Cellular homeostasis requires a balance between cell production, cell survival, and cell death. Production of natural killer (NK) cells from bone marrow precursor cells requires interleukin 15 (IL-15); however, very little is known about the factors controlling survival of mature NK cells in vivo. Because mice deficient in IL-15 (IL-15−/− mice) fail to develop NK cells, it is not known whether mature NK cells can survive in an environment lacking IL-15. We hypothesized that IL-15 might indeed be required for survival of mature NK cells in vivo. Freshly isolated NK cells labeled with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) were adoptively transferred into IL-15−/− mice and littermate control (IL-15+/−) mice. Within 36 hours after transfer, NK cells were detected in both IL-15−/− and IL-15+/− mice; however, significantly more (P < .003) CFSE-positive (CFSE+) NK cells were found in control mice than in IL-15−/− mice. By 5 days, similar numbers of CFSE+ NK cells were still easily detected in IL-15+/− mice, whereas no CFSE+ NK cells survived in IL-15−/− mice. Furthermore, mice with severe combined immunodeficiency treated with the Fab fragment of a blocking antibody recognizing a signaling subunit of the IL-15 receptor, IL-2/15Rβ, had a significant (∼90%) loss of NK cells compared with control mice. Finally, NK cells from Bcl-2 transgenic mice that were adoptively transferred into IL-15−/− mice did survive. These results show conclusively that IL-15 is required for mature NK cell survival in vivo and suggest that IL-15 mediates its effect on NK cell survival by means of Bcl-2.
Introduction
Natural killer (NK) cells are innate immune lymphocytes that provide at least 2 critical functions during early host defense: cytolysis of infected or transformed cells regulated by a unique repertoire of newly defined receptors, and early provision of cytokines and chemokines for activation of innate and adaptive immune responses.1-5 NK cells develop in the bone marrow microenvironment, where their differentiation requires the actions of the stromal cell–derived cytokine interleukin 15 (IL-15).6 7 Very little is known about the factors controlling survival and homeostasis of mature NK cells in vivo after their release from the bone marrow.
IL-15 is a widely expressed, pleiotropic cytokine first described in 1994 on the basis of its ability to stimulate proliferation of an IL-2–dependent cell line.8,9 It was subsequently found to share 2 receptor subunits with IL-2. Both cytokines signal through the shared IL-2/15Rβ and common γ chain ([γc] also shared by IL-4, IL-7, IL-9, and IL-21).10,11 IL-15 also uses its own unique receptor, IL-15Rα, which confers high-affinity binding of this cytokine and may, in certain cases, also transduce an intracellular signal.12-14 Studies in murine models have shown that NK cell differentiation is dependent on IL-15 signaling. Because of defects in production, NK cells are deficient or absent in mice with targeted disruptions of IL-15 (IL-15−/−),15IL-15 receptor subunits (IL-15Rα−/−, IL-2/15Rβ−/−, and γc−/−),16-18 or IL-15 signaling components (Jak3−/− and STAT5a/b−/−).19,20 Likewise, mice generated to overexpress IL-15 have large expansions of NK cells.21,22 The discovery that a patient with an NK-deficient form of severe combined immunodeficiency (SCID) lacked expression of the IL-2/15Rβ chain23 further highlights the importance of IL-15 signaling for NK cell production in vivo. In contrast, mice lacking IL-2, which shares signaling components with IL-15 and also can induce NK cell differentiation in vitro, were shown to have normal NK cell function,24 a finding that distinguishes IL-15 as the critical in vivo signaling molecule necessary for NK cell development.
Although it is known that IL-15 is required for NK cell development, the factors necessary for maintaining NK cell homeostasis in the periphery are unknown. Because cellular homeostasis requires a balance between cell production, cell survival, and cell death, it is reasonable to assume that a common factor might control more than one of these processes. We hypothesized that, given the wide expression of IL-15 and the absolute requirement of this cytokine for the production of NK cells, IL-15 might also regulate NK cell survival. We previously reported that IL-15 can sustain human NK cell survival in vitro.25 In this study, we investigated the survival of NK cells labeled with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) that were adoptively transferred into mice lacking IL-15 (IL-15−/−) or littermate control mice (IL-15+/−). Our results show for the first time that IL-15 is absolutely required for maintenance of mature NK cell survival in vivo.
Materials and methods
Mice and reagents
IL-15−/− mice (C57BL/6 background) were provided by Immunex (Seattle, WA)15 and bred at Ohio State University. Wild-type (wt), Ly5.1-positive (Ly5.1+), and SCID mice (all on C57BL/6 background) were purchased from Jackson Laboratories (Bar Harbor, ME). These mice were maintained in specific pathogen–free conditions at Ohio State University, and all experiments were carried out with approval from the Institutional Animal Care and Use Committee. Bcl-2 transgenic (Bcl-2tg) mice (C57BL/6 background), described previously,26 were provided by Dr H. L. Aguila (University of Connecticut). Bcl-2tg mice were maintained at the University of Connecticut Health Sciences Animal Facility, and all experiments with them were conducted in accordance with the university's institutional guidelines. Phycoerythrin (PE)–conjugated or fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (mAbs) recognizing the following antigens were purchased from Pharmingen (San Diego, CA): DX5 (PE), CD45.1 (Ly5.1, PE), NK1.1 (PE, FITC, and allophycocyanin [APC]), murine Bcl-2 (PE), and isotype-control antibodies (Abs). The CFSE was purchased from Molecular Probes (Eugene, OR). Recombinant murine IL-15 (muIL-15) was a gift from Immunex. Antimouse IL-2/15Rβ (TM-β1, rat IgG2b) was isolated from hybridoma supernatants27 (a gift from T. Tanaka), and control Ab (rat IgG) was purchased from Sigma (St Louis, MO).
Isolation of murine NK cells
NK cells were isolated from the spleens of wt, Ly5.1+, SCID, or Bcl-2tg mice. Single-cell suspensions of splenocytes were made, red blood cells lysed, and viable lymphocytes isolated by density-gradient centrifugation using Lympholyte-M (Cedarlane Laboratories, Hornby, ON, Canada). Cells from wt, Ly5.1+, and SCID mice were resuspended in phosphate-buffered saline (PBS) plus 2% fetal-calf serum (FCS), and NK cells were negatively selected by using a labeling kit from StemCell Technologies (Vancouver, BC, Canada) that included biotinylated Abs recognizing CD3, CD5, CD22, T-cell receptor (TCR)αβ, GR-1, TER119, and F4/80. This was followed by secondary labeling of Ab-coated cells with magnetic beads in accordance with the manufacturer's recommendations (StemCell Technologies). For enrichment of Bcl-2tg NK cells, lymphocytes were labeled with biotinylated Abs recognizing CD22 (Pharmingen), F480 (Caltag, Burlingame, CA), CD19, CD3, TCR, CD5, Ter119, and 8C5 (Biosource, Camarillo, CA), and this was followed by secondary labeling with anti-biotin magnetic beads (Miltenyi Biotec, Germany). All cells were then passed through magnetically activated cell-sorter LS columns (Miltenyi Biotec), and NK cells were obtained by negative selection. The eluted cells were stained with DX5 or NK1.1 and isotype-control mAbs and analyzed for NK cell purity by flow cytometry. Purity ranged from 50% to 80% for wt, Ly5.1+, and SCID NK cells and was about 20% for Bcl-2tg NK cells.
Adoptive transfer of wt and Bcl-2tg NK cells into IL-15−/−mice
NK cells were purified from wt or Ly5.1 mice (6-10 weeks old) as described above. NK cells were labeled for 15 minutes at room temperature with CFSE (1 μM) in PBS, washed twice with 10% FCS, once with PBS, and resuspended in sterile PBS for injection. For experiments with wt NK cells, equal numbers of NK cells (7 × 105 to 2.5 × 106 cells/mouse) were injected into the tail veins of IL-15−/− and littermate control IL-15+/−mice (7-18-weeks old; mean age, 13.2 weeks). For experiments with Bcl-2tg cells, 1 to 1.5 × 106 NK cells were injected into the orbital vein of IL-15−/− and littermate control mice (8-10 weeks old). Mice were killed 36 hours, 5 days, or 7 days after transfer, and lymphocytes from the blood, liver, or spleen were isolated. Liver cells were first digested with collagenase IV (0.02%) and DNase I (10 U/mL) at 37°C for 40 minutes. Then, both digested liver cells and splenocytes were processed into single-cell suspensions. This was followed by density centrifugation with Lympholyte-M and collection of viable cells. Samples were stained with DX5-PE, CD45.1 (Ly5.1)–PE, and/or NK1.1–PE or NK1.1–APC and the appropriate isotype-control mAb. Flow cytometry was then used to analyze CFSE-positive (CFSE+) transferred NK cells. At least 100 000 events were collected for each organ, with as many as 1 000 000 events analyzed, depending on the number of cells available. For all experiments, similar numbers of events were analyzed for tissues from IL-15−/− and control mice.
Calculation of NK cell recovery
NK cell recovery is expressed as the percentage of NK cells originally transferred. To determine this value, total cell counts from the spleen, liver, and blood were multiplied by the percentage of CFSE+ NK cells found in each tissue and then divided by the original number of NK cells transferred (equal to the total number of cells transferred times the percentage of NK cells). This value represents the NK cell recovery from an individual tissue. To determine differences in total NK cell recovery, the numbers of NK cells recovered from all 3 tissues were added and then divided by the original number of NK cells transferred.
Administration of an Ab to the muIL-2/15Rβ chain
SCID mice (3 per group) were injected intraperitoneally twice daily with 500 μg Fab fragments of TM-β1 (anti–IL-2/15Rβ) or control Ab (rat IgG) for 7 days. To obtain Fab fragments, whole Ab was digested at a concentration of 10 mg/mL for 4 hours at 37°C in a buffer containing 20 mM sodium phosphate (pH 7.0), 2 mM EDTA (ethylenediaminetetraacetic acid), 10 mM cysteine, and 2% wt/vol mercuripapain (Sigma). Cleavage was arrested by the addition of iodoacetamide to a final concentration of 20 mM for 30 minutes at room temperature. Iodoacetamide was removed by overnight dialysis in PBS. The resultant Fab fragments were analyzed for purity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, quantitated, and sterile filtered for injection.
Measurement of Bcl-2 expression in NK cells
To determine initial Bcl-2 levels, enriched NK cells were stained immediately for intracellular Bcl-2. The remaining NK cells were plated in 96-well, U-bottomed plates in RPMI 1640 plus 10% FCS at a density of 2.3 to 3.0 × 105 cells/well and cultured with muIL-15 (10 ng/mL) or medium alone. Cells were harvested after 12 and 24 hours of culture and stained for intracellular Bcl-2. Briefly, for experiments in which NK cells were less than 80% pure, cells were first stained with NK1.1-FITC or isotype-control mAb. For higher-purity NK cell preparations or after staining with NK1.1, cells were permeabilized by washing with 0.03% saponin (Sigma) and incubated for 20 minutes at 4°C with a PE-conjugated Ab recognizing murine Bcl-2. Bcl-2 protein levels were then measured by flow cytometry.
Assessment of nuclear degradation of NK cells by propidium iodide (PI) staining
Enriched NK cells were plated in 96-well, U-bottomed plates at a density of 2.3 to 3.0 × 105 cells/well in medium (RPMI 1640 plus 10% FCS). Cells were cultured with muIL-15 or medium alone. After 24 hours, cell membranes were permeabilized with 0.25% saponin (Sigma), and this was followed by addition of 100 μg/mL RNase A (10 mg/mL stock) and 50 μg/mL PI solution (1 mg/mL stock). PI incorporation was measured by flow cytometry.
Statistical and flow cytometric analyses
The Student t test was used for statistical analysis, with a P value of .05 or less considered to represent significance. All flow cytometric analyses except those in the Bcl-2tg experiments were done with an XL flow cytometer (Beckman Coulter, Fullerton, CA), and data were analyzed with WinMDI software (Joseph Trotter, Scripps Research Institute, La Jolla, CA). For the Bcl-2tg experiments, an FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) was used and data were analyzed with CellQuest (BD Biosciences) and WinMDI software.
Results
Adoptive transfer of NK cells Into IL-15−/− and control mice
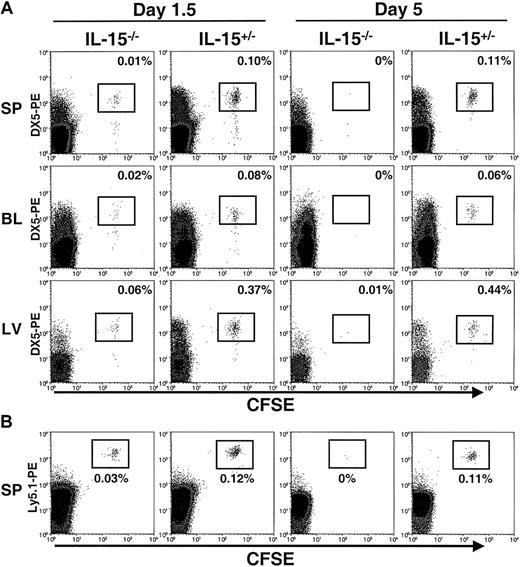
Freshly isolated murine NK cells were enriched from wt or Ly5.1+ mice and labeled with CFSE. Equal numbers of cells were then adoptively transferred into IL-15−/− mice (Ly5.2+), which lack NK cells, and littermate control IL-15+/− mice (Ly5.2+), which have functional NK cells.15 The DX5 antigen was used to track transferred NK cells because of the bright staining obtained with this Ab; however, more than 98% of the enriched NK cells were consistently positive for both DX5 (DX5+) and NK1.1 (NK1.1+) before the adoptive transfer (data not shown). At 1.5 days, transferred CFSE+DX5+ NK cells were detected in the spleen, blood, and liver of both IL-15−/− and IL-15+/− mice (Figure 1A). However, assessment of total recovery of NK cells from all 3 tissues revealed that greater than 7-fold more transferred NK cells were recovered from control mice than from mice lacking IL-15 (P < .003; Figure 1A and Table1). Therefore, NK cells could successfully be transferred into IL-15−/− mice but did not appear to survive. By 5 days, transferred NK cells had disappeared completely from IL-15−/− mice, whereas a substantial population of CFSE+DX5+ NK cells was still present in littermate control (IL-15+/−) mice (Figure 1A and Table 1). In adoptive transfer experiments in which NK cells were isolated from Ly5.1+ mice, transferred cells could be further distinguished as CFSE+Ly5.1+ and were identified in the spleens of both IL-15−/− and control mice 1.5 days after the transfer but were undetectable in IL-15−/− mice by 5 days (Figure 1B).
Detection of transferred NK cells in IL-15−/− and control mice.
CFSE+ NK cells were assessed by flow cytometry 1.5 and 5 days after transfer into IL-15−/− and control (IL-15+/−) mice. (A) The spleen (SP), blood (BL), and liver (LV) were analyzed for the presence of CFSE+DX5+ NK cells. Cells were gated on viable lymphocytes. Transferred cells are highlighted in boxes, and the percentages for each organ are shown. At day 1.5, transferred cells were detectable in both IL-15−/− and control mice, with significantly higher numbers of NK cells recovered from the controls (7.4 ± 0.7 fold; P < .003; total NK cells). At day 5, transferred NK cells were undetectable in IL-15−/− mice but still present in control mice. Results are representative of 4 independent experiments at 1.5 days and 4 independent experiments at 5 days. (B) For 3 of the 1.5-day and 5-day adoptive transfer experiments, NK cells were isolated from Ly5.1+ mice, labeled with CFSE, and transferred into IL-15−/− and control mice (both of which are Ly5.2+). Consistent with the results shown in panel A, transferred cells could be detected as CFSE+Ly5.1-PE+ in the spleens of IL-15−/− and control mice killed at day 1.5 but were undetectable in IL-15−/− mice at day 5.
Detection of transferred NK cells in IL-15−/− and control mice.
CFSE+ NK cells were assessed by flow cytometry 1.5 and 5 days after transfer into IL-15−/− and control (IL-15+/−) mice. (A) The spleen (SP), blood (BL), and liver (LV) were analyzed for the presence of CFSE+DX5+ NK cells. Cells were gated on viable lymphocytes. Transferred cells are highlighted in boxes, and the percentages for each organ are shown. At day 1.5, transferred cells were detectable in both IL-15−/− and control mice, with significantly higher numbers of NK cells recovered from the controls (7.4 ± 0.7 fold; P < .003; total NK cells). At day 5, transferred NK cells were undetectable in IL-15−/− mice but still present in control mice. Results are representative of 4 independent experiments at 1.5 days and 4 independent experiments at 5 days. (B) For 3 of the 1.5-day and 5-day adoptive transfer experiments, NK cells were isolated from Ly5.1+ mice, labeled with CFSE, and transferred into IL-15−/− and control mice (both of which are Ly5.2+). Consistent with the results shown in panel A, transferred cells could be detected as CFSE+Ly5.1-PE+ in the spleens of IL-15−/− and control mice killed at day 1.5 but were undetectable in IL-15−/− mice at day 5.
In all the tissues assessed (spleen, blood, and liver), significantly more adoptively transferred NK cells were recovered from control mice than from IL-15−/− mice (Table 1). Similar to the distribution of NK cells in wt mice, most transferred NK cells were found in the spleen, with lower numbers recovered from the blood and liver (Table 1). Interestingly, there was no significant difference in the percentages of CFSE+ NK cells recovered in tissues from control mice at 1.5 and at 5 days, suggesting that transferred NK cells were maintained in vivo at constant levels during the time points examined. CFSE labeling can also detect proliferation, since the fluorescence of this dye is halved with each cellular division. At the time points examined in the current study, the transferred NK cells appeared uniform in their CFSE intensity and thus did not appear to have cycled in vivo. This result suggests that the observed differences in NK cell recovery were not due to NK cell proliferation in control (IL-15+/−) mice. Furthermore, transferred cells were still detectable in control mice for at least 10 days after adoptive transfer (data not shown), indicating that the lack of IL-15, rather than technical difficulties in detecting cells, accounted for the inability to detect cells in IL-15−/− mice after 5 days.
In vivo blockade of the IL-2/15Rβ chain results in a significant loss of NK cells
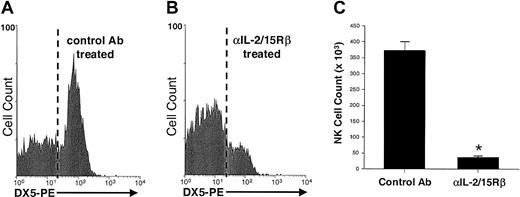
The role of IL-15 in NK cell survival was again examined in vivo, this time by using mice with an intact NK cell compartment. SCID mice, which lack T and B cells but have normal NK cell function, were treated for one week with the Fab fragment of a blocking Ab that recognizes the muIL-2/15Rβ chain (TM-β1) or with control Fab. IL-15 and IL-2 both can signal through the shared IL-2/15Rβ chain, but because of their absence of T cells, SCID mice lack expression of IL-2, thus allowing exclusive examination of IL-15 signaling with the anti–IL-2/15Rβ Ab. After 7 days of Ab treatment, there was a significant difference in the number of NK cells recovered from mice treated with anti–IL-2/15Rβ and controls, with about 90% fewer splenic NK cells recovered from mice given anti–IL-2/15Rβ (P < .0004; Figure2). This disappearance of NK cells was not due to Fc, immune-mediated clearance of cells, since a single injection of whole TM-β1 Ab completely cleared NK cells within 24 hours, whereas one injection of the Fab fragment used in these experiments had no effect on NK cell numbers at this early time point (data not shown). Thus, the failure of NK cells to survive in mice given anti–IL-2/15Rβ, similar to the disappearance of NK cells in IL-15−/− mice, can be attributed solely to the inhibition of IL-15 signaling.
NK cells recovered from SCID mice treated with an Ab against the IL-2/15Rβ chain or control Ab.
SCID mice (3 per group) were treated twice daily (500 μg/dose) with the Fab fragment of a blocking Ab against the IL-2/15Rβ chain (TM-β1) or control Fab (rat IgG). Shown is a flow histogram gated on splenic lymphocytes and stained for DX5-PE after 7 days of treatment with control Fab (A) or anti–IL-2/15Rβ Fab (B). Results from 2 representative animals are shown. (C) Significantly fewer splenic NK cells were recovered from mice treated with anti–IL-2/15Rβ than from controls (38.2 ± 3.8 × 103 versus 372.1 ± 28.2 × 103 NK cells/spleen). The asterisk indicates P less than .0004. Error bars indicate SEM.
NK cells recovered from SCID mice treated with an Ab against the IL-2/15Rβ chain or control Ab.
SCID mice (3 per group) were treated twice daily (500 μg/dose) with the Fab fragment of a blocking Ab against the IL-2/15Rβ chain (TM-β1) or control Fab (rat IgG). Shown is a flow histogram gated on splenic lymphocytes and stained for DX5-PE after 7 days of treatment with control Fab (A) or anti–IL-2/15Rβ Fab (B). Results from 2 representative animals are shown. (C) Significantly fewer splenic NK cells were recovered from mice treated with anti–IL-2/15Rβ than from controls (38.2 ± 3.8 × 103 versus 372.1 ± 28.2 × 103 NK cells/spleen). The asterisk indicates P less than .0004. Error bars indicate SEM.
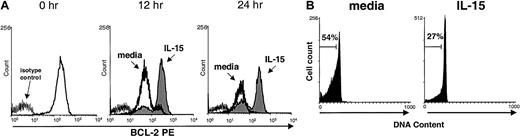
IL-15 maintains murine NK cell Bcl-2 expression
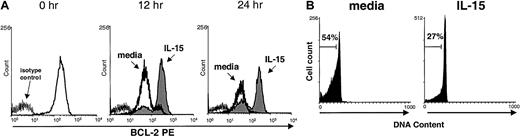
We reported previously that IL-15 activation of human NK cells induces up-regulation of the lymphocyte antiapoptotic factor Bcl-2 in vitro, resulting in a survival benefit.25 In the current study, we found that murine NK cells cultured in medium alone were not able to maintain Bcl-2 expression, whereas cells cultured with IL-15 did (Figure 3A). Likewise, consistent with results of previous studies in humans25 and mice,28 PI nuclear staining showed that murine NK cells cultured in medium underwent apoptosis more quickly than cells cultured with IL-15 (Figure 3B). This suggests one possible mechanism by which IL-15 may prevent apoptosis and maintain survival of mature NK cells in vivo.
Maintenance of Bcl-2 protein and survival of NK cells cultured with IL-15.
(A) Freshly isolated murine NK cells were stained for baseline (0 hour) expression of Bcl-2 protein by using intracellular flow cytometry and again after culture for 12 and 24 hours with 10 ng/mL IL-15 (shaded area) or medium alone (dark line). NK cells cultured in IL-15 maintained significantly higher mean fluorescence intensity (MFI) of Bcl-2 staining than those cultured in medium alone (158.9 ± 80.4 versus 58.1 ± 17.6; P = .05; n = 4 at 12 hours and 152.4 ± 58.3 versus 28.9 ± 21.0; P = .03; n = 3 at 24 hours). Results are representative of four 12-hour and three 24-hour experiments. (B) Viability of enriched NK cells cultured in the presence of IL-15 (10 ng/mL) for 24 hours was assessed with PI staining. NK cells cultured with IL-15 had 50% less cell death than cells cultured in medium alone (n = 3). Results from one representative experiment are shown.
Maintenance of Bcl-2 protein and survival of NK cells cultured with IL-15.
(A) Freshly isolated murine NK cells were stained for baseline (0 hour) expression of Bcl-2 protein by using intracellular flow cytometry and again after culture for 12 and 24 hours with 10 ng/mL IL-15 (shaded area) or medium alone (dark line). NK cells cultured in IL-15 maintained significantly higher mean fluorescence intensity (MFI) of Bcl-2 staining than those cultured in medium alone (158.9 ± 80.4 versus 58.1 ± 17.6; P = .05; n = 4 at 12 hours and 152.4 ± 58.3 versus 28.9 ± 21.0; P = .03; n = 3 at 24 hours). Results are representative of four 12-hour and three 24-hour experiments. (B) Viability of enriched NK cells cultured in the presence of IL-15 (10 ng/mL) for 24 hours was assessed with PI staining. NK cells cultured with IL-15 had 50% less cell death than cells cultured in medium alone (n = 3). Results from one representative experiment are shown.
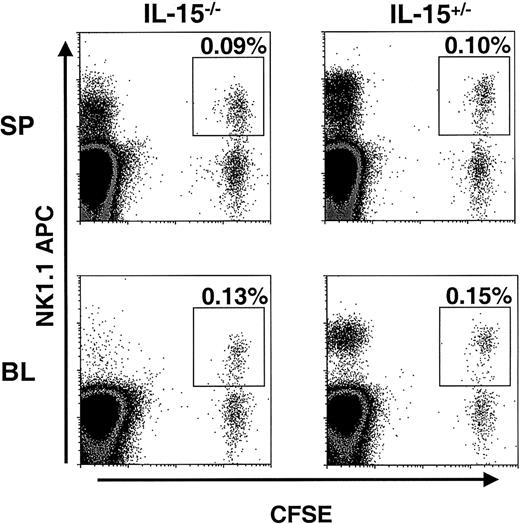
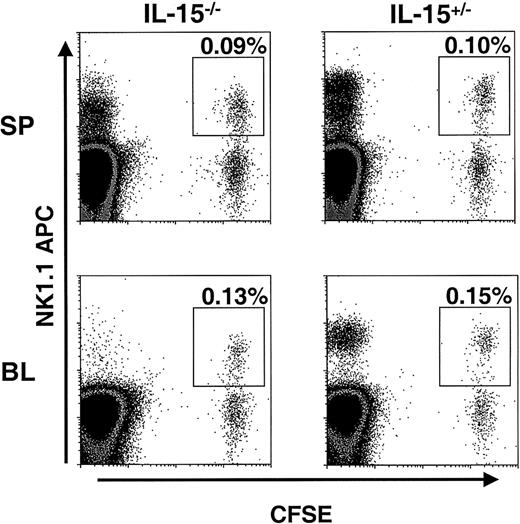
NK cells from Bcl-2tg mice survive in IL-15−/−mice
To assess the importance in vivo of our finding that IL-15 can maintain Bcl-2 levels in murine NK cells in vitro, NK cells from mice overexpressing Bcl-2 (Bcl-2tg mice) were adoptively transferred into IL-15−/− mice and littermate controls. The Bcl-2tg donor mice used in these experiments globally overexpress human Bcl-2 in all hematopoietic cells, and NK cells from these mice have prolonged survival in vitro.26 Enriched Bcl-2tg NK cells were first labeled with CFSE and then transferred into IL-15−/− and control mice (2 pairs). At 1.5 days, spleen, liver, and blood samples from these mice were examined for the presence of CFSE+ NK cells. In contrast to the results of the adoptive transfer experiments with wt NK cells, similar numbers of Bcl-2tg NK cells were detected in IL-15−/− and control mice (Figure4). One week after transfer, Bcl-2tg splenic NK cells could still be detected in equal numbers in both IL-15−/− and control mice (average, 0.025% and 0.03% CFSE+NK1.1+ cells, respectively; data not shown; n = 2). These findings further support the idea that Bcl-2 is a key survival factor for NK cells and, together with our in vitro data, suggest that IL-15 mediates its effect on NK cell survival by means of Bcl-2.
Bcl-2tg NK cells are maintained in IL-15−/− mice.
CFSE-labeled, enriched NK cells from Bcl-2tg mice (purity about 20%) were adoptively transferred into IL-15−/−and control mice (n = 2 pairs). At 1.5 days after transfer, the spleen (SP; upper panels) and blood (BL; lower panels) were analyzed for the presence of NK1.1+CFSE+ NK cells. Cells were gated on viable lymphocytes, and transferred NK cells are highlighted. Similar numbers of Bcl-2tg NK cells were detected in both IL-15−/− (left) and IL-15+/− (right) mice. Data from one experiment are shown. Transferred Bcl-2tg NK cells were also detected in similar numbers in IL-15−/− and control mice 7 days after transfer (data not shown).
Bcl-2tg NK cells are maintained in IL-15−/− mice.
CFSE-labeled, enriched NK cells from Bcl-2tg mice (purity about 20%) were adoptively transferred into IL-15−/−and control mice (n = 2 pairs). At 1.5 days after transfer, the spleen (SP; upper panels) and blood (BL; lower panels) were analyzed for the presence of NK1.1+CFSE+ NK cells. Cells were gated on viable lymphocytes, and transferred NK cells are highlighted. Similar numbers of Bcl-2tg NK cells were detected in both IL-15−/− (left) and IL-15+/− (right) mice. Data from one experiment are shown. Transferred Bcl-2tg NK cells were also detected in similar numbers in IL-15−/− and control mice 7 days after transfer (data not shown).
Discussion
In this study, we used 2 separate models to demonstrate conclusively that IL-15 is necessary for the survival of NK cells in vivo. Furthermore, we showed that IL-15 can maintain Bcl-2 expression in vitro and that NK cells overexpressing Bcl-2 survive longer than wt NK cells when transferred into IL-15−/− mice. Thus, these results suggest a possible mechanism by which IL-15 can sustain murine NK cell survival in vivo.
It is conceivable that the observed in vivo effects of IL-15 on NK cell survival were indirect; however, the data presented here and our previous in vitro studies with human NK cells25 suggest strongly that IL-15 can directly promote NK cell survival. IL-15 protein is expressed by a variety of immune and nonimmune cell types, such as bone marrow stroma, antigen-presenting cells including monocytes/macrophages and dendritic cells, epithelial cells, and fibroblasts.7 This wide expression of IL-15 in vivo is consistent with its role as an NK cell survival factor. After viral and bacterial infection of peripheral blood mononuclear cells in vitro, expression of IL-15 is up-regulated and activates NK cell cytotoxicity and cytokine production.7,29,30 In vivo neutralization of IL-15 in SCID mice before administration of lipopolysaccharide significantly reduced production of interferon-γ by NK cells and mortality associated with the Shwartzman reaction, a fatal cytokine-induced shock.31 In the current study, we showed that in addition to activation of NK cell cytotoxicity and cytokine production, IL-15 is critical for survival of mature NK cells in vivo. Thus, up-regulation of IL-15 early during infection likely also alters NK cell homeostasis to favor an efficient innate immune response before the organism can mount an adaptive, T-cell–mediated response. Evidence has shown that IL-15 is also important for the survival and expansion of memory CD8+ T cells, in distinct contrast to IL-2, which promotes activation-induced cell death of these lymphocytes.32 The regulation of innate and adaptive immune lymphocyte homeostasis by the same cytokine indicates that IL-15 is essential for an efficient immune response and suggests a possible coordination of these two cell-mediated arms of the immune system.
In the current study, we used IL-15−/− mice to demonstrate that adoptively transferred wt NK cells do not survive in an IL-15–deficient environment. This effect likely depends primarily on the absence of IL-15 rather than other deficiencies, because treatment of IL-15−/− mice with exogenous IL-15 for one week can expand or differentiate and maintain the survival of endogenous NK cells in such mice.15 It is also possible, although unlikely, that transferred NK cells in IL-15−/− mice might traffic to areas other than the major NK cell compartments examined here, and this possibility cannot be excluded. Adoptive transfer experiments also revealed that wt NK cells did not undergo substantial proliferation in vivo 5 days after adoptive transfer. This is probably because the NK cells transferred in this study were mature resting lymphocytes that were not exposed to an activation signal. It would be interesting to use this system to evaluate NK cell proliferation in vivo after activation with stimuli such as administration of exogenous cytokines or during infection.
We found that signaling through the IL-2/15Rβ chain is clearly important for regulation of NK cell survival by IL-15, because blocking this receptor in vivo resulted in a loss of NK cells of about 90%. The complete IL-15R heterotrimeric complex consists of a private high-affinity subunit, IL-15Rα, and the IL-2/15Rβ and γc, which signal through Jak/STAT pathways as well as additional intracellular pathways similar to IL-2.7,33 Because IL-15Rα−/−, IL-2/15Rβ−/−, and γc−/− mice all have severe NK cell deficiencies as the result of a failure to produce NK cells,16-18 it is difficult to assess the relative contributions of these receptor subunits to IL-15–induced NK cell survival. An in vivo study by Li et al34 examined the effects of IL-15R subunits on T-cell survival and showed that down-regulation of the γc is correlated with decreased expression of Bcl-2 and increased apoptosis. Other studies demonstrated activation of Bcl-2 by means of the IL-2/15Rβ chain.35,36 Therefore, the maintenance of Bcl-2 that we observed in vitro could have been the result of signaling of IL-15 by means of the γc in concert with the IL-2/15Rβ and might have contributed to the effects of IL-15 on NK cell survival. Previous evidence suggested that IL-15Rα is also capable of transducing an intracellular signal, and IL-15 binding of this receptor was shown to promote the survival of several cell lines, possibly by sequestering the TRAF2 death-cascade adapter or by activating nuclear factor-κB.12-14 Thus, the failure of NK cells to develop in IL-15Rα−/− mice may be due to the fact that the IL-15Rβγ heterodimer lacks sufficient affinity for the usually low concentration of endogenous IL-15 to bind and signal, or because signaling through the isolated IL-15Rα chain is absent. Our data showing the disappearance of about 90% of mature NK cells with blockade of IL-15Rβ signaling support the idea that the role of IL-15Rα in mature NK cell survival in vivo could be to confer high-affinity binding between the heterotrimeric complex and its ligand.
It is likely that IL-15 regulates NK cell differentiation and NK cell survival through distinct pathways. IL-15 is required for NK cell differentiation, but Bcl-2 does not appear to be required, because lymphocytes seem to develop normally in Bcl-2−/−mice,37 and global overexpression of Bcl-2 in IRF-1−/− mice (which lack inducible IL-15) or γc−/− mice does not restore NK cell development.38,39 However, Bcl-2 probably does function as a survival factor, because Bcl-2−/− mice rapidly lose their lymphocytes several weeks after birth37 and mature NK cells from mice globally overexpressing Bcl-2 were resistant to apoptosis induced by growth-factor starvation.26 In this study, we showed that IL-15 maintains murine NK cell levels of Bcl-2, and in a previous study, we found that IL-15–induced survival of human NK cells was lost when Bcl-2 was blocked in vitro.25 These results, along with our finding that Bcl-2tg NK cells are maintained in IL-15−/− mice, suggest that IL-15 activation of Bcl-2 is important for mature NK cell survival.
Our findings in this study provide the first in vivo evidence that IL-15 is necessary for NK cell survival and highlight the critical importance of this cytokine in overall NK cell homeostasis. On the basis of these results and what is already known about the role of IL-15 in NK cell development, we hypothesize that IL-15 may be a master regulator of NK cells, directing NK cell differentiation, homeostasis, and survival in vivo.
We thank Kellie Archer for statistical analysis and Ivan K. Lukic, Charlene Mao, and Xiaotie Bu for excellent technical assistance.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2001-12-0293.
Supported by grants CA-68458, CA-95426, and P30CA-16058 (M. A. Caligiuri) and RO1 AI46708 (H.L.A.) from the National Institutes of Health. M. A. Cooper, T.A.F., and J.B.V. are recipients of Medical Scientist Program Fellowships from The Ohio State University College of Medicine and Public Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael A. Caligiuri, The Ohio State University, 458A Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210; e-mail: caligiuri-1@medctr.osu.edu.