TO THE EDITOR:
The European Leukemia Net (ELN) genetic risk classifications for acute myeloid leukemia (AML) guide prognosis and considerations for allogeneic hematopoietic cell transplantation. The 2017 and 2022 ELN risk stratifications were primarily developed based on patient outcomes from intensive chemotherapy-based approaches.1,2 Given the development of less-intensive therapeutic strategies, such as hypomethylating agents (HMA) combined with the oral B-cell lymphoma 2 (BCL2) inhibitor venetoclax (VEN) and small molecule inhibitors, the ELN 2024 criteria and its refined version were introduced to more accurately prognosticate and risk-stratify outcomes for this subset of patients.3-5 While accelerated-phase (AP) or blast-phase (BP) myeloproliferative neoplasms (MPNs) are frequently treated with AML-based therapies, they exhibit a distinct molecular profile compared to de novo AML.6,7 In addition, survival outcomes in MPN-AP/BP remain limited even in the current era of myeloid therapies, with no significant difference between intensive and less-intensive therapies.8 Therefore, we aimed to evaluate the various iterations of the ELN AML risk classification criteria in a cohort of patients with MPN-AP/BP who were treated with less-intensive therapy.
Our study comprises 211 adult patients diagnosed with MPN-AP/BP in 2017 or later from 9 academic centers. It also includes patients from a previously published cohort with additional follow-up.8 We included both patients with MPN-AP and those with MPN-BP, given the high-risk nature of the disease once blasts are ≥10%.9,10 Of these, 126 patients underwent next-generation sequencing of a targeted gene panel at diagnosis and received less-intensive therapy as their first-line treatment for MPN-AP/BP. Less-intensive therapy was defined as HMA-based therapy, HMA + VEN-based therapy, or targeted therapy with or without HMA. The population was predominantly male (61%), with a median age of 71 (range, 33-94) years. During chronic phase, patients received hydroxyurea (n = 79), JAK inhibitors (n = 44), HMA (n = 7), and/or interferon (n = 2). The median time to progression to AP or BP was 54.3 months. There were 81 patients diagnosed with MPN-BP (blast count of ≥20% within the peripheral blood or bone marrow) and 45 diagnosed with MPN-AP (blast count of 10%-19% in the peripheral blood or bone marrow) with no observed difference in overall survival (OS) (P = .49). The most common first-line treatment regimens were HMA + VEN (n = 55), HMA monotherapy (n = 36), HMA + JAK inhibitor (n = 16), and isocitrate dehydrogenase (IDH)1/2 inhibitors (n = 7). Additionally, 33 patients (26.4%) subsequently underwent allogeneic hematopoietic cell transplantation.
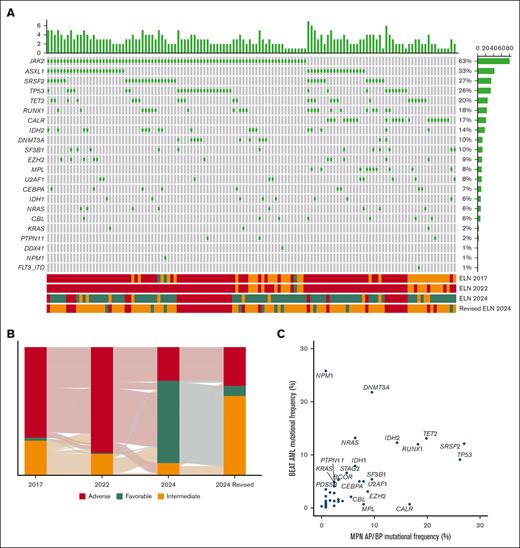
Sixty-three percent of patients had a JAK2 mutation, 17% had a CALR mutation, 8% had an MPL mutation, and 11% were triple-negative (Figure 1A). Following JAK2, the most frequently mutated genes included ASXL1 (33%), SRSF2 (27%), and TP53 (26%) (Figure 1A). The prior AML risk criteria, along with the potential inclusion of patients with MPN-BP within these criteria, are described in supplemental Table 1.1-4 The ELN 2017 and 2022 criteria classified patients similarly, with a high percentage assigned to the intermediate and adverse risk categories, and few in the favorable-risk group (Figure 1B). The ELN 2024 criteria assigned the most patients to the favorable-risk group (n = 81), while the refined ELN 2024 criteria identified the largest proportion of intermediate-risk patients (n = 78) (Figure 1B). In contrast, the ELN 2022 criteria placed most patients into the adverse risk group (n = 104) (Figure 1B). The ELN 2024 criteria distributed patients most evenly among the risk groups (Figure 1B). Adverse risk mutations, including ASXL1, TP53, and SRSF2, were significantly higher in MPN-AP/BP compared to a previously published cohort of de novo AML (Figure 1C).11 Furthermore, mutations associated with favorable-risk disease, such as NPM1, were notably less common in MPN-AP/BP (0.7% vs 25.8%) (Figure 1C).11
Mutational and ELN risk profile of patients with MPN-AP/BP. (A) Oncoprint shows an integrated annotation of mutations and ELN risk categories. Patients are organized in columns with genes labeled along the rows. Bar plots illustrate the mutations per patient (top) and the frequency of mutations in the cohort (right). ELN risk classifications are color-coded (bottom). The green square marks a mutated gene. (B) The Sankey plot displays the changes in risk assignment across the 2017, 2022, 2024, and refined 2024 ELN criteria. (C) Dot plot representation of the mutation frequency in the BEAT AML cohort of patients and MPN AP/BP patients. Overlapping sequenced genes are depicted.
Mutational and ELN risk profile of patients with MPN-AP/BP. (A) Oncoprint shows an integrated annotation of mutations and ELN risk categories. Patients are organized in columns with genes labeled along the rows. Bar plots illustrate the mutations per patient (top) and the frequency of mutations in the cohort (right). ELN risk classifications are color-coded (bottom). The green square marks a mutated gene. (B) The Sankey plot displays the changes in risk assignment across the 2017, 2022, 2024, and refined 2024 ELN criteria. (C) Dot plot representation of the mutation frequency in the BEAT AML cohort of patients and MPN AP/BP patients. Overlapping sequenced genes are depicted.
Due to the heterogeneous nature of this real-world patient cohort, response rates were assessed at various time points following the initiation of therapy. The best response to initial treatment was utilized for analysis to account for this variability. Complete response or complete response with incomplete hematologic recovery (CR/CRi) according to ELN response criteria was achieved in 30.1% of patients (n = 38). Within the adverse risk groups, no difference in the rates of CR/CRi was observed across different criteria (P = .85); CR/CRi was achieved in 28% of adverse risk patients using ELN 2017, 30% using ELN 2022, 33% using ELN 2024%, and 34% using the refined ELN 2024 criteria (supplemental Figure 1). Similarly, in the intermediate-risk group, no significant differences in CR/CRi rates were noted (P = .58), with CR/CRi achieved in 38% of patients under the 2017 criteria, 33% under the ELN 2022, 33% under the 2024 criteria, and 27% under the refined 2024 criteria (supplemental Figure 1). However, in the favorable-risk group, where patient numbers were limited, CR/CRi rates showed significant differences (P < .0001). No patients in the favorable-risk cohort met CR/CRi criteria under the ELN 2017 or 2022 classifications (supplemental Figure 1). In contrast, CR/CRi was achieved in 28% of patients under the ELN 2024 criteria and 40% under the refined ELN 2024 criteria (supplemental Figure 1). Multivariate logistic regression indicated significantly higher rates of CR/CRi in patients with IDH1 mutations (odds ratio, 0.16; 95% confidence interval (CI), 0.03-0.9; P = .04) despite no patient receiving an IDH1 inhibitor as frontline therapy (supplemental Figure 2). No gene was significantly associated with failure to achieve CR/CRi (supplemental Figure 2).
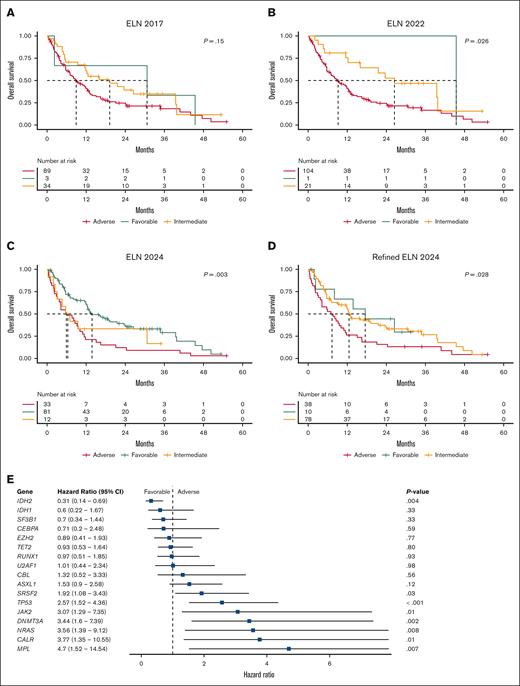
OS was analyzed using Kaplan-Meier methodology. The reverse Kaplan-Meier median follow-up was 31.5 months, and the median OS was 11.5 months (95% CI, 8.1-13.2). For the ELN 2017 classifications, the median OS was 30.7, 19.2, and 8.9 months for favorable, intermediate, and adverse risk groups, respectively (Figure 2A). According to the ELN 2022 criteria, the median OS was 45.4, 26.5, and 9.2 months, respectively (Figure 2B). Utilizing the ELN 2024 criteria, the median OS was 13.8, 6.4, and 5.9 months, respectively (Figure 2C). Finally, the refined ELN 2024 criteria yielded a median OS of 17.5, 12.6, and 7.3 months across the favorable, intermediate, and adverse risk groups, respectively (Figure 2D). Among the models, the ELN 2024 criteria demonstrated the most robust predictive performance for OS (C-index, 0.60), compared to ELN 2017 (C-index, 0.56), ELN 2022 (C-index, 0.57), and refined ELN 2024 (C-index, 0.58). Multivariate Cox proportional hazard modeling identified patients with an IDH2 mutation to have significantly improved OS (hazard ratio, 0.31; 95% CI, 0.14-0.69; P = .004) (Figure 2E). In contrast, mutations in SRSF2, TP53, JAK2, DNMT3A, NRAS, CALR, and MPL were associated with shorter OS (Figure 2E). However, OS did not differ significantly by the total number of mutations (P = .81). In addition, OS outcomes between patients with a canonical driver mutation and triple-negative MPN-AP/BP were not significantly different, much like what has been reported in previous studies (P = .06) (supplemental Figure 3).12-14
OS and multivariate analysis. Kaplan-Meier OS curves for (A) ELN 2017, (B) ELN 2022, (C) ELN 2024, and (D) refined ELN 2024 risk criteria. The global P value is shown. A vertical tick mark indicates censoring. (E) Forest plot summarizing the results from a multivariate Cox proportional hazards model adjusted for genes mutated at a frequency >5%. The hazard ratio (HR) is represented as a blue square, while the 95% CI is displayed as a horizontal line. An HR of <1 indicates that mutated genes confer a longer OS than the reference group (wildtype). Conversely, an HR of >1 suggests a shorter OS than the reference group.
OS and multivariate analysis. Kaplan-Meier OS curves for (A) ELN 2017, (B) ELN 2022, (C) ELN 2024, and (D) refined ELN 2024 risk criteria. The global P value is shown. A vertical tick mark indicates censoring. (E) Forest plot summarizing the results from a multivariate Cox proportional hazards model adjusted for genes mutated at a frequency >5%. The hazard ratio (HR) is represented as a blue square, while the 95% CI is displayed as a horizontal line. An HR of <1 indicates that mutated genes confer a longer OS than the reference group (wildtype). Conversely, an HR of >1 suggests a shorter OS than the reference group.
Because there are currently no consensus risk stratification criteria for MPN-AP/BP, we aimed to evaluate the performance of various ELN AML risk classification criteria, highlighting their application in risk stratification and prognostication. Among the ELN models, the ELN 2024 criteria demonstrated the strongest predictive accuracy for OS and most evenly balanced the risk groups, surpassing the ELN 2017, ELN 2022, and refined ELN 2024 criteria. Although there was no significant difference in OS between adverse and intermediate risk, the ELN 2024 criteria may be an appropriate tool for risk stratification of patients with MPN-AP/BP receiving less-intensive therapy.
This study highlighted the distinct clinical and genetic profile of patients with MPN-AP/BP, characterized by a high prevalence of adverse risk mutations such as TP53, ASXL1, and SRSF2, along with most patients classified as intermediate or adverse risk according to various ELN criteria. However, the low occurrence of cytogenetic abnormalities, including translocations and inversions, precluded the evaluation of their prognostic significance. A study by Gangat et al15 examined 47 patients with MPN-BP who were uniformly treated with HMA + VEN as initial therapy or upon relapse; multivariate analysis indicated that the absence of complex karyotypes and N/KRAS mutations had a prognostic impact. Notably, patients with MPN-BP were not included in the VIALE-A trial.16 However, considering the uncertain benefit of adding VEN to HMA for this population and that MPN-AP behaves similarly to MPN-BP in terms of clinical progression, developing a comprehensive risk stratification tailored for MPN-AP/BP patients undergoing less-intensive therapy will be crucial.
Each institution received approval from the institutional review board to conduct this retrospective project.
Contribution: A.A.P. and R.S. designed the study plan, performed data analysis, and wrote the manuscript; and all other authors collected data and reviewed/revised the manuscript.
Conflict-of-interest disclosure: A.A.P. received honoraria from AbbVie and Bristol Myers Squibb and institutional research funding from Pfizer and Kronos Bio. R.M.S. received honoraria from Bristol Myers Squibb, Kura Oncology, Gilead Sciences, Rigel, and Servier. E.C.C. received consulting fees from AbbVie and Rigel. S.G.I. received honoraria from Medical Logix (medical education) and reports serving on an advisory board with MorphoSys. R.K.R. received research funding from Incyte, Constellation, Zentalis, Stemline, and Ryyu, as well as consulting fees from Celgene-Bristol Myers Squibb, Kartos, Zentalis, Karyopharm, Dainippon, GlaxoSmithKline-Sierra, Galecto, PharmaEssentia, Incyte, CTI BioPharma, Servier, MorphoSys/Constellation, and Sumitomo. T.B. reports membership on the advisory committee for Novartis, Geron Corporation, and Gilead, as well as participation in the speakers’ bureau for Novartis. Y.A. received research funding from Biomea, Curis, BioSight, ALX Oncology Novartis; and honoraria from Servier, Pfizer, Bristol Myers Squibb, Kite, Astellas, and Rigel. J.S.G. received research funding from AbbVie, Genentech, New Wave, Pfizer, and Prelude; and served on the steering committees and scientific advisory boards of AbbVie, Bristol Myers Squibb, Genentech, and Servier. V.G. received research funding from AbbVie and Novartis; reports consulting fees from Novartis, Bristol Myers Squibb/Celgene, Keros, AbbVie, Constellation Biopharma, Pfizer, GlaxoSmithKline, and CTI BioPharma; reports honoraria from Novartis, Bristol Myers Squibb/Celgene, and AbbVie; and serves on the data safety monitoring or advisory board for Bristol Myers Squibb/Celgene, Roche, AbbVie, Pfizer, GlaxoSmithKline, and CTI BioPharma. K.M.P. reports consulting fees from Protagonist Therapeutics and AbbVie; received research funding from Protagonist Therapeutics, Merck, and AbbVie; and reports speakers bureau membership with Merck. O.O. reports receiving consulting fees from AbbVie, Blueprint Medicines, Bristol Myers Squibb, CTI, Impact Biomedicines, Kymera, Novartis, Servier, Taiho Pharmaceutical, and Treadwell Therapeutics. Additionally, funding for research (to institution) has been received from AbbVie, Agios, Aprea AB, Astex Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, Celgene, CTI BioPharma Corp, Daiichi Sankyo, Incyte, Janssen Oncology, Kartos Therapeutics, Loxo, Novartis, NS Pharma, and OncoTherapy Science. The remaining authors declare no competing financial interests.
Correspondence: Anand A. Patel, Section of Hematology/Oncology, University of Chicago, 5841 S Maryland Ave, MC 2115, Chicago, IL 60637; email: anand.patel@bsd.uchicago.edu.
References
Author notes
Data are available on reasonable request from the corresponding author, Anand A. Patel (anand.patel@bsd.uchicago.edu).
The full-text version of this article contains a data supplement.