Abstract
Thymus-dependent reconstitution of the peripheral T-cell compartment is critical for the successful outcome of bone marrow transplantation. However, graft-versus-host disease (GVHD) affects thymic stromal function and thus prevents normal T-cell maturation and selection. To determine whether cytoprotection of thymic epithelial cells (TECs) by keratinocyte growth factor (KGF) averts GVHD-related injury to the thymus, a nonirradiated murine parent→F1 transplantation model was investigated. Administration of KGF between days −3 and +3 of GVHD induction preserved normal thymic size, cellularity, and thymocyte phenotype when measured 2 weeks after transplantation and compared with saline-treated parent→F1 mice that received allogeneic transplants. Moreover, the characteristic GVHD-induced impairment in cell cycle progression of pro- and pre-T cells was prevented by KGF. However, the normal phenotypic and functional status of the thymus did not correlate with the higher number of GVHD-inducing mature donor T cells in thymi of KGF-treated mice. Importantly, extensive analysis of the different TEC populations within the thymic cortex and medulla revealed an almost normal stromal architecture and composition in GVHD mice treated with KGF. These observations are likely to reflect an indirect effect of KGF on thymopoiesis as KGF-receptor expression was demonstrated to be restricted to TECs. Thus, pharmacologic doses of KGF appear to exert a potent effect on TEC function, which in turn allows for normal T lymphopoiesis to occur during acute GVHD.
Introduction
Allogeneic bone marrow transplantation (BMT) is the treatment of choice for a number of malignant and nonmalignant disorders.1,2 The outcome of BMT is dependent on a successful and comprehensive reconstitution of the immune system with a broad T-cell receptor (TCR) repertoire, which, in turn, requires the de novo generation of T cells in the thymus.3-9 In this regard, effective development and normal repertoire selection of T cells are critically dependent on the seeding of hematopoietic precursor cells to a regularly structured thymic microenvironment.10 T-cell precursors enter the thymus at the cortico-medullary junction as cells with a CD3−CD4−CD8− (triple negative [TN]) phenotype. During subsequent maturation, the CD44+TN cells (triple-negative stage I [TNI]) acquire CD25 (TNII), before CD44 (TNIII) and later CD25 (TNIV) expression is lost from the cell surface.11 These phenotypic changes are paralleled by a robust proliferation during all stages of T-cell lymphopoiesis, finally resulting in a 4000-fold cellular expansion within the TN compartment. Succeeding this stage, thymocytes acquire a CD4+CD8+ (double-positive [DP]) phenotype. The assembly of a complete TCRαβ/CD3 complex renders DP cells subject to thymic selection generating a repertoire of CD4 and CD8 single positive (SP) cells able to distinguish between vital self and injurious nonself. During the obligatory postselection maturation, which takes place in the medulla and may last as little as one day, thymocytes acquire full functional competence and finally emigrate to the periphery.12
The thymic stromal compartment is mainly composed of a complex 3-dimensional network of epithelial cells with distinct phenotypes.10 These cells effect discrete functions including attraction of hematopoietic precursors to the thymus, promotion of early thymocyte differentiation,13,14selection of DP cells, and functional maturation of newly generated T cells. Unique subtypes of cortical and medullary thymic epithelial cells (TECs) have been distinguished by use of scanning electron microscopy15 and immunohistology,16-18suggesting that each cell type plays a distinct role in providing the above functions. In the absence of a regularly structured and composed thymic microenvironment, these essential functions are severely altered and frequently preclude normal thymocyte development to occur.19,20 Mesenchymal-epithelial cell interactions are crucial for TEC morphogenesis with growth regulated in a paracrine fashion by keratinocyte growth factor (KGF, aka fibroblast growth factor 7 [FGF-7]), FGF-10, and possibly other factors.21-32 Both KGF and FGF-10 regulate epithelial cell proliferation and differentiation, the former frequently in synergy with other growth factors.33 The biologic activities of KGF and FGF-10 are mediated through the KGF receptor (KGFR), that is, the FGFR2IIIb splice variant of the FGF-2 receptor.34,35Direct involvement of the KGFR in thymic development was recently demonstrated in KGFR-deficient mice and animals mutant for FGF-10, both revealing a developmental arrest in thymic organogenesis at day 12.5 of gestation.22
Graft-versus-host disease (GVHD) represents a major transplant-related complication initiated by host-reactive donor T cells.36,37 Morphologic and functional alterations have been documented for the thymus that identify this organ as a specific target of acute GVHD, with thymocytes, TECs, and bone marrow–derived thymic stroma cells each being affected. In consequence, these severe changes lead to dysplasia, thymic involution, disappearance of Hassall bodies, and a loss in the distinction of cortex and medulla, ultimately disturbing regular thymocyte development.38-44Specifically, in the affected thymic microenvironment pro- and pre-T cells fail to a large extent to enter the cell cycle, DP cells undergo enhanced apoptosis, and aberrant repertoire selection results in the persistence of T cells with a self-reactive specificity.45-49 The molecular basis for the profound effects on the thymic microenvironment remains to be determined. It is, however, likely that the appropriate physiologic signals critical for homing of hematopoietic precursors to the thymus and for subsequent T-cell development cannot be sufficiently provided by TECs affected by GVHD.
Protecting thymic epithelium from a harmful alloimmune response following BMT may ameliorate GVHD-associated deficits in transplant recipients. The purpose of this study was therefore to determine whether stimulation of KGFR with pharmacologic doses of KGF prevents the injury inflicted on the thymic microenvironment during the course of acute GVHD. To this end, a nonconditioned murine parent→F1 haploidentical transplantation model was studied45,46 50-54 in which thymic injury is independent of damage to TECs caused by γ-irradiation.
Materials and methods
Mice
C57BL/6 (B6, H-2b) and [C57BL/6 × DBA/2]F1 (B6D2F1, H-2bxd) were obtained from Iffa Credo (Charles River, France), and from a breeding colony at the University Hospital, Basel, Switzerland. The C57BL/6 congenic mouse strain B6.SJL-PtprcaPep3b/BoyJ(Ly5.1) was obtained from The Jackson Laboratories, ME. Mice were housed in a specific pathogen–free (SPF) facility. All animals were kept in accordance with Swiss guidelines and regulations. Female mice used for this study were between 6 and 10 weeks of age.
Reagents
For 4-color flow cytometric (FACS) analyses, the following monoclonal antibodies (moAbs) (conjugated to biotin, fluorescein isothiocyanate [FITC], phycoerythrin [PE], or CyChrome) were used: anti-CD3 (clone 145-2C11), anti-CD8 (53-6.7), anti-CD4 (RM4-5), anti-TCRβ (H57-592), anti-CD44 (clone IM7), anti-CD25 (PC61), anti-Ly5.1 (CD45.1; A20), and anti-CD16/CD32 (2.4G2) (all from Pharmingen, San Diego, CA); streptavidin-tricolor (Caltag, Burlingame, CA); and streptavidin-Cy5 (Zymed Laboratories, San Francisco, CA). FITC-conjugated anti-BrdU moAb (3D4) was purchased from Becton Dickinson (Mountain View, CA). For immunohistology, polyclonal anti–cytokeratin-5 Ab (Progene GmbH, Heidelberg, Germany), biotinylated anti–cytokeratin-18 moAb (Ks 18.04; Progene GmbH), biotinylated UEA-1 lectin (Vector Laboratories, Lausanne, Switzerland), MTS10 (Pharmingen), polyclonal anti-FGFR2 Ab (unpublished; a generous gift from Dr Sabine Werner, Zürich, Switzerland), and biotinylated anti–CD80-moAb (16-10A1; Pharmingen), were used. The medullary thymic epithelial cell line mTEC2-3 was a gift from Dr M. Kasai (Tokyo, Japan).55
Graft-versus-host disease
The transplantation model used has been previously described in detail.45,46,50,51 53 In brief, acute GVHD was induced by transplantation of nonirradiated B6D2F1 mice (Ly5.2+;H-2bxd) with 25 × 106unseparated parental C57BL/6 splenocytes (Ly5.2+; H-2b), or with congenic B6.CD45.1 (Ly5.1+; H-2b) cells. Donor cells were administered in a volume of 400 μL Leibovitz-15 media (Invitrogen AG, Basel, Switzerland) via tail vein injection. Mice that received syngeneic transplants (B6D2F1→B6D2F1) served as non-GVHD controls and received 25 × 106 donor splenocytes.
KGF treatment
Mice were injected subcutaneously for a period from days −3 to +3 after induction of GVHD (day 0) with Hanks balanced salt solution (HBSS) or recombinant human KGF (rhKGF; solubilized in HBSS) at a dose of 5 mg/kg per day. rhKGF was produced in Escherichia coliand had a median effective dose (ED50) of 40.02 ng/mL (kindly provided by Amgen, Thousand Oaks, CA). B6D2F1 control mice that received syngeneic transplants were treated either with HBSS or with KGF. Significant differences between these 2 latter treatment groups were not observed except where indicated in “Results.”
Flow cytometric analysis
Cells (0.5-1 × 106) were washed, resuspended in 1% fetal calf serum (FCS)/phosphate buffered saline (PBS)/sodium azide and incubated for 15 minutes at 4°C with unlabeled 2.4G2 moAb to prevent unspecific binding of the Fcγ receptors. For 3-color flow cytometry, cells were first stained with fluorochrome- and biotin-conjugated moAbs and were subsequently labeled with streptavidin-Cy5 (Zymed Laboratories). Washed cells were immediately analyzed using a 2-laser FACS calibur (Becton Dickinson, Mountain View, CA).
Analysis of cell proliferation in vivo
At 3 hours and one hour before they were killed, mice that had received transplants were injected intraperitoneally (ip) with 5′-bromo-2′-deoxyuridine (BrdU; 1 mg in 0.2 mL PBS; Sigma, Buchs, Switzerland). Thymi were isolated and DNA-synthesizing cells were detected by 4-color flow cytometry, as described previously.46 56 Briefly, thymocytes (1 × 106) were stained with a mixture of PE-conjugated anti-CD3,-CD8,-CD4 moAbs, CyChrome-conjugated anti-CD44 moAb, and biotinylated anti-CD25 moAb for 30 minutes on ice. Cells were then washed and incubated with streptavidin-Cy5 for 15 minutes. Cells were subsequently permeabilized with ice-cold 0.15M NaCl/95% ethanol for 30 minutes at 4°C and fixed for another 30 minutes at room temperature in 1% paraformaldehyde/PBS with 0.01% Tween. Cells were then treated with 50 U/mL DNAse I in a 0.15 M NaCl/4.2mM MgCl2 buffer for 10 minutes at room temperature. After washing, cells were stained with FITC-conjugated anti–BrdU moAb for 30 minutes at room temperature. Washed cells were analyzed using a FACScalibur (Becton Dickinson, Franklin Lakes, NJ).
Detection of donor/host chimerism
To discern donor-derived T cells (Ly5.1+) from host T cells (Ly5.2+), thymocytes and splenocytes were isolated from mice that received transplants and control mice and stained with biotin-conjugated anti–CD45.1 moAb and revealed with streptavidin-CyChrome.
Cell separation
Freshly isolated thymocytes were stained with the appropriate moAbs and then sorted into CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ subpopulations with the use of a FACSvantage cell sorter (Becton Dickinson, Franklin Lakes, NJ). Thymic epithelial cells were isolated using a previously published method.57 Briefly, thymi were digested with collagenase D and thymocytes were removed thereafter from the cell suspension. Adherent cells were stained with a combination of anti-Iab(major histocompatibility [MHC] class II) and CD45 moAbs. Ia+CD45− cells were sorted on a FACSvantage.
Histopathology and immunohistology
For detection of KGFR and CD80 surface expression, thymi were isolated and embedded in cryoembedding media (Tissue-Tek, Sakura Finetec, Netherlands). Frozen samples were cut into 6-μm-thick sections, fixed with 4% paraformaldehyde/PBS, and stained with biotinylated anti–FGFR-2 or anti-CD80 antibodies for one hour. After washing, sections were incubated with streptavidin-conjugated horseradish peroxidase. Sections were then incubated with 3′-amino-9′-ethylcarbazole (AEC; Sigma) and counterstained with hemalaun. For analysis of thymic morphology, fixed paraffin sections were stained with hematoxylin and eosin.
Panels of antibodies and lectins have previously been used to characterize different TEC subsets.16-18 In brief, TEC subsets were identified using combinations of anti–cytokeratin 18 moAb and UEA-1 lectin, polyclonal anti–cytokeratin 5 antibody, and the epithelial cell–specific MTS-10 antibody. The particular staining protocol was adapted from Klug and coworkers18 and is listed in more detail in Table 1. The 2- and 3-color immunoflourescent sections were analyzed using a confocal microscope (Carl-Zeiss AG, Feldbach, Switzerland).
Polymerase chain reaction
For end-point polymerase chain reaction (PCR) analysis of KGFR messenger RNA (mRNA) expression, total RNA was isolated from whole thymic tissue, freshly isolated thymic epithelial cells, established thymic epithelial cell lines, or thymocyte subpopulations (where indicated). After reverse transcription, the complementary DNA (cDNA) was amplified for 38 cycles.
For quantitative PCR analysis, total RNA was isolated from unseparated thymic tissue at day 13 after transplantation. RNA was reverse transcribed and the resulting cDNA was amplified in a total volume of 25 μL buffer containing 20 ng cDNA, 1 x SYBR Green PCR Master Mix (PE Biosystems, Rotkreuz, Switzerland), and 300 nM forward and reverse primer each. Primers for real-time PCR were designed according to published mouse RNA sequences. As an internal reference for thymic epithelial cells, the gene for the epithelial V–like antigen (EVA)58 was amplified, whereas glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal reference for all cells. The cycle conditions were as follows: 50°C for 2 minutes followed by 95°C for 10 minutes, after which 40 cycles of amplification were carried out (95°C for 15 seconds, 60°C for 1 minute). The PCR reaction was performed in a GeneAmp 5700 SDS Real Time PCR machine (PE Biosystems). Samples were analyzed in triplicates, and the result was averaged. Primers used are as follows: EVA: sense GTGCCGCCTGCTCGTC, antisense CCGAACATCTGTCCCGTTGA; GAPDH: sense ACCATGTAGTTGAGGTCAATGAAGG, antisense GGTGAAGGTCGGTGTGAACG; Mip-1β: sense TGCTCGTGGCTGCCTTCT, antisense CAGGAAGTGGGAGGGTCAGA; Mip-1α: sense TTTTGAAACCAGCAGCCTTTG, antisense TCTTTGGAGTCAGCGCAGATC; TECK: sense CAGCACAGGATCAAATGGAATG, antisense GGTTGCAGCTTCCACTCACTT; IL7: sense GGGAGTGATTATGGGTGGTGAG, antisense TGCGGGAGGTGGGTGTAG;Aire: sense GGTTCTGTTGGACTCTGCCCTG, antisense TGTGCCACGACGGAGGTGAG; granzyme B: sense GACCAGCTCTGTCCTTGGCA, antisense ATGTCAGTTGGGTTGTCACAGC; CD80: sense GAGTCTGGAACCCATCTGCA, antisense GAAGCGAGGCTTTGGGAAAC; KGFR: sense CACTCGGGGATAAATAGCTC, antisense GTCCTTCTCT- GTGGCATCAT.
Statistical analysis
Groups were compared by one-way analysis of variance (ANOVA). Where ANOVA revealed a significant difference, the Bonferroni/Dunn post hoc test was performed. The overall statistical significance level was set to 5%. StatView from SAS Institute (Cary, NC) was used for statistical analysis.
Results
Treatment of transplant recipients with KGF diminishes thymic GVHD
To investigate a role for KGF in the prevention of thymic GVHD, a murine haploidentical transplantation model was investigated. The model was independent of total body irradiation or other cytotoxic preconditioning regimens that may adversely affect thymocytes and thymic epithelial cells. Severe acute GVHD was elicited in B6D2F1 mice by transfer of 25 × 106unseparated splenocytes from C57BL/6 donors. In addition, the mice that received transplants (B6→B6D2F1) were treated subcutaneously from days −3 to day +3 of transplantation with either HBSS or KGF (5 mg/kg per day). HBSS-treated B6D2F1recipients of 25 × 106 syngeneic B6D2F1splenocytes served as controls.
The effect of KGF on thymic weight, cellularity, and function was investigated on day 13 after transplantation. As demonstrated in Figure1A-B, mice that had received allogeneic transplants and had been injected with HBSS suffered a significant loss in thymic weight and cellularity when compared with syngeneic controls. However, treatment with KGF fully inhibited this GVHD-induced thymic injury. Thymocytes of mice that had received syngeneic transplants and had been treated with KGF displayed normal thymocyte maturation comparable to B6D2F1→B6D2F1 mice treated with HBSS (data not shown). Notwithstanding, thymic weights were increased by approximately 50% in the former for reasons yet to be defined. Thus, KGF treatment of GVHD mice did not return cellularity to levels of KGF-treated control mice.
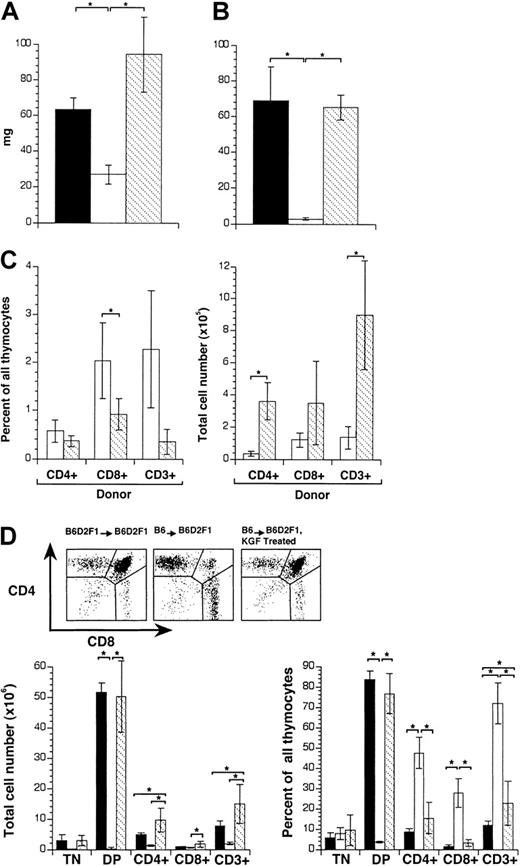
Treatment of mice with KGF diminishes thymic GVHD.
Acute GVHD was induced by transfer of 25 × 106 parental B6 splenocytes to unirradiated B6D2F1 mice. Recipients were analyzed on day 13 after transplantation. Mice were treated from days −3 to +3 of transplantation with KGF (ip, 5.0 mg/kg per mouse per day KGF; ▧) or HBSS (■). B6D2F1 mice that received transplants with syngeneic splenocytes served as non-GVHD controls and received HBSS (▪). (A) Thymus weight (mg) and (B) cellularity (total cell number × 106) were determined. (C) Infiltration of donor T cells into the thymus (in percent, left; total cell number × 105, right). Donor-derived T cells were distinguished from host cells (CD45.2+) by the expression of CD45.1. (D) Cell surface expression of CD3, CD4, and CD8 on thymic cells was analyzed by flow cytometry and the major thymocyte subsets were quantified (% of total cells and total cell numbers × 106, respectively). TN indicates CD3, CD4, CD8 triple-negative thymocytes; DP indicates CD4, CD8 double-positive thymocytes. The graph represents pooled data from 3 independent experiments, with 10 mice analyzed for each group. Analysis by ANOVA; *P < .05.
Treatment of mice with KGF diminishes thymic GVHD.
Acute GVHD was induced by transfer of 25 × 106 parental B6 splenocytes to unirradiated B6D2F1 mice. Recipients were analyzed on day 13 after transplantation. Mice were treated from days −3 to +3 of transplantation with KGF (ip, 5.0 mg/kg per mouse per day KGF; ▧) or HBSS (■). B6D2F1 mice that received transplants with syngeneic splenocytes served as non-GVHD controls and received HBSS (▪). (A) Thymus weight (mg) and (B) cellularity (total cell number × 106) were determined. (C) Infiltration of donor T cells into the thymus (in percent, left; total cell number × 105, right). Donor-derived T cells were distinguished from host cells (CD45.2+) by the expression of CD45.1. (D) Cell surface expression of CD3, CD4, and CD8 on thymic cells was analyzed by flow cytometry and the major thymocyte subsets were quantified (% of total cells and total cell numbers × 106, respectively). TN indicates CD3, CD4, CD8 triple-negative thymocytes; DP indicates CD4, CD8 double-positive thymocytes. The graph represents pooled data from 3 independent experiments, with 10 mice analyzed for each group. Analysis by ANOVA; *P < .05.
Because the extent of thymic damage had been previously correlated with the presence of mature donor T cells entering the thymus,46 we next determined the frequencies and absolute numbers of infiltrating T cells of donor origin in these mice. Thymic single-cell suspensions of Ly5.2+ recipients were analyzed by flow cytometry for the in situ presence of mature Ly5.1+cells. KGF treatment diminished the relative percentage of thymus-infiltrating T cells, and in particular that of mature CD8+ cells, when compared with HBSS-treated mice (Figure1C). However, the absolute number of donor-derived T cells was several-fold higher in KGF-treated hosts when compared with control transplant recipients (ie, those injected with HBSS). Thus, the protective effect of KGF during GVHD on thymocyte development was not due to a decrease in thymus-infiltrating mature T cells.
Thymocytes of mice that had received syngeneic and allogeneic transplants and had been treated with either HBSS or KGF were further analyzed for expression of CD3, CD4, and CD8. Comparing the 3 experimental groups, significant changes in the relative number of TN thymocytes were not observed (Figure 1D). However, the DP population was severely diminished whereas SP mature thymocytes were greatly increased in B6→B6D2F1 recipients treated with HBSS. These changes were typical for GVHD-mediated thymic pathology and are accounted for by both enhanced apoptosis of DP thymocytes and the presence of mature donor T cells in the thymus, as recently published.46 In contrast, mice injected with allogeneic T cells and KGF displayed a normal thymocyte maturation with the notable exception of an increased number of CD3+ T cells, likely reflecting the presence of mature T cells of donor origin. Taken together, these results demonstrated that treatment with KGF prevented the GVHD-associated thymic changes in weight, cellularity, and cell maturation, independently of effects on the thymic recruitment of donor-derived T cells.
KGF treatment maintains normal cell cycle progression of resident CD3−CD4−CD8− thymocytes despite acute GVHD
At 2 weeks after transplantation, the absolute cellularities within each of the 4 phenotypically distinct TN thymocyte subpopulations were significantly decreased in mice injected with saline (Figure 2, upper panel). In contrast, treatment with KGF preserved cell numbers for each of the TN subpopulations. Thus, KGF prevented the loss of the most immature pro- and pre-T cells in the presence of alloreactive T cells. Interestingly, KGF increased cellularity of TN stage IV cells to supranormal levels in mice that received syngeneic transplants (data not shown). Thus, KGF did not fully return cellularity of this subpopulation in GVHD mice when the cell numbers of KGF-treated control mice were referenced as 100%.
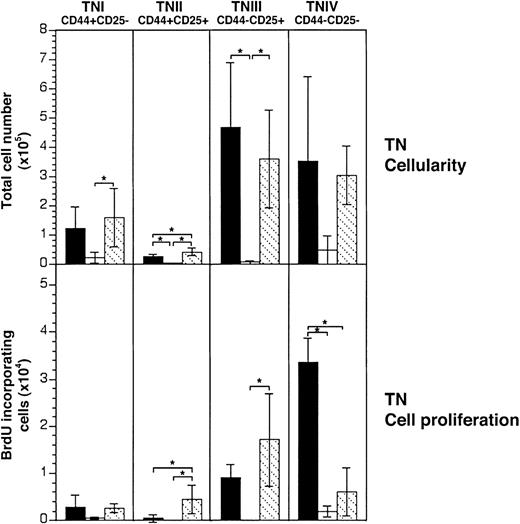
KGF treatment maintains normal cell cycle progression of the most immature thymocyte subsets.
Acute GVHD was induced in unirradiated B6D2F1 mice as in Figure 1. CD3, CD4, CD8 triple-negative (TN) thymocytes were analyzed 13 days after transplantation for surface expression of CD44 and CD25 and for BrdU incorporation. Upper panels: TN cellularity (total TN cell number × 105) in mice treated with KGF (▧) or with HBSS (■) was ascertained and compared with HBSS-treated B6D2F1 mice that received syngeneic transplants (▪). Lower panels: the absolute cell numbers (× 104) of BrdU+ cells among TN thymocytes were determined. Each graph represents pooled data from 2 independent experiments, with 7 mice analyzed for each group. Analysis by ANOVA; *P < .05.
KGF treatment maintains normal cell cycle progression of the most immature thymocyte subsets.
Acute GVHD was induced in unirradiated B6D2F1 mice as in Figure 1. CD3, CD4, CD8 triple-negative (TN) thymocytes were analyzed 13 days after transplantation for surface expression of CD44 and CD25 and for BrdU incorporation. Upper panels: TN cellularity (total TN cell number × 105) in mice treated with KGF (▧) or with HBSS (■) was ascertained and compared with HBSS-treated B6D2F1 mice that received syngeneic transplants (▪). Lower panels: the absolute cell numbers (× 104) of BrdU+ cells among TN thymocytes were determined. Each graph represents pooled data from 2 independent experiments, with 7 mice analyzed for each group. Analysis by ANOVA; *P < .05.
Because the loss of TN cells during GVHD is the consequence of impaired cellular proliferation, we investigated whether KGF averted this functional deficiency. BrdU incorporation was used to determine cell cycle progression56 which normally occurs at all phenotypic TN stages and in particular within stages II and IV for each TN subpopulation. In B6→B6D2F1 recipients injected with HBSS, viable TN cells of stages II, III, and IV were severely affected by GVHD in their proliferative capacity (Figure 2, lower panel). However, in KGF-treated mice with GVHD, the TN subpopulations II and III proliferated at normal (TNIII) or even increased (TNII) levels. For TNIV cells, the GVHD-induced inhibition of cell cycle progression was only partially reversed by KGF.
KGF treatment fails to modulate splenic GVHD
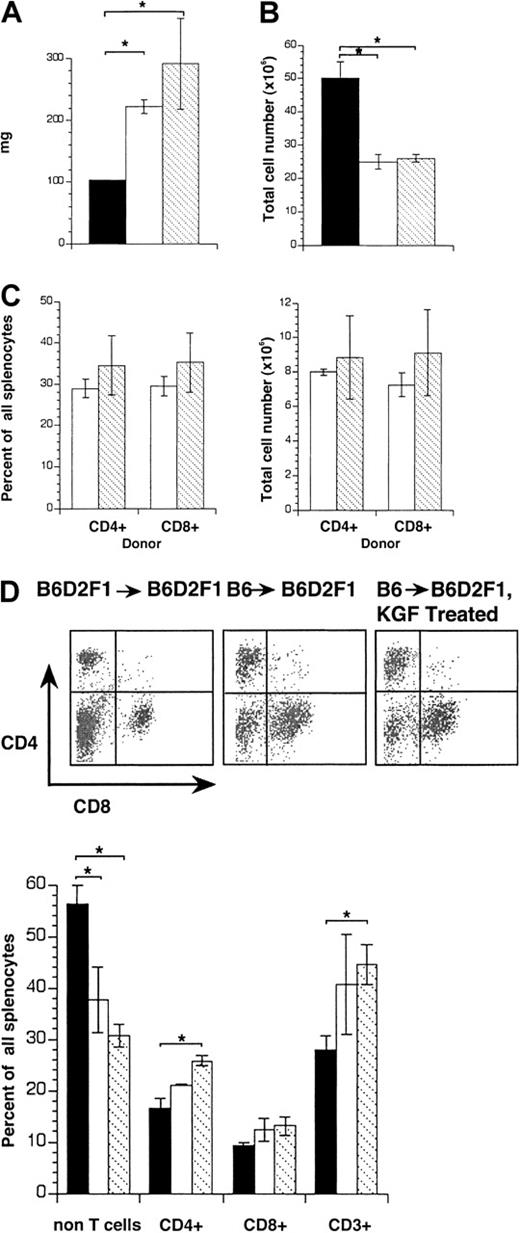
Because the spleen serves as a typical target of acute GVHD, we next assessed the extent of splenic damage in B6→B6D2F1mice treated either with KGF or HBSS. KGF influenced neither weights nor absolute cellularities of the spleens in mice that received allogeneic transplants when compared with mice injected with HBSS (Figure 3A-B). Moreover, treatment with KGF had no effect on the degree of donor T-cell infiltration (Figure3C). Phenotypic analysis by flow cytometry of splenocytes revealed for both groups of recipients a loss in non-T cells (eg, B lymphocytes) in lieu of CD4+CD8− and CD4−CD8+ T cells (Figure 3D). Thus, in vivo administration of KGF did not affect the severity of splenic GVHD.
KGF treatment does not affect the severity of splenic GVHD.
Acute GVHD was induced in unirradiated B6D2F1 mice as in Figure 1 and recipients were analyzed on day 13 after transplantation. (A) Spleen weights (mg) and (B) cellularities (cells × 106) in transplant recipients treated with KGF (▧) or HBSS (■). HBSS-treated B6D2F1 mice that received syngeneic transplants served as non-GVHD controls (▪). (C) Infiltration of donor T cells into the spleen (in percent, left panel; total cell number × 106 right panel) was determined. Donor-derived T cells were distinguished from host cells (CD45.2+) by their expression of CD45.1. (D) The surface expression of CD4 and CD8 on splenocytes was analyzed by flow cytometry and quantified (% of total splenocytes). The graphs represent pooled data from 3 independent experiments, with 10 mice analyzed for each group. Analysis by ANOVA; *P < .05.
KGF treatment does not affect the severity of splenic GVHD.
Acute GVHD was induced in unirradiated B6D2F1 mice as in Figure 1 and recipients were analyzed on day 13 after transplantation. (A) Spleen weights (mg) and (B) cellularities (cells × 106) in transplant recipients treated with KGF (▧) or HBSS (■). HBSS-treated B6D2F1 mice that received syngeneic transplants served as non-GVHD controls (▪). (C) Infiltration of donor T cells into the spleen (in percent, left panel; total cell number × 106 right panel) was determined. Donor-derived T cells were distinguished from host cells (CD45.2+) by their expression of CD45.1. (D) The surface expression of CD4 and CD8 on splenocytes was analyzed by flow cytometry and quantified (% of total splenocytes). The graphs represent pooled data from 3 independent experiments, with 10 mice analyzed for each group. Analysis by ANOVA; *P < .05.
The receptor for KGF is expressed on TECs but not on thymocytes
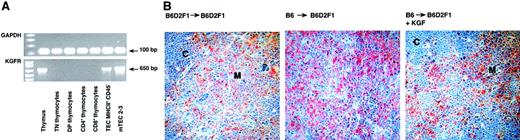
The effect of KGF on thymic T-cell maturation may either be brought about directly by binding of KGF to its specific receptor on thymocytes or, alternatively, may be accomplished indirectly via an effect on thymic stromal cells. To define the thymic target cell(s) of KGF, thymocytes at all maturational stages and TECs were analyzed by end-point reverse transcription (RT)–PCR for the expression of KGFR. As demonstrated in Figure 4A, transcripts for KGFR were exclusively detected in freshly isolated thymic epithelial cells (CD45−MHC II+), in an established thymic medullary epithelial cell line (mTEC 2-3)55 and in unseparated thymic tissue. In contrast, none of the purified thymocyte subpopulations displayed mRNA specific for the KGFR. The selective expression of the receptor on TECs was further ascertained by immunohistology using a polyclonal antibody directed against FGFR-2. This antibody was raised against the YDINRVPEEQMTFKDLVS sequence and specifically recognizes all splice variants of FGFR-2 including FGFR2IIIb (aka KGFR; Dr S. Werner, personal communication, July 2001). Since KGFR is expressed exclusively on epithelial cells,33 simultaneous staining with anti–cytokeratin antibodies will unambiguously identify KGFR on TECs. Our data show that KGFR was expressed exclusively on thymic epithelial cells of both cortex and medulla (Figure 4B) as consecutive sections stained for cytokeratins displayed an identical pattern and KGFR was not expressed on thymocytes (data not shown), corroborating the PCR data shown in Figure 4A. Therefore, the observed effects of KGF on intrathymic T-cell development were likely accomplished via binding of this cytokine to its specific receptor on TECs.
KGF receptor is expressed on thymic epithelial cells.
(A) End-point RT-PCR analysis with cDNA isolated from unseparated thymic tissue of a naive mouse, from flow cytometrically purified thymocyte subsets and TECs (MHCII+CD45−), and from the immortal thymic epithelial cell line mTEC2-3. (B) Thymic tissue sections of the 3 transplant groups were analyzed for expression of KGFR using an anti–FGFR2 antibody. Adjacent sections (not shown) were stained for cytokeratins in order to verify the presence of TECs. Original magnification × 200. C indicates cortex; M, medulla.
KGF receptor is expressed on thymic epithelial cells.
(A) End-point RT-PCR analysis with cDNA isolated from unseparated thymic tissue of a naive mouse, from flow cytometrically purified thymocyte subsets and TECs (MHCII+CD45−), and from the immortal thymic epithelial cell line mTEC2-3. (B) Thymic tissue sections of the 3 transplant groups were analyzed for expression of KGFR using an anti–FGFR2 antibody. Adjacent sections (not shown) were stained for cytokeratins in order to verify the presence of TECs. Original magnification × 200. C indicates cortex; M, medulla.
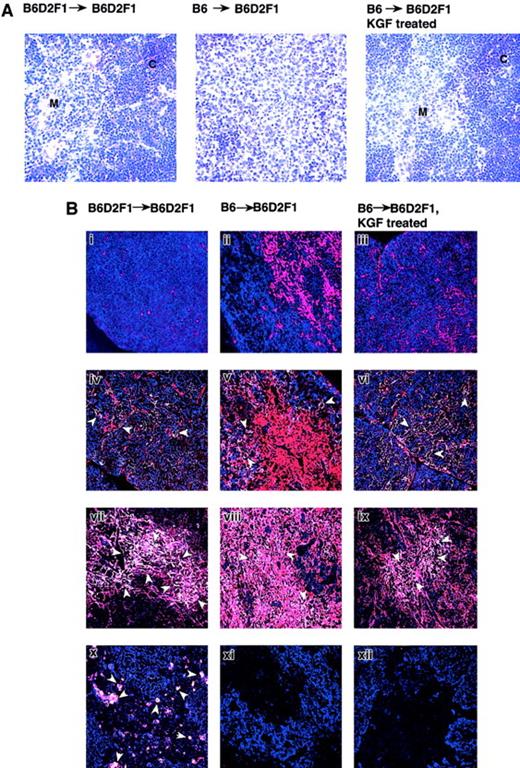
KGF treatment essentially preserves the thymic microenvironment despite GVHD
The loss of normal thymic morphology is a typical feature of GVHD and has recently been linked to the alloimmune response directed against thymic stromal components (W.K. and G.A.H., in preparation, 2002). Because normal T-cell development was preserved by KGF despite acute GVHD (as judged by splenic alterations) and since KGF receptor expression was detected exclusively on TECs, the cellular stromal composition and architecture of the thymic microenvironment was investigated in B6→B6D2F1 recipients. Figure5A shows that the clear separation between cortex and medulla was lost in acute GVHD in mice treated with HBSS but was clearly maintained in recipients injected with KGF. Immunohistology using UAE-1 lectin, MTS-10, and antibodies to cytokeratin (K) 5 and K18 distinguished TECs into 4 distinct subpopulations: the major cortical (K18+K5−UEA-1−MTS10−), the minor cortical (K18+K5+), the major medullary (K5+MTS10+), and the minor medullary epithelial cells (K18+UEA-1+)18(see Table 1). In HBSS-treated B6→B6D2F1 mice, the major cortical epithelial cells were severely diminished in cell number in response to acute GVHD, contributing to a smaller cortex (Figure 5Bii). In contrast, treatment with KGF preserved the cellularity of this subpopulation to a degree indistinguishable to that of syngeneic transplantation controls (Figure 5Bi,iii). The frequencies of minor cortical and major medullary epithelial cells also remained unchanged in mice injected with KGF when compared with syngeneic transplantation controls, whereas recipients injected with HBSS sustained a substantial loss of both of these subpopulations (Figure 5Biv-ix). Only minor medullary epithelial cells appeared to be unaffected by KGF treatment because both treatment groups of B6→B6D2F1 recipients were shown to display an almost complete loss of this subpopulation (Figure 5Bx-xii). However, this subpopulation of epithelial cells did express the KGFR in naı̈ve thymi (data not shown).
KGF treatment preserves the thymic microenvironment during acute GVHD.
GVHD was induced in KGF- or HBSS-treated B6D2F1 mice, as described in Figure 1, and mice were analyzed on day 13 after transplantation. HBSS-treated B6D2F1 mice that received syngeneic transplants served as non-GVHD controls. (A) Frozen thymic sections (6 μm) from the 3 transplant groups were analyzed for histopathology (Hematoxylin + Eosin stain; original magnification × 200). (B) Immunohistology of 4 distinct subpopulations of thymic epithelial cells in mice that received allogeneic and syngeneic transplants. Thymic epithelial cells were identified using a panel of antibodies according to Table 1. Two-color immunoflourescent analyses were performed using a confocal microscope (Carl-Zeiss). Major cortical cells appear blue (i-iii), whereas minor cortical TEC (iv-vi), major medullary TEC (vii-ix), and minor medullary TEC appear white (x-xii) (ie, altered color by computer-assisted management of confocal microscopy data, see Table 1). The arrows denote individual positive cells. Original magnification × 200. The data substantiate that KGF treatment preserves in the presence of GVHD the normal structure of cortical and major medullary TECs but not of the subpopulation of minor medullary TECs. C indicates cortex; M, medulla.
KGF treatment preserves the thymic microenvironment during acute GVHD.
GVHD was induced in KGF- or HBSS-treated B6D2F1 mice, as described in Figure 1, and mice were analyzed on day 13 after transplantation. HBSS-treated B6D2F1 mice that received syngeneic transplants served as non-GVHD controls. (A) Frozen thymic sections (6 μm) from the 3 transplant groups were analyzed for histopathology (Hematoxylin + Eosin stain; original magnification × 200). (B) Immunohistology of 4 distinct subpopulations of thymic epithelial cells in mice that received allogeneic and syngeneic transplants. Thymic epithelial cells were identified using a panel of antibodies according to Table 1. Two-color immunoflourescent analyses were performed using a confocal microscope (Carl-Zeiss). Major cortical cells appear blue (i-iii), whereas minor cortical TEC (iv-vi), major medullary TEC (vii-ix), and minor medullary TEC appear white (x-xii) (ie, altered color by computer-assisted management of confocal microscopy data, see Table 1). The arrows denote individual positive cells. Original magnification × 200. The data substantiate that KGF treatment preserves in the presence of GVHD the normal structure of cortical and major medullary TECs but not of the subpopulation of minor medullary TECs. C indicates cortex; M, medulla.
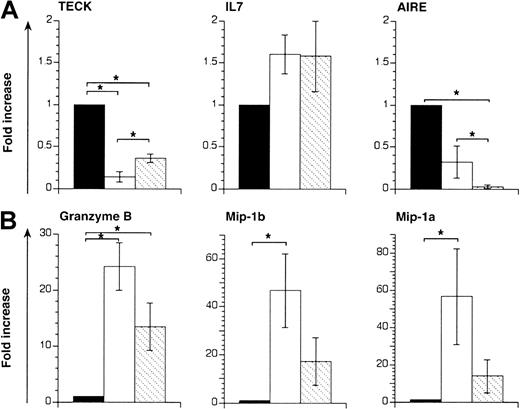
KGF affects TEC function
The effect of KGF on thymic epithelial cells may include alterations in gene expression that result in enhanced biologic functions, thus maintaining normal thymocyte maturation despite the presence of allogeneic T cells. In normal thymic development, epithelial cells produce necessary chemotactic and activating stimuli to lymphoid precursors, (eg, thymic epithelial chemokine; TECK,59 and macrophage inflammatory protein-1 [Mip-1]60). Moreover, these cells provide crucial survival and proliferation signals for TN cells (eg, IL-7),61 and unique molecules necessary for repertoire selection of DP thymocytes (eg, autoimmune regulator [Aire]).62,63 To investigate whether KGF affected expression of these molecules during acute GVHD, quantitative RT-PCR analysis was performed on thymic tissues. TEC-specific transcripts were normalized to the expression of the EVA.58 Expression of the chemokine TECK was significantly diminished in epithelial cells during GVHD but was partially restored by KGF treatment (Figure6A). In contrast, IL-7 expression was increased among thymic epithelial cells during GVHD albeit not to a statistically significant degree. KGF treatment had no further effect. Analysis for the expression of the transcription factor Aire, which is usually expressed in minor medullary epithelial cells,62revealed a decrease of specific mRNA following GVHD. KGF treatment did not restore Aire expression, which is in agreement with a lack of minor medullary epithelial cells in KGF-treated mice with GVHD (Figure5B).
KGF modulates gene expression in thymi from mice that received transplants.
Acute GVHD was induced in unirradiated B6D2F1 mice, as described in Figure 1. Recipients of allogeneic T cells were treated with either KGF (▧) or HBSS (■). HBSS-treated B6D2F1mice that received syngeneic transplants served as non-GVHD controls (▪). RNA isolated from whole thymic tissue was analyzed 13 days after transplantation by quantitative RT-PCR for transcripts specific for IL-7, TECK, and Aire (A) and for Mip-1α, Mip-1β, and granzyme B (B), respectively. TEC-derived transcripts (TECK, IL-7, Aire) were normalized for EVA expression whereas hematopoietic cell–specific transcripts (granzyme B, Mip-1α, and Mip-1β) were normalized for GAPDH expression. Each graph represents pooled data from one experiment, with 3 mice analyzed for each group. Analysis by ANOVA; *P < .05.
KGF modulates gene expression in thymi from mice that received transplants.
Acute GVHD was induced in unirradiated B6D2F1 mice, as described in Figure 1. Recipients of allogeneic T cells were treated with either KGF (▧) or HBSS (■). HBSS-treated B6D2F1mice that received syngeneic transplants served as non-GVHD controls (▪). RNA isolated from whole thymic tissue was analyzed 13 days after transplantation by quantitative RT-PCR for transcripts specific for IL-7, TECK, and Aire (A) and for Mip-1α, Mip-1β, and granzyme B (B), respectively. TEC-derived transcripts (TECK, IL-7, Aire) were normalized for EVA expression whereas hematopoietic cell–specific transcripts (granzyme B, Mip-1α, and Mip-1β) were normalized for GAPDH expression. Each graph represents pooled data from one experiment, with 3 mice analyzed for each group. Analysis by ANOVA; *P < .05.
Because donor T cells mediate a strong inflammatory response and TEC dysfunction in this nonirradiation model of GVHD, we examined whether KGF altered the thymic microenvironment and resulted in decreased inflammation. The mRNA species for granzyme B, Mip-1α, and Mip-1β were therefore measured by quantitative PCR and normalized to GAPDH expression. Transcripts for granzyme B, a cellular activation marker of cytotoxic T cells, were greatly increased in thymic tissue affected by GVHD (Figure 6B). Treatment with KGF reduced the number of granzyme B–specific transcripts by 2-fold, despite the fact that the absolute number of donor T cells was increased in these mice when compared with saline-treated animals with GVHD. The expression of Mip-1α and Mip-1β, that is, important mediators of inflammation,64was up-regulated during acute thymic GVHD (Figure 6B and L. Piali, S.R., W.K., and G.H., manuscript in preparation, 2002). Treatment with KGF reduced specific transcripts of these chemokines by 3- to 4-fold, despite the increased number of infiltrating donor T cells in the thymus.
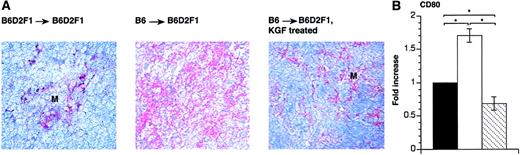
KGF reduces CD80 expression in thymic GVHD
CD80 (B7.1) is a critical costimulatory molecule for thymocyte development and mature T-cell function.65 66 We therefore determined CD80 expression in thymic tissue sections of mice that received syngeneic and allogeneic transplants. To this end, consecutive thymic tissue sections were analyzed for the expression of CD80 and cytokeratin 18 (CK18), respectively. The comparison of the staining revealed a pattern compatible with CD80 expression on thymic epithelial cells (data not shown). We found that CD80 expression was up-regulated in the thymus in the presence of acute GVHD (Figure7A). Treatment with KGF diminished this expression in both the cortical and medullary compartment to a degree comparable to syngeneic transplant recipients despite an increase in the absolute number of mature donor-derived T cells. These immunohistologic findings were further corroborated by quantitative PCR analysis (Figure 7B). Thus, KGF treatment reduces the cell surface expression of the costimulatory molecule CD80 on stromal cells (and possibly other cells, including activated T cells) and may thus hamper their allogeneicity.
KGF reduces CD80 expression on thymic stromal cells in recipients with GVHD.
Acute GVHD was induced in KGF-treated or HBSS-treated B6D2F1 mice, as described in Figure 1. (A) At 13 days after transplantation, frozen thymic sections (6 μm) from the 3 transplant groups were stained with anti–CD80 moAb. Slides were then counterstained with hemalaun. Adjacent sections (not shown) were stained for cytokeratins in order to verify the presence of TECs. Original magnification × 200. (B) RNA isolated from whole thymic tissue was analyzed 13 days after transplantation by end-point RT-PCR for transcripts specific for CD80. Transcripts were normalized for EVA expression. The graph represents data from 3 mice analyzed for each group. Analysis by ANOVA; *P < .05.
KGF reduces CD80 expression on thymic stromal cells in recipients with GVHD.
Acute GVHD was induced in KGF-treated or HBSS-treated B6D2F1 mice, as described in Figure 1. (A) At 13 days after transplantation, frozen thymic sections (6 μm) from the 3 transplant groups were stained with anti–CD80 moAb. Slides were then counterstained with hemalaun. Adjacent sections (not shown) were stained for cytokeratins in order to verify the presence of TECs. Original magnification × 200. (B) RNA isolated from whole thymic tissue was analyzed 13 days after transplantation by end-point RT-PCR for transcripts specific for CD80. Transcripts were normalized for EVA expression. The graph represents data from 3 mice analyzed for each group. Analysis by ANOVA; *P < .05.
Discussion
KGF preserved normal thymic development as revealed by typical cellularity, frequency, and cellular proliferation of the different immature and mature thymocyte subsets despite the systemic presence of acute GVHD (Figures 1-3). The structure of the thymic microenvironment following exposure to KGF was almost normal, as assessed by the regular cellular compositions of several TEC compartments (Figure 5). We hypothesize that this effect was a direct consequence of KGF on thymic epithelial cells as TECs but not thymocytes or other stromal elements specifically express the KGF receptor (Figure 4). Treatment with KGF also reduced CD80 expression, possibly rendering TECs less likely targets to allogeneic T-cell recognition (Figure 7). Interestingly, the absolute cell number of mature donor T cells in B6→B6D2F1recipients treated with KGF was, however, significantly increased (Figure 1). Finally, KGF treatment was shown to affect the transcriptional activity of several genes ascribed to play an important role in TEC function when measured under these conditions (Figure 6). We therefore conclude that TECs are targets of GVHD and hypothesize that prevention or reversal of TEC injury by KGF has a beneficial effect on developing thymocytes by allowing a normal cross-talk to occur between thymic epithelia and thymocytes.
Recent investigations concerning issues of the mesenchymal-epithelial cell interactions, the morphogenesis of epithelium, and the mechanisms operational in cutaneous wound repair have identified KGF as a highly specific and potent mitogen.21,25-27 Although KGF transcripts appear to be restricted to cells of mesenchymal origin, factor-responsive cells are exclusively of epithelial cell type. The mitogenic activity of KGF is mediated through the KGF receptor, a splice variant of FGF-2 receptor.34,35 Thymic epithelial cells have been reported to bear cell surface markers common with epithelia of other organs, in particular the epidermal keratinocytes in the skin.67 Despite these phenotypic similarities it is important to recognize that the capacity to efficiently support development and selection of T cells is a unique feature of TECs.68 We now demonstrate that KGFR is expressed in freshly isolated thymic epithelial cells (ie, MHCII+, CD45−) as well as in established thymic epithelial cell lines (Figure 4A). In contrast, none of the thymocyte subpopulations expressed this receptor. These observations strongly imply that treatment with KGF affected TECs and the effects observed in this GVHD model were due to changes in thymic epithelia. Although we cannot exclude a minor contribution of peripheral effects by KGF, several types of data indicate that KGF does have a central thymic effect: (1) clinical assessment of GVHD comparing the HBSS and KGF treatment groups did not reveal significant changes in this model, (2) a role for glucocorticoids as effectors of the observed thymic changes (ie, loss of DP cells and hypocellularity) has been ruled out in this model,45 (3) the receptor for KGF is expressed on TECs, (4) KGF is produced by TECs, (5) TECs respond in vitro to the ligand and support thymocyte survival, (6) KGF administration causes a supranormal level of thymocytes at least 3 months after discontinuing KGF administration in irradiated recipients of congenic as well as allogeneic T-cell–depleted bone marrow grafts, neither of which will experience GVHD, and (7) the number of TECs is strikingly higher in KGF-treated as compared with control recipients receiving allogeneic cells prior to any evidence of peripheral manifestations of GVHD including weight curves or B-cell lymphopenia, the 2 most sensitive indicators of subacute GVHD. These data have been independently replicated in 3 separate laboratories (G.A.H., B.R.B., and K.I.W.) and are not shown here.
Cytoprotection constitutes a promising approach to ameliorate epithelial injury inflicted by GVHD. KGF has recently been recognized as an agent for epithelial cell repair in different GVHD target organs. Administered to mice before extensive conditioning and bone marrow transplantation, KGF ameliorated both survival and GVHD related pathologies in liver, lung, and skin but not in spleen, colon, and ileum.69 Similarly, in our experiments, splenic GVHD was not alleviated (Figure 3). In other experimental systems, an increased survival of transplant recipients was also observed when KGF was administered prior to total body irradiation, either alone or in combination with chemotherapy.70,71 Here, KGF treatment protected the gastrointestinal epithelium from radiation- and immune-mediated injury, reflecting variations in the clinical outcome depending on the choice of the experimental model used and the duration of KGF administered. Despite these differences, the central biologic response to pharmacologic doses of KGF was due to a potent trophic effect that may very well be specific for individual tissues. For example, the KGF effect on intestinal epithelium72included the survival of crypt stem cells,73 improved DNA repair,74 and an enhanced thickness of the entire mucosa71 with an increased formation of goblet cells75 and their secretory products.76,77Conversely, the decreased pulmonary damage observed after KGF treatment was secondary to enhanced epithelialization and the attenuation of immune-mediated injury.78 Thus, the pharmacologic effects of KGF treatment are documented for tissues where KGF receptor expression has been convincingly demonstrated, such as the intestinal epithelium, hepatocytes, skin keratinocytes, and alveolar type II cells.72,79 80
Although the molecular mechanisms of KGF-mediated protection remain to be defined, the beneficial effects of KGF on TECs—and consequently on thymocyte development—may be effected by different mechanisms. First, KGF may promote homeostatic TEC proliferation and differentiation ensuring normal thymopoiesis. KGF may also expedite the process of epithelial cell recovery following direct damage by cellular and humoral effector mechanisms of GVHD-mediated inflammation. In this context, it has recently been shown that KGF mediates a suppressive effect on inflammation-induced gene expression as it prevents the interferon-stimulated trafficking of STAT1 from the cytosol to the nucleus.81 Second, KGF may modulate the allogeneicity of TECs and thus prevent activation of donor cytotoxic T cells, which in turn directly injure TECs and thus indirectly inhibit thymocyte development. A decreased allogeneicity of TECs exposed to KGF may be supported by our observation of diminished expression of the costimulatory molecule CD80 on these cells (Figure 7). In this experiment, the staining pattern of CD80 antigen resembled that of epithelial cells although we cannot formally exclude additional CD80 expression on other cells such as activated donor T cells. CD80 has been shown to be necessary to costimulate T cells for full activation to nominal antigens and alloantigens.66 The observed up-regulation of this molecule on thymic stromal cells during GVHD implies that thymic epithelial cells exert a potent allostimulatory activity to infiltrating donor-derived T cells. Reduction of CD80 expression as a consequence of KGF treatment may therefore render these cells less efficient stimulators and targets of allorecognition. As a result, donor-derived T cells—albeit present in substantial numbers—failed to be fully activated within the thymus as verified by granzyme B and Mip-1 expression (Figure 6). Mip-1 expression is only minimally detected in mature intrathymic T cells but constitutes a typical hallmark of donor-derived, activated T cells in the transplantation model used here (L. Piali and G.A.H., data not shown). Furthermore, administration of KGF to animals prior to conditioning by chemotherapy and/or radiation had a long-lasting beneficial effect on thymic function,69 underscoring a cytoprotective mechanism for this factor. Both mechanisms discussed, that is, enhancement of TEC function and modulation of TEC allogeneicity, are not mutually exclusive and will result in continued thymic function despite the presence of GVHD. The former mechanism appears to encompass the production of the chemokine TECK (Figure 6). TECK is predominantly expressed by epithelial cells, with cortical epithelium being the most prominent source.82 TECK chemotactic activity is maximal for DP and immature SP cells,83 that is, for developing thymocytes which migrate from the outer aspects of the thymus to the center of the organ. Importantly, these thymocytes receive along their migratory pathway distinct developmental signals provided from diverse epithelial microenvironments.14
KGF treatment did not prevent damage to all thymic epithelial cell subpopulations (Figure 5B). Although KGFR could be detected on minor medullary epithelial cells by immunohistology, we failed to detect these cells in allogeneic transplant recipients despite KGF treatment. A loss of these cells correlated with a severe decrease of mRNA specific for the transcription factor autoimmune regulator, Aire. This molecule is typically (but not exclusively) expressed in minor medullary epithelial cells.62 A homozygous deficiency for Aire is the etiologic cause of the autoimmune-polyendocrinopathy-candidiasis ectodermal dystrophy (APECED), an autosomal recessive disease without known human leukocyte antigen (HLA) association. The almost complete loss of airetranscripts in allogeneic transplant recipients treated with either HBSS or KGF may therefore have a functional bearing on thymic repertoire selection of T cells despite the normal thymocyte development as judged by cell numbers and phenotype. Experiments are presently underway to address this issue.
With its defined role as a cytoprotective agent for epithelial cells, enhanced production of endogenous KGF may thus constitute an adjunct strategy for GVHD treatment following allogeneic BMT. For example, expression of KGF is subject to negative regulation such as glucocorticoids, a standard component of GVHD therapy that decreases KGF mRNA in a time- and concentration-dependent manner.84In consequence, it may be of clinical benefit to administer exogenous KGF in a pharmacologic dose prior to conditioning and in the presence of thymic GVHD.
The authors thank V. Wyss for excellent technical help and Drs L. Piali and M. Keller for helpful discussions.
Supported by the Swiss National Science Foundation grants 31-555820.98 (G.A.H.) and 0031-61782.00 (W.K.), and National Institutes of Health (NIH) grants RO1 HL54729 and RO1 HL54850 (K.I.W.), and NIH grants RO1 AI34459, RO1 CA72669, RO1 HL55209, and RO1 HL63452 (B.R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Georg A. Holländer, Pediatric Immunology, Lab #406, Department of Research, Kantonsspital, Hebelstrasse 20, CH-4031 Basel, Switzerland; e-mail: georg-a.hollaender@unibas.ch.