Key Points
Compared with the general Swedish population, individuals with CLL had increased risks for severe COVID-19 also after Omicron emerged.
All-cause mortality increased by 55% among individuals with CLL in the capital (Stockholm) at the onset of the pandemic vs the year before.
Visual Abstract
Individuals with chronic lymphocytic leukemia (CLL) face an increased risk for severe COVID-19. This study from Sweden, a country that only had a few mandatory restrictions at the onset of the pandemic, used 10 nationwide registers to compare the risks for severe COVID-19 outcomes of polymerase chain reaction–verified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections through February 2023 in individuals with and those without CLL. From a population of 8 275 839 (6653 CLL) individuals born between 1930 and 2003, 2 088 163 first infections (1289 CLL) were included. The 90-day all-cause mortality rate and adjusted relative risk (aRR; 95% confidence interval) for individuals with CLL vs the general population was 24.8% (1.95; 1.58-2.41) during wild-type, 17.2% (2.38; 1.58-3.57) during Alpha, 4.1% (0.71; 0.24-2.08) during Delta, and 12.6% (1.49; 1.24-1.78) during Omicron infections. Their mortality during Omicron was 0.6% (<65 years), 5.4% (65-74 years), and 19.7% (≥75 years). Small molecule inhibitors (1.56; 1.03-2.37) and corticosteroid usage (1.45; 1.04-2.02) was associated with increased mortality. Next, we analyzed the all-cause mortality in the capital (Stockholm), widely affected by SARS-CoV-2 at the onset of the pandemic. Mortality in individuals with CLL increased by 55% during the first 6 months of 2020 vs 2019, and the age- and sex-aRR by 30 June was 1.53 (1.09-2.15) for individuals with CLL (P = .02) and 1.29 (1.25-1.33) for the general population (P < .001). Collectively, a significantly increased risk for severe COVID-19 and death was observed among individuals with CLL in Sweden, particularly at the onset of the pandemic when few national protective measures were introduced and also after Omicron emerged, emphasizing the need for a more pro-active pandemic strategy for CLL.
Introduction
Individuals with chronic lymphocytic leukemia (CLL) face an increased risk for severe outcomes from infections.1,2 The immune dysfunction caused by the disease and/or its treatments adversely affect the degree of serologic responses to vaccination.1,3-6 When severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged, health care providers anticipated that individuals with CLL might be particularly vulnerable,7 which was confirmed in early studies of hospitalized patients with COVID-19 and CLL.8-10 Sweden, the setting of this study, implemented only a few public health interventions during the early phases of the pandemic, and there was an absence of general lockdowns and recommendations for societal facemasks use.11-13 A better understanding of the outcomes of patients with CLL in such open society settings is warranted to inform future SARS-CoV-2 vaccination campaigns and epidemic preparedness for medical high-risk groups. Furthermore, data on mortality among individuals with CLL in comparison with the general population during the Omicron era remain limited.14,15
The treatment of CLL has shifted from chemoimmunotherapy to small molecule inhibitors, including Bruton tyrosine kinase inhibitors (BTKis), B-cell lymphoma 2 inhibitors (Bcl-2is), and phosphoinositide 3-kinase inhibitors (PI3Kis). However, their effects on immune responses and risks for severe infections have been less predictable. Individuals with CLL, particularly if they are receiving BTKis, mount significantly lower serologic responses to vaccination than healthy individuals and patients with other types of immunodeficiencies.16-18 However, an Australian study demonstrated that multiple COVID-19 vaccine doses led to adequate anti-spike levels that were associated with very low mortality and hospitalization rates.15
In this nationwide study, the aim was to compare the risk for severe outcomes in polymerase chain reaction (PCR)-verified SARS-CoV-2 infections in individuals with CLL and in the general population and to study factors that affect the risk for severe disease and mortality among individuals with CLL. Furthermore, for the capital region (Stockholm), all-cause mortality was analyzed at the outbreak of the pandemic and was compared with periods before and after the pandemic for both individuals with and those without CLL.
Methods
Study design and data sources
We conducted a nationwide cohort study using data from the following 10 national registers with high coverage: the Total Population Register, the Multi-Generation Register, the Longitudinal Integrated Database for Health Insurance and Labor Market Studies, SmiNet, the National Vaccination Register, the National Patient Register, the National Prescribed Drug Register (NPDR), the National Cause of Death Register, the Swedish Intensive Care Registry, and the CLL register within the Information Network for Cancer Care (INCA CLL register).19,20 Data from the Total Population Register, Multi-Generation Register, and Longitudinal Integrated Database for Health Insurance and Labor Market Studies were used for sociodemographic data. SmiNet and National Vaccination Register were used to identify all PCR tests that were positive for SARS-CoV-2 and COVID-19 vaccinations in Sweden. The National Patient Register, NPDR, National Cause of Death Register, and Swedish Intensive Care Registry were used for classification of comorbidities and outcomes of the PCR-verified SARS-CoV-2 infection (mortality, hospitalization, and intensive care unit [ICU] admission). The INCA CLL register was used to identify all individuals diagnosed with CLL. INCA is part of the Regional Cancer Centers in Sweden’s quality control registries for improved cancer care throughout Sweden. In this register, all patients diagnosed with CLL in Sweden are registered (∼550 per year) to assimilate treatment strategies and outcomes in CLL care throughout Sweden.21
Study population and exposure
Individuals born between 1930 and 2003 who were living in Sweden any time from 1 February 2020 to 31 March 2023 were included, and all PCR-verified SARS-CoV-2 infection episodes through 27 February 2023 were identified. An infection episode was defined as all SARS-CoV-2–positive PCR tests within 90 days (2 consecutive positive tests with >90 days in between were defined as 2 separate episodes). Until June 2020 and from February 2022, SARS-CoV-2 PCR testing in Sweden was restricted to health care settings.22 Among people with infection episodes, we classified the exposure of interest, CLL, using the date of CLL diagnosis. Individuals with CLL who had their date of diagnosis up until 90 days after the date of the first positive test of the infection episode were considered exposed. Because very few re-infections were identified in individuals with CLL (n = 83), we only analyzed the first PCR-verified infection per individual.
Outcomes
Primary outcomes were all-cause mortality and COVID-19 related mortality (a U07.1 or U07.2 International Statistical Classification of Diseases and Related Health Problems 10th Revision diagnosis as underlying cause of death in the death certificate). These were presented both for the entire population (irrespective of SARS-CoV-2 infection) and for individuals with SARS-CoV-2 infections (mortality data available through 31 December 2023). For individuals with a PCR-verified SARS-CoV-2 infection, the 90-day all-cause mortality and 90-day COVID-19 mortality from the date of the first positive SARS-CoV-2 test were presented.
Secondary outcomes were COVID-19 hospitalization and ICU admission. COVID-19 hospitalization was defined as a hospitalization if the SARS-CoV-2 positive PCR test was performed any time from 14 days before the date of hospital admission up until the date of hospital discharge, and a COVID-19 diagnosis (U07.1 or U07.2) was registered as a primary or secondary discharge code. A COVID-19 ICU admission was defined as an ICU admission that occurred during such a COVID-19 hospitalization and a COVID-19 diagnosis (U07.1 or U07.2) was registered as a primary or secondary discharge code at the ICU.
Other variables
Data were also collected for age, sex, region of birth, residential region in Sweden, age-standardized income quartile, prescription drug use before the infection episode, CLL treatments, time since CLL diagnosis in relation to the SARS-CoV-2 infection, COVID-19 vaccination status, and SARS-CoV-2 variant period. Prescribed medications included drugs prescribed for conditions such as diabetes, cardiac disease, cardiovascular diseases, respiratory diseases, and conditions warranting immunosuppressive treatments. The considered CLL treatments were Bcl-2i, BTKi, and PI3Ki. We also addressed the impact of corticosteroids (corresponding to a daily dose of ≥10 mg of prednisone).16 Only these types of prescription treatments were considered to have full coverage from the NPDR. As such, treatments administered in specialist care settings, such as CD20 monoclonal antibodies and chemotherapy, were not included because of a lack of complete coverage in the data sources. COVID-19 vaccination status was assessed at 14 days before the positive PCR test for SARS-CoV-2. The variant period of the included SARS-CoV-2 infection episodes was either based on a sequenced sample or the national variant distribution (wild type, 31 January 2020 through 14 February 2021; Alpha, 15 February 2021 through 27 June 2021; Delta, 28 June 2021 through 26 December 2021; Omicron, 27 December 2021 through 27 February 2023).23
All-cause mortality at the outbreak of the pandemic
To understand how mortality rates in individuals with CLL evolved from before the pandemic to the early prevaccination phase of the pandemic and onward, we also analyzed the crude cumulative incidence and age- and sex-adjusted relative risk of all-cause mortality on 30 June and 31 December for each year from 2017 through 2022. This was done for both individuals with and those without CLL in Stockholm County, the capital region of Sweden, with a population of ∼2.4 million people who were widely affected by SARS-CoV-2 early in 2020 and for whom we had access to prepandemic data (methods described in more detail in supplemental Table 1).
Statistical methods
Continuous variables were presented as median (interquartile interval [IQI]) and categorical variables were reported as frequencies (percentage). Kruskal Wallis tests were used for continuous variables and χ2 tests were used for categorical variables.
Regarding the primary outcome, the 90-day mortality, crude mortality rates, and adjusted risk ratios (RRs) with 95% confidence intervals (CIs) were presented. Modified Poisson regression models were used to obtain the RRs for individuals with CLL in comparison with individuals without CLL. The 95% CIs were derived from a sandwich variance estimator. The fully adjusted model included age, sex, region of birth, income quartile, residential region, days since the start of the study period, SARS-CoV-2 variant period, COVID-19 vaccination status, and all investigated prescribed drugs separately. Age and time were modeled with restricted cubic splines with 4 knots set at the fifth, 35th, 65th, and 95th quantiles among individuals who experienced the investigated mortality outcome.24 The Kaplan-Meier estimates for 90-day survival and log-rank tests for individuals with and without CLL by age group (<65 years, 65-74 years, ≥75 years) were also presented. To better understand the mortality across different populations, 18 predefined subgroups were defined and used for the outcome analyses (<65 years, 65-74 years, ≥75 years, male sex, female sex, income quartile 1, income quartiles 2-4, born in Sweden, born abroad, unvaccinated, ≥2 vaccine doses, >3 doses, >4 doses, >5 doses, wild type, Alpha, Delta, Omicron). Regarding the secondary outcomes of COVID-19 hospitalization and ICU admission, the crude rates and adjusted RRs with 95% CIs were also presented. The same models and subgroup analyses were used as for mortality.
The characteristics of individuals with CLL who experienced and those who did not experienced 90-day all-cause mortality, COVID-19 hospitalization, and COVID-19 ICU admission were then compared. Furthermore, outcome rates, unadjusted RRs, and adjusted RRs were presented for 3 predefined exposures of interest, namely (1) treatment with Bcl-2i, BTKi, or PI3Ki, (2) treatment with corticosteroids (corresponding to a daily dose of ≥10 mg of prednisone), and (3) SARS-CoV-2 variant period. The COVID-19 ICU admission rate was not included in these analyses because the number of individuals who experienced this outcome were considered too few for the adjusted analyses. Bcl-2i, BTKi, or PI3Ki treatment and treatment with corticosteroids, age, residential region, anti-coagulant use, immunosuppressive drug use, number of prescribed drugs, time from CLL diagnosis to PCR test, COVID-19 vaccination status, and time since start of the pandemic were considered potential confounders and were thus adjusted for in the adjusted analyses. For SARS-CoV-2 variant period, biologic sex, region of birth, income quartile, antidiabetic drug use, antihypertensive drug use, heart disease drug use, lipid-modifier drug use, obstructive lung disease drug use, Bcl-2i, BTKi, or PI3Ki use, and corticosteroid use were also adjusted for.
Only low levels of missing data were observed for region of birth (<1%), residential region (<1%), and income quartile (<1%); all other variables had complete data. An alpha level of .05 was used in the study. All data processing and analyses were performed using R version 4.3.1.
Ethics approval
Ethical approval for this study was granted by the Swedish Ethical Review Authority (Dnr 2022-01793-01, 2023-05877-02, Dnr 2018/1030-31, Dnr 2020-01385, 2020-02145, 2020-04069, and 2022-02127-02).
Results
Study population
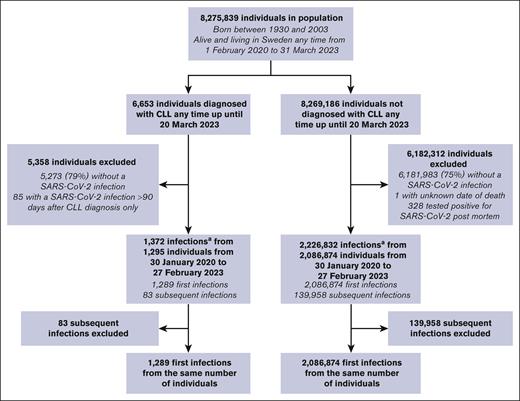
From a population of 8 275 839 individuals (6653 CLL, 8 269 186 no CLL), 2 088 163 first (1289 CLL, 2 086 874 no CLL) and 140 041 subsequent PCR-verified SARS-CoV-2 infections (83 CLL, 139 958 no CLL) were identified (Figure 1). Characteristics of the included (first PCR-verified SARS-CoV-2 infection) and excluded (no PCR-verified SARS-CoV-2 infection or excluded for other reasons) individuals in the CLL and no CLL groups are presented in Table 1. Included individuals with CLL more often had Bcl-2i (P < .001), BTKi (P < .001), and corticosteroids (P < .001) prescribed than excluded individuals. A larger proportion of individuals without CLL (n = 749 945; 36%) were infected during the first Omicron surge (December 2021 and January 2022) than individuals with CLL (n = 242; 19%; supplemental Figure 1). When looking at the number of individuals with and without CLL with a first positive SARS-CoV-2 PCR test by age group per 1000 persons at risk for each calendar month, an increased incidence was also observed for individuals with CLL from February 2022 (supplemental Figure 2). A total of 20% (1335/6653) of individuals with CLL died during the study period (through 31 December 2023) of which 12.3% (164/1335) had a COVID-19 diagnosis registered in the death certificate and 10.4% (139/1335) had a COVID-19 diagnosis registered as the underlying cause of death.
Study flowchart.aIf >90 days had passed between 2 positive consecutive PCR tests, these were classified as 2 different infection episodes.
Study flowchart.aIf >90 days had passed between 2 positive consecutive PCR tests, these were classified as 2 different infection episodes.
Characteristics of included (SARS-CoV-2 infected) and excluded individuals by CLL status
| Variable . | CLL (N = 6653) . | No CLL (N = 8 269 186) . | ||||
|---|---|---|---|---|---|---|
| Included (n = 1295) . | Excluded (n = 5358) . | P value . | Included (n = 2 086 874) . | Excluded (n = 6 182 312) . | P value . | |
| Age, median (IQI), y∗ | 73.0 (64.0-80.0) | 74.0 (68.0-80.0) | <.001 | 41.0 (30.0-54.0) | 51.0 (34.0-68.0) | <.001 |
| Age category, y, n (%) | ||||||
| ≤39 | 4 (0.3) | 5 (0.1) | <.001 | 958 861 (45.9) | 2 060 048 (33.3) | <.001 |
| 40-49 | 34 (2.6) | 76 (1.4) | 439 206 (21.0) | 860 816 (13.9) | ||
| 50-59 | 172 (13.3) | 414 (7.7) | 353 490 (16.9) | 958 894 (15.5) | ||
| 60-69 | 295 (22.8) | 1133 (21.1) | 169 099 (8.1) | 943 644 (15.3) | ||
| 70-79 | 454 (35.1) | 2334 (43.6) | 93 806 (4.5) | 931 770 (15.1) | ||
| ≥80 | 336 (25.9) | 1396 (26.1) | 72 412 (3.5) | 427 140 (6.9) | ||
| Male sex, n (%) | 848 (65.5) | 3281 (61.2) | .005 | 962 863 (46.1) | 3 194 079 (51.7) | <.001 |
| Region of birth, n (%) | ||||||
| Sweden | 1081 (83.5) | 4742 (88.5) | <.001 | 1 613 953 (77.3) | 4 832 511 (78.2) | <.001 |
| Outside Sweden | 214 (16.5) | 615 (11.5) | 472 921 (22.7) | 1 349 801 (21.8) | ||
| Missing | 0 | 1 (0.0) | 227 (0.0) | 803 (0.0) | ||
| Income quartile, n (%)† | ||||||
| 1 | 267 (20.6) | 1094 (20.4) | .192 | 391 326 (18.8) | 1 634 555 (26.4) | <.001 |
| 2 | 271 (20.9) | 1261 (23.5) | 575 938 (27.6) | 1 511 967 (24.5) | ||
| 3 | 345 (26.6) | 1414 (26.4) | 574 194 (27.5) | 1 512 831 (24.5) | ||
| 4 | 412 (31.8) | 1589 (29.7) | 545 405 (26.1) | 1 522 601 (24.6) | ||
| Missing | 0 | 0 | 11 (0.0) | 358 (0.0) | ||
| Prescription drug use, n (%)‡ | ||||||
| Anticoagulant | 500 (38.6) | 1960 (36.6) | .185 | 149 702 (7.2) | 775 764 (12.5) | <.001 |
| Antidiabetic | 233 (18.0) | 785 (14.7) | .003 | 91 607 (4.4) | 399 578 (6.5) | <.001 |
| Antihypertensive | 746 (57.6) | 3258 (60.8) | .038 | 323 753 (15.5) | 1 626 465 (26.3) | <.001 |
| Heart disease drug | 105 (8.1) | 458 (8.5) | .649 | 39 391 (1.9) | 183 285 (3.0) | <.001 |
| Immunosuppressive drug | 153 (11.8) | 489 (9.1) | .004 | 35 861 (1.7) | 114 131 (1.8) | <.001 |
| Lipid-modifier | 438 (33.8) | 1772 (33.1) | .630 | 150 848 (7.2) | 836 068 (13.5) | <.001 |
| Obstructive lung disease drug | 196 (15.1) | 721 (13.5) | .127 | 174 340 (8.4) | 528 354 (8.5) | <.001 |
| CLL treatments, n (%)§ | ||||||
| BCL-2i | 153 (11.8) | 323 (6.0) | <.001 | NA | NA | NA |
| BTKi | 312 (24.1) | 853 (15.9) | <.001 | NA | NA | NA |
| PI3Ki | 12 (0.9) | 32 (0.6) | .262 | NA | NA | NA |
| Corticosteroids | 626 (48.3) | 1989 (37.1) | <.001 | NA | NA | NA |
| Any of the above treatments | 769 (59.4) | 2458 (45.9) | <.001 | NA | NA | NA |
| COVID-19 vaccination status at end of follow-up, n (%)‖ | ||||||
| Unvaccinated | 123 (9.5) | 455 (8.5) | <.001 | 246 911 (11.8) | 827 531 (13.4) | <.001 |
| 1 dose | 15 (1.2) | 36 (0.7) | 37 613 (1.8) | 94 769 (1.5) | ||
| 2 doses | 100 (7.7) | 275 (5.1) | 501 715 (24.0) | 1 055 549 (17.1) | ||
| 3 doses | 189 (14.6) | 523 (9.8) | 802 961 (38.5) | 1 857 814 (30.1) | ||
| 4 doses | 301 (23.2) | 1097 (20.5) | 348 429 (16.7) | 1 059 259 (17.1) | ||
| 5 doses | 567 (43.8) | 2972 (55.5) | 149 245 (7.2) | 1 287 390 (20.8) | ||
| All-cause mortality, n (%)¶ | 341 (26.3) | 994 (18.6) | <.001 | 58 575 (2.8) | 245 223 (4.0) | <.001 |
| COVID-19 mortality, n (%)# | 151 (11.7) | 13 (0.2) | <.001 | 16 573 (0.8) | 1062 (0.0) | <.001 |
| COVID-19 as underlying cause of mortality, n (%)∗∗ | 128 (9.9) | 11 (0.2) | <.001 | 13 858 (0.7) | 796 (0.0) | <.001 |
| Variable . | CLL (N = 6653) . | No CLL (N = 8 269 186) . | ||||
|---|---|---|---|---|---|---|
| Included (n = 1295) . | Excluded (n = 5358) . | P value . | Included (n = 2 086 874) . | Excluded (n = 6 182 312) . | P value . | |
| Age, median (IQI), y∗ | 73.0 (64.0-80.0) | 74.0 (68.0-80.0) | <.001 | 41.0 (30.0-54.0) | 51.0 (34.0-68.0) | <.001 |
| Age category, y, n (%) | ||||||
| ≤39 | 4 (0.3) | 5 (0.1) | <.001 | 958 861 (45.9) | 2 060 048 (33.3) | <.001 |
| 40-49 | 34 (2.6) | 76 (1.4) | 439 206 (21.0) | 860 816 (13.9) | ||
| 50-59 | 172 (13.3) | 414 (7.7) | 353 490 (16.9) | 958 894 (15.5) | ||
| 60-69 | 295 (22.8) | 1133 (21.1) | 169 099 (8.1) | 943 644 (15.3) | ||
| 70-79 | 454 (35.1) | 2334 (43.6) | 93 806 (4.5) | 931 770 (15.1) | ||
| ≥80 | 336 (25.9) | 1396 (26.1) | 72 412 (3.5) | 427 140 (6.9) | ||
| Male sex, n (%) | 848 (65.5) | 3281 (61.2) | .005 | 962 863 (46.1) | 3 194 079 (51.7) | <.001 |
| Region of birth, n (%) | ||||||
| Sweden | 1081 (83.5) | 4742 (88.5) | <.001 | 1 613 953 (77.3) | 4 832 511 (78.2) | <.001 |
| Outside Sweden | 214 (16.5) | 615 (11.5) | 472 921 (22.7) | 1 349 801 (21.8) | ||
| Missing | 0 | 1 (0.0) | 227 (0.0) | 803 (0.0) | ||
| Income quartile, n (%)† | ||||||
| 1 | 267 (20.6) | 1094 (20.4) | .192 | 391 326 (18.8) | 1 634 555 (26.4) | <.001 |
| 2 | 271 (20.9) | 1261 (23.5) | 575 938 (27.6) | 1 511 967 (24.5) | ||
| 3 | 345 (26.6) | 1414 (26.4) | 574 194 (27.5) | 1 512 831 (24.5) | ||
| 4 | 412 (31.8) | 1589 (29.7) | 545 405 (26.1) | 1 522 601 (24.6) | ||
| Missing | 0 | 0 | 11 (0.0) | 358 (0.0) | ||
| Prescription drug use, n (%)‡ | ||||||
| Anticoagulant | 500 (38.6) | 1960 (36.6) | .185 | 149 702 (7.2) | 775 764 (12.5) | <.001 |
| Antidiabetic | 233 (18.0) | 785 (14.7) | .003 | 91 607 (4.4) | 399 578 (6.5) | <.001 |
| Antihypertensive | 746 (57.6) | 3258 (60.8) | .038 | 323 753 (15.5) | 1 626 465 (26.3) | <.001 |
| Heart disease drug | 105 (8.1) | 458 (8.5) | .649 | 39 391 (1.9) | 183 285 (3.0) | <.001 |
| Immunosuppressive drug | 153 (11.8) | 489 (9.1) | .004 | 35 861 (1.7) | 114 131 (1.8) | <.001 |
| Lipid-modifier | 438 (33.8) | 1772 (33.1) | .630 | 150 848 (7.2) | 836 068 (13.5) | <.001 |
| Obstructive lung disease drug | 196 (15.1) | 721 (13.5) | .127 | 174 340 (8.4) | 528 354 (8.5) | <.001 |
| CLL treatments, n (%)§ | ||||||
| BCL-2i | 153 (11.8) | 323 (6.0) | <.001 | NA | NA | NA |
| BTKi | 312 (24.1) | 853 (15.9) | <.001 | NA | NA | NA |
| PI3Ki | 12 (0.9) | 32 (0.6) | .262 | NA | NA | NA |
| Corticosteroids | 626 (48.3) | 1989 (37.1) | <.001 | NA | NA | NA |
| Any of the above treatments | 769 (59.4) | 2458 (45.9) | <.001 | NA | NA | NA |
| COVID-19 vaccination status at end of follow-up, n (%)‖ | ||||||
| Unvaccinated | 123 (9.5) | 455 (8.5) | <.001 | 246 911 (11.8) | 827 531 (13.4) | <.001 |
| 1 dose | 15 (1.2) | 36 (0.7) | 37 613 (1.8) | 94 769 (1.5) | ||
| 2 doses | 100 (7.7) | 275 (5.1) | 501 715 (24.0) | 1 055 549 (17.1) | ||
| 3 doses | 189 (14.6) | 523 (9.8) | 802 961 (38.5) | 1 857 814 (30.1) | ||
| 4 doses | 301 (23.2) | 1097 (20.5) | 348 429 (16.7) | 1 059 259 (17.1) | ||
| 5 doses | 567 (43.8) | 2972 (55.5) | 149 245 (7.2) | 1 287 390 (20.8) | ||
| All-cause mortality, n (%)¶ | 341 (26.3) | 994 (18.6) | <.001 | 58 575 (2.8) | 245 223 (4.0) | <.001 |
| COVID-19 mortality, n (%)# | 151 (11.7) | 13 (0.2) | <.001 | 16 573 (0.8) | 1062 (0.0) | <.001 |
| COVID-19 as underlying cause of mortality, n (%)∗∗ | 128 (9.9) | 11 (0.2) | <.001 | 13 858 (0.7) | 796 (0.0) | <.001 |
Kruskal Wallis tests were used for continuous variables and χ2 tests were used for categorical variables.
ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision; NA, not applicable.
Age at the start of the COVID-19 pandemic.
In 2019, if not available, region from years 2020, 2021, or 2022 were used.
From 30 January 2019 to 30 January 2020.
CLL treatment data available through 30 November 2023.
COVID-19 vaccination data available until 1 March 2023.
Death any time through 31 December 2023.
Death any time through 31 December 2023 with a U07.1 or U07.2 ICD-10 diagnosis code registered in the death certificate.
Death any time through 31 December 2023 with a U07.1 or U07.2 ICD-10 diagnosis code registered in the death certificate as underlying cause of death.
Characteristics of SARS-CoV-2 infected individuals with and without CLL
The median (IQI) age was 75 (66-82) years among individuals with CLL and 43 (31-55) years among individuals without CLL (Table 2). A total of 66% (n = 844) and 46% (n = 962 863) were male among individuals with and those without CLL, respectively. A total of 30% (n = 381) of individuals with CLL were unvaccinated before their infection as opposed to 52% of individuals without CLL (n = 1 088 607). Among both individuals with and those without CLL, Comirnaty was the most administered COVID-19 vaccine for all 5 doses (supplemental Table 2). The characteristics of infected individuals with CLL by variant period are presented in supplemental Table 3. A total of 38% (315/835) of individuals infected during Omicron were aged >80 years as opposed to 18% to 26% for the other 3 variant periods.
Characteristics of included (PCR-verified SARS-CoV-2 infection) individuals by CLL status
| Variable . | CLL (n = 1289) . | No CLL (n = 2 086 874) . |
|---|---|---|
| Age, median (IQI), y | 75.0 (66.0-82.0) | 43.0 (31.0-55.0) |
| Age category, y, n (%) | ||
| ≤39 | 2 (0.2) | 900 776 (43.2) |
| 40-49 | 25 (1.9) | 444 167 (21.3) |
| 50-59 | 145 (11.2) | 373 164 (17.9) |
| 60-69 | 271 (21.0) | 189 714 (9.1) |
| 70-79 | 426 (33.0) | 95 892 (4.6) |
| ≥80 | 420 (32.6) | 83 161 (4.0) |
| Male sex, n (%) | 844 (65.5) | 962 863 (46.1) |
| Region of birth, n (%) | ||
| Sweden | 1075 (83.4) | 1 613 953 (77.3) |
| Outside Sweden | 214 (16.6) | 472 694 (22.7) |
| Missing | 0 | 227 (0.0) |
| Income quartile, n (%) | ||
| 1 | 414 (32.1) | 528 062 (25.3) |
| 2 | 398 (30.9) | 514 511 (24.7) |
| 3 | 173 (13.4) | 512 839 (24.6) |
| 4 | 234 (18.2) | 520 426 (24.9) |
| Missing | 70 (5.4) | 11 036 (0.5) |
| Prescription drug use, n (%) | ||
| Anticoagulant | 571 (44.3) | 167 379 (8.0) |
| Antidiabetic | 260 (20.2) | 105 115 (5.0) |
| Antihypertensive | 797 (61.8) | 358 682 (17.2) |
| Heart disease drug | 108 (8.4) | 41 722 (2.0) |
| Immunosuppressive drug | 321 (24.9) | 42 678 (2.0) |
| Lipid modifier | 446 (34.6) | 171 087 (8.2) |
| Obstructive lung disease drug | 211 (16.4) | 182 877 (8.8) |
| CLL treatments, n (%) | ||
| BCL-2i | 70 (5.4) | NA |
| BTKi | 239 (18.5) | NA |
| PI3Ki | 7 (0.5) | NA |
| Corticosteroids | 139 (10.8) | NA |
| Any of the above treatments | 368 (28.5) | NA |
| Time from CLL diagnosis to PCR test, n (%) | ||
| During SARS-CoV-2 infection | 73 (5.7) | NA |
| <1 year | 127 (9.9) | NA |
| 1-5 years | 456 (35.4) | NA |
| >5 years | 633 (49.1) | NA |
| COVID-19 vaccination status 14 days before first infection, n (%) | ||
| Unvaccinated | 381 (29.6) | 1 088 607 (52.2) |
| 1 dose | 29 (2.2) | 48 498 (2.3) |
| 2 doses | 133 (10.3) | 645 648 (30.9) |
| 3 doses | 435 (33.7) | 251 709 (12.1) |
| 4 doses | 202 (15.7) | 33 429 (1.6) |
| 5 doses | 109 (8.5) | 18 983 (0.9) |
| SARS-CoV-2 variant, n (%) | ||
| Wild type | 258 (20.0) | 551 711 (26.4) |
| Alpha | 122 (9.5) | 389 996 (18.7) |
| Beta | 1 (0.1) | 2441 (0.1) |
| Gamma | 0 | 189 (0.0) |
| Delta | 73 (5.7) | 141 316 (6.8) |
| Omicron | 835 (64.8) | 1 001 221 (48.0) |
| Variable . | CLL (n = 1289) . | No CLL (n = 2 086 874) . |
|---|---|---|
| Age, median (IQI), y | 75.0 (66.0-82.0) | 43.0 (31.0-55.0) |
| Age category, y, n (%) | ||
| ≤39 | 2 (0.2) | 900 776 (43.2) |
| 40-49 | 25 (1.9) | 444 167 (21.3) |
| 50-59 | 145 (11.2) | 373 164 (17.9) |
| 60-69 | 271 (21.0) | 189 714 (9.1) |
| 70-79 | 426 (33.0) | 95 892 (4.6) |
| ≥80 | 420 (32.6) | 83 161 (4.0) |
| Male sex, n (%) | 844 (65.5) | 962 863 (46.1) |
| Region of birth, n (%) | ||
| Sweden | 1075 (83.4) | 1 613 953 (77.3) |
| Outside Sweden | 214 (16.6) | 472 694 (22.7) |
| Missing | 0 | 227 (0.0) |
| Income quartile, n (%) | ||
| 1 | 414 (32.1) | 528 062 (25.3) |
| 2 | 398 (30.9) | 514 511 (24.7) |
| 3 | 173 (13.4) | 512 839 (24.6) |
| 4 | 234 (18.2) | 520 426 (24.9) |
| Missing | 70 (5.4) | 11 036 (0.5) |
| Prescription drug use, n (%) | ||
| Anticoagulant | 571 (44.3) | 167 379 (8.0) |
| Antidiabetic | 260 (20.2) | 105 115 (5.0) |
| Antihypertensive | 797 (61.8) | 358 682 (17.2) |
| Heart disease drug | 108 (8.4) | 41 722 (2.0) |
| Immunosuppressive drug | 321 (24.9) | 42 678 (2.0) |
| Lipid modifier | 446 (34.6) | 171 087 (8.2) |
| Obstructive lung disease drug | 211 (16.4) | 182 877 (8.8) |
| CLL treatments, n (%) | ||
| BCL-2i | 70 (5.4) | NA |
| BTKi | 239 (18.5) | NA |
| PI3Ki | 7 (0.5) | NA |
| Corticosteroids | 139 (10.8) | NA |
| Any of the above treatments | 368 (28.5) | NA |
| Time from CLL diagnosis to PCR test, n (%) | ||
| During SARS-CoV-2 infection | 73 (5.7) | NA |
| <1 year | 127 (9.9) | NA |
| 1-5 years | 456 (35.4) | NA |
| >5 years | 633 (49.1) | NA |
| COVID-19 vaccination status 14 days before first infection, n (%) | ||
| Unvaccinated | 381 (29.6) | 1 088 607 (52.2) |
| 1 dose | 29 (2.2) | 48 498 (2.3) |
| 2 doses | 133 (10.3) | 645 648 (30.9) |
| 3 doses | 435 (33.7) | 251 709 (12.1) |
| 4 doses | 202 (15.7) | 33 429 (1.6) |
| 5 doses | 109 (8.5) | 18 983 (0.9) |
| SARS-CoV-2 variant, n (%) | ||
| Wild type | 258 (20.0) | 551 711 (26.4) |
| Alpha | 122 (9.5) | 389 996 (18.7) |
| Beta | 1 (0.1) | 2441 (0.1) |
| Gamma | 0 | 189 (0.0) |
| Delta | 73 (5.7) | 141 316 (6.8) |
| Omicron | 835 (64.8) | 1 001 221 (48.0) |
NA, not applicable.
The 90-day mortality
The 90-day all-cause mortality was 15.0% (n = 193) among individuals with CLL and 1.2% (n = 24 277) among individuals without CLL; of those, 84 and 13 025, respectively, were from infections that occurred through 30 June 2021 (when most individuals with CLL had received 2 doses25). Of the individuals who died within 90 days, 75% (n = 144) of individuals with CLL and 66% (n = 15 934) of individuals without CLL had a COVID-19 diagnosis code registered in the death certificate. When restricting the diagnosis codes to the underlying reason of death, the corresponding figures were 64% (n = 124) and 56% (n = 13 510). Individuals with CLL had a significantly lower survival probability in all 3 investigated age groups (<65 years, 65-74 years, ≥75 years; P < .001 for all age groups; supplemental Figure 3).
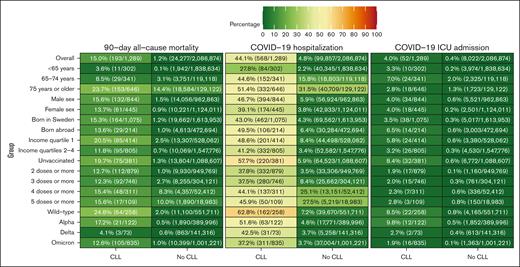
The 90-day all-cause mortality rates overall and in the 18 subgroups in individuals with and those without CLL are presented in Figure 2. Among individuals <65 years, the 90-day all-cause mortality was 3.6% (11/302) among individuals with CLL and 0.1% (1942/1 838 634) among individuals without CLL. The mortality rate for all individuals with CLL was 24.8% (64/258) during the wild-type period, 17.2% (21/122) during the Alpha period, 4.1% (3/73) during the Delta period, and 12.6% (105/835) during the Omicron period. The 90-day all-cause mortality rate for individuals with CLL during the Omicron period when PCR testing for SARS-CoV-2 was readily available for the public (December 2021 and January 2022) was 8.0% (17/213). The corresponding mortality rate among individuals with CLL during the Omicron period ranged from 0.6% (1/160) among individuals <65 years to 5.4% (11/202) among individuals 65 to 74 years and 19.7% (93/473) among individuals ≥75 years (supplemental Figure 4).
The 90-day all-cause mortality rates, COVID-19 hospitalization rates, and COVID-19 ICU admission rates in individuals with and those without CLL.
The 90-day all-cause mortality rates, COVID-19 hospitalization rates, and COVID-19 ICU admission rates in individuals with and those without CLL.
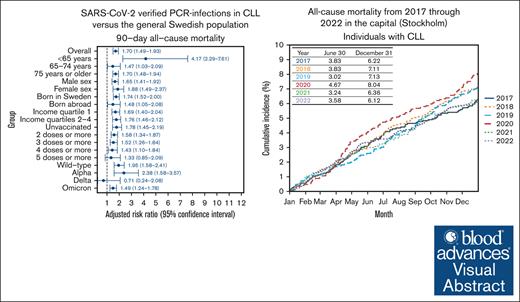
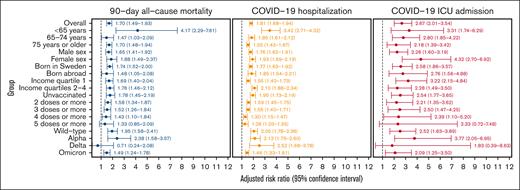
The overall RR (95% CI) for the 90-day all-cause mortality was 1.70 (1.49-1.93) among individuals with CLL when compared with those without CLL (Figure 3). The strongest association was observed in the subgroup analysis restricted to individuals <65 years (4.17; 2.29-7.61). A significantly increased risk for 90-day all-cause mortality among individuals with versus those without CLL was observed in all subgroup analyses except when the analyses were restricted to individuals who received ≥5 doses of a COVID-19 vaccine and to infections that occurred during the Delta variant period. The RR (95% CI) for mortality among individuals with CLL when compared with the general population was 1.95 (1.58-2.41) for wild type, 2.38 (1.58-3.57) for Alpha, 0.71 (0.24-2.08) for Delta, and 1.49 (1.24-1.78) for Omicron. A dose-response relationship was observed for COVID-19 vaccination doses with the highest RR for unvaccinated individuals and the lowest RR for individuals who received 5 doses or more.
Forest plot of fully adjusted RRs for 90-day all-cause mortality, COVID-19 hospitalization, and COVID-19 ICU admission in individuals with CLL compared with those without CLL.
Forest plot of fully adjusted RRs for 90-day all-cause mortality, COVID-19 hospitalization, and COVID-19 ICU admission in individuals with CLL compared with those without CLL.
Secondary outcomes
Overall, the adjusted RR (95% CI) was 1.81 (1.68-1.94) for COVID-19 hospitalization and 2.67 (2.01-3.54) for COVID-19 ICU admission among individuals with CLL when compared with those without CLL (Figure 3). Individuals with CLL had an increased risk for COVID-19 hospitalization in all subgroup analyses, whereas for ICU admission, no significant difference could either be demonstrated or excluded for individuals who received ≥5 doses of a COVID-19 vaccine and those with infections during the Delta variant period. For the Omicron period, the adjusted RRs (95% CIs) for COVID-19 hospitalization and COVID-19 ICU admission were 1.46 (1.33-1.61) and 2.09 (1.25-3.50), respectively, in individuals with CLL when compared with those without CLL.
Factors associated with the outcomes in individuals with CLL
Supplemental Tables 4-6 show the characteristics of individuals with CLL by 90-day all-cause mortality (supplemental Table 4), hospitalization (supplemental Table 5), and ICU admission (supplemental Table 6). The median (IQI) age was 82 (75-86) years among individuals who died within 90 days and 73 (64-80) years among individuals who did not die within 90 days (P < .001). A total of 44.0% (85/193) of individuals who died within 90 days were in income quartile 1 as opposed to 30.0% (329/1096) of individuals who did not die within 90 days (P<.001). The mortality and hospitalization rates and RRs with 95% CIs for CLL treatment status and SARS-CoV-2 variant period are presented in Table 3. Individuals who were treated with Bcl-2i, BTKi, or PI3Ki had an increased risk for both 90-day all-cause mortality (1.56; 1.03-2.37) and COVID-19 hospitalization (adjusted RR, 1.25; 95% CI, 1.02-1.54) when compared with individuals who were not treated with these drugs. Similar adjusted RRs were observed for corticosteroid treatment (1.45; 1.04-2.02 and 1.23; 1.05-1.44). The adjusted RR (95% CI) for Omicron vs wild type was 0.28 (0.13-0.61) for 90-day all-cause mortality and 0.62 (0.46-0.84) for COVID-19 hospitalization.
Hospitalization and 90-day all-cause mortality rates and RRs for individuals with CLL, by CLL treatment status and SARS-CoV-2 variant period
| Variable . | COVID-19 hospitalization . | 90-day all-cause mortality . | ||||
|---|---|---|---|---|---|---|
| Hospitalization rate % (n/N) . | Unadjusted RR (95% CI) . | Adjusted RR (95% CI) . | Mortality rate % (n/N) . | Unadjusted RR (95% CI) . | Adjusted RR (95% CI) . | |
| Bcl-2i, BTKi, or PI3Ki∗ | ||||||
| Not treated | 42.6 (435/1022) | Ref | Ref | 14.5 (148/1022) | Ref | Ref |
| Treated | 49.8 (133/267) | 1.17 (1.02-1.35) | 1.25 (1.02-1.54) | 16.9 (45/267) | 1.16 (0.86-1.58) | 1.56 (1.03-2.37) |
| Corticosteroids† | ||||||
| Not treated | 42.4 (488/1150) | Ref | Ref | 13.8 (159/1150) | Ref | Ref |
| Treated | 57.6 (80/139) | 1.36 (1.16-1.59) | 1.23 (1.05-1.44) | 24.5 (34/139) | 1.77 (1.28-2.45) | 1.45 (1.04-2.02) |
| SARS-CoV-2 variant period‡,§ | ||||||
| Wild type | 62.8 (162/258) | Ref | Ref | 24.8 (64/258) | Ref | Ref |
| Alpha | 51.6 (63/122) | 0.82 (0.68-1.00) | 0.85 (0.69-1.04) | 17.2 (21/122) | 0.69 (0.45-1.08) | 0.63 (0.34-1.15) |
| Delta | 42.5 (31/73) | 0.68 (0.51-0.90) | 0.86 (0.60-1.24) | 4.1 (3/73) | 0.17 (0.05-0.51) | 0.14 (0.04-0.47) |
| Omicron | 37.2 (311/835) | 0.59 (0.52-0.67) | 0.62 (0.46-0.84) | 12.6 (105/835) | 0.51 (0.38-0.67) | 0.28 (0.13-0.61) |
| Variable . | COVID-19 hospitalization . | 90-day all-cause mortality . | ||||
|---|---|---|---|---|---|---|
| Hospitalization rate % (n/N) . | Unadjusted RR (95% CI) . | Adjusted RR (95% CI) . | Mortality rate % (n/N) . | Unadjusted RR (95% CI) . | Adjusted RR (95% CI) . | |
| Bcl-2i, BTKi, or PI3Ki∗ | ||||||
| Not treated | 42.6 (435/1022) | Ref | Ref | 14.5 (148/1022) | Ref | Ref |
| Treated | 49.8 (133/267) | 1.17 (1.02-1.35) | 1.25 (1.02-1.54) | 16.9 (45/267) | 1.16 (0.86-1.58) | 1.56 (1.03-2.37) |
| Corticosteroids† | ||||||
| Not treated | 42.4 (488/1150) | Ref | Ref | 13.8 (159/1150) | Ref | Ref |
| Treated | 57.6 (80/139) | 1.36 (1.16-1.59) | 1.23 (1.05-1.44) | 24.5 (34/139) | 1.77 (1.28-2.45) | 1.45 (1.04-2.02) |
| SARS-CoV-2 variant period‡,§ | ||||||
| Wild type | 62.8 (162/258) | Ref | Ref | 24.8 (64/258) | Ref | Ref |
| Alpha | 51.6 (63/122) | 0.82 (0.68-1.00) | 0.85 (0.69-1.04) | 17.2 (21/122) | 0.69 (0.45-1.08) | 0.63 (0.34-1.15) |
| Delta | 42.5 (31/73) | 0.68 (0.51-0.90) | 0.86 (0.60-1.24) | 4.1 (3/73) | 0.17 (0.05-0.51) | 0.14 (0.04-0.47) |
| Omicron | 37.2 (311/835) | 0.59 (0.52-0.67) | 0.62 (0.46-0.84) | 12.6 (105/835) | 0.51 (0.38-0.67) | 0.28 (0.13-0.61) |
Ref, reference.
The adjusted model also included age, residential region, anticoagulant use, immunosuppressive drug use, number of prescribed drugs, time from CLL diagnosis to PCR test, COVID-19 vaccination status, and time since 29 January 2020.
The adjusted model also included age, residential region, anticoagulant use, immunosuppressive drug use, number of prescribed drugs, time from CLL diagnosis to PCR test, COVID-19 vaccination status, and time since 29 January 2020.
The adjusted model also included age, biologic sex, region of birth, residential region, income quartile, anticoagulant use, antidiabetic drug use, antihypertensive drug use, heart disease drug use, immunosuppressive drug use, lipid-modifier use, obstructive lung disease drug use, number of prescribed drugs, time from CLL diagnosis to PCR test, COVID-19 vaccination status, Bcl-2/BTK/PI3K inhibitor use, and corticosteroids use.
One individual with a SARS-CoV-2 Beta variant infection was excluded from these analyses.
Mortality at the onset of the pandemic in the capital (Stockholm)
By 30 June, the cumulative incidence of all-cause mortality among individuals with CLL who lived in the capital region (Stockholm) was 3.83% in 2017, 3.83% in 2018 (±0% compared with 2017), 3.02% in 2019 (−21%), 4.67% in 2020 (+55%), 3.24% in 2021 (−31%), and 3.58% in 2022 (+10%; Figure 4). An increase in mortality, albeit numerically lower than in individuals with CLL by 30 June was also observed for the general population in 2020 when compared with 2019 (0.55% vs 0.42%, +31%). By 31 December, the cumulative incidence of mortality was 7.13% in 2019 and 8.04% in 2020 (+13%) for individuals with CLL and 0.82% in 2019 and 0.97% in 2020 (+18%) for the general population. The age- and sex-adjusted RR (95% CI) for all-cause mortality by 30 June in 2020 vs 2019 was 1.53 (1.09-2.15) for individuals with CLL (P = .02) and 1.29 (1.25-1.33) for the general population (P < .001; supplemental Table 7).
Cumulative incidence of all-cause mortality from 2017 through 2022 in individuals with and those without CLL in Stockholm County.
Cumulative incidence of all-cause mortality from 2017 through 2022 in individuals with and those without CLL in Stockholm County.
Discussion
In this nationwide cohort study set in Sweden, a country considered unique by implementing only a few mandatory societal restrictions at the onset of the pandemic, we demonstrated increased risks for mortality, hospitalization, and ICU admission among individuals with PCR-verified SARS-CoV-2 infections with versus without CLL overall and across subgroup analyses (sociodemographic factors, COVID-19 vaccination status, and SARS-CoV-2 variant period). Importantly, we also demonstrated that in the capital region of Sweden, a region much affected by SARS-CoV-2 during the early phase of the pandemic when few societal restrictions were implemented, patients with CLL had a 55% increase in the cumulative incidence of all-cause mortality from 1 January through 30 June 2020 when compared with the same period in 2019. Notably, a continued increased risk for mortality was observed among Swedish individuals with versus those without CLL during the Omicron period. Furthermore, we demonstrated that different CLL treatment types were associated with increased risks for COVID-19 hospitalization and 90-day all-cause mortality.
Data on COVID-19 outcomes among individuals with CLL in comparison with the general population are scarce. A Danish study that included 499 individuals (22 from electronic health records and 477 from a population-based data source) with CLL infected during the Omicron BA.2 period (1-28 January 2022) observed a 30-day mortality of 1.8% (9/499).14 The corresponding 30-day all-cause mortality for the same period in our study was 6.9% (12/174). It should, however, be noted that there were methodologic differences between the studies. Our results indicate that Omicron infections were still associated with substantial mortality among individuals with CLL in Sweden who underwent PCR testing, although the limited testing from February 2022 and onward might have led to enrichment of more severe disease presentations during the later phases of the study period based on the observed increase in the mortality rate after general public testing was stopped. Among individuals who received ≥5 doses of a COVID-19 vaccination, no significant increase in the 90-day all-cause mortality could be demonstrated or excluded. This is mainly in line with an Australian report that demonstrated that multiple vaccine doses led to a 94% rate of seroconversion in participants with CLL.5 Importantly, another Australian study that included 241 participants with CLL who were subject to general lockdown at the onset of the pandemic and who then received multiple vaccine doses, reported that only 1 patient died.15
We observed worse relative risks for outcomes among individuals with CLL who were <65 years with adjusted RRs for mortality being higher (4.17; 2.29-7.61) when compared with those in the age groups 65 to 74 years (1.47; 1.03-2.09) and >75 years (1.70; 1.48-1.94). An overall mortality rate of 3.6% among individuals <65 years highlights the CLL-related vulnerability. Old age was, however, not associated with an increased ICU admission rate likely because individuals of advanced age may be more likely to receive ICU treatment limitations during scenarios with overcrowded ICUs and tougher admission prioritizations.26,27
Treatment with Bcl-2i, BTKi, or PI3Ki was associated with an increased risk for both 90-day all-cause mortality and COVID-19 hospitalization among individuals with CLL. The impact of BTKi therapy on COVID-19 outcomes among individuals with CLL has been controversial.28-30 The effects of Bcl-2i therapy (venetoclax) on COVID-19 outcomes have been even less well understood.31,32 Furthermore, treatment with corticosteroids was associated with an increased risk for 90-day all-cause mortality among individuals with CLL, in line with findings from previous studies not focused on CLL.33,34
Importantly, we demonstrated a 55% and 31% increase in all-cause mortality in the capital region through 30 June 2020, when Stockholm was particularly affected by the pandemic, when compared with the same period in 2019 among individuals with CLL and the general population, respectively. The age- and sex-adjusted RR (95% CI) was 1.53 (1.09-2.15) for CLL and 1.29 (1.25-1.33) for the general population. A previous study that used weekly all-cause mortality data from Nordic countries found 75 (95% prediction interval, 29 to 122) excess deaths per 100 000 population when compared with 1 (−38 to 40) in Denmark, 15 (−34 to 65) in Finland, and 6 (−35 to 47) in Norway during 2020.35 These results, along with the reports from Denmark and Australia,14,15 indicate that the death rates among patients with CLL in our study were higher at the very onset of the pandemic than in similar countries where lockdown and other preventive measures were applied early at the spread of SARS-CoV-2.36 It should be noted that the mortality among individuals with CLL was lower in 2019 than in both 2017 and 2018 in our study, which may possibly be because of the increasing usage of BTKi (ie, ibrutinib) in Sweden.
This study has several strengths, including the inclusion of close to all individuals diagnosed with CLL in Sweden, a country known for its unique open-society COVID-19 strategy, because of the high coverage of the National CLL Register, and having the entire population born from 1930 to 2003 as comparators. By using data from 10 national registers with high coverage, we were able to adjust for potential confounders, such as comorbidities, demographic and socioeconomic factors, vaccination, and small molecule–based CLL therapy. Data from national registers on PCR-testing, hospital admission, and deaths, including cause of death, also ensured high accuracy in the outcomes. Because of the long period of follow-up (2020-2023), we were also able to compare outcomes during different periods of the pandemic. Importantly, we were also able to study the all-cause mortality among patients with CLL at the very outbreak of the pandemic in the widely affected capital region and compare the outcomes with the years before and after.
However, there are also limitations to this study. CLL-related factors, such as clinical stage, genomic aberrations, and immunoglobulin levels, were not available in the registers and could therefore not be considered. We did not have access to results from self-administered SARS-CoV-2 antigen tests, although emergency unit admitted/examined or hospitalized patients (ie, the main target population of the study) always underwent a PCR test. It is likely that individuals with CLL were more likely to undergo PCR testing than individuals without CLL, particularly during periods with more limited PCR testing, which possibly led to selection bias. Collectively, the limitations of not having access to rapid antigen test and the limited PCR-based testing in Sweden during periods of the pandemic means that our study was more focused on severe infections that warranted health care admission and thus PCR testing. Because no national register of all SARS-CoV-2 negative PCR tests exists in Sweden, we were not able to describe testing intensities across different age groups and periods of the pandemic in individuals with and those without CLL.
In conclusion, the finding of a 55% increase in the all-cause mortality in the widely affected capital region from 1 January through 30 June 2020 when compared with the same period in 2019 is numerically higher than what has been reported for other countries and calls for further studies from settings that implemented different governance and policy measures in the prevaccination period of the pandemic. Collectively, our study is valuable in the continued COVID-19 era and also provides important information for future viral outbreaks or pandemics.
Acknowledgments
This study was funded by grants from the Swedish Research Council (2021-04779_VR), the Swedish Cancer Society (22 0553 SCIA 03 H and 22 2220 Pj 01H), the Cancer Society in Stockholm (224143), Region Stockholm (Centrum för Innovativ MEDicin [FoUI-963338] and Avtal om Läkarutbildning och Forskning [FoUI-987366]), and the Swedish state under an agreement between the Swedish government and the county councils.
Authorship
Contribution: P.H. was responsible for data curation and formal analysis, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis; L.H., S.A., and C.C. were responsible for funding acquisition; C.C., P.N., A.Ö., and L.H. contributed resources; P.H. was responsible for the software and visualization; A.Ö. and L.H. were responsible for supervision; P.H., L.B., A.Ö., and L.H. were responsible for writing the original draft of the manuscript; and all authors conceptualized the study, were responsible for validation, were involved in the investigation, methodology development, and project administration, have read and approved the manuscript, and are responsible for reviewing and editing of the manuscript.
Conflict-of-interest disclosure: F.K. reports funding grants from CSL Behring. P.B. reports receiving speaking and lecture fees from CSL Behring and Takeda. C.C. reports funding grants from Gilead Sciences Inc; serving as a consultant or in an advisory role for Gilead Sciences Inc and ViiV Healthcare; and speaking and lecture fees from Gilead Sciences Inc and ViiV Healthcare. S.A. reports funding grants from AbbVie and Gilead Sciences Inc, and speaking and lecture fees from AbbVie, Biogen Inc, Gilead Sciences Inc, and Merck Sharp & Dohme. A.Ö. reports funding grants from BeiGene Ltd, Lilly/Loxo Inc, and Merck Sharp & Dohme. L.H. reports funding grants from IQVIA. The remaining authors declare no competing financial interests.
Correspondence: Lotta Hansson, Department of Hematology, Karolinska University Hospital, Eugeniavägen 3, Solna, 171 76 Stockholm, Sweden; email: lotta.hansson@regionstockholm.se.
References
Author notes
Presented, in part, at the 66th annual meeting of the American Society of Hematology, San Diego, CA, 7 to 10 December 2024.
The individual participant data underlying this article were subject to ethical approval and cannot be shared publicly. Data from the de-identified administrative health registries are not freely available because of protection of the personal integrity of the participants.
The full-text version of this article contains a data supplement.