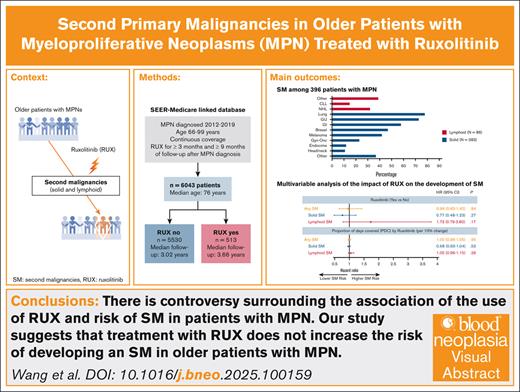
Key Points
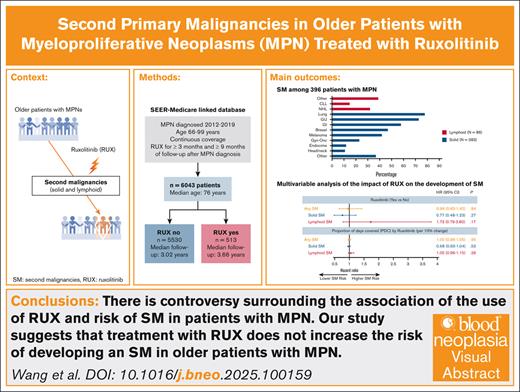
There is controversy surrounding the association between RUX use and the risk of SM in patients with MPN.
Our study suggests that treatment with RUX does not increase the risk of developing an SM in older patients with MPN.
Visual Abstract
Polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF) are classical Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs) associated with an increased risk of development of second primary malignancies (SMs). The reasons for solid and lymphoid SMs are unclear, but a therapy-related effect has been invoked. Although an increased risk of nonmelanoma skin cancers is associated with the use of ruxolitinib (RUX), its influence on the development of other forms of SMs remains controversial. We conducted a retrospective cohort analysis to assess associations between RUX and SMs among older patients diagnosed with MPN from 2012 to 2019 in the Surveillance, Epidemiology, and End Results–Medicare–linked database. We identified 6043 patients (2396 with PV, 2944 with ET, 703 with MF) with a median age of 76 years (interquartile range [IQR], 71-82). After a median follow-up of 3.66 (IQR, 2.25-5.17) and 3.02 years (IQR, 1.84-4.75) for 513 RUX users and 5530 nonusers, respectively, 469 patients developed an SM: 383 solid and 86 lymphoid. In the multivariable proportional subdistribution hazard regression model with death as a competing risk, the risk of developing any SM did not differ by RUX use status (hazard ratio [HR], 0.96; 95% confidence interval [CI], 0.65-1.42; P = .84) or by proportion of days covered by RUX (every 10% increase; HR, 1.00; 95% CI, 0.96-1.05; P = .95). RUX exposure did not affect the risk of solid SM (HR, 0.77; 95% CI, 0.48-1.23; P = .27) or lymphoid SM (HR, 1.73; 95% CI, 0.79-3.80; P = .17). Our results suggest that RUX use does not increase the risk of SM in older patients with MPN.
Introduction
Polycythemia vera (PV), essential thrombocythemia (ET), and primary and secondary myelofibrosis (MF) are Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs) associated with driver mutations in the Janus kinase–signal transducer and activator of transcription (JAK-STAT) signaling pathway, including mutations in JAK2, calreticulin (CALR), or thrombopoietin receptor (MPL) genes.1 The risk of clonal evolution to more aggressive myeloid neoplasms, including development of MF, myelodysplastic syndrome/neoplasms (MDS), and transformation to acute myeloid leukemia (AML), among patients with MPN is well established.2 In addition, MPNs also carry an increased risk of developing second primary malignancies (SMs), including lymphoproliferative disorders and solid organ malignancies.3,4
Aberrant activation of the JAK-STAT signaling pathway has been associated with the development of solid tumors,5 lymphoid malignancies,3,6 and progression to other myeloid neoplasms.7 Certain cytotoxic therapies used historically for MPNs, such as pipobroman, radioactive phosphorus, and chlorambucil, have been associated with therapy-related SMs in this patient population.8,9 The association of hydroxyurea (HU) with SMs, beyond the increased incidence of nonmelanoma skin cancers (NMSCs), has been debated.10 Ruxolitinib (RUX), a selective JAK1/2 inhibitor, was approved by the US Food and Drug Administration (FDA) in 2011 for treating symptomatic MF and, since 2014, for treating HU-refractory or -intolerant PV.11 Although RUX is not approved for ET, RUX after HU therapy was compared to best available therapy and showed similar rates of complete response at the end of 1 year of treatment and improved some disease-related symptoms.12 Its administration is useful in certain circumstances as recommended by the National Comprehensive Cancer Network.13 RUX effectively reduces hematocrit in patients with PV and controls symptoms and splenomegaly in both PV and MF.14-16 Additionally, RUX improves survival in MF as well as event-free survival, thromboembolic-free survival, and molecular responses among patients with PV.17,18
RUX use has been conclusively linked to an increased risk of NMSCs.19-21 However, the relationship between JAK inhibitors and the development of other SMs has not been clearly elucidated, partly due to the limited follow-up time in prospective studies.16,22-24 The JAK-STAT signaling pathway is a critical intracellular cascade that regulates gene expression involved in immunity, hematopoiesis, and cell survival. The immunosuppressive effects of RUX can lead to JAK-STAT pathway inhibition and impairment of immune surveillance, potentially resulting in an increased risk of SM.25 The expanded-access program study evaluating the safety and efficacy of RUX in MF reported that SMs developed in 6.1% of the study population, raising concerns for RUX-associated oncogenicity.26 Nevertheless, of 137 patients who developed SMs, 60 (43.8%) had NMSCs.27 A previous study reported that 4 of 69 patients (5.8%) on JAK1/2 inhibitor treatment (RUX, gandotinib, fedratinib, or momelotinib) and 2 of 557 conventionally treated patients (0.36%) developed aggressive B-cell lymphomas.6 Increased interest in the potential association between RUX and incidence of lymphoid malignancies led to additional retrospective analysis with diverging conclusions.28,29
The association between JAK inhibitors and SMs was recently explored in a large prospective, randomized, safety end point trial of tofacitinib, a JAK inhibitor indicated for rheumatoid arthritis, aimed at evaluating the incidence of major adverse cardiovascular events and cancer. The risk of developing cancer was higher in the patients who received tofacitinib than tumor necrosis factor inhibitors, especially in patients aged ≥65 years in a prespecified subgroup analyses.30 The FDA issued a black box warning after the results of this trial of JAK inhibitors used for the management of rheumatoid arthritis (tofacitinib, upadacitinib, and baricitinib) to highlight the risk of SM, which has been considered a class effect.31 In fact, all currently approved JAK inhibitors used for PV (RUX) and MF (RUX, fedratinib, pacritinib, and momelotinib) carry the warning regarding SMs on their labels for that reason.11,32-34
Population-based studies evaluating the relationship between RUX and SMs in patients with MPN are limited and differ in their methods and findings. Our study goal was to evaluate the effect of RUX on the development of SM among a large, contemporarily treated group of older patients with MPN in the United States.
Methods
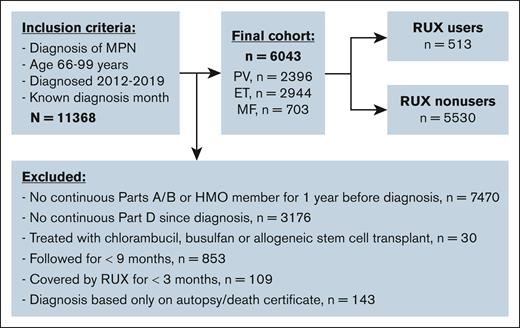
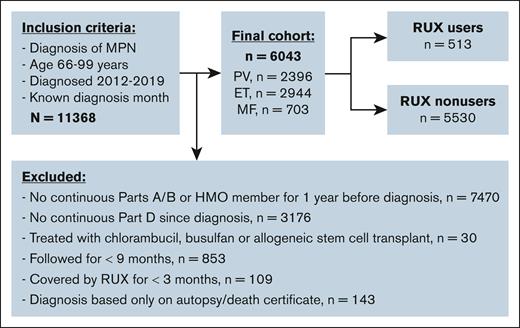
We conducted a retrospective cohort study of older adults with MPN in the Surveillance, Epidemiology, and End Results (SEER)–Medicare–linked database. We identified patients with MPN including PV, ET, and MF according to the International Classification of Diseases (ICD) for Oncology third edition (codes 9950, 9961, and 9962). Patients were required to (1) be diagnosed between 2012 and 2019; (2) be aged 66 to 99 years at the time of diagnosis; (3) have continuous enrollment in Medicare parts A and B from 1 year before MPN diagnosis through the end of follow-up; and (4) have continuous part D coverage from diagnosis to the end of follow-up. Patients were excluded from analysis if they (1) were members of health maintenance organizations; (2) were treated with chlorambucil, busulfan, or allogeneic stem cell transplant; (3) were followed for <9 months after MPN diagnosis; (4) had been covered by RUX for <3 months; and (5) were diagnosed only based on death certificate or autopsy findings. We followed patients from MPN diagnosis to 31 December 2020, SM diagnosis, death, or change of insurance status, whichever came first.
We established the use of RUX after the diagnosis of MPN via Medicare part D claims. Reverse causality was addressed by excluding patients who started RUX within the 6-month period before SM diagnoses or the end of follow-up. RUX proportion of days covered (PDC) was calculated as the ratio of the number of days the patient was covered by RUX to the number of days from RUX initiation to 6 months before the end of follow-up, provided the patient had been covered by RUX for at least 3 months. According to this definition, a patient who never received RUX would have a PDC of 0%, whereas a patient taking RUX daily from initiation of therapy to 6 months before the end of follow-up would have a PDC of 100%.
Our outcome of interest was the first SM, which was defined as the first new malignancy other than a myeloid malignancy or NMSC, diagnosed at least 6 months after cohort entry. Evolution to a more aggressive myeloid malignancy including MDS and AML were excluded, because patients with MF selected for RUX treatment usually have a higher-risk disease prone to progression. Because cancer information reported to SEER registries was only available through the end of 2019, we used SEER data from 2012 to 2019 and Medicare claims from 2020 to identify the first SM. To confirm a new SM diagnosis from Medicare claims, we used ICD, 10th revision, Clinical Modification codes and required that patients to not have the cancer reported from SEER before 2020 and have either 1 inpatient claim or 2 outpatient claims with SM diagnosis at least 30 days apart. The date of SM diagnosis was defined as the date of the first qualifying SM claim, and a 12-month period without a similar claim was required to ensure the SM was truly a new diagnosis. We categorized SMs as solid or lymphoid malignancies.
We obtained patient sociodemographic characteristic data, including age at diagnosis, sex, race and ethnicity, marital status, SEER region, cancer history, frailty status,35,36 Elixhauser comorbidity score,37 census tract Yost index,38 and state buy-in. To construct the Elixhauser score, we searched for ICD-9 and ICD-10 diagnosis codes in the 12 months before MPN diagnosis that appeared on any inpatient claim or on at least 2 outpatient/physician claims >30 days apart.39 The frailty index by Kim et al, which has been validated in Medicare beneficiaries aged ≥65 years, uses a cumulative deficit approach to estimate frailty, with beneficiaries being categorized as nonfrail or frail during the 12 months before diagnosis.35,36 State buy-in was a proxy for individual socioeconomic status.
Descriptive statistics were used to summarize baseline patient characteristics. Categorical variables were presented using frequencies and percentages and compared between RUX users and nonusers using Pearson χ2 tests. Medians and interquartile ranges (IQRs) were calculated for continuous variables. The cumulative incidence function (CIF) of SMs was computed via a competing risk model. Death and development of an SM other than the malignancy of interest (solid or lymphoid) were considered as competing events. Gray test was used to assess differences across strata. The impact of RUX on the development of an SM was assessed by multivariable proportional subdistribution hazard regression models with competing risks. We first analyzed RUX as a binary variable and then as a continuous variable by every 10% change of PDC. RUX and HU use were investigated as time-varying variates. Patients were initially considered nonusers before treatment initiation and users thereafter for the remainder of follow-up. The models were adjusted for age at MPN diagnosis, sex, race, marital status, SEER region, history of prior malignancy, frailty status,35,36 Elixhauser comorbidity score,37 census tract Yost index,38 state buy-in, and prior HU exposure. We also assessed the association of RUX use with solid and lymphoid SMs separately. For each specific subtype of SMs, besides death, other SM types were also considered as competing events. Sensitivity analyses were conducted by further restricting RUX users to those who had used RUX for at least 6 months and had been followed for 12 months. All statistical tests were 2-sided, with a type I error of 0.05 to achieve statistical significance, and were performed with SAS version 9.4 (SAS Inc, Cary, NC). The Yale University Human Investigation Committee determined that there was no direct involvement of human participants in the conduction of this project.
Results
The final cohort included 6043 patients (2396 with PV, 2944 with ET, and 703 with MF) with a median age at diagnosis of 76 years (IQR, 71-82; Figure 1). A total of 513 patients (9.8%) initiated RUX after a median of 0.53 years (IQR, 0.13-1.98) from diagnosis. Patients received RUX for a median of 1.33 years (IQR, 0.71-2.51) and had a median PDC of 94.4% (IQR, 79.9%-99.3%). RUX users were more likely than nonusers to be younger, male, have MF and a history of prior malignancy, and reside in metropolitan areas (Table 1). In addition, fewer RUX users were frail or used HU. The study followed patients for up to 9.0 years (median, 3.09 [IQR, 1.92-4.83]) and 1524 patients (25.2%; 82 RUX users and 1342 nonusers) died during follow-up. The median follow-up time was similar among RUX users (3.66 years [IQR, 2.25-5.17]) and nonusers (3.02 years [IQR, 1.84-4.75]).
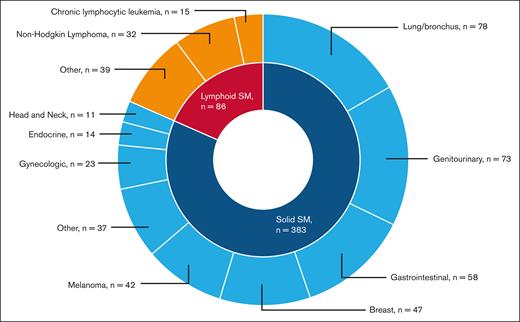
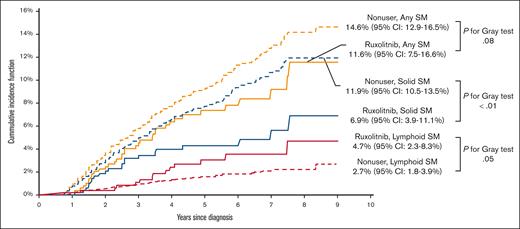
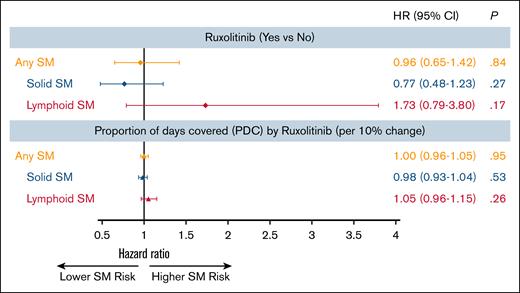
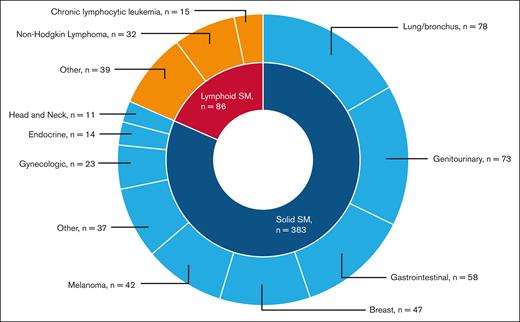
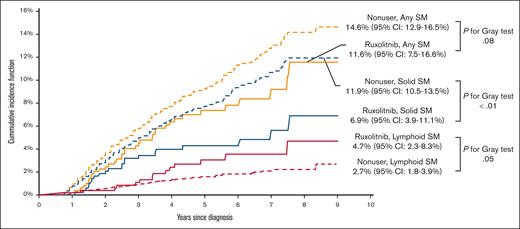
A total of 469 patients (7.8%) developed an SM: 383 (6.3%) had solid SMs, and 86 (1.4%) had lymphoid SMs. The 3 most common types of solid SMs were lung and bronchus cancer (n = 78 [20.4%]), breast cancer (n = 47 [12.3%]), and prostate cancer (n = 43 [11.2%]). Among patients with lymphoid SMs, 32 (37.2%) were diagnosed with non-Hodgkin lymphomas (Figure 2). Approximately 7.0% of RUX users and 7.8% of nonusers developed an SM. As shown in Figure 3, the CIF of any SM was 11.6% (95% confidence interval [CI], 7.6-16.6) and 14.6% (95% CI, 12.9-16.5) among RUX users and nonusers, respectively (Gray test, P = .08). In the multivariable competing risk model, after adjusting for sociodemographic characteristics and comorbidities, the risk of developing any SM did not differ by RUX use status (hazard ratio [HR], 0.96; 95% CI, 0.65-1.42; P = .84) or RUX PDC (every 10% increase; HR, 1.00; 95% CI, 0.96-1.05; P = .95; Table 2; Figure 4). There was no difference in the risk of SM development among patients with different MPNs (PV, ET, and MF) by RUX use status or RUX PDC (Table 2).
Types of malignancies among 396 patients with MPN who developed a second malignancy.
Types of malignancies among 396 patients with MPN who developed a second malignancy.
Multivariable analysis of the impact of RUX on the development of SMs among patients with MPN∗. ∗Multivariable models were adjusted for age at MPN diagnosis, sex, race, marital status, SEER region, history of previous malignancy, frailty status, Elixhauser comorbidity score, census tract Yost index, state buy-in, and previous HU exposure.
Multivariable analysis of the impact of RUX on the development of SMs among patients with MPN∗. ∗Multivariable models were adjusted for age at MPN diagnosis, sex, race, marital status, SEER region, history of previous malignancy, frailty status, Elixhauser comorbidity score, census tract Yost index, state buy-in, and previous HU exposure.
Of 383 patients who developed a solid SM, 22 patients received RUX. The CIF of solid SMs was lower among RUX users (6.9%; 95% CI, 3.9-11.1) than nonusers (11.9%; 95% CI, 10.5-13.5; Gray test, P < .01; Figure 3). In the multivariable competing risk model, the risk of developing a solid SM was not influenced by RUX use status (HR, 0.77; 95% CI, 0.48-1.23; P = .27) or RUX PDC (every 10% increase; HR, 0.98; 95% CI, 0.93-1.04; P = .53; Figure 4).
Of 86 patients who developed a lymphoid SM, only 14 (16.3%) ever received RUX. The CIF of lymphoid SMs was 4.7% (95% CI, 2.3-8.3) and 2.7% (95% CI, 1.7-3.9) for RUX users and nonusers, respectively (Gray test, P = .05; Figure 3). The multivariable competing risk model also showed no difference between RUX users and nonusers (RUX ever use [HR, 1.73; 95% CI, 0.79-3.80; P = .17]; every 10% increase in RUX PDC [HR, 1.05; 95% CI, 0.96-1.15; P = .26]; Figure 4). In an exploratory analysis of RUX users vs nonusers by MPN subgroup, we observed that RUX users in the combined PV/ET subgroup, but not in the MF subgroup, had a higher risk of lymphoid SM than nonusers (HR, 3.47; 95% CI, 1.57-7.66; P < .01). The risk of solid SM was not increased among RUX users in both MPN subgroups.
Sensitivity analysis
Among 5751 patients followed for at least 12 months, 32 of 441 patients (7.3%) who received RUX for >6 months and 395 of 5310 patients (7.4%) who never used RUX developed an SM. There was no significant difference in the incidence of any SM between both groups (HR, 0.97; 95% CI, 0.64-1.47; P = .90), nor in the risk of specific SM subtypes (solid [HR, 0.85; 95% CI, 0.52-1.38; P = .50] or lymphoid [HR, 1.47; 95% CI, 0.63-3.41; P = .37]).
Discussion
We conducted a large, comprehensive, US-based cancer registry analysis of 6043 older adults diagnosed with MPN from 2012 through 2019 and identified 469 individuals (7.8%) who developed a lymphoid or solid SM after a median follow-up of 3.0 years. Our definition of SM excluded MPN progression to more aggressive myeloid malignancies, such as MDS and AML, because patients with MF selected for treatment with RUX typically have a higher-risk disease that is prone to such progression. The CIF for developing any SM was similar among RUX users and nonusers: 11.6% and 14.6%, respectively (P = .08). The risk of any SM, including solid or lymphoid subtypes, did not differ by RUX utilization status or by RUX PDC in multivariable analyses. Sensitivity analyses were performed to address any potential for lead-time bias with patients followed for at least 12 months with RUX users on RUX for at least 6 months, and we did not observe a difference in the incidence of SM or its subtypes.
The overall risk of developing an SM in our study is slightly higher than what has been reported in the literature for individuals with MPNs (7.8%). Two observational studies showed a cumulative incidence of any SM of up to 9.9% at 5 years and 15.2% at 10 years, with an increasing risk of a new SM among patients aged >60 years.40,41 In a SEER study, Brunner et al reported a 5- and 10-year SM CIF of 8% and 12.7%, respectively.41 These studies included individuals who developed additional myeloid malignancies. An observational study of 820 patients diagnosed with PV and ET at a median age of 59 years reported a cumulative risk of developing a lymphoid SM of 0.93% at 5 years and 2.96% at 10 years,3 comparable to our observations. The above studies included younger patient populations, whereas our study was limited to only older patients (age ≥66 years at diagnosis). The higher CIF of SM observed in our cohort may be attributable to the older age of the patient population.
The most common solid SM in our cohort was lung and bronchus cancer, consistent with previous studies excluding NMSCs.41 Breast cancer and melanoma were the next most prevalent SMs, which differ in frequency across the literature.4,42 Non-Hodgkin lymphoma was the most frequently observed lymphoid SM identified, consistent with prior studies showing an increased risk of lymphoid SM in patients with MPNs.43 This pattern raised concerns about a potential link to JAK inhibitors, particularly after a report by Porpaczy et al describing a 16-fold increased risk of aggressive B-cell lymphomas in patients treated with JAK1/2 inhibitors.6 In an exploratory subgroup analysis, we observed that RUX users with PV/ET but not MF had a higher risk of lymphoid SM than nonusers. However, the median follow-up for patients with PV/ET (3.17 years [IQR, 1.92-4.92]) was longer than for patients with MF (2.59 years [IQR, 1.57-4.19]; P < .01). In addition, the small number of patients in the PV/ET subgroup limits the reliability of this finding. Additional studies have not demonstrated a significant difference in the incidence of lymphoid SM in patients with MPNs after RUX exposure, suggesting that the increased risk may be related to the underlying MPN biology.28,44 These studies focused on patients with MF, and additional investigation of RUX effect on incidence of lymphoid SM among patients with PV/ET is needed. NMSCs are often reported in patients receiving cytoreductive therapies with HU and RUX15,21,45; however, we were unable to assess for this risk given the limitations in NMSC reporting within the SEER database.
In alignment with our findings, Barbui et al did not describe an excess risk of carcinomas or hematologic SMs among RUX-exposed compared with unexposed patients with MPN.46 Similarly, Thomas et al reported no difference in the incidence of non-AML SM in patients with MF treated with RUX.44 Separate observational studies also failed to show a significant difference in the incidence of SM in RUX-naïve patients with MF compared to those treated with RUX, after excluding patients diagnosed with NMSCs.22,29 Furthermore, a qualitative review of the 5-year follow-up of 2 large, randomized, phase 3 clinical trials in MF (COMFORT-I and COMFORT-II), 2 phase 3 trials in PV (RESPONSE and RESPONSE-2), and 1 phase 2 trial in PV (MAJIC-PV) showed inconsistent increased incidence of SM in RUX-exposed patients.15,17,47-49 Overall, the lower incidence of SM in these randomized studies than our cohort may be related to differences in patients’ median age: 60 to 70 years vs 76 years. Data assessing the risk of SM exclusively in a population of patients with ET are limited, because RUX is not FDA approved for this indication.11
Although fedratinib received FDA approval for MF treatment in August 2019,50 its use remained limited within the time frame of our study (2012-2020). In our cohort, only 23 individuals received fedratinib for a short period of time, precluding meaningful subgroup analysis.
An important strength of this study is the utilization of a large, real-world cohort of older patients with MPN who were diagnosed between 2012 and 2019, after RUX approval. Previous studies have shown that the risk of developing a SM increases over time in the MPN population as well as with increasing patient age.40 Our study was focused on an older population (66-99 years), which may have higher incidence of SM than younger patients with MPN; however, no increased incidence of SM was observed among patients treated with RUX.
Our study has several limitations. The median follow-up period on the study was 3 years, with a median RUX treatment duration of 1.33 years, which could limit our ability to detect the potential role of RUX in the long-term risk of SM development. Notably, prior studies have reported median times to SM from RUX exposure up to 3.8 years (IQR, 2.0-7.0)51 with similar observation periods when excluding NMSC,22 suggesting that a longer follow-up may be necessary to fully capture these events. We were unable to account for the incidence of NMSC in our analysis as SEER does not consistently collect data on this subgroup of skin cancers. The SEER database does not contain laboratory, pathology and molecular testing results, hindering our ability to confirm the SEER-reported diagnosis and perform analyses based on driver mutation status. Our results have limited applicability to younger patients and Medicare-insured patients participating in health maintenance organizations, and do not apply to other JAK inhibitors.
In this large real-world study of older patients with MPN, the risk of SM was similar among RUX users and nonusers suggesting that RUX does not increase the incidence of SM in this population. Longer follow-up studies including younger patients are warranted to further explore the risk for acquiring an SM by patients with MPN.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
J.M.S. is supported by the National Cancer Institute of the National Institutes of Health (award T32CA233414). J.P.B. is supported by the Edwards P. Evans Foundation. The collection of the California cancer incidence data used in this study were supported by the California Department of Public Health, as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s SEER Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries under agreement number U55/CCR921930-02 awarded to the Public Health Institute. This study was supported by the Frederick A. Deluca Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The ideas and opinions expressed herein are those of the author(s) and do not imply endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Authorship
Contribution: R.W., X.M., and N.A.P. conceived the study; R.W. conducted statistical analysis, with input from J.M.S., R.M.S., X.M., and N.A.P.; R.W. and J.M.S. wrote the initial draft of the manuscript; X.M. and N.A.P. supervised all aspects the project; and all authors contributed to study design and interpretation of results, reviewed, critically edited, and approved the final manuscript for publication.
Conflict-of-interest disclosure: R.W. receives funding from Flatiron Health. J.M.S. participated in the advisory board of Sobi. R.M.S. received consulting fees from Bristol Myers Squibb (BMS), Gilead Sciences, Kura Oncology, Rigel, Servier, Syndax, and TScan; and participated in a steering committee for Servier. S.F.H. reports consultancy fees from Janssen, Pharmacyclics, AbbVie, AstraZeneca, Flatiron Health Inc, Novartis, Seagen, Genetech, Merck, TG Therapeutics, ADC Therapeutics, Epizyme, Servier, and Thyme Inc; research funding from Celgene, Zhejiang DTRM Biopharma, and TG Therapeutics; and honoraria form Pharmacyclics, AstraZeneca, and Bayer. A.M.Z. reports consultancy fees and research funding from AbbVie, Acceleron, Amgen, Aprea, Boehringer Ingelheim, Cardiff Oncology, BMS, Incyte, and Novartis; research funding from ADC Therapeutics, Astex, and Pfizer; consultancy fees from Agios Pharmaceuticals, Astellas, BeyondSpring, Daiichi Sankyo, Epizyme, Genentech, Gilead Sciences, Kura, Ionis, Loxo Oncology, Janssen, AstraZeneca, Jasper, and Jazz; serving on clinical trial committees at AbbVie, BioCryst, BMS, Geron, Gilead Sciences, Kura, Loxo Oncology, and Novartis; and travel support from Cardiff Oncology, Novartis, and Pfizer. N.N. reports research funding from GlaxoSmithKline and Janssen; and consultancy fees from CellsBin. X.M. received research funding (institutional) from Celgene/BMS and consults for BMS. N.A.P. received consulting fees from Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene/BMS, CTI BioPharma/Sobi, PharmaEssentia, Constellation Pharmaceuticals/MorphoSys, Aptose Biosciences, AbbVie, Aptose Biosciences, Karyopharm Therapeutics, and Syndax Pharmaceuticals; and other financial support for serving on an independent data review committee for Cogent Biosciences. The remaining authors declare no competing financial interests.
Correspondence: Jessica M. Stempel, Division of Medical Oncology and Hematology, Department of Internal Medicine, Yale School of Medicine, 333 Cedar St, New Haven, CT 06520-8020; email: jessica.stempel@yale.edu.
References
Author notes
R.W. and J.M.S. are joint first authors.
X.M. and N.A.P. are joint senior authors.
The data used in this study were provided through a collaboration between the National Cancer Institute and the Centers for Medicare and Medicaid Services. The Data Use Agreement precludes us from sharing these data.