Key Points
Voruciclib administered on days 1 to 14 in 28-day cycles was well tolerated with no DLT up to the target dose of 200 mg.
Voruciclib modulated MCL1 messenger RNA, RNA polymerase 2 phosphorylation, and MYC and NF-κB transcriptional programs in patient samples.
Visual Abstract
The antiapoptotic protein, myeloid cell leukemia-1 (Mcl-1), contributes to the pathophysiology of acute myeloid leukemia (AML) and certain B-cell malignancies. Tumor dependence on Mcl-1 is associated with resistance to venetoclax. Voruciclib, an oral cyclin-dependent kinase (CDK) inhibitor targeting CDK9, indirectly decreases Mcl-1 protein expression and synergizes with venetoclax in preclinical models. This dose escalation study evaluated voruciclib in patients with previously treated hematologic malignancies. Initially, voruciclib was administered daily, continuously, on a 28-day cycle (group 1). After 2 patients with prior allogeneic stem cell transplantation had a dose-limiting toxicity (DLT) of interstitial pneumonitis at 100 mg, voruciclib administration was changed to days 1 to 14 of a 28-day cycle (group 2). Forty patients, 21 with AML and 19 with B-cell malignancies, were enrolled. Patients had a median of 3 prior lines of therapy (range, 1-8). Dose escalation in group 2 was stopped at 200 mg, a dose that achieved plasma concentrations sufficient for target inhibition, without DLTs observed. The most common adverse events were diarrhea (30%), nausea (25%), anemia (22%), fatigue (22%), constipation (17%), dizziness (15%), and dyspnea (15%). In AML, 1 patient achieved a morphologic leukemia-free state, and 2 had stable disease. Voruciclib treatment led to a decrease in MCL1 messenger RNA expression, downregulation of myelocytomatosis (MYC) and NF-κB transcriptional gene sets, and reduced phosphorylation of RNA polymerase 2. Voruciclib on intermittent dosing was well tolerated, with no DLTs, paving the way for evaluation of the combination of voruciclib with venetoclax for patients with previously treated AML. This trial was registered at www.clinicaltrials.gov as #NCT03547115.
Introduction
B-cell leukemia/lymphoma-2 (Bcl-2) family proteins are important regulators of apoptosis in normal and malignant cells of hematopoietic origin, including acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and B-cell non-Hodgkin lymphomas (NHL). The myeloid cell leukemia-1 (Mcl-1) protein, a member of the Bcl-2 family, interacts with other proteins in this family and is dynamically regulated at both the messenger RNA (mRNA) and protein level. Inhibition of Mcl-1 is sufficient to induce apoptosis in AML and CLL, and patients with AML and CLL with lower MCL1 mRNA and protein expression levels have improved outcome.1-3
Increased tumor dependence on Mcl-1 is a common mechanism of resistance to venetoclax, which can be reversed by targeting Mcl-1.4-6 Several BCL2 homology 3 domain mimetics have been evaluated in the clinic to directly inhibit Mcl-1.7,8 However, development of these compounds has been challenging because of on-target cardiovascular toxicity associated with Mcl-1 expression in cardiac tissues.9
Mcl-1 protein levels can also be decreased indirectly by inhibiting transcriptional cyclin dependent kinases (CDK7 and CDK9). CDK7/cyclin H, components of the general transcription factor TFIIH, and CDK9/cyclin T, the catalytic core of the positive transcription elongation factor b, phosphorylate RNA polymerase 2 (RNAP2) at Ser 5 and Ser 2 sites to facilitate initiation and elongation of RNA transcription, respectively.10-15 Early studies with pan-CDK inhibitors for patients with various hematologic malignancies have shown antitumor activity but were associated with off-target toxicities because of a narrow therapeutic index.16 Preclinical studies have shown that specific CDK9 inhibition can decrease Mcl-1 protein expression in various tumor models.17,18 Preferential targeting of CDK9 could be a more effective and possibly safer anticancer therapy approach than pan-CDK inhibitors.
Expression of the transcription factor myelocytomatosis (MYC), which regulates cell growth and proliferation, is also mediated by RNAP2.19 Deregulated expression of MYC in vivo is indispensable for both tumor initiation and progression, and overexpression of MYC induces lymphoid malignancies in transgenic mice.20-23 Furthermore, Mcl-1 and MYC cooperatively drive tumorigenesis in some tumor models.24 MYC overexpression is associated with chemoresistance and poor outcomes in lymphoma, whereas elevated MYC-immunopositivity in bone marrow is associated with poor outcomes in untreated AML.25-27 Thus, targeting MYC is an attractive therapeutic strategy.
Voruciclib is a potent, oral CDK inhibitor with greater binding affinity toward CDK9 than CDK4, CDK6, and CDK1.28 In preclinical studies in AML, CLL, and diffuse large B-cell lymphoma (DLBCL) models, voruciclib decreased MCL1 transcripts and proteins in a dose-dependent manner, with maximal effect achieved at in vitro concentrations of 1 μM, and synergized with venetoclax as evidenced by increased apoptosis, decreased tumor growth rate, and improved survival in murine xenograft models.29-31 In CLL, voruciclib increased apoptosis in a dose-dependent manner and synergized with the Bcl-2/Bcl-xL inhibitor navitoclax in primary CLL cells in stromal coculture models mimicking the lymph node microenvironment.32
Two phase 1 dose escalation and expansion studies in patients with solid tumors evaluated voruciclib administered once daily either continuously or on days 1 to 14 of 21-day cycles.33,34 The most common adverse events were nausea and diarrhea, which were generally manageable. The maximum tolerated dose (MTD) was 350 mg on continuous dosing and 600 mg on intermittent dosing. Voruciclib pharmacokinetics were dose proportional, with a mean half-life of 28 hours, and a large volume of distribution indicating preferential distribution into tissues compared with plasma.
With the recognition of the preferential effect of voruciclib on CDK9, and the understanding of the role of CDK9 in Mcl-1 and MYC regulation, we conducted this phase 1 study to evaluate the safety, preliminary efficacy, pharmacokinetics, and pharmacodynamics of voruciclib for patients with relapsed or refractory hematologic malignancies.
Methods
Patients
Patients aged ≥18 years of age with a diagnosis of AML, CLL, or B-cell NHL (DLBCL, mantle cell lymphoma, follicular lymphoma, and marginal zone lymphoma) were eligible if they had disease progression after ≥2 prior standard therapies, no prior CDK9 inhibitor exposure, Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate renal and hepatic function. Patients were required to have a neutrophil count of ≥0.5 × 109/L and a platelet count of ≥50 × 109/L at study entry, unless due to disease infiltration in the marrow. Further details on eligibility criteria are available in the protocol (protocol; supplemental Material).
Study design
Dose escalation followed a 3+3 design with an optional expansion cohort of 6 to 12 patients at a dose selected for further evaluation. Initially dose escalation proceeded in cohorts that concurrently enrolled patients with B-cell malignancies and AML. Dose-limiting toxicity (DLT) was assessed in cycle 1 and included grade ≥3 nonhematologic toxicity, grade ≥3 tumor lysis syndrome regardless of prophylaxis, grade ≥3 nonhematologic laboratory abnormalities, febrile neutropenia, and prolonged myelosuppression in AML (details in the protocol in the online supplement). The MTD was defined as the dose level with ≤1 DLT in up to 6 evaluable patients and 1 dose level lower than the dose at which ≥2 DLTs were reported.
Voruciclib was administered once a day and continued until disease progression or intolerability. Initially voruciclib was administered daily continuously in 28-day cycles at the starting dose of 50 mg (group 1). Based on toxicities observed in group 1, the protocol was amended to change voruciclib administration to days 1 to 14 in a 28-day cycle (group 2), exclude prior allogeneic stem cell transplantation, and conduct dose escalation in separate cohorts for AML and B-cell malignancies. The intermittent dosing schedule was selected empirically based on the voruciclib mean half-life of 28 hours, with the first 14 days aimed at achieving steady state plasma concentrations for at least 10 days, then ∼7 days for drug clearance from plasma and tissue, followed by 7 days free of drug exposure.
Assessments
The schedule of patient visits and safety assessments is found in the protocol (online supplement). Tumor lysis syndrome monitoring in patients with CLL and AML was specified and treatment, if required, followed institutional guidelines. Use and type of antimicrobial prophylaxis was not mandated and left to the investigator’s choice, except for azoles that were not permitted in cycle 1. Disease response assessment was performed using the International Workshop on CLL 2008 criteria in CLL, Lugano 2014 criteria in NHL, and the European LeukemiaNet (ELN) 2017 criteria in AML, and reported based on the investigator’s assessment.35-37 Blood samples for pharmacokinetic analysis and correlative studies were obtained in cycle 1.
Correlative studies
Pharmacodynamic analyses were performed on longitudinal blood samples collected during cycle 1: day 1 predose and 6 hours postvoruciclib dosing, day 2 predose, day 8 predose, and end of treatment. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll gradient and resuspended in ammonium-chloride-potassium lysing buffer (Life Technologies) for red blood cell lysis. Cells were viably frozen in fetal bovine serum with 10% dimethyl sulfoxide and stored in liquid nitrogen until use.
Mcl-1 and pRNAP2Ser2 proteins in PBMCs were quantified by flow cytometry. Gene expression studies included quantitative real-time polymerase chain reaction and RNA sequencing (RNA-seq) with gene set enrichment analysis. Detailed methods can be found in the correlative studies methods; supplemental Methods. RNA-seq data are available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=.
Analysis
All statistical analyses were descriptive and performed in the intent to treat population. Ratios were calculated with their 95% confidence intervals. Pharmacokinetic parameters were calculated by noncompartmental analysis. Correlative study analyses were performed in GraphPad Prism.
Study conduct and oversight
This study (registered at www.clinicaltrials.gov as #NCT03547115) was conducted in accordance with applicable regulatory requirements and in accordance with updated Declaration of Helsinki and International Conference on Harmonization good clinical practice guidelines. The protocol was approved by each study site’s independent ethics committee, and all patients provided written informed consent before enrolling in the study. The study was designed with input from the investigators and sponsored by MEI Pharma (San Diego, CA). Data collection and trial procedures were overseen by the investigators. Data were verified by the sponsor, analyzed by sponsor statisticians, and interpreted by academic authors and sponsor representatives. The manuscript was prepared by the authors, and all authors had final responsibility for content and the decision to submit for publication.
Results
Patients
Between August 2018 and August 2022, 40 patients were enrolled at 11 centers, including 21 with AML and 19 with B-cell malignancies. Demographics and disease characteristics are summarized in Table 1. The median age was 70 years (range, 40-90) and 73% of patients were aged ≥65 years. Baseline Eastern Cooperative Oncology Group performance status was 1 in 73% of patients. In the 19 patients with B-cell malignancies, the median number of prior therapies was 4 (range, 1-7), 4 patients (21%) had prior chimeric antigen receptor T-cell therapy, and 3 (16%) prior allogeneic stem cell transplant. In the 21 patients with AML, the median number of prior lines of therapy was 3 (range, 2-8), 19 patients (90%) had prior venetoclax, 12 (57%) prior anthracyclines, and 2 (10%) prior allogeneic stem cell transplant. In patients with AML, the 2017 ELN genetic risk was classified as adverse in 15 patients (71%) and intermediate in 6 (29%), with the most common adverse mutation affecting TP53 in 6 patients (29%), and RUNX1 and ASXL1 in 4 (19%) each. Enrollment in the 200-mg dose level in B-cell malignancies in group 2 was closed after only 1 patient was enrolled because of slow accrual in this cohort during the COVID-19 pandemic. There were no DLTs in 4 patients with AML enrolled in the 200-mg dose level, and an additional 6 patients were enrolled in an expansion cohort at this dose.
Demographics and disease characteristics
| Parameter . | B-cell malignancies/AML continuous dosing . | B-cell malignancies intermittent dosing . | . | AML intermittent dosing . | Total (N = 40) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 8) . | 100 mg (n = 8) . | 100 mg (n = 3) . | 150 mg (n = 3) . | 200 mg (n = 1) . | 100 mg (n = 3) . | 150 mg (n = 4) . | 200 mg (n = 4) . | 200 mg expansion (n = 6) . | |||
| Sex, n (%) | |||||||||||
| Female | 4 (50.0) | 6 (75.0) | 0 | 2 (66.7) | 0 | 0 | 1 (25.0) | 1 (25.0) | 5 (83.3) | 19 (47.5) | |
| Race, n (%) | |||||||||||
| Asian | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.5) | |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 1 (16.7) | 2 (5.0) | |
| White | 7 (87.5) | 8 (100.0) | 2 (66.7) | 3 (100.0) | 1 (100.0) | 3 (100.0) | 3 (75.0) | 4 (100.0) | 5 (83.3) | 36 (90.0) | |
| Other | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.5) | |
| Age (y) | |||||||||||
| Median (range) | 77 (53-90) | 70 (40-84) | 68 (59-73) | 63 (63-78) | 67 | 71 (61-72) | 77 (65-83) | 60 (57-70) | 74 (63-80) | 70 (40-90) | |
| ≥65, n (%) | 6 (75.0) | 7 (87.5) | 2 (66.7) | 1 (33.3) | 1 (100.0) | 2 (66.7) | 4 (100.0) | 1 (25.0) | 5 (83.3) | 29 (72.5) | |
| ECOG PS, n (%) | |||||||||||
| 0 | 1 (12.5) | 3 (37.5) | 2 (66.7) | 0 | 0 | 0 | 1 (25.0) | 1 (25.0) | 1 (16.7) | 9 (22.5) | |
| 1 | 7 (87.5) | 5 (62.5) | 1 (33.3) | 3 (100.0) | 1 (100.0) | 3 (100.0) | 3 (75.0) | 3 (75.0) | 5 (83.3) | 31 (77.5) | |
| Tumor type, n (%) | |||||||||||
| AML | 0 | 4 (50.0) | 0 | 0 | 0 | 3 (100.0) | 4 (100.0) | 4 (100.0) | 6 (100.0) | 21 (52.5) | |
| CLL | 1 (12.5) | 0 | 1 (33.3) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 3 (7.5) | |
| DLBCL | 5 (62.5) | 2 (25.0) | 0 | 1 (33.3) | 1 (100.0) | 0 | 0 | 0 | 0 | 9 (22.5) | |
| FL | 1 (12.5) | 1 (12.5) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (7.5) | |
| MCL | 1 (12.5) | 1 (12.5) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (7.5) | |
| MZL | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (2.5) | |
| Prior therapies | |||||||||||
| Median (range) | 4 (2-6) | 4 (1-8) | 2 (2-6) | 3 (2-8) | 4 | 3 (3-7) | 3.50 (2-5) | 2.50 (2-3) | 3 (2-6) | 3 (1-8) | |
| ≥3, n (%) | 7 (87.5) | 6 (75.0) | 1 (33.3) | 2 (66.7) | 1 (100.0) | 3 (100.0) | 3 (75.0) | 2 (50.0) | 5 (83.3) | 30 (75.0) | |
| Prior HSCT | 1 (12.5) | 3 (37.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (10.0) |
| Parameter . | B-cell malignancies/AML continuous dosing . | B-cell malignancies intermittent dosing . | . | AML intermittent dosing . | Total (N = 40) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 8) . | 100 mg (n = 8) . | 100 mg (n = 3) . | 150 mg (n = 3) . | 200 mg (n = 1) . | 100 mg (n = 3) . | 150 mg (n = 4) . | 200 mg (n = 4) . | 200 mg expansion (n = 6) . | |||
| Sex, n (%) | |||||||||||
| Female | 4 (50.0) | 6 (75.0) | 0 | 2 (66.7) | 0 | 0 | 1 (25.0) | 1 (25.0) | 5 (83.3) | 19 (47.5) | |
| Race, n (%) | |||||||||||
| Asian | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.5) | |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 1 (16.7) | 2 (5.0) | |
| White | 7 (87.5) | 8 (100.0) | 2 (66.7) | 3 (100.0) | 1 (100.0) | 3 (100.0) | 3 (75.0) | 4 (100.0) | 5 (83.3) | 36 (90.0) | |
| Other | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.5) | |
| Age (y) | |||||||||||
| Median (range) | 77 (53-90) | 70 (40-84) | 68 (59-73) | 63 (63-78) | 67 | 71 (61-72) | 77 (65-83) | 60 (57-70) | 74 (63-80) | 70 (40-90) | |
| ≥65, n (%) | 6 (75.0) | 7 (87.5) | 2 (66.7) | 1 (33.3) | 1 (100.0) | 2 (66.7) | 4 (100.0) | 1 (25.0) | 5 (83.3) | 29 (72.5) | |
| ECOG PS, n (%) | |||||||||||
| 0 | 1 (12.5) | 3 (37.5) | 2 (66.7) | 0 | 0 | 0 | 1 (25.0) | 1 (25.0) | 1 (16.7) | 9 (22.5) | |
| 1 | 7 (87.5) | 5 (62.5) | 1 (33.3) | 3 (100.0) | 1 (100.0) | 3 (100.0) | 3 (75.0) | 3 (75.0) | 5 (83.3) | 31 (77.5) | |
| Tumor type, n (%) | |||||||||||
| AML | 0 | 4 (50.0) | 0 | 0 | 0 | 3 (100.0) | 4 (100.0) | 4 (100.0) | 6 (100.0) | 21 (52.5) | |
| CLL | 1 (12.5) | 0 | 1 (33.3) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 3 (7.5) | |
| DLBCL | 5 (62.5) | 2 (25.0) | 0 | 1 (33.3) | 1 (100.0) | 0 | 0 | 0 | 0 | 9 (22.5) | |
| FL | 1 (12.5) | 1 (12.5) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (7.5) | |
| MCL | 1 (12.5) | 1 (12.5) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (7.5) | |
| MZL | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (2.5) | |
| Prior therapies | |||||||||||
| Median (range) | 4 (2-6) | 4 (1-8) | 2 (2-6) | 3 (2-8) | 4 | 3 (3-7) | 3.50 (2-5) | 2.50 (2-3) | 3 (2-6) | 3 (1-8) | |
| ≥3, n (%) | 7 (87.5) | 6 (75.0) | 1 (33.3) | 2 (66.7) | 1 (100.0) | 3 (100.0) | 3 (75.0) | 2 (50.0) | 5 (83.3) | 30 (75.0) | |
| Prior HSCT | 1 (12.5) | 3 (37.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (10.0) |
ECOG PS Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; HSCT, hematopoietic stem cell transplant; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma.
Exposure and patient disposition
All patients have discontinued voruciclib, 23 (57.5%) because of disease progression, 9 (22.5%) because of an adverse event, 6 (15%) because of investigator’s decision, and 2 (5%) after withdrawal of consent. The median duration of therapy was 5.3 weeks (range, 0.9-22.3), with no meaningful differences in treatment duration in the daily and intermittent dosing groups. Five patients, 4 in group 1 and 1 in group 2, temporarily interrupted voruciclib dosing for adverse event management. Dose escalation in group 2 was stopped at 200 mg in the AML and B-cell malignancies groups without reaching the MTD because this dose achieved trough plasma concentrations considered sufficient for target inhibition based on preclinical models.
Safety
In the 100-mg continuous administration dose, 2 patients developed a DLT of interstitial pneumonitis, which precluded further dose escalation on this schedule and led to the switch to intermittent dosing. The first case was observed in a 40-year-old female with secondary AML after myelodysplastic syndrome and relapsed disease after 7 lines of therapy including allogeneic stem cell transplant and subsequent acute graft-versus-host disease. The patient developed a grade 2 differentiation syndrome (fever and hypoxia) on day 5 of cycle 1, which was treated with corticosteroids and hydroxyurea. On day 13, the patient was diagnosed with grade 3 interstitial pneumonitis without respiratory failure, constituting a DLT, which subsequently resolved with corticosteroid treatment. The second case occurred in a 67-year-old female with secondary AML after myelodysplastic syndrome and relapsed disease after 2 lines of therapy including allogeneic stem cell transplantation and subsequent chronic graft-versus-host disease. On day 26 of cycle 1 the patient developed grade 3 radiologically confirmed interstitial pneumonitis accompanied by hypoxemic respiratory failure, constituting a DLT, which resolved within 4 weeks. Of note, although it did not meet formal criteria for a DLT, a third patient, a 90-year-old female with DLBCL and relapsed disease after 3 prior lines of therapy, developed grade 4 acute hypoxemia respiratory failure with bilateral diffuse radiographic opacities during cycle 4, which was treated was high dose corticosteroids and recovered 3 weeks later.
No DLTs were reported on voruciclib with intermittent dosing of voruciclib at either 100, 150, or 200 mg dosing levels.
The most common adverse events (all grades, grade ≥3) were diarrhea (30%, 2.5%), nausea (25%, 0%), anemia (22.5%, 17.5%), fatigue (22.5%, 0%), constipation (17.5%, 0%), and anorexia (17.5%, 0%), with incidence by disease and dose level shown in Table 2. The most common grade 3/4 adverse events were anemia (17.5%), sepsis (12.5%), febrile neutropenia, hypotension, lymphopenia, neutropenia, and thrombocytopenia (7.5% each), with incidence by disease and dose level shown in Table 3. There was no grade 3/4 aspartate aminotransferase/alanine aminotransferase elevation in any patient, and no grade 3/4 neutropenia in 19 patients with B-cell malignancies.
Adverse events in 10% or more of patients
| . | B-cell malignancies/AML continuous dosing . | B-cell malignancies intermittent dosing . | AML intermittent dosing . | Total N = 40 . | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 8) . | 100 mg (n = 8) . | 100 mg (n = 3) . | 150 mg (n = 3) . | 200 mg (n = 1) . | 100 mg (n = 3) . | 150 mg (n = 4) . | 200 mg (n = 10) . | Total (N = 40) . | |
| Diarrhea | 2 (25.0) | 1 (12.5) | 1 (33.3) | 1 (33.3) | 0 | 1 (33.3) | 1 (25.0) | 5 (50.0) | 12 (30.0) |
| Nausea | 2 (25.0) | 1 (12.5) | 1 (33.3) | 0 | 1 (100.0) | 0 | 1 (25.0) | 4 (40.0) | 10 (25.0) |
| Anemia | 2 (25.0) | 0 | 0 | 0 | 1 (100.0) | 1 (33.3) | 2 (50.0) | 3 (30.0) | 9 (22.5) |
| Fatigue | 0 | 2 (25.0) | 1 (33.3) | 0 | 0 | 1 (33.3) | 1 (25.0) | 4 (40.0) | 9 (22.5) |
| Constipation | 1 (12.5) | 1 (12.5) | 0 | 1 (33.3) | 0 | 0 | 2 (50.0) | 2 (20.0) | 7 (17.5) |
| Anorexia | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 6 (60.0) | 7 (17.5) |
| Dizziness | 1 (12.5) | 1 (12.5) | 0 | 1 (33.3) | 1 (100.0) | 0 | 1 (25.0) | 1 (10.0) | 6 (15.0) |
| Dyspnea | 1 (12.5) | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 2 (30.0) | 6 (15.0) |
| Sepsis | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 2 (50.0) | 2 (20.0) | 5 (12.5) |
| Vomiting | 1 (12.5) | 1 (12.5) | 0 | 0 | 1 (100.0) | 0 | 1 (25.0) | 1 (10.0) | 5 (12.5) |
| Chills | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 2 (20.0) | 4 (10.0) |
| Epistaxis | 0 | 0 | 0 | 0 | 0 | 0 | 2 (50.0) | 2 (20.0) | 4 (10.0) |
| Hypertension | 1 (12.5) | 1 (12.5) | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (10.0) | 4 (10.0) |
| Hypotension | 1 (12.5) | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 4 (10.0) |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (25.0) | 2 (20.0) | 4 (10.0) |
| Pyrexia | 0 | 1 (12.5) | 0 | 0 | 0 | 2 (66.7) | 1 (25.0) | 0 | 4 (10.0) |
| . | B-cell malignancies/AML continuous dosing . | B-cell malignancies intermittent dosing . | AML intermittent dosing . | Total N = 40 . | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 8) . | 100 mg (n = 8) . | 100 mg (n = 3) . | 150 mg (n = 3) . | 200 mg (n = 1) . | 100 mg (n = 3) . | 150 mg (n = 4) . | 200 mg (n = 10) . | Total (N = 40) . | |
| Diarrhea | 2 (25.0) | 1 (12.5) | 1 (33.3) | 1 (33.3) | 0 | 1 (33.3) | 1 (25.0) | 5 (50.0) | 12 (30.0) |
| Nausea | 2 (25.0) | 1 (12.5) | 1 (33.3) | 0 | 1 (100.0) | 0 | 1 (25.0) | 4 (40.0) | 10 (25.0) |
| Anemia | 2 (25.0) | 0 | 0 | 0 | 1 (100.0) | 1 (33.3) | 2 (50.0) | 3 (30.0) | 9 (22.5) |
| Fatigue | 0 | 2 (25.0) | 1 (33.3) | 0 | 0 | 1 (33.3) | 1 (25.0) | 4 (40.0) | 9 (22.5) |
| Constipation | 1 (12.5) | 1 (12.5) | 0 | 1 (33.3) | 0 | 0 | 2 (50.0) | 2 (20.0) | 7 (17.5) |
| Anorexia | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 6 (60.0) | 7 (17.5) |
| Dizziness | 1 (12.5) | 1 (12.5) | 0 | 1 (33.3) | 1 (100.0) | 0 | 1 (25.0) | 1 (10.0) | 6 (15.0) |
| Dyspnea | 1 (12.5) | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 2 (30.0) | 6 (15.0) |
| Sepsis | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 2 (50.0) | 2 (20.0) | 5 (12.5) |
| Vomiting | 1 (12.5) | 1 (12.5) | 0 | 0 | 1 (100.0) | 0 | 1 (25.0) | 1 (10.0) | 5 (12.5) |
| Chills | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 2 (20.0) | 4 (10.0) |
| Epistaxis | 0 | 0 | 0 | 0 | 0 | 0 | 2 (50.0) | 2 (20.0) | 4 (10.0) |
| Hypertension | 1 (12.5) | 1 (12.5) | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (10.0) | 4 (10.0) |
| Hypotension | 1 (12.5) | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 4 (10.0) |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (25.0) | 2 (20.0) | 4 (10.0) |
| Pyrexia | 0 | 1 (12.5) | 0 | 0 | 0 | 2 (66.7) | 1 (25.0) | 0 | 4 (10.0) |
Data presented as n (%).
Grade 3/4 adverse events in 5% or more of patients
| . | B-cell malignancies/AML continuous dosing . | B-cell malignancies intermittent dosing . | AML intermittent dosing . | Total N = 40 . | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 8) . | 100 mg (n = 8) . | 100 mg (n = 3) . | 150 mg (n = 3) . | 200 mg (n = 1) . | 100 mg (n = 3) . | 150 mg (n = 4) . | 200 mg (n = 10) . | Total (N = 40) . | |
| Anemia | 2 (25.0) | 0 | 0 | 0 | 0 | 0 | 2 (50.0) | 3 (30.0) | 7 (17.5) |
| Sepsis | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 2 (50.0) | 2 (20.0) | 5 (12.5) |
| Febrile neutropenia | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 3 (7.5) |
| Hypotension | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 3 (7.5) |
| Lymphopenia | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 3 (7.5) |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 2 (20.0) | 3 (7.5) |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (25.0) | 1 (10.0) | 3 (7.5) |
| Epistaxis | 0 | 0 | 0 | 0 | 0 | 0 | 2 (50.0) | 0 | 2 (5.0) |
| Hyperglycemia | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (5.0) |
| Hypoalbuminemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 2 (5.0) |
| Pneumonia | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 2 (5.0) |
| Rectal abscess | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (10.0) | 2 (5.0) |
| Respiratory failure | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (5.0) |
| Syncope | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 2 (5.0) |
| Urinary incontinence | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 2 (5.0) |
| Leukopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (10.0) | 2 (5.0) |
| . | B-cell malignancies/AML continuous dosing . | B-cell malignancies intermittent dosing . | AML intermittent dosing . | Total N = 40 . | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 8) . | 100 mg (n = 8) . | 100 mg (n = 3) . | 150 mg (n = 3) . | 200 mg (n = 1) . | 100 mg (n = 3) . | 150 mg (n = 4) . | 200 mg (n = 10) . | Total (N = 40) . | |
| Anemia | 2 (25.0) | 0 | 0 | 0 | 0 | 0 | 2 (50.0) | 3 (30.0) | 7 (17.5) |
| Sepsis | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 2 (50.0) | 2 (20.0) | 5 (12.5) |
| Febrile neutropenia | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 3 (7.5) |
| Hypotension | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 3 (7.5) |
| Lymphopenia | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 3 (7.5) |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 2 (20.0) | 3 (7.5) |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (25.0) | 1 (10.0) | 3 (7.5) |
| Epistaxis | 0 | 0 | 0 | 0 | 0 | 0 | 2 (50.0) | 0 | 2 (5.0) |
| Hyperglycemia | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (5.0) |
| Hypoalbuminemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 2 (5.0) |
| Pneumonia | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 2 (5.0) |
| Rectal abscess | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (10.0) | 2 (5.0) |
| Respiratory failure | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (5.0) |
| Syncope | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 2 (5.0) |
| Urinary incontinence | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (10.0) | 2 (5.0) |
| Leukopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (10.0) | 2 (5.0) |
Data presented as n (%).
Voruciclib was discontinued because of an adverse event in 9 patients, including 3 cases of pneumonitis as detailed above, which were all considered to be related to voruciclib and 6 cases where the adverse event was considered unrelated to voruciclib (1 case each of sepsis, mucormycosis, hypercalcemia, traumatic intracerebral hemorrhage, dyspnea due to pleural effusion, and metastatic neuroendocrine carcinoma).
Seven patients had an adverse event with a fatal outcome, including 2 patients with AML with sepsis, 2 patients with AML with intestinal complications from their disease (intestinal ischemia and diverticular perforation), 1 patient with AML with aspiration pneumonia, 1 patient with CLL with traumatic intracranial hemorrhage after a mechanical fall, and 1 patient with CLL with newly diagnosed metastatic neuroendocrine carcinoma.
Efficacy
Of 21 patients with AML, 1 in the 100 mg continuous dosing group achieved a morphologic leukemia-free state that was ongoing when the patient was discontinued from the study in cycle 4 because of the need for a concomitant medication prohibited by the protocol. This was an 81-year-old female with adverse risk AML, with complex karyotype and TP53 mutation, and relapsed disease after 4 prior lines of therapies, including venetoclax. Bone marrow count was 6% at baseline and 4% at the end of cycle 1 and upon discontinuation from the study in cycle 4. In addition, 9 patients had stabilization of bone marrow blasts, which lasted ≥3 months in 2 patients and met the criteria for stable disease by 2017 ELN criteria. Of 19 patients with B-cell malignancies, 4 patients had a reduction in sum of the product of the dimensions of lymph nodes, ranging from 4% to 49% (Table 4), but objective responses were not observed by formal criteria.
Characteristics of patients with B-cell malignancies experiencing tumor reduction
| Patient . | Diagnosis . | No. of prior therapies . | Therapy duration, d . | Baseline SPD, cm2 . | Nadir SPD, cm2 . | Change SPD, % . |
|---|---|---|---|---|---|---|
| 1 | FL | 2 | 126 | 49.8 | 25.4 | −49% |
| 2 | DLBCL | 3 | 110 | 14.5 | 10.5 | −28% |
| 3 | CLL | 5 | 155 | 74.5 | 69.5 | −7% |
| 4 | MZL | 4 | 154 | 28.4 | 27.2 | −4% |
| Patient . | Diagnosis . | No. of prior therapies . | Therapy duration, d . | Baseline SPD, cm2 . | Nadir SPD, cm2 . | Change SPD, % . |
|---|---|---|---|---|---|---|
| 1 | FL | 2 | 126 | 49.8 | 25.4 | −49% |
| 2 | DLBCL | 3 | 110 | 14.5 | 10.5 | −28% |
| 3 | CLL | 5 | 155 | 74.5 | 69.5 | −7% |
| 4 | MZL | 4 | 154 | 28.4 | 27.2 | −4% |
SPD, sum perpendicular diameters.
Pharmacokinetics
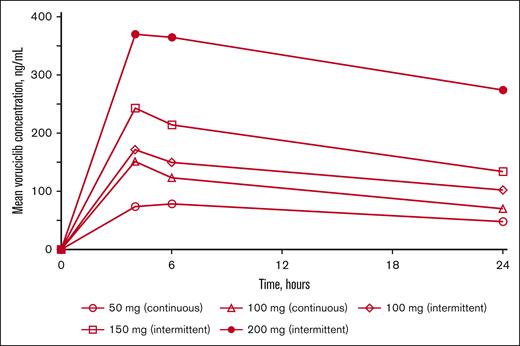
Voruciclib plasma concentration profiles are shown in Figure 1 and pharmacokinetic parameters are presented in supplemental Table 1. Maximum plasma concentrations of voruciclib were achieved typically at ∼4 hours after dose, and voruciclib plasma exposure was nearly dose-proportional in the dose range studied. Overall, plasma concentration profiles were consistent with historical data reflecting an average half-life of ∼28 hours. At the 200 mg dose, based on expected twofold accumulation after once-daily dosing, mean steady state voruciclib Ctrough exceeded 450 ng/mL, a concentration shown to have synergistic activity with venetoclax in an AML preclinical model.31
Mean voruciclib plasma concentration–time profiles after single-dose administration on day 1.
Mean voruciclib plasma concentration–time profiles after single-dose administration on day 1.
Treatment with voruciclib downmodulates oncogenic pathways
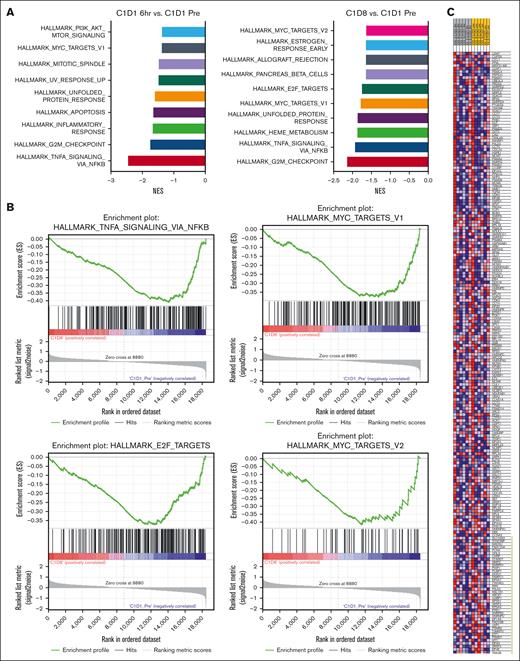
To identify gene expression changes in response to voruciclib, transcriptomics analysis was conducted by bulk RNA-seq on pretreatment and posttreatment PBMC samples from 6 patients with AML who were dosed with 100 to 200 mg of voruciclib. Principal component analysis revealed that samples clustered by patient rather than by time point, indicating significant heterogeneity in baseline activity of signaling pathways between individual patients (supplemental Figure 1). We detected expression of 22 055 protein coding genes. Using a cutoff of at least 1.5-fold change we identified 952 genes whose expression was affected by voruciclib. Among these samples, 11 genes were significantly deregulated at 6 hours (adjusted P < .05) and 50 genes were significantly deregulated after 8 days of treatment. We further analyzed the downregulated genes for functional significance and found that several oncogenic pathways were significantly associated with the downregulated genes as early as 6 hours and after 8 days after initial voruciclib exposure, including MYC transcriptional targets, tumor necrosis factor signaling via NF-κB, and cell cycle early region 2 binding factor targets and G2M checkpoint (Figure 2A-B). We determined that expression of genes known to be regulated by MYC was significantly affected by voruciclib at 6 hours (P < .05; 41 genes were enriched at 6 hours vs cycle 1, day 1 pretreatment; Figure 2C). RNA-seq analysis of PBMCs demonstrated a trend toward reduction in MYC, and MCL1 mRNA transcript levels in AML samples, whereas CDK9 levels remained unchanged (data not shown).
Gene set enrichment analyses (GSEA) from patients with AML after voruciclib treatment. RNA-seq was performed on PBMCs from 6 patients with AML treated with 100 to 200 mg voruciclib. Normalized gene counts were compiled from bulk RNA-seq analysis and processed using GSEA to identify NES for the top 10 Hallmark pathways by P value (A). (B) GSEA enrichment plots of significantly downregulated oncogenic pathways. (C) Heat map depicting the ranked list of genes with core enrichment for the Hallmark MYC targets V1 pathway. The range of expression values are visualized as red (high) to blue (low). Green bar indicates enriched genes. All data sets shown are represented as on-treatment vs cycle 1 day 1 (C1D1) pretreatment and statistical significance is represented as P < .05. NES, normalized enrichment scores.
Gene set enrichment analyses (GSEA) from patients with AML after voruciclib treatment. RNA-seq was performed on PBMCs from 6 patients with AML treated with 100 to 200 mg voruciclib. Normalized gene counts were compiled from bulk RNA-seq analysis and processed using GSEA to identify NES for the top 10 Hallmark pathways by P value (A). (B) GSEA enrichment plots of significantly downregulated oncogenic pathways. (C) Heat map depicting the ranked list of genes with core enrichment for the Hallmark MYC targets V1 pathway. The range of expression values are visualized as red (high) to blue (low). Green bar indicates enriched genes. All data sets shown are represented as on-treatment vs cycle 1 day 1 (C1D1) pretreatment and statistical significance is represented as P < .05. NES, normalized enrichment scores.
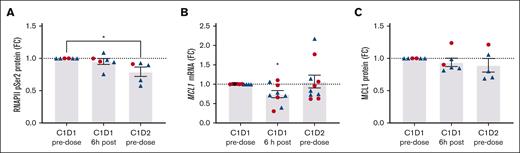
Flow cytometry was used to evaluate protein levels and posttranslational modifications in circulating blasts and PBMCs from patients with AML dosed with 100 to 200 mg of voruciclib. Reduced levels of pRNAP2Ser2 were observed in AML blasts after voruciclib treatment (Figure 3A; supplemental Figure 2A), with a significant decrease observed between the pretreatment sample and start of cycle 2 pretreatment time points. MCL1 mRNA in PBMCs in on-treatment samples was significantly decreased compared with pretreatment; however, the decrease in Mcl-1 protein in AML blasts did not reach significance (Figure 3B-C; supplemental Figure 2B).
Voruciclib-induced pharmacodynamic changes in pRNAP2Ser2 and Mcl-1 in samples from patients with AML. Voruciclib dose of 100 to 150 mg (blue) or 200 mg (red). (A) Levels of pRNAP2Ser2 protein in circulating malignant cells were analyzed by flow cytometry. Data are shown as pRNAP2Ser2 mean fluorescent intensity fold change (FC; P = .042; n = 6). (B) mRNA expression of MCL1 gene in PBMCs was quantified by quantitative real-time polymerase chain reaction. Data are shown as mRNA FC (n = 10). (C) Analysis of Mcl-1 protein by flow cytometry in circulating blasts from patients with AML expressed as FC of mean fluorescent intensity (n = 6). For panels A-C, data shown are FC for samples taken at C1D1 at 6 hours after voruciclib dose and C2D1 before dose vs C1D1 before dose. ∗P ≤ .05.
Voruciclib-induced pharmacodynamic changes in pRNAP2Ser2 and Mcl-1 in samples from patients with AML. Voruciclib dose of 100 to 150 mg (blue) or 200 mg (red). (A) Levels of pRNAP2Ser2 protein in circulating malignant cells were analyzed by flow cytometry. Data are shown as pRNAP2Ser2 mean fluorescent intensity fold change (FC; P = .042; n = 6). (B) mRNA expression of MCL1 gene in PBMCs was quantified by quantitative real-time polymerase chain reaction. Data are shown as mRNA FC (n = 10). (C) Analysis of Mcl-1 protein by flow cytometry in circulating blasts from patients with AML expressed as FC of mean fluorescent intensity (n = 6). For panels A-C, data shown are FC for samples taken at C1D1 at 6 hours after voruciclib dose and C2D1 before dose vs C1D1 before dose. ∗P ≤ .05.
RNA-seq analysis was also performed on pretreatment and posttreatment PBMC samples from patients with CLL/NHL. Consistent with the pharmacodynamic effects observed in AML samples, we detected downregulation of tumor necrosis factor signaling via NF-κB. In addition, we observed a decrease in the oxidative phosphorylation gene set in response to voruciclib treatment (supplemental Figure 3A). There was also a trend toward reduced mRNAs for MYC, MCL1, and BCL2A1 in samples from patients with CLL while on treatment (supplemental Figure 3B).
Discussion
In this phase 1 study conducted in patients with relapsed/refractory AML and NHL, we found that single-agent voruciclib administered at doses up to 200 mg for 14 consecutive days on a 28-day cycle was well tolerated, with no DLTs, and achieved target inhibition based on our correlative studies. Although single-agent activity was minimal, the study successfully identified the voruciclib dose to take forward into a combination study with venetoclax.
Venetoclax has emerged as a highly effective new standard of care for treating CLL and AML; however, a common resistance mechanism is Mcl-1 overexpression.38,39 Overexpression of Mcl-1 is associated with poor prognosis in AML and B-cell malignancies.1-3 CDK9 inhibitors could synergize with venetoclax by downregulating expression of Mcl-1 and thereby reduce venetoclax resistance.
Early-generation small-molecule pan-CDK inhibitors such as flavopiridol/alvocidib and dinaciclib have shown activity in hematologic malignancies, as single agent or in combination with chemotherapy. However, their development has been challenged by toxicities because of off target effects, including a high incidence of serious adverse events and discontinuations because of toxicities.40-43 In addition, tumor lysis syndrome has been reported in CLL and AML, requiring prophylactic measures and careful monitoring.44,45
Our aim in this single-agent dose escalation study was to evaluate the safety and pharmacodynamic activity of voruciclib at doses that achieve plasma concentrations in the 1 μM range shown to inhibit CDK9, MCL1, and MYC in preclinical models, paving the way for the evaluation of voruciclib in combination with venetoclax.31 Based on pharmacokinetic results from phase 1 studies in solid tumors, we anticipated to reach these concentrations at voruciclib doses of ∼200 mg.33,34 The continuous dosing schedule evaluated initially resulted in 2 DLTs of interstitial pneumonitis in patients with AML administered voruciclib at 100 mg, a dose lower than our target dose, and substantially lower than 350 mg that was identified as the MTD with continuous daily dosing in a phase 1 study in solid tumors. Interstitial pneumonitis had not been reported in >70 patients with solid tumors who received voruciclib at doses up to 800 mg. It is unclear whether the 2 cases were direct drug toxicity or were confounded by prior intensive therapy and allogeneic stem cell transplantation with graft-versus-host disease in both cases, differentiation syndrome in 1, or other factors unique to AML.
The intermittent schedule evaluated in our study was selected empirically based on the voruciclib mean half-life that was longer than 1 day, with the first 14 days aimed at achieving steady state plasma concentrations for at least 10 days, then ∼7 days for drug clearance from plasma and tissue, followed by 7 days free of drug exposure. Voruciclib administered on days 1 to 14 of 21-day cycles had been evaluated in patients with solid tumors and identified 600 mg as the MTD. Rather than using this schedule in our study, we opted for a 14 days on/14 days off dosing to match venetoclax administration schedule in 28-day cycles. Unlike other CDK9 inhibitors that are administered intravenously, voruciclib oral administration is not only convenient to patients but also provides flexibility to evaluate various dosing schedules for schedule optimization.
This intermittent dosing schedule was well tolerated, with no DLTs observed at the doses evaluated. The most common nonhematologic adverse events were gastrointestinal or constitutional, and all were low-grade events. No significant neutropenia was observed in patients with B-cell NHL at the doses studied, which supports future studies of voruciclib in combination with venetoclax, because the latter is known to frequently cause neutropenia.
Voruciclib antitumor activity in the heavily pretreated patients enrolled in the study was modest, with 1 of 3 patients with AML at 100 mg achieving morphologic leukemia free state, and 2 of 10 patients with AML at 200 mg achieving stable disease lasting at least 3 months. It is possible that more frequent voruciclib dosing in a cycle, for example on days 1 to 21, would have achieved greater efficacy by reducing the duration of treatment-free intervals to 7 days. It is also possible that doses of >200 mg may have resulted in more objective responses. However, because single-agent CDK9 inhibitors on their own are not expected to induce significant antileukemic effects, we decided to stop dose escalation at a safer dose before reaching the MTD to focus on exploring the combination with venetoclax, with a more comprehensive dose-range evaluation ongoing.
Pharmacodynamic correlative studies suggested that doses up to 200 mg decreased RNAP2Ser2 phosphorylation, and the RNA-seq data demonstrated downregulation of MYC transcriptional targets and oxidative phosphorylation pathways. The effects of voruciclib on Mcl-1 protein expression were more modest. We observed that MCL1 RNA levels were significantly decreased at 6 hours after dose on day 1. Given rapid Mcl-1 protein turnover, this might be sufficient time to observe protein suppression. However, voruciclib does not reach its maximal plasma concentration until ∼4 hours, and therefore 6 hours after dose may still be too early to observe downstream protein-level changes. In future trials we plan to also test after several days of dosing to evaluate more pronounced Mcl-1 protein suppression.
Although single-agent activity of voruciclib was modest, this aligns with the expectation that CDK9 inhibitors alone may not provide sufficient antileukemic efficacy. However, the scientific rationale for combining voruciclib with venetoclax remains strong. Resistance to venetoclax in AML and B-cell malignancies is frequently driven by increased dependence on Mcl-1 in response to Bcl-2 inhibition. CDK9 inhibition downregulates Mcl-1 transcription, and although voruciclib did not significantly lower baseline Mcl-1 protein levels, we hypothesize that in the presence of venetoclax-induced Mcl-1 dependence, voruciclib will reverse this adaptive resistance mechanism, enhancing the efficacy of venetoclax. This is consistent with previous findings that combinations of venetoclax with a FLT3 inhibitor, such as gilteritinib, or with a mitogen-activated extracellular kinase inhibitor, such as cobimetinib, can suppress the compensatory upregulation of Mcl-1, contributing to the observed synergy in preclinical models.46,47
Given the compelling scientific rationale of indirectly targeting Mcl-1, numerous other CDK9 inhibitors are in early stages of development, although published data at this time are limited.48 The infusional AZD4573 showed some activity as a single agent in peripheral T-cell lymphoma and various B-cell malignancies, and promising objective response rate in combination with acalabrutinib in previously treated DLBCL; however, the combination was associated with a high rate of grade ≥3 toxicity, hence its development was terminated.49,50 Other infusional CDK9 inhibitors include PRT-2527 currently under evaluation in solid tumors and lymphomas, enitociclib currently being studied in combination with venetoclax in lymphoid malignancies, and GFH-009 studied in various hematologic malignancies and solid tumors.51-53 Orally administered CDK9 inhibitors include KB-0742 being studied in MYC-addicted tumors, TP-1287 in solid tumors, and fadraciclib in solid tumors and hematologic malignancies.54 It is unlikely that comparative data between these various new agents will be available. Therefore, we believe that careful evaluation of dose and schedule informed by rigorous pharmacokinetic and correlative pharmacodynamic analyses as performed in our study will be critical to understand the potential benefits and risks of each of these drugs.
In summary, our phase 1 study defined an intermittent voruciclib dosing schedule that is well tolerated at doses that achieve pharmacodynamic evidence of decreased RNAP2Ser2 phosphorylation and inhibition of MCL1 and MYC transcription, thereby setting the stage for a combination trial of voruciclib plus venetoclax that is currently underway.
Acknowledgments
The authors thank patients for their participation in the study. Ingrid Koo contributed to manuscript preparation.
M.S.D. is supported by the National Institutes of Health award R01CA266298. A.V.D. is supported by Leukemia & Lymphoma Society Translational Research Program award 6517-22.
Authorship
Contribution: M.S.D., Y.A.-V., A.V.D., and R.G.G. were responsible for protocol development; M.S.D., D.M.B., Y.A.-V., C.S.D., D.N.E., S.N.D., M.M.A.M., K.H.B., V.R.B., S.A., R.J.C., and A.V.D. were responsible for patient management and data collection; R.G.G., S.E.W., M.S.D., N.J.-S., and A.V.D. were responsible for study analyses; P.R. was responsible for pharmacokinetic analysis; M.S.D., N.J.-S., M.C.C., C.R., E.C.D., and A.V.D. were responsible for correlative studies; and all authors contributed to data review and manuscript preparation.
Conflict-of-interest disclosure: M.S.D. has received institutional research funding from AbbVie, AstraZeneca, Ascentage Pharma, Genentech, MEI Pharma, Novartis, and Surface Oncology, and reports personal consulting income from AbbVie, Adaptive Biosciences, Ascentage Pharma, AstraZeneca, BeiGene, Bristol Myers Squibb, Eli Lilly, Genentech, Genmab, Janssen, Merck, MEI Pharma, Nuvalent, Secura Bio, TG Therapeutics, and Takeda. D.M.B has received consultancy fees from AbbVie, Genentech, Pharmacyclics, Pfizer, Verastem, ArQule/Merck, and TG Therapeutics, and research funding from ArQule, Ascentage, AstraZeneca, BeiGene, DTRM, Genentech, Juno, Loxo, Novartis, Pharmacyclics, and TG Therapeutics. Y.A.-V. has received research funding from Jazz, BerGenBio, MEI Pharma, Astex, Sun Pharma, FibroGen and Daiichi-Sankyo, and consultancy fees from CytomX and Sun Pharma. C.S.D. is equity holder of Gilead and OverT Therapeutics; serves on the advisory board of Genmab, AbbVie, Regeneron, F. Hoffman-La Roche Ltd/Genentech Inc, Seattle Genetics, and Merck; and has received research funding from Bristol Myers Squibb/Celgene, Merck, AbbVie, Novartis, Cargo, and Nektar. D.N.E. has received research funding from Novartis, Bristol Myers Squibb, Pfizer, Rigel, and Kite/Gilead. S.N.D. has received consultancy fees from Pfizer, Rigel, and Kite/Gilead. M.M.A.M. has received consultancy fees from and was on the advisory boards of Hansa Biopharma, CareDx, NMDP, Incyte, Gilead, NexImmune, and Stemline Therapeutics; and has received research funding from Gilead and NexImmune. K.H.B. served on the advisory board of Novartis. V.R.B. reports participating in the safety monitoring committee for Protagonist; serves as an associate editor for the journal, Current Problems in Cancer, and as a contributor for BMJ Best Practice; reports receiving consulting fees from Imugene, Sanofi, and Taiho; reports research funding (institutional) from MEI Pharma, Actinium Pharmaceutical, Sanofi US Services, AbbVie, Pfizer, Incyte, Jazz, and National Marrow Donor Program; and reports drug support (institutional) from Chimerix for a trial. S.A. has received consultancy fees from AbbVie, Daiichi-Sankyo, and Servier, and reports research funding from Incyte, AltruBio, and Actinium Pharmaceutical. P.R. is a consultant to MEI Pharma. S.E.W. is an employee of MEI Pharma. R.G.G. is an employee of, and holds equity shares in, MEI Pharma. A.V.D has received consulting fees from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Genmab, Incyte, Janssen, Lilly Oncology, MEI Pharma, Merck, Nurix, and Prelude, and has ongoing research funding from AbbVie, AstraZeneca, Bayer Oncology, BeiGene, Bristol Myers Squibb, Cyclacel, Genmab, Incyte, Lilly Oncology, MEI Pharma, Merck, MorphoSys and Nurix. The remaining authors declare no competing financial interests.
Correspondence: Matthew S. Davids, Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: matthew_davids@dfci.harvard.edu; and Alexey V. Danilov, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; email: adanilov@coh.org.
References
Author notes
Deidentified individual participant data that underlie the reported results are available on request from the author, Richard G. Ghalie (rghalie@meipharma.com). The data will be made available for a period of 1 year after the publication date. If available, the data will be provided electronically in Excel worksheets, within a reasonable timeframe. The study protocol is included as a data supplement available with the online version of this article.
The full-text version of this article contains a data supplement.