
Next, we determined the impact of IKZF1 status on survival and relapse outcomes. There was no evidence of any impact on EFS of ΔIKZF1 detected by PCR (hazard ratio [HR], 0.93 [0.69-1.26]; P = .64) or when ΔIKZF1 was detected by MLPA (HR, 1.07 [0.81-1.40]; P = .63). Although a different effect was again seen between BCR-ABL1− and BCR-ABL1+ ALL, neither the difference nor the interaction were statistically significant. Kaplan-Meier curves for EFS and OS in the presence or absence of ΔIKZF1 or IKZF1plus for BCR-ABL1− and BCR-ABL1+ ALL are shown in supplemental Figure 2.
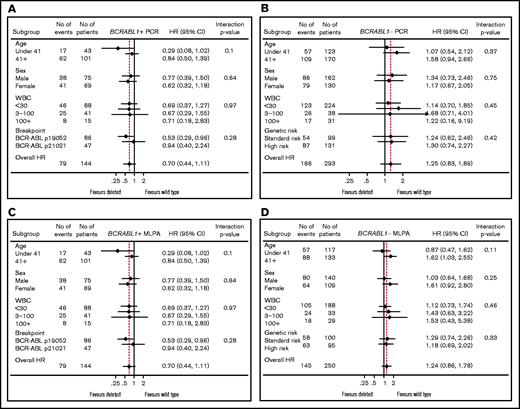
All subsequent analyses were performed within BCR-ABL1− and BCR-ABL1+ cohorts separately. Table 5 shows univariable and multivariable EFS and OS HRs for the effect of PCR ΔIKZF1 deletion, including deletion type, that is, DN or LOF IKZF1 lesions, split by BCR-ABL1 status. In all analyses, there was no evidence of an increased risk of an event for patients with any type of deletion. Due to a significant interaction between IKZF1 and the BCR-ABL1 breakpoint, an interaction term was also included in the multivariable model for BCR-ABL1+ ALL, with HRs presented for each breakpoint. Here, significant results were seen for the p190 breakpoint, although paradoxically, they appear to show a protective effect of ΔIKZF1 deletion. The MLPA analysis also allowed evaluation of the IKZF1plus profile. Here, patients were analyzed in 3 groups: no deletion (reference group), ΔIKZF1 deleted but without the IKZF1plus profile, and IKZF1plus. Results for EFS and OS in this group are shown in supplemental Table 2. Again, no significant negative effects were seen within the whole population or within BCR-ABL1− cases. There was 1 borderline significant result for p210 breakpoint DN BCR-ABL1+ ALL, but this was based on just 6 events in 11 patients. All multivariable analyses were rerun, including interactions between ΔIKZF1 and randomized treatment arms (rituximab plus standard of care or standard of care alone), all showing no evidence that treatment with rituximab had modified the effect of ΔIKZF1. Similarly, there was no impact of any ΔIKZF1, any subtype of ΔIKZF1, or IKZF1plus on relapse risk in univariable or multivariable analyses (supplemental Table 3) including a model also adjusted for end-of-induction MRD. To ensure that we had not introduced bias by excluding the patients with the worst outcome who did not achieve remission at all, the EFS analysis was repeated, including primary refractory disease as an event. Even in this case, ΔIKZF1 detected by either method did not have a statistically significant relationship with outcome (data not shown).
Subgroup analyses
Forest plots for EFS for both PCR- and MLPA-determined IKZF1 status are shown in Figure 3. The plots show the impact of IKZF1 status within key variables for BCR-ABL1+ and BCR-ABL1− ALL, separately. There are no subgroups that showed a statistically significant negative impact of IKZF1 status. The same was seen for OS (data not shown).
Forest plots. Shown is the relationship between major variables and BCR-ABL1+ ALL PCR-determined IKZF1 status (A), BCR-ABL1− ALL PCR-determined IKZF1 status (B), BCR-ABL1+ ALL MLPA-determined IKZF1 status (C), and BCR-ABL1− ALL MLPA-determined IKZF1 status (D).
Forest plots. Shown is the relationship between major variables and BCR-ABL1+ ALL PCR-determined IKZF1 status (A), BCR-ABL1− ALL PCR-determined IKZF1 status (B), BCR-ABL1+ ALL MLPA-determined IKZF1 status (C), and BCR-ABL1− ALL MLPA-determined IKZF1 status (D).
Discussion
We screened more than two-thirds of our trial population by >1 method to detect ΔIKZF1. MLPA expectedly detects a larger range of lesions than the PCR approach. However, our data show that the failure rate of MLPA is considerably higher than for PCR. MLPA takes longer to carry out and needs a greater degree of skill to interpret correctly: gray areas where interpretation is unclear are not uncommon. On the other hand, the information obtained is greater because the full spectrum of IKZF1 deletions and copy-number alterations affecting other genes can be assessed. PCR is rapid and reliable, as well as being more sensitive than MLPA, but risks missing ΔIKZF1 lesions that have deleted the PCR primer sites. Fifty-three ΔIKZF1 lesions were identified by MLPA but not PCR. We believe this finding is worthy of note because it is typical for one technique to be chosen for both trial and clinical screening purposes. Our data demonstrate that neither technique can be considered a “gold standard” for IKZF1 deletion detection.
We did not detect any relationship between ΔIKZF1 and survival outcomes, which is interesting and perhaps somewhat surprising. Our prior work from the UKALL12 study did not demonstrate a relationship between ΔIKZF1 in multivariable analysis,3 raising the question of whether specific element(s) particular to our therapeutic approach in the United Kingdom negate the relationship.
Because we assessed a larger population of both BCR-ABL1+ and BCR-ABL1− ALL than many prior studies, we think it unlikely that our findings stem from having insufficient power to detect an impact of ΔIKZF1 on outcome, but rather that the effect sizes seen in our cohort are genuinely much smaller: in multivariable analysis for BCR-ABL1− ALL, the EFS HR (compared with no deletion) was 1.23 for ΔIKZF1 by PCR and 1.33 for IKZF1plus, with HRs for time to relapse of just 1.03 and 0.87, respectively.
We did note a significant relationship between ΔIKZF1 and early treatment response, namely MRD at the end of induction, but in patients with BCR-ABL1− ALL only, and this did not translate into any survival difference. This finding stands in sharp contrast to observations in childhood BCR-ABL1+ ALL where there was a highly unfavorable outcome in the presence of IKZF1 deletions.13 Even the relationship we found between MRD and ΔIKZF1 is distinct from that in the DCOG-ALL10 pediatric cohort,14 where IKZF1 deletion remained predictive for inferior outcome in DCOG-ALL10 cases treated even in the medium-risk arm, indicating that the prognostic value of ΔIKZF1 was independent of the early treatment response as monitored by MRD.
In an adult population, data from the GRAALL studies reported by Beldjord and colleagues15 reported an HR of 2.65 (95% confidence interval, 1.48-4.73; P = .001) for CIR 260 patients with BCR-ABL1− ALL with a median age of 34 years (range, 15-59 years). Indeed, MRD and ΔIKZF1 status were the only factors that impacted CIR.
As a result of our finding being at variance with one of the key studies in the field, we speculated that the lack of impact of ΔIKZF1 on EFS may have stemmed from the assignment to the “high-risk” treatment stratification and resultant receipt of alloSCT; thus, it was important to note that among both PCR- and MLPA-determined IKZF1 WT there was no significant difference in the number of patients receiving alloSCT than in the ΔIKZF1 population (61% vs 70% and 60% vs 61%, respectively). The transplant rates did not differ between IKZF1 WT and ΔIKZF1 when analyzed separately by BCR-ABL1 status. For completeness, we also ensured there were no differences between the groups in those assigned to transplant but relapsing or dying before receiving it, and we performed an analysis of time to relapse censoring for alloSCT in first complete remission. Also, as described, we did not find any impact by randomized assignment to the rituximab treatment arm. It is noteworthy that this trial had a much higher rate of alloSCT than would be typical because the role of reduced-intensity conditioned alloSCT in those over 40 years of age was one of the main trial questions.
Having found no relationship between ΔIKZF1 and survival outcomes, we nonetheless expect that we may see some relationship with outcome within specific subgroups. Kobitzsch and colleagues characterized 482 cases from adults aged 16 to 65 years with BCR-ABL− ALL who participated in GMALL studies. Those with LOF lesions had a worse outcome, suggesting a clear distinction between LOF and DN ΔIKZF1.7 We did not find any such relationship, nor did we find a relationship between the IKZF1plus profile as described by Stanulla and colleagues in childhood ALL.16 Similarly, our data are distinct from the GIMEMA study by Fedullo and colleagues wherein ΔIKZF1, alongside CDKN2A/B and/or PAX5, led to significantly lower disease-free survival (24.9% vs 43.3%; P = .026) as well as DN IKZF1 lesions independently impacting outcome.8
The most obvious difference between all of the prior reports described herein and our own is the age of the patients. With a median age of 46 years and a lower age limit of 23 years, our study enrolled the largest number and proportion of older patients in whom the impact of ΔIKZF1 has been reported to date, although we saw no suggestion of worse outcomes within patients aged ≤41 years. There were very few young patients in our study as patients below the age of 25 years with BCR-ABL1− ALL are recruited to the pediatric study in the United Kingdom. As such, we suggest that the hypothesis that the adverse impact of ΔIKZF1 may be age-related should be tested in large, combined data sets that include greater numbers of young adults than our study.
In conclusion, our study, carefully conducted within a uniformly treated trial population, demonstrates clearly that ΔIKZF1 is not necessarily a universal biomarker of poor outcome in adult ALL. We are currently contributing data to an EWALL-generated HARMONY study that will investigate the prognostic effect of ΔIKZF1 in at least 10 European ALL studies to determine whether difference in effect relates to age, treatment, or background genetics.
Acknowledgments
The authors thank UK National Health Service trial sites and patients for samples.
This work was supported by Cancer Research UK (CRUK) grant C27995/A9609 for UKALL14 (A.K.F.), a Blood Cancer UK Gordon Piller Studentship (R.J.M.), and CRUK program grant C27995/A21019 (A.K.F. and A.V.M.). UKALL14 was coordinated by the CRUK and University College London Cancer Trials Centre, and supported by the Comprehensive Local Research Network (CLRN).
Authorship
Contribution: R.J.M., E.B., and N.Z. performed IKZK1 analyses by PCR and MLPA; R.J.M. performed additional experiments; S.L. and K.Z.A. performed MRD analyses; L.C.-H., E.L., and P.P. coordinated the UKALL14 trial and gathered and helped to interpret data; A.A.K. performed statistical analyses; D.I.M., C.J.R., T.F.M., A.K.M., and B.P. managed the trial and performed clinical interpretation of the data; E.P., D.L., and B.P. designed and interpreted laboratory studies; R.J.M., A.K.F., and A.V.M. wrote the paper; A.K.F. and A.V.M. conceived and designed the study and obtained funding; and all authors contributed to manuscript writing and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adele K. Fielding, UCL Cancer Institute, University College London, 72 Huntley St, London, WC1E 6DD United Kingdom; e-mail: a.fielding@ucl.ac.uk.
References
Author notes
A.V.M. and A.K.F. contributed equally.
Please contact the corresponding author with any requests for data sharing at a.fielding@ucl.ac.uk.
The full-text version of this article contains a data supplement.