Recent interest in bone marrow (BM) transplantation in nonconditioned or minimally conditioned recipients warrants investigation of homing patterns of transplanted hematopoietic progenitor cells (HPCs) in irradiated and nonirradiated recipients. To this end, phenotypically defined populations of BM cells were tracked in lethally irradiated or nonirradiated mice at 1, 3, 6, and 24 hours after transplantation. Recovery of transplanted cells at all time points was higher in BM of nonirradiated mice, similar to earlier suggestions. The percentage of lineage-negative Sca-1+cells and Sca-1+ cells expressing CD43, CD49e, and CD49d steadily increased in BM of nonirradiated mice up to 24 hours, while fluctuating in irradiated mice. Cell cycle status and BrdU incorporation revealed that less than 20% of Sca-1+ cells and fewer Sca-1+lin− cells had cycled by 24 hours after transplantation. To more directly examine trafficking of primitive HPCs, purified grafts of CD62L− or CD49e+ subfractions of Sca-1+lin−cells, previously shown to be enriched for long-term repopulating cells, also were tracked in vivo. Recovery of purified cells was similarly increased in BM of nonirradiated mice. When 50 to 100 of these BM-homed cells were examined in serial transplantation studies, BM-homed cells from initially nonirradiated mice were enriched 5- to 30-fold for cells capable of long-term hematopoiesis in secondary recipients. Collectively, these data suggest that homing or survival of transplanted cells in irradiated recipients is less efficient than that in nonirradiated recipients, implicating an active role of radiation-sensitive microenvironmental cues in the homing process. These results may have important clinical implications in the design of BM transplantation protocols.
Introduction
Homing, engraftment, and fate of transplanted hematopoietic stem cells (HSCs) remain poorly understood phenomena. While some studies support the specificity of certain components of the homing process, such as chemotaxis, intravasation, anchorage, survival, and proliferation of transplanted HSCs,1-8 other studies show no differences in the seeding efficiencies of mature9 and primitive bone marrow (BM) stem cell subsets10 or in the distribution of BM-homed cells in secondary recipients,11 suggesting that homing is not specific.
The majority of in vivo tracking studies have focused on examining the trafficking and cycling activity of phenotypically undefined populations of BM cells, with little regard for phenotypic characterization of donor cells homing to specific organs. It is possible that HSCs, which constitute less than 1% of graft cells, may proficiently home to BM, but that their specific homing is concealed by the massive movement of the large cohort of other, more mature BM cells. Studies examining homing of colony-forming cells1-4,6,7 may not accurately reflect the homing of long-term repopulating cells.12-15 Examinations of populations of cells defined on the basis of stem cell phenotype8 are beginning to show a different, possibly more specific, picture of homing. Identification of the adhesion molecule repertoire of primitive hematopoietic progenitor cells (HPCs) homing to the BM shortly after transplantation may provide evidence of adhesion molecules potentially involved in trafficking, homing, and lodging of transplanted HSCs in the BM and may outline a sequential requirement of different adhesion molecules at different stages of homing.
Investigations into the homing of HSCs following transplantation have most commonly been performed in myeloablated recipients. Increased vascular permeability resulting from radiation damage of endothelial and stromal cells16 may lead to nonspecific seeding of transplanted cells in various organs based largely on tissue mass and vascularity. Studies showing broader tissue distribution of donor cells in irradiated recipients11,17,18 and greater recovery of donor cells in BM of nonmyeloablated murine recipients2,11suggest more efficient homing or better survival of primitive HPCs in nonmyeloablated marrow. Homing patterns of primitive HPCs in nonmyeloablated recipients may better portray natural trafficking patterns of HSCs in vivo and, as such, may serve as tools to study natural movement of HPCs between the marrow and periphery. Furthermore, the notion that marrow spaces or “niches” need to be created for successful engraftment of donor HPCs has been recently challenged by studies demonstrating successful engraftment in nonconditioned or minimally conditioned murine and human recipients.19-24These studies warrant further investigation of the fate and homing patterns of transplanted HPCs in nonmyeloablated or minimally myeloablated recipients.
In the present work, trafficking patterns of primitive HPCs were mapped in the first 24 hours after transplantation of grafts consisting of either unfractionated low-density BM cells or purified Sca-1+lin− subfractions. We examined the in vivo distribution, recovery, cell cycle status, adhesion molecule expression patterns, and secondary long-term engraftment potential of cells homing to the BM of irradiated or nonirradiated mice. The results presented herein demonstrate that transplanted HSCs capable of long-term secondary hematopoiesis home more efficiently or survive better in nonirradiated recipients, which may have important clinical implications for BM transplantation in nonablated or minimally ablated patients.
Materials and methods
Mice
C57BL/6 female mice (CD45.2 allele) were purchased from Jackson Laboratories (Bar Harbor, ME) at 8 to 10 weeks of age and allowed to acclimate for 1 to 2 weeks prior to their use in these studies. B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ) congenic mice (CD45.1 allele) were either purchased from Jackson Laboratories or maintained in our breeding colony and used between 8 to 12 weeks of age. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Donor cells
Bone marrow grafts consisted of low-density BM (LDBM) cells or purified Sca-1+lin− cells subfractionated on the basis of CD49e or CD62L expression into fractions enriched for long-term engraftment potential ([ENG], Sca-1+lin−CD49e+ or Sca-1+lin−CD62L−) as previously described.25 Adhesion molecules CD49e and CD62L were chosen in these studies so that grafts would consist of phenotypes where both positive expression (CD49e+) and negative expression (CD62L−) are enriched for long-term repopulating cells, as previously described.25 Donor LDBM or ENG cells were isolated from C57BL/6 or B6.BoyJ mice and stained with either PKH26 or PKH2 (Sigma ImmunoChemicals, St Louis, MO) as previously described,26 27 or with 0.5-1.0 μM CFSE-1 (Molecular Probes, Eugene, OR) according to manufacturer's instructions. After staining, cells were washed extensively and resuspended in complete medium (CM) (Iscove modified Dulbecco medium [IMDM] supplemented with 10% fetal calf serum [FCS; Hyclone, Logan, UT], 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin). All of the CM ingredients (except for FCS) were obtained from BioWhittaker (Walkersville, MD). When available, congenic donor cells were not labeled with any dye and were identified by appropriate CD45.1 or CD45.2 expression by flow cytometry. The choice of the green fluorescent dyes CFSE and PKH2 or the red fluorescent dye PKH26 was based on the nature of the analyses to be performed on the cells after staining. The different tracking methods used in different experiments were evenly distributed along the time course of analyses after transplantation and were equally effective in detecting donor cells. Since CFSE-1–stained cells exhibit aberrant cell surface staining of some antigens for 1 to 2 hours following CFSE-1 staining (C.M.O.-T., unpublished data, 2000), cells were not tracked with CFSE-1 at the 1- and 3-hour time points.
Primary short-term (1°ST) cell tracking
Female recipient C57BL/6 or B6.BoyJ mice between 10 and 12 weeks of age were lethally irradiated with 950 cGy administered in a single dose from a 137Cs gamma irradiator (GammaCell 40; Nordion International, Kanata, ON, Canada) 17.5 to 20 hours prior to transplantation (mean = 18.5 hours). Irradiated or nonirradiated mice received transplants via tail vein injections of 1 × 104to 1 × 108 LDBM donor cells or 4 × 104 to 3 × 105 purified ENG cells. In experiments where BM-homed ENG cells were isolated and assayed for long-term engraftment potential, 1°ST-irradiated recipients were injected with at least 1 × 105 graft cells, while nonirradiated mice received at least 2 × 105 purified cells to facilitate donor cell isolation. Mice were killed 1, 3, 6, and 20 to 24 hours later, and BM, spleen, peripheral blood (PB), lung, and liver were collected and single-cell suspensions prepared and lysed. Lysed cells from LDBM transplants were analyzed for donor cell recovery, primitive phenotype, adhesion molecule expression, cell cycle status, and proliferation history, while lysed cells from ENG transplants were analyzed for donor cell recovery and engraftment potential in serial transplantations.
Calculation of recovery of donor cells
The frequency of donor cells falling within a light scatter gate including lymphocytes and large granular cells, was determined for each harvested tissue, based on the background fluorescence of cells from unmanipulated mice. This frequency was multiplied by the total number of cells in each tissue, then divided by the number of cells in the original graft to calculate the recovery of total transplanted cells. The number of BM cells harvested from both tibias and femurs was considered to represent 18.7% of total murine marrow, or 40% if humeri and pelvic bones were also included.28 The total number of PB cells was calculated assuming the total PB volume to be 2 mL per mouse.29 Because of the low frequency of detectable donor cells when ENG cells were injected, flow cytometric files containing 2 × 105 to 1 × 106 events were saved and the frequency of donor cells calculated manually using event count.
Adhesion molecule and lineage analysis of harvested donor cells
Harvested BM cells from recipients of LDBM grafts were stained with Sca-1 and biotinylated antibodies to either CD11a (clone 2D7), CD43 (clone S7), CD44 (clone IM7), CD49d (clone 9C10), CD49e (clone 5H10-27; MFR5), CD31 (clone MEC 13.3), CD62L (clone MEL-14), or CD3 and CD45R/B220. Biotinylated antibodies were developed with streptavidin-allophycocyanin (APC; Molecular Probes). Donor cells were distinguished from recipient by PKH2, PKH26, CFSE, or appropriate CD45.1 or CD45.2 staining as described above. All antibodies were from BD PharMingen (San Diego, CA). Donor cells exhibiting small light scatter properties characteristic of primitive cells were gated and examined for Sca-1 expression and primitive phenotype (CD3−CD45R/B220−) or adhesion molecule expression using a FACScan or FACSCalibur (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA). Between 1 × 104 and 5 × 105 events were acquired per sample. To avoid saving very large files, in some cases only donor-positive events were saved during flow cytometric acquisition.
Serial transplantation studies
BM-homed donor cells from 1°ST recipients of ENG cells were isolated by flow cytometry, and 50 to 200 of these cells, along with 1 × 105 competitor LDBM cells of recipient origin, were transplanted into primary long-term (1°LT) congenic recipients within 3 hours of receiving lethal irradiation in a split dose of 700 cGy + 350 cGy 4 hours apart. 1°LT recipients were bled from the tail vein monthly for 6 to 7 months for analysis of donor-derived hematopoiesis by determining the percentage of CD45.1+ or CD45.2+ PB leucocytes. In some experiments, 1°LT recipients were killed 6 to 7 months after transplantation, and 2-5 × 106 LDBM cells were transplanted into lethally irradiated (700 cGy + 350 cGy) secondary LT (2°LT) recipients without competitor cells. 2°LT recipients were bled from the tail vein monthly for 6 to 7 months for analysis of donor-derived hematopoiesis. In some experiments, donor-derived cells from 1°LT and 2°LT recipients were analyzed 6 to 7 months after transplantation for the percentage of lineage cells using phycoerythrin (PE)–conjugated antibodies specific for CD3+, CD45/B220+, Gr-1+, and Mac-1+cells.
Cell cycle status
Fresh cells or donor-derived Sca-1+ cells from harvested BM and spleen of transplant recipients were isolated by cell sorting using a FACStarplus or FACSVantage SE flow cytometer (BDIS) and analyzed for cell cycle position using propidium iodide (PI) as previously described.30 The low number of donor Sca-1+ cells attainable from PB precluded cell cycle analyses of these cells.
BrdU administration
Mice received 4 intraperitoneal injections of 100 μg/g BrdU in 200 μL H2O at 36, 24, 12, and 1 hour before receiving transplants of LDBM cells. Mice destined for 24-hour homing studies received an additional injection of BrdU 12 hours after BM transplantation.
BrdU staining
Donor-derived cells from mice administered BrdU were assayed for BrdU uptake by 2 different methods. In the first, donor Sca-1+ cells from BM or spleen at 1, 3, 6, or 24 hours after transplantation were isolated by flow cytometric cell sorting and then stained for BrdU as previously described.31 Briefly, sorted Sca-1+ cells were fixed with 1% formaldehyde (Tousimis, Rockville, MD) and 0.2% Tween 20 (Sigma) in phosphate-buffered saline (PBS) for at least 10 minutes but no longer than 24 hours. Cells were then treated with 4 M HCl in 0.2% Tween 20/PBS for 30 minutes at 37°C, washed with 0.1 M sodium borate (Sigma), and then washed with 0.2% Tween 20/PBS. Cells were then stained with fluorescein isothiocyanate (FITC)–conjugated anti-BrdU antibody (BD PharMingen). Prior to acquisition, PI was added to a final concentration of 10 μg/mL for 60 minutes.
In the second method, bulk unsorted BM or spleen cells were analyzed flow cytometrically using 4-color analysis for donor origin, surface phenotype, and BrdU incorporation simultaneously as previously described32 with slight modifications. Briefly, cells were stained with the appropriate anti-CD45.1–PE or anti-CD45.2–PE to identify donor cells, biotinylated Sca-1, anti-CD3–cychrome, and anti-B220–cychrome, followed by streptavidin-APC, then fixed with 1% formaldehyde overnight. In some experiments Sca-1–cychrome was used with biotinylated lineage markers developed with streptavidin-APC. All antibodies were from BD PharMingen. Cells were permeabilized with 0.1% saponin/PBS (Perm Buffer) plus 2% formaldehyde for 10 minutes at room temperature, pelleted, then permeabilized with 0.2% Tween 20/PBS for 10 minutes at room temperature, washed with Perm Buffer, and incubated with 100 to 300 Kunitz units of DNAseI (Sigma) in Hanks balanced salt solution for 60 minutes at 37°C. Cells were washed in Perm Buffer and then stained with anti-BrdU–FITC (BD PharMingen).
Statistical analysis
Data are expressed as the mean ± SEM where applicable. Differences between groups were analyzed using an unpaired 2-sidedt test. Differences in chimerism were analyzed by repeated measures analysis of variance using an arcsine transformation. A probability value of less than 0.05 was considered significant for all tests.
Results
Identification of graft sizes allowing for adequate detection of homed cells
As a first step in examining the distribution of donor cells in vivo following transplantation, the number of donor cells homing to BM, spleen, or remaining in PB after transplantation was determined. Log increasing doses of donor LDBM cells from 1 × 104 to 1 × 108 cells were injected into single irradiated mice and allowed to home for 3 hours. The frequency of donor cells detected at 3 hours in all 3 tissues examined positively correlated with the number of graft cells injected (Table 1). Based on these results, subsequent experiments were designed to deliver between 17 and 90 × 106 cells per graft.
Recovery of donor cells in LDBM transplants
Total recovery (BM + spleen + PB) of graft cells was calculated based on the frequency of donor cells detected in BM, spleen, and PB, and the total number of nucleated cells within each tissue, as described in “Materials and methods.” The frequency of donor cells ranged from 0.3% in nonirradiated spleen at 24 hours to up to 39% of total cells in 1-hour irradiated PB. The cellularity of irradiated BM, spleen, and PB in recipient mice ranged between 24-55 × 106, 3-8 × 106, and 1-4 × 106, respectively, depending on the time of analysis after irradiation (range, 19.5 hours for 1-hour time points to 42.5 hours for 20- to 24-hour time points). Cellularity of nonirradiated BM, spleen, and PB averaged 212 × 106, 72 × 106, and 14 × 106, respectively.
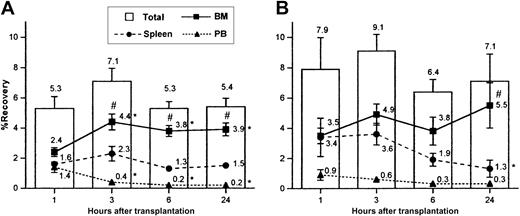
Total recovery of donor cells did not vary significantly between 1 and 24 hours in either transplant setting but was, however, slightly higher in nonirradiated recipients at each time point compared with irradiated mice (bars in Figure 1A-B), in agreement with previous suggestions.2 11 Total recovery in nonirradiated mice was significantly higher than that in irradiated mice when data from all 4 time points were pooled together (7.6 ± 0.6, n = 18; and 5.6 ± 0.3, n = 38, respectively,P < .05). When individual tissues were examined in both transplant settings, recovery of donor cells was highest in BM from 3 to 24 hours after transplantation (Figure 1A-B), reflecting either the specificity of BM homing of graft cells or the relatively larger mass of this tissue, or both. Distribution of donor cells differed with time in the 2 transplant settings. While recovery of donor cells in BM increased rapidly and reached a plateau by 3 hours in irradiated mice, recovery in nonirradiated marrow remained fairly constant. Recovery in nonirradiated BM was significantly greater than that in irradiated BM when data from all 4 time points were pooled together (5.7 ± 0.5%, n = 18; and 3.70% ± 0.2, n = 38; respectively,P < .05). Interestingly, while recovery in irradiated spleen did not change significantly with time, donor cells accumulated initially in nonirradiated spleen and then declined at later time points (Figure 1A-B). As expected, cell recovery was highest in PB at 1 hour but declined thereafter. In initial experiments, lung and liver were also examined for the recovery of donor cells, but since total recovery in the 2 tissues combined was typically less than 0.1%, these tissues were omitted from subsequent analyses.
Primitive phenotype and adhesion molecule phenotype of BM-homed Sca-1+ cells
To gain insight into trafficking of primitive HPCs and adhesion molecules possibly involved in HPC homing, donor-derived Sca-1+ cells homing to BM were phenotyped for lineage and adhesion molecule expression at 1, 3, 6, and 20 to 24 hours after transplantation. Figure 2 shows representative dot plots and histograms for a typical adhesion molecule analysis of donor Sca-1+ cells found in BM 1 hour after transplantation. All phenotypic analyses were performed on donor Sca-1+ cells exhibiting light scatter properties characteristic of primitive HPCs (low side and forward scatter). Frequency of lineage-negative, CD43+, CD49e+, CD49d+, and CD62L+ BM-homed donor Sca-1+ cells increased in nonirradiated mice from 1 to 24 hours after transplantation, while these frequencies mostly fluctuated in irradiated mice (Figure 3A-E). CD11a and CD44 were present on 95% to 100% of donor Sca-1+cells in the graft and all tissues examined and did not differ in irradiated and nonirradiated mice (n = 3-11, data not shown). At 20 to 24 hours, a slightly higher frequency of Sca-1+ cells in nonirradiated mice expressed CD43, CD49e, and CD49d and were CD3−CD45R/B220− (Figure 3), matching a phenotype enriched for long-term engrafting cells.25Interestingly, while approximately 45% to 55% of donor Sca-1+ graft cells and those remaining in PB at 1 hour expressed CD62L, the majority of Sca-1+ cells recovered from BM at 1 hour lacked CD62L expression (Figure 3E).
Percent recovery of total donor cells in BM, spleen, and PB at 1, 3, 6, and 24 hours following transplantation in irradiated or nonirradiated recipients.
Irradiated (A) or nonirradiated (B) mice received 17-90 × 106 LDBM cells, killed at the indicated time points, and analyzed for recovery of donor cells in BM, spleen, and PB as described in “Materials and methods.” Bars represent mean ± SEM recovery of total donor cells (sum of recoveries in BM + spleen + PB), and lines represent mean ± SEM recoveries in BM, spleen, or PB. Numbers at each time point represent mean recoveries. For each time point, n = 7-12 in panel A and n = 3-5 in panel B for each tissue. *P < .05 when compared with 1-hour mean of same tissue, #P < .05 when compared with spleen and PB of same time point. In order to provide an SEM value for total recovery at each time point, total recoveries were not calculated by adding the means of the recoveries from each tissue, but rather by averaging the total recoveries from each individual experiment.
Percent recovery of total donor cells in BM, spleen, and PB at 1, 3, 6, and 24 hours following transplantation in irradiated or nonirradiated recipients.
Irradiated (A) or nonirradiated (B) mice received 17-90 × 106 LDBM cells, killed at the indicated time points, and analyzed for recovery of donor cells in BM, spleen, and PB as described in “Materials and methods.” Bars represent mean ± SEM recovery of total donor cells (sum of recoveries in BM + spleen + PB), and lines represent mean ± SEM recoveries in BM, spleen, or PB. Numbers at each time point represent mean recoveries. For each time point, n = 7-12 in panel A and n = 3-5 in panel B for each tissue. *P < .05 when compared with 1-hour mean of same tissue, #P < .05 when compared with spleen and PB of same time point. In order to provide an SEM value for total recovery at each time point, total recoveries were not calculated by adding the means of the recoveries from each tissue, but rather by averaging the total recoveries from each individual experiment.
Flow cytometric analysis of adhesion molecule expression on donor Sca-1+ cells in irradiated and nonirradiated recipient BM 1 hour after transplantation.
In this representative experiment, 50 × 106 PKH2-stained LDBM cells were transplanted into irradiated (A) or nonirradiated (B) mice. Mice were killed 1 hour later, and BM cells were lysed and stained with Sca-1–PE and CD43-biotin, followed by streptavidin-APC. Cells were analyzed flow cytometrically by collecting listmode files containing 300 to 1000 donor Sca-1+ events exhibiting low side and forward angle scatter, properties characteristic of primitive hematopoietic cells. In this representative experiment, 1 × 105 (A) and 1.1 × 104 (B) total events were collected, which contained 664 and 597 Sca-1+events in histograms A and B, respectively. The frequencies of PKH2+ cells were 26% (A) and 1.2% (B) and the frequencies of Sca-1+ cells within donor cells were 31% (A) and 25% (B), while the frequencies of CD43+ cells within donor Sca-1+ cells were 24% (A) and 17% (B). The frequencies of donor cells when all nucleated cells were included in the analysis were 3.8% (A) and 1.1% (B). To avoid collecting very large files, files were sometimes acquired to exclude recipient cells, as shown in panel B.
Flow cytometric analysis of adhesion molecule expression on donor Sca-1+ cells in irradiated and nonirradiated recipient BM 1 hour after transplantation.
In this representative experiment, 50 × 106 PKH2-stained LDBM cells were transplanted into irradiated (A) or nonirradiated (B) mice. Mice were killed 1 hour later, and BM cells were lysed and stained with Sca-1–PE and CD43-biotin, followed by streptavidin-APC. Cells were analyzed flow cytometrically by collecting listmode files containing 300 to 1000 donor Sca-1+ events exhibiting low side and forward angle scatter, properties characteristic of primitive hematopoietic cells. In this representative experiment, 1 × 105 (A) and 1.1 × 104 (B) total events were collected, which contained 664 and 597 Sca-1+events in histograms A and B, respectively. The frequencies of PKH2+ cells were 26% (A) and 1.2% (B) and the frequencies of Sca-1+ cells within donor cells were 31% (A) and 25% (B), while the frequencies of CD43+ cells within donor Sca-1+ cells were 24% (A) and 17% (B). The frequencies of donor cells when all nucleated cells were included in the analysis were 3.8% (A) and 1.1% (B). To avoid collecting very large files, files were sometimes acquired to exclude recipient cells, as shown in panel B.
Frequency of lineage-negative, CD43+, CD49e+, CD49d+, and CD62L+ cells among donor Sca-1+ cells in BM and PB at 1, 3, 6, and 20 to 24 hours following transplantation in irradiated or nonirradiated recipients.
Irradiated or nonirradiated mice received 17-90 × 106LDBM cells, killed at the indicated time points, and donor Sca-1+ cells in BM and PB analyzed for lineage expression (A), expression of CD43 (B), CD49e (C), CD49d (D), and CD62L (E) by flow cytometry as described in “Materials and methods.” PB samples were only analyzed at 1 hour because of infrequency of donor cells at later time points. Data are expressed as the mean ± SEM percent of donor Sca-1+ cells that have light scatter properties characteristic of primitive hematopoietic cells and that express each adhesion molecule. Horizontal lines on each graph represent the mean percent of Sca-1+ cells in the original graft that express each adhesion molecule. For each time point, n = 6-11 for irradiated BM, n = 3-5 for nonirradiated BM, n = 3-7 for PB, and n = 4-7 for graft cells. #P < .05 when compared with earlier time points of same transplant group, *P < .05 when compared with 1-hour BM values.
Frequency of lineage-negative, CD43+, CD49e+, CD49d+, and CD62L+ cells among donor Sca-1+ cells in BM and PB at 1, 3, 6, and 20 to 24 hours following transplantation in irradiated or nonirradiated recipients.
Irradiated or nonirradiated mice received 17-90 × 106LDBM cells, killed at the indicated time points, and donor Sca-1+ cells in BM and PB analyzed for lineage expression (A), expression of CD43 (B), CD49e (C), CD49d (D), and CD62L (E) by flow cytometry as described in “Materials and methods.” PB samples were only analyzed at 1 hour because of infrequency of donor cells at later time points. Data are expressed as the mean ± SEM percent of donor Sca-1+ cells that have light scatter properties characteristic of primitive hematopoietic cells and that express each adhesion molecule. Horizontal lines on each graph represent the mean percent of Sca-1+ cells in the original graft that express each adhesion molecule. For each time point, n = 6-11 for irradiated BM, n = 3-5 for nonirradiated BM, n = 3-7 for PB, and n = 4-7 for graft cells. #P < .05 when compared with earlier time points of same transplant group, *P < .05 when compared with 1-hour BM values.
Recovery and serial transplantation of donor cells in ENG cell transplants
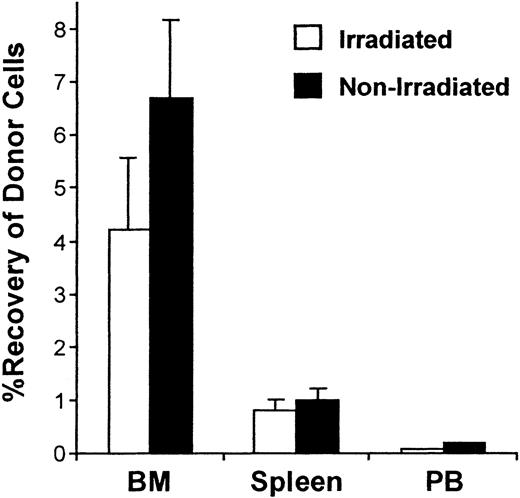
To examine the trafficking of primitive HPCs more directly, grafts composed of 4 × 104 to 3 × 105 sorted cells enriched for stem cell phenotype (Sca-1+lin−CD49e+ or Sca-1+lin−CD62L−) were tracked at 20 hours in irradiated and nonirradiated 1°ST mice. Figure4 shows representative dot plots of typical analyses of donor cells found in BM and spleen tissues and the range of frequencies of donor cells detected in all experiments in BM, spleen, and PB. Similar to increased recovery of graft cells in BM of nonirradiated recipients when LDBM grafts were transplanted, recovery of purified ENG phenotypes was higher in nonirradiated mice, while recovery in spleen and PB was similar in the 2 settings (Figure5).
Flow cytometric analysis of donor cells in BM and spleen 20 hours after transplantation of purified Sca-1+lin−CD62L− cells into irradiated or nonirradiated 1°ST mice.
In this representative experiment, irradiated or nonirradiated C57BL/6 mice (CD45.2+) received 1-3 × 105 sorted Sca-1+lin− CD62L− cells of B6.BoyJ origin (CD45.1+), killed 20 hours later, and donor cells in harvested BM and spleen detected by flow cytometry as described in “Materials and methods.” The frequency of donor cells is given in the upper left quadrant of each dot plot and was calculated manually using event count from listmode files containing between 5 × 105 to 1 × 106 events. The range of frequencies of detected donor cells in all experiments is given in the table.
Flow cytometric analysis of donor cells in BM and spleen 20 hours after transplantation of purified Sca-1+lin−CD62L− cells into irradiated or nonirradiated 1°ST mice.
In this representative experiment, irradiated or nonirradiated C57BL/6 mice (CD45.2+) received 1-3 × 105 sorted Sca-1+lin− CD62L− cells of B6.BoyJ origin (CD45.1+), killed 20 hours later, and donor cells in harvested BM and spleen detected by flow cytometry as described in “Materials and methods.” The frequency of donor cells is given in the upper left quadrant of each dot plot and was calculated manually using event count from listmode files containing between 5 × 105 to 1 × 106 events. The range of frequencies of detected donor cells in all experiments is given in the table.
Percent recovery of donor cells in BM, spleen, and PB 20 hours after transplantation of purified Sca-1+lin− CD62L− or Sca-1+lin− CD49e+ cells into irradiated or nonirradiated 1°ST mice.
Irradiated or nonirradiated mice received 4 × 104 to 3 × 105 sorted Sca-1+lin−CD62L− or CD49e+ cells, killed 20 hours later, and percent recovery of donor cells in harvested BM, spleen, and PB was calculated as described in “Materials and methods.” Bars represent mean ± SEM recovery of cells in each tissue; n = 11 for irradiated, n = 4 for nonirradiated.
Percent recovery of donor cells in BM, spleen, and PB 20 hours after transplantation of purified Sca-1+lin− CD62L− or Sca-1+lin− CD49e+ cells into irradiated or nonirradiated 1°ST mice.
Irradiated or nonirradiated mice received 4 × 104 to 3 × 105 sorted Sca-1+lin−CD62L− or CD49e+ cells, killed 20 hours later, and percent recovery of donor cells in harvested BM, spleen, and PB was calculated as described in “Materials and methods.” Bars represent mean ± SEM recovery of cells in each tissue; n = 11 for irradiated, n = 4 for nonirradiated.
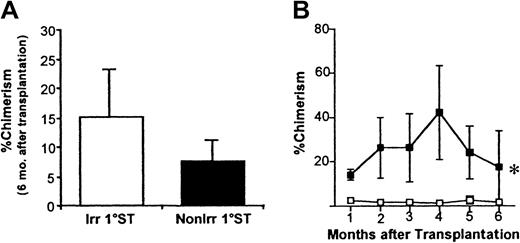
We next defined the in vivo trafficking patterns of HSCs in irradiated or nonirradiated mice by examining the long-term repopulating potential of BM-homed donor cells 20 hours after injection of ENG cells. To this end, BM-homed donor cells were isolated from irradiated or nonirradiated 1°ST recipients, and 50 to 200 of these cells transplanted competitively into 1°LT-irradiated recipients. No significant differences in chimerism were detected in 1°LT recipients of BM-homed donor cells from irradiated or nonirradiated 1°ST mice (Figure 6A) up to 6 months after transplantation. However, when 2-5 × 106 LDBM cells from 1°LT recipients were transplanted into 2°LT mice, donor-derived chimerism was significantly greater in recipients of BM-homed cells from nonirradiated 1°ST recipients (Figure 6B), suggesting more efficient homing or better survival of primitive HPCs in nonmyeloablated marrow. BM-homed cells from both irradiated and nonirradiated 1°ST mice were equally effective in providing multilineage engraftment in both 1°LT and 2°LT recipients and did not apparently differ in their relative contribution to either myeloid or lymphoid lineages (data not shown).
Percent donor-derived chimerism in 1°LT and 2°LT recipients of BM-homed donor cells from irradiated or nonirradiated 1°ST recipients.
Irradiated or nonirradiated 1°ST recipients received 1-3 × 105 Sca-1+lin−CD62L− or Sca-1+lin−CD49e+ cells, killed 20 hours later, and 50 to 200 BM-homed donor cells were transplanted competitively into lethally irradiated 1°LT recipients. Percent donor-derived chimerism in 1°LT recipients 6 months after transplantation is shown in panel A, where bars represent mean ± SEM chimerism; n = 13 for irradiated, n = 5 for nonirradiated. In some experiments, 2-5 × 106 LDBM cells from 1°LT recipients 7 months after transplantation were transplanted without competition into lethally irradiated 2°LT recipients (panel B). In panel B, 1°LT recipients had received 50 or 100 BM-homed cells of Sca-1+lin−CD62L− origin. Data in panel B represent mean ± SEM chimerism; n = 3-5 mice at each time point. Data in both A and B were analyzed using repeated measures analysis of variance. *P < .05. Source of graft cells: ■ indicates irradiated 1°ST; ▪, nonirradiated 1°ST.
Percent donor-derived chimerism in 1°LT and 2°LT recipients of BM-homed donor cells from irradiated or nonirradiated 1°ST recipients.
Irradiated or nonirradiated 1°ST recipients received 1-3 × 105 Sca-1+lin−CD62L− or Sca-1+lin−CD49e+ cells, killed 20 hours later, and 50 to 200 BM-homed donor cells were transplanted competitively into lethally irradiated 1°LT recipients. Percent donor-derived chimerism in 1°LT recipients 6 months after transplantation is shown in panel A, where bars represent mean ± SEM chimerism; n = 13 for irradiated, n = 5 for nonirradiated. In some experiments, 2-5 × 106 LDBM cells from 1°LT recipients 7 months after transplantation were transplanted without competition into lethally irradiated 2°LT recipients (panel B). In panel B, 1°LT recipients had received 50 or 100 BM-homed cells of Sca-1+lin−CD62L− origin. Data in panel B represent mean ± SEM chimerism; n = 3-5 mice at each time point. Data in both A and B were analyzed using repeated measures analysis of variance. *P < .05. Source of graft cells: ■ indicates irradiated 1°ST; ▪, nonirradiated 1°ST.
Cell cycle status
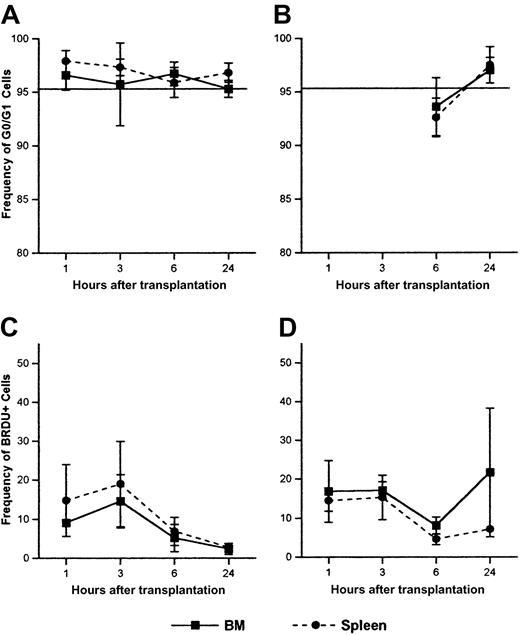
To examine the activation of primitive HPCs following transplantation, donor Sca-1+ cells from BM and spleen were analyzed for cell cycle status by PI staining of sorted cells or by BrdU incorporation. Figure 7 shows representative BrdU and cell cycle analysis of BM-homed Sca-1+ and Sca-1+lin− cells from irradiated and nonirradiated recipients 6 hours after injection of LDBM. More than 95% of donor Sca-1+ cells isolated from BM or spleen were in G0/G1 regardless of time of analysis or irradiation status, a frequency similar to that of Sca-1+ cells in the original graft (Figure 8A-B). No significant differences in G0/G1 status were noted between irradiated and nonirradiated mice or at different times of analysis.
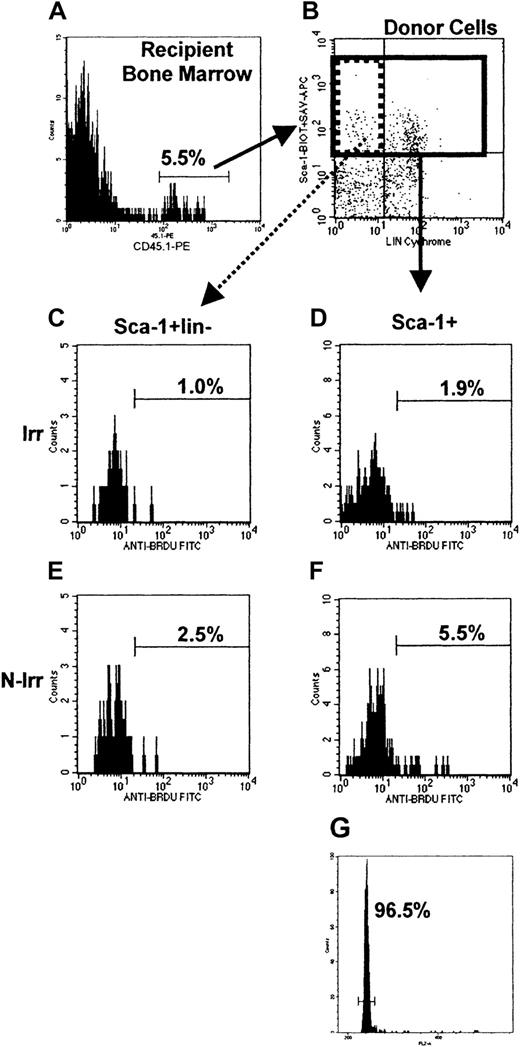
Flow cytometric analysis of BrdU incorporation in donor Sca-1+ and Sca-1+lin− cells in irradiated and nonirradiated recipient BM, and cell cycle analysis of donor Sca-1+ cells in irradiated recipient BM 6 hours after transplantation.
55 × 106 LDBM cells of B6.BoyJ origin were transplanted into irradiated or nonirradiated C57Bl/6 mice. Mice were killed 6 hours later, and BM cells lysed and stained with CD45.1-PE, Sca-1–biotin, CD3-cychrome, B220-cychrome, followed by streptavidin-APC. Cells were permeabilized and stained with anti-BrdU–FITC as described in “Materials and methods.” (A) and (B) represent typical analyses where donor cells (5.5%) are gated from all nucleated cells and analyzed for Sca-1 and lineage expression (B). Donor Sca-1+ cells (27% of donor cells; solid region in B) were then analyzed for their BrdU incorporation in irradiated (D) or nonirradiated (F) mice, or cell cycle status by PI staining (typical cell cycle histogram shown in G). Donor Sca-1+lin− cells (21% of donor Sca-1+ cells; dotted region in B) were likewise analyzed for BrdU incorporation (C,E). Sca-1+lin−histograms contain between 75 and 85 events, whereas Sca-1+histograms contain between 256 and 271 events. Frequencies of BrdU+ cells and cells in G0/G1 are given on each histogram.
Flow cytometric analysis of BrdU incorporation in donor Sca-1+ and Sca-1+lin− cells in irradiated and nonirradiated recipient BM, and cell cycle analysis of donor Sca-1+ cells in irradiated recipient BM 6 hours after transplantation.
55 × 106 LDBM cells of B6.BoyJ origin were transplanted into irradiated or nonirradiated C57Bl/6 mice. Mice were killed 6 hours later, and BM cells lysed and stained with CD45.1-PE, Sca-1–biotin, CD3-cychrome, B220-cychrome, followed by streptavidin-APC. Cells were permeabilized and stained with anti-BrdU–FITC as described in “Materials and methods.” (A) and (B) represent typical analyses where donor cells (5.5%) are gated from all nucleated cells and analyzed for Sca-1 and lineage expression (B). Donor Sca-1+ cells (27% of donor cells; solid region in B) were then analyzed for their BrdU incorporation in irradiated (D) or nonirradiated (F) mice, or cell cycle status by PI staining (typical cell cycle histogram shown in G). Donor Sca-1+lin− cells (21% of donor Sca-1+ cells; dotted region in B) were likewise analyzed for BrdU incorporation (C,E). Sca-1+lin−histograms contain between 75 and 85 events, whereas Sca-1+histograms contain between 256 and 271 events. Frequencies of BrdU+ cells and cells in G0/G1 are given on each histogram.
Cell cycle status and BrdU incorporation of donor Sca-1+ cells in BM and spleen at 1, 3, 6, and 24 hours following transplantation in irradiated or nonirradiated recipients.
Irradiated (A,C) or nonirradiated (B,D) mice received 17-90 × 106 LDBM cells, killed at the indicated time points, and donor Sca-1+ cells in BM and spleen analyzed for cell cycle status by PI (A-B) or BrdU incorporation (C-D) by flow cytometry as described in “Materials and methods.” Data are expressed as the mean ± SEM percent of Sca-1+ donor cells in G0/G1 phase of cell cycle (A-B) and the mean ± SEM percent of Sca-1+ BrdU+ donor cells (C-D) at different time points after transplantation. Horizontal lines on panels A and B represent the mean percent of Sca-1+ cells in the original graft in G0/G1. The low number of donor Sca-1+cells attainable from PB precluded cell cycle analyses of these cells. For each time point, n = 3-9 for panel A, n = 3 for panel B, n = 2-4 for panel C, and n = 3-4 for panel D.
Cell cycle status and BrdU incorporation of donor Sca-1+ cells in BM and spleen at 1, 3, 6, and 24 hours following transplantation in irradiated or nonirradiated recipients.
Irradiated (A,C) or nonirradiated (B,D) mice received 17-90 × 106 LDBM cells, killed at the indicated time points, and donor Sca-1+ cells in BM and spleen analyzed for cell cycle status by PI (A-B) or BrdU incorporation (C-D) by flow cytometry as described in “Materials and methods.” Data are expressed as the mean ± SEM percent of Sca-1+ donor cells in G0/G1 phase of cell cycle (A-B) and the mean ± SEM percent of Sca-1+ BrdU+ donor cells (C-D) at different time points after transplantation. Horizontal lines on panels A and B represent the mean percent of Sca-1+ cells in the original graft in G0/G1. The low number of donor Sca-1+cells attainable from PB precluded cell cycle analyses of these cells. For each time point, n = 3-9 for panel A, n = 3 for panel B, n = 2-4 for panel C, and n = 3-4 for panel D.
Less than 22% of BM- and spleen-homed Sca-1+ cells from irradiated or nonirradiated mice were found to contain BrdU when analyzed between 1 and 24 hours, although this frequency declined by 24 hours in irradiated mice (Figure 8C-D). The more primitive Sca-1+lin− cells were also analyzed for BrdU incorporation and found to contain less than 10% BrdU+ cells regardless of irradiation status (data not shown). Of interest is that in 6 of 9 experiments, donor Sca-1+ cells homing to BM of nonirradiated recipients contained between 2- and 10-fold more BrdU+ cells than BM-homed Sca-1+ cells in irradiated marrow (Figure 8C-D). A similar trend of increased cycling in nonirradiated marrow was noted for the more primitive Sca-1+lin− cells and the more mature Sca-1–negative cells (3 of 5 and 7 of 7 experiments, respectively; data not shown).
Discussion
In this report, we define the trafficking patterns and adhesion molecule repertoire of classes of phenotypically defined primitive BM cells early after their transplantation into lethally irradiated or nonirradiated recipient mice. Higher recovery of transplanted cells and increased incidence of CD43+, CD49e+, CD49d+, and lin− BM-homed Sca-1+ cells in nonirradiated mice relative to irradiated mice suggests more efficient homing or better survival of primitive HPCs in a nonirradiated environment. The primitive nature of these cells was further substantiated in serial transplantation experiments, where BM-homed cells in nonirradiated mice were found to be relatively enriched for long-term engrafting cells capable of sustaining long-term hematopoiesis for 2 generations. Our results are in agreement with those recently reported by Bubnic and Keating,33 who documented the homing of long-term repopulating cells to BM of nonirradiated mice by 24 hours after transplantation in a similar transplant model.
The increase in CD43, CD49e, and CD49d expression on BM-homed Sca-1+ cells in nonirradiated mice correlates with the enhanced engraftment potential of BM-homed cells in nonirradiated mice and supports our earlier studies25 and those of others,33,34 suggesting an importance of these molecules in homing or engraftment of primitive HPCs. Whether these molecules are involved in homing and/or anchorage of primitive HPCs to BM or in modulating a yet-to-be-identified parameter important in engraftment remains to be determined. Nevertheless, these data suggest that while homing in myeloablated recipients may represent a random process due to radiation damage of stroma and/or endothelial cells, homing in nonmyeloablated recipients may not only be more specific, but may also better portray natural trafficking patterns of HSCs in vivo. Recent studies in parabiotic mice36 support the notion that a small number of HSC naturally traverse between blood and marrow in normal mice, further suggesting the existence of established migratory pathways for HSC between blood and BM in nonablated hosts. Our data suggest that these pathways are at least partially disrupted after lethal irradiation, such that fewer HSC home to, or survive within, an irradiated BM microenvironment. The reported generalized up-regulation of adhesion molecule counterreceptors, such as VCAM,37,38ICAM,38,39 and PECAM39 after irradiation may contribute to nonspecific seeding of transplanted HSC to sites other than BM. Preliminary studies in our laboratory suggest that shortened time intervals between radiation dosing and transplantation may have favorable outcomes for homing and possibly engraftment, supporting the notion that radiation-sensitive microenvironmental cues may be involved in the homing process and fate of transplanted cells. Whether radiation-induced bystander effects (reviewed in Mothersill and Seymour40) negatively impact the function of transplanted primitive HPC and account for some of our observed differences in homing and engraftment in irradiated and nonirradiated mice is unknown. Nevertheless, our finding that graft cells home more efficiently to nonirradiated BM may partially explain the recent successes of BM transplantation in this scenario.19-24 Better understanding of homing mechanisms may open these areas to manipulation and the possible design of protocols aimed at enhancing trafficking of transplanted HSCs to BM, which may be especially beneficial to those patients undergoing minimally ablated BM transplantation.
This report examined the adhesion molecule repertoire of phenotypically defined Sca-1+ cells early after transplantation. Although analysis of adhesion molecule expression on Sca-1+lin− cells would have yielded more complete knowledge of the homing patterns of primitive HPCs, analysis of such rare donor cell populations in recipient mice is technically difficult. However, the finding that CD62L−Sca-1+ cells, known to be enriched for long-term engraftment potential,25 rapidly homed to BM within 1 hour while the more mature CD62L+ cells remained in PB suggests the rapid trafficking of primitive HPCs in both irradiated and nonirradiated hosts. The dynamic changes in adhesion molecule expression observed on BM-homed donor Sca-1+ cells between 1 and 24 hours after transplantation suggest either continual trafficking of different phenotypes of transplanted cells to BM at different time points after transplantation, or modulation of adhesion molecule expression following transplantation. It is likely that the increase in CD62L expression on BM-homed Sca-1+ donor cells between 1 and 24 hours represents up-regulation of this molecule, possibly to facilitate anchorage of these cells in BM niches. Similar increases in CD62L expression have been observed on human CD34+ cells homing to NOD/SCID BM by 48 hours after transplantation (C.M.O.-T., unpublished data, 2000) and may be analogous to the reversible expression of CD34 on murine HSCs.35
Analyses of cycling activity of transplanted cells within the first 48 hours of transplantation have yielded varying results,2,5-7,11,36,37 possibly due to differences in the methodology used for cell cycle determination. While assays examining loss of fluorescence of membrane dyes2,6,11 examine proliferation history of transplanted cells and are subject to dye loss by means other than proliferation, those using DNA-specific stains such as PI36 reveal a snapshot of cycling status at time of analysis. We have attempted to assay cycling status of transplanted cells through the use of both BrdU labeling and PI analysis to document both proliferation history and instantaneous cell cycle position. While our own data and those of others using PI show more than 93% of BM- and spleen-homed murine5 and human36 donor cells in G0/G1 up to 24 hours after transplantation, we found that up to 20% of these cells had incorporated BrdU. That PI and BrdU cell cycle analyses do not always correspond is known and possibly is due to incorporation of BrdU into G1-phase cells. Alternatively, if the decreased percentage of BrdU+ cells observed in irradiated mice at 24 hours represents true loss of cycling cells, these data may represent proliferation inhibition and subsequent apoptosis of transplanted cells in active phases of cell cycle, as we have previously documented.36 The slightly higher fraction of cycling cells detected in nonirradiated mice, together with the increased recovery of long-term repopulating cells in BM of nonirradiated mice, suggests the possibility of enhanced survival of transplanted primitive HPCs in a nonirradiated environment. Whether proliferation inhibition and apoptosis of transplanted cycling cells are secondary to effects of radiation remains unknown, but are intriguing possibilities to explain the observed increase in recovery of transplantable HSCs in the BM of nonirradiated mice 20 hours after transplantation.
Supported in part by National Institutes of Health grant RO1 HL62200.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christie M. Orschell-Traycoff, Department of Medicine, Indiana University School of Medicine, 1044 W Walnut St, R4-202, Indianapolis, IN 46202-5254; e-mail:corschel@iupui.edu.