Abstract
Dendritic cells (DCs) are considered the principal initiators of immune response because of their ability to migrate into peripheral tissues and lymphoid organs, process antigens, and activate naive T cells. There is evidence that extracellular nucleotides regulate certain functions of DCs via G-protein–coupled P2Y receptors (P2YR) and ion-channel–gated P2X receptors (P2XR). Here we investigated the chemotactic activity and analyzed the migration-associated intracellular signaling events such as actin reorganization and Ca++ transients induced by common P2R agonists such as adenosine 5′-triphosphate (ATP) and 2-methylthioadenosine triphosphate, the P2YR agonists UTP and adenosine 5′-diphosphate (ADP), or the P2XR agonists αβ-methylenadenosine-5′-triphosphate and 2′,3′-(4-benzoyl)benzoyl-ATP. The common P2R agonists and the selective P2YR agonists turned out to be potent chemotactic stimuli for immature DCs, but not for mature DCs. In contrast, P2XR agonists had only marginal chemotactic activity in both DC types. Chemotaxis was paralleled by a rise in the intracellular Ca++concentration and by actin polymerization. Studies with pertussis toxin implicated that intracellular signaling events such as actin polymerization, mobilization of intracellular Ca++, and migration induced by nucleotides was mediated via Gi/oprotein–coupled P2YR. Moreover, functional studies revealed selective down-regulation of this Gi/oprotein–coupled chemotactic P2YR responsiveness during maturation, although immature and mature DCs expressed similar amounts of mRNA for the P2R subtypes (P2Y2R, P2Y4R, P2Y5R, P2Y7R, P2Y11R and P2X1R, P2X4R, P2X7R), and no major differences in respect to the mRNA expression of these receptors could be observed by semiquantitative reverse transcription and polymerase chain reaction (RT-PCR). In summary, our data describe a differential chemotactic response of immature and mature DCs to nucleotides, and lend further support to the hypothesis that P2R are a novel class of immunomodulatory plasma membrane receptors suitable for pharmacological intervention.
Introduction
Dendritic cells (DCs) are antigen-presenting cells specialized to activate naive T lymphocytes and initiate primary immune responses.1-3 They originate from hemopoietic stem cells and migrate into peripheral sites. Immature DCs reside in most unperturbed tissues, where they are adapted to capture antigens and alert for danger signals such as microorganisms, dying cells, and inflammatory cytokines.4 Upon exposure to these factors, DCs undergo maturation, a process that involves acquisition of high levels of membrane major histocompatibility complex (MHC) and costimulatory molecules, and the production of a broad panel of cytokines.1 As part of the maturation program, DCs acquire a propensity to migrate to secondary lymphoid organs for T-cell priming.3
Adenosine 5′-triphosphate (ATP) is a well-known extracellular mediator in the nervous and cardiovascular systems.5,6Neurons, platelets, macrophages, T lymphocytes, and epithelial and endothelial cells are able to secrete high amounts of ATP via nonlytic pathways.7-14 In addition, any agents causing plasma-membrane damage may trigger ATP release and thus raise its extracellular concentration in the tissues.15 Cell responses elicited by ATP are due to binding to 2 different P2 receptor (P2R) subfamilies16: P2X and P2Y receptors (P2XR and P2YR).17 P2XR are ligand-gated ion channels permeable to Ca++, Na+, and K+.18Agonists for the P2X receptors are ATP, adenosine 5′-O-(3-thiotriphosphate) (ATPγS), and αβ-methyladenosine-5′-triphosphate (αβ-meATP). In addition, 2′3′-(4-benzoyl)benzoyl ATP (BzATP) is considered a selective P2X7R agonist.15,17 P2YR receptors, on the contrary, belong to the family of serpentine receptors. These receptors interact at the cytoplasmatic side of the plasma membrane with G-proteins coupled to phospholipase-C activation, diacylglycerol and inositol triphosphate formation, as well as Ca++mobilization from intracellular stores.19 High-affinity ligands for P2YR are ATP, adenosine 5′-diphosphate (ADP), and uridine 5′-triphosphate (UTP) as well as the synthetic analogs adenosine 5‘-0-(3-thiotriphosphate) (ATPγS) and 2-methylthioadenoside triphosphate (2-MeSATP).19
Expression of P2YR and P2XR has been demonstrated in both mouse and human DCs.20-22 Depending on the protocol of stimulation, ATP can have widely differing effects on DCs. At low concentrations (20-200 μM), this nucleotide increases membrane expression of CD54, CD80, CD86, and CD83, while it inhibits LPS- and CD40-ligand–dependent production of interleukin (IL)-1α, IL-1β, tumor necrosis factor (TNF)-α, IL-6, and IL-12.23 Furthermore, T cells primed with DCs matured in the presence of low ATP doses display an impaired type 1 polarization due to reduced production of IL-12.23 At high concentrations, ATP causes striking morphological alterations followed by cell death.21,24,25Moreover, a recent paper by Liu et al26 reported that ATP applied in the vicinity of DCs caused dendrite reorientation via P2YR, suggesting a possible chemotactic activity of this nucleotide. However, direct proof of chemotactic activity of ATP in DCs is missing. In the present study, we examined the pharmacological basis of nucleotide-dependent chemotactic activity using nucleotides with different selectivity for P2YR and P2XR, respectively, and unveiled an unexpected different behavior of immature versus mature DCs.
Materials and methods
Reagents
ATP, UTP, 2′-3′-(4-benzoyl)benzoylATP (BzATP), ADP, αβ-methyladenosine-5-triphoshate (αβ-meATP), recombinant human complement fragment 5a (C5a), lysophosphatidylcholine, and pertussis toxin (PT) were obtained from Sigma (Deisenhofen, Germany); adenosine 5′-O-(3-thiotriphosphate) (ATPγS) from Boehringer (Mannheim, Germany); adenosine 5′-O-(3-thiotriphosphate) (2-MeSATP) from Amersham (Braunschweig, Germany); anti-CD14 MicroBeads, separation columns, and magnetic MultiStand from Miltenyi Biotec (Bergisch Gladbach, Germany); N-(7-nitrobenz-2-oxa-1,3-diazol-4yl)-phalla-cidin (NBD-phallacidin) from Becton Dickinson (Heidelberg, Germany); fura-2/acetoxymethyl ester (fura-2/am) Adenosine 5′-O-(3-thiotriphosphate) from Calbiochem (La Jolla, CA); macrophage inflammatory protein (MIP)–3β/CCL19 from PeproTech (London, United Kingdom); and IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) were from Natutec (Frankfurt, Germany).
Preparation of human DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats by Ficoll-Paque separation and lysis.27After centrifugation, the leukocyte-containing pellet was resuspended in 2 mL phosphate buffered saline (PBS) containing 0.15% ethylenediaminetetraacetic acid (EDTA) and 0.5% bovine serum albumin (BSA). The cells were separated by CD14 mAb-coated MicroBeads using Macs single-use separation columns. The CD14-positive cells (purity > 96%) were suspended in RPMI 1640 medium containing 10% fetal calf serum (FCS), 1% glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, 100 U/mL IL-4, and 1000 U/mL GM-CSF. After 5 days of culture at 37°C with 5% CO2, cells were harvested for experiments. Monocyte-derived DCs were CD14−(< 5%) and were more than 95% CD1a+, CD80low, CD83low, CD86low, and CD115high. These cells show a high capability for phagocyte dextran and were characterized by a low effectivity to induce T-cell activation. Further differentiation into mature DCs was induced by treatment with 3 μg/mL lipopolysaccharide (LPS) (E coli, serotype 0111:B4, Sigma) for 48 hours. Mature DCs were more than 95% CD80high, CD83high, CD86high, and CD115low (monoclonal antibodies and respective isotype controls were from Coulter-Immunotech, Krefeld, Germany). These cells showed a low phagozytose activity, but were effective in inducing T-cell activation.
Actin polymerization
The content of filamentous actin was analyzed by flow cytometry with NBD-phallacidin staining.28 Briefly, aliquots (50 μL) of DC suspensions (5 × 105 cells/mL) were withdrawn at the indicated time intervals and fixed in a 7.4% formaldehyde buffer. After 1 hour, cells were mixed with the staining cocktail containing 7.4% formaldehyde, 0.33 μM NBD-phallacidin, and 1 mg/mL lysophosphatidylcholine. The mean channel number of the fluorescence intensity of each sample was measured by flow cytometry. The relative f-actin content in comparison to the medium control was calculated.
Intracellular Ca++ measurements
Intracellular free Ca++ was measured in Fura-2–labeled DCs with the digital fluorescence microscope unit Attofluor (Zeiss, Oberkochen, Germany). Briefly, DCs were incubated with 2 μmol Fura-2/am for 30 minutes at 37°C in Ca++- and Mg2+-free buffer. Cells were washed twice and resuspended in the same buffer containing 1.5 mM CaCl2 and MgCl2. Fluorescence traces after stimulation with nucleotides were followed spectrofluorometrically, and the ratio between 340 nm and 380 nm was calculated.
Migration assay
Experiments were performed in triplicate using 48-well Transwell chambers (Nuclepore, Tübingen, Germany). Buffer or stimuli were added into the lower compartment wells. Thereafter a 10-μm polycarbonate membrane with a pore size of 5 μm (Nuclepore) was placed over the wells. DCs (105 cells/well) were added to the upper compartment and incubated at 37°C for 90 minutes in a humidified atmosphere. After removing the cells from the upper side of the membrane by wiping over a profiled rubber, migrated DCs on the lower side of the membrane were fixed in methanol and stained with hematoxylin. Migrated DCs were counted in 5 randomly chosen high-power (×400) fields, and a mean value for each sample was calculated. The chemotactic index was calculated as the ratio between DCs migrated in the presence and in the absence of stimuli.
TNF-α release assay
DCs were incubated for 2 hours with PT (4 μg/mL)–containing medium or medium alone before treatment with the 10-5 M of the indicated nucleotides together with LPS (3 μg/mL) for 24 hours. At the end of the treatment, cell-free supernatants were collected and TNF-α was measured using OptiEIA kits from PharMingen (San Diego, CA).
Detection of nucleotide receptor mRNA by RT-PCR
The mRNA was isolated by using QIAshredder and RNeasy kits (QIAGEN, Hilden, Germany). The cDNA was obtained using mRNA, pd(N)6 primers and M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA). All oligonucleotides used as primers for PCR were designed to recognize a unique sequence exclusive for each target cDNA. Sequences of the specific primers were as follows: P2Y1R 5′-TGC CGC CGT CTC CTC GTC GTT C-3′ (sense) and 5′-CGC CAC CAC CAC AAT GAG CCA CAC-3′ (antisense); P2Y2R 5′-CCT CAA GAC CTG GAA TGC GT-3′ (sense) and 5′-TGA CTG AGC TGT AGG CCA CG-3′ (antisense); P2Y4R 5′-GCC ATG GCC AGT ACA GAG TC-3′ (sense), and 5′-GTG GTT GTG GGC TGC ATA AT-3′ (antisense); P2Y6R 5′-GTG GCT GGC CCG TGA CAA CC-3′ forward, and 5′-CCG CTG CAA AGC CCT CCA ATA C-3′; P2Y11R 5′-GTG GTT GAG TTC CTG GTG GC-3′ (sense), and 5′-CCA GCA GGT TGC AGG TGA AG-3′ and (antisense); P2X1R 5′-CGC CTT CCT CTT CGA GTA TG-3′ (sense), and 5′-GGA AGA CGT AGT CAG CCA CA-3′ (antisense); P2X4R 5′-CCT GTT CGA GTA CGA CAC GC-3′ (sense), and 5′-GTG TGT GTC ATC CTC CAC CG-3′ (antisense); P2X7R 5′-AGA TCG TGG AGA ATG GAG TG-3′ (sense), and 5′-TTC TCG TGG TGT AGT TGT GG-3′ (antisense); β2-microglobulin 5′-CCT TGA GGC TAT CCA GCG TA-3′ (sense), and 5′-GTT CAC ACG GCA GGC ATA CT-3′ (antisense). PCR was carried out with 30 cycles of denaturation (94°C, 1 minute), ramped annealing (60°C, 1 minute), and extension (72°C, 1 minute). The obtained PCR products were subjected to electrophoresis on a 1.5% agarose gel and were visualized by ethidium bromide staining. The intensity of bands in PCR gels was quantified by measuring the optical density with the ONEDscan computer software package (Scanalytics, Fairfax, VA). To compare the mRNA expression of immature and mature DCs, the signals of P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2X1R, P2X4R, and P2X7R were normalized to β2-microglobulin. To ensure linear cDNA amplification, increasing numbers of cycles were checked. Linear amplification was obtained between 22 and 34 cycles. The identity of the generated products was proven by sequencing after cloning using pCRII vectors.
Statistical analysis
Where indicated, the Mann-Whitney test was used to compare differences. P values ≤ .05 were considered significant.
Results
Intracellular Ca++ transients induced by nucleotides in immature and mature DCs
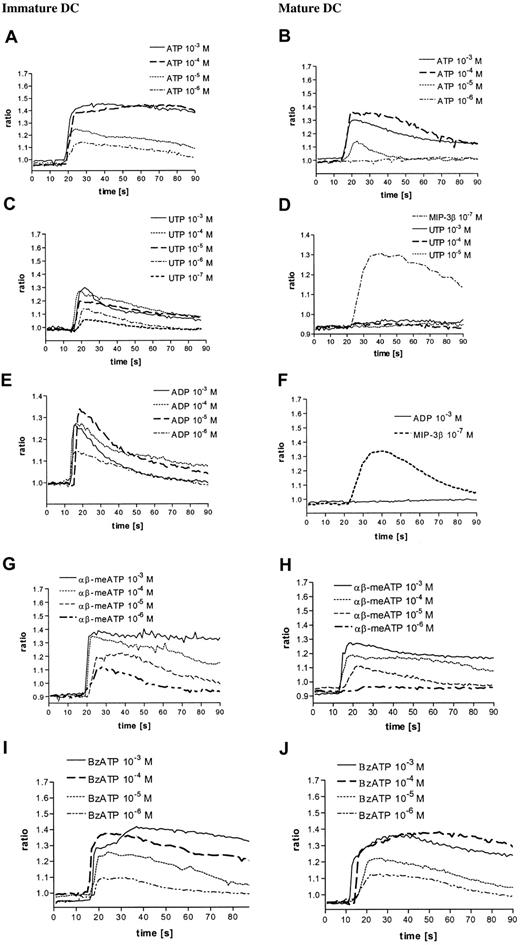
It has been previously reported that extracellular nucleotides elicit Ca++ transients in human DCs.25 Here we show that the common P2R agonist ATP triggered a fast and dose-dependent Ca++ signal in immature DCs. The ATP-induced Ca++ increase was rapid and followed by a slowly declining phase (10−5 M and 10−6 M ATP) or a long-lasting plateau (10−4 and 10−3 M ATP) (Figure 1A). The Ca++response was basically fully saturated at 10−4 M ATP. ATP triggered a Ca++ signal also in mature DCs, though with different potency and kinetics. While the initial Ca++spike was of comparable amplitude, the following sustained phase declined more rapidly (Figure 1A-B). To rule out the possibility that differences in the shape and amplitude of the Ca++ signal could be due to a different ATP-degrading activity of the 2 cell populations, we used the nonhydrolizable ATP analog ATPγS that triggered Ca++ transients comparable to those elicited by ATP (data not shown). UTP is an agonist at different P2YR subtypes (P2Y2R, P2Y4R, and P2Y6R) that lack agonist activity at P2XR. Figure 1C shows that stimulation of immature DCs with UTP induced a dose-dependent fast and transient Ca++ increase, but of smaller amplitude compared to that caused by ATP. In contrast to immature DCs, mature DCs did not respond to UTP (Figure 1D). Unresponsiveness was not due to a generalized defect in Ca++ responses as the chemokine MIP-3β, whose receptor is unregulated during DC maturation, triggered a strong Ca++ response in mature DCs (Figure 1D). ADP, an additional agonist at certain P2YR subtypes (P2Y1R and P2Y12R), showed a similar pattern of responses (Figure 1E-F). To check whether P2XR-dependent responses also differed in immature and mature DCs, we used 2 P2XR agonists, αβ-meATP and BzATP. Both αβ-meATP and BzATP caused a [Ca++]i increase in both cell populations, although the former analog was less potent in mature DCs.
Nucleotides induce intracellular Ca++ transients in immature and mature DCs.
Immature (A,C,E,G,I) and mature (B,D,F,H,J) DCs were loaded with fura-2/am as reported in “Materials and methods” and stimulated with nucleotides at the indicated concentrations. MIP-3β was used as a positive control for mature DCs. Data from 1 representative experiment of 5 similar are shown.
Nucleotides induce intracellular Ca++ transients in immature and mature DCs.
Immature (A,C,E,G,I) and mature (B,D,F,H,J) DCs were loaded with fura-2/am as reported in “Materials and methods” and stimulated with nucleotides at the indicated concentrations. MIP-3β was used as a positive control for mature DCs. Data from 1 representative experiment of 5 similar are shown.
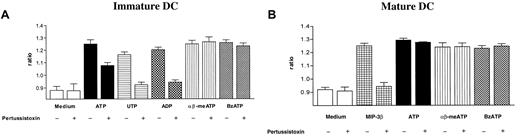
The [Ca++]i increase was variably affected by chelation of extracellular Ca++, depending on the agonist nucleotide employed (Table 1). Using ATP as an agonist, the [Ca++]i increase was reduced by about 30%, markedly affected in response to αβ-meATP or BzATP, while it was not altered with UTP and ADP. These results suggest that UTP- and ADP-mediated responses are almost exclusively due to P2YR, whereas αβ-meATP– and BzATP-mediated responses are due to P2XR activation, and ATP-mediated responses might depend on the activation of both receptor subtypes. Mobilization of Ca++from intracellular stores by serpentine receptors is often mediated via PT-sensitive Gi/o proteins.29 30 To test the participation of PT-inhibitable G-proteins in P2YR-mediated signaling, DCs were preincubated with PT and then stimulated with the nucleotides. In immature DCs, PT partially inhibited the ATP-triggered Ca++ increase, completely abrogated that induced by the selective P2YR agonists ADP and UTP, and had no effect on the P2XR agonist αβ-meATP– and BzATP-mediated [Ca++]i (Figure2A). In mature DCs, PT had no effect on ATP, αβ-meATP–, and BzATP-mediated Ca++ transients, while it inhibited that due to MIP-3β, a well-known G-protein–coupled stimulus (Figure 2B).
The influence of PT on intracellular Ca++transients in immature and mature DCs.
Cells were incubated in the presence or in the absence of 4 μg/mL PT for 2 hours and then triggered with the indicated stimuli. Peak intracellular Ca++ was measured 10 seconds after addition of the stimulus. (A) Immature DC; (B) mature DC. Nucleotide concentration was 10−4 M, while MIP-3β was 10−7 M. Data are expressed as mean ± SEM (n = 5).
The influence of PT on intracellular Ca++transients in immature and mature DCs.
Cells were incubated in the presence or in the absence of 4 μg/mL PT for 2 hours and then triggered with the indicated stimuli. Peak intracellular Ca++ was measured 10 seconds after addition of the stimulus. (A) Immature DC; (B) mature DC. Nucleotide concentration was 10−4 M, while MIP-3β was 10−7 M. Data are expressed as mean ± SEM (n = 5).
Nucleotides stimulate actin polymerization and chemotaxis in immature DCs, but not in mature DCs
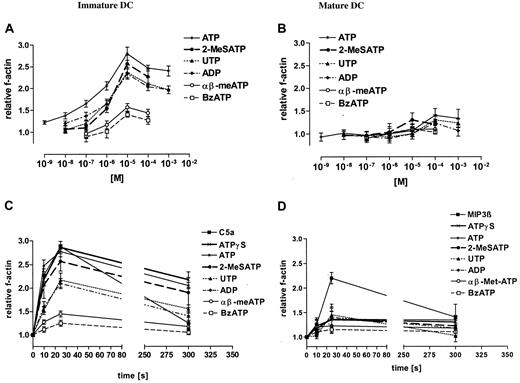
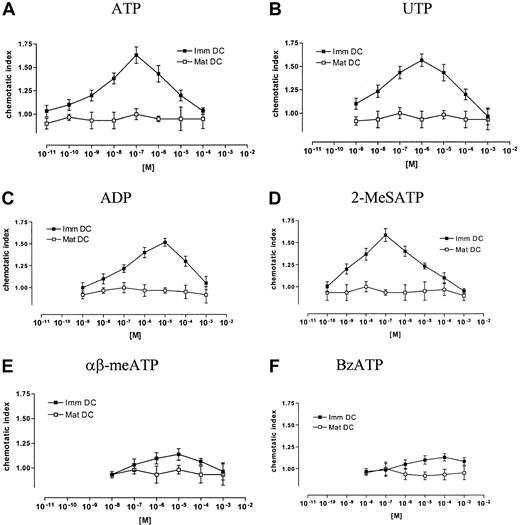
Nucleotides are known to be potent chemotactic stimuli for leukocytes. Figure 3A shows that they caused a rapid and transient polymerization of the actin network in immature DCs, with an increase of the f-actin content of about 50% within 25 seconds. The common P2R agonists ATP and 2-MeSATP induced a good response, with a half-maximal responses in the low μM range (Figure 3A). The selective P2YR agonists UTP and ADP induced slightly lower responses. Half-maximal and maximal responses for these nucleotides were seen with 10−6 and 10−5 M, respectively. The P2XR agonists BzATP and αβ-meATP had only minimal effects on actin reorganization (Figure 3A). Nucleotide-stimulated actin polymerization reached maximum level within 25 to 30 seconds, and then declined slowly (not shown). Actin polymerization caused by an optimal ATP dose was similar to that caused by a full stimulatory C5a dose, a potent chemotactic stimulus for immature DCs (Figure 3C). In contrast to immature DCs, all nucleotides tested induced only a small actin response in mature DCs (Figure 3B,D), whereas MIP-3β was used here as a positive control (Figure 3D). Next, DCs were stimulated with different nucleotides, and oriented migration was evaluated. The common P2R agonists ATP and 2-MeSATP as well as the selective P2YR agonists UTP and ADP elicited a typical bell-shaped dose-dependent chemotactic response in immature DCs (Figure 4A-D). Maximal chemotactic responses were observed upon stimulation with 10−7 M ATP and 2-MeSATP, while UTP and ADP were slightly less potent and elicited the maximum effect at 10−6 or 10−5 M, respectively. The maximal chemotactic response to 10−7 M ATP was comparable with that caused by 10−8 M C5a. Negligible chemotaxis was seen by stimulation with αβ-meATP and BzATP (Figure 4E-F). Nucleotide-stimulated actin polymerization as well as migration was almost abolished by pretreatment of DCs with PT (Table2), showing that the 2 processes were Gi/o-protein–dependent. Mature DCs did not show chemotactic response to any of the nucleotides tested (Figure 4A-F), while they migrate to MIP-3β, a potent chemotactic stimulus for mature DCs. However, mature DCs did not lose their ability to generate other responses to nucleotide stimulation, for example, nucleotides profoundly inhibited LPS-dependent secretion of TNF-α in mature DCs (Figure 5).
Stimulation with nucleotides induces actin polymerization in immature but not mature DCs.
(A) Nucleotide dose-dependency of f-actin polymerization in immature DCs. (B) Nucleotide dose-dependency of f-actin polymerization in mature DCs. (C) Time course of f-actin polymerization in immature DCs (nucleotide concentration was 10−4 M; C5a was 10−8 M). (D) Time course of f-actin polymerization in mature DCs (nucleotide concentration was 10−4 M; MIP-3β was 10−8 M). The relative f-actin content was determined at the indicated time points by flow cytometry. Data are expressed as mean ± SEM (n = 4).
Stimulation with nucleotides induces actin polymerization in immature but not mature DCs.
(A) Nucleotide dose-dependency of f-actin polymerization in immature DCs. (B) Nucleotide dose-dependency of f-actin polymerization in mature DCs. (C) Time course of f-actin polymerization in immature DCs (nucleotide concentration was 10−4 M; C5a was 10−8 M). (D) Time course of f-actin polymerization in mature DCs (nucleotide concentration was 10−4 M; MIP-3β was 10−8 M). The relative f-actin content was determined at the indicated time points by flow cytometry. Data are expressed as mean ± SEM (n = 4).
Nucleotides are chemotactic for immature but not mature DCs.
Immature and mature DCs were exposed for 90 minutes at 37°C in a Transwell chamber to the indicated concentrations of ATP (A), UTP (B), ADP (C), 2-MeSATP (D), αβ-meATP (E), or BzATP (F). C5a (10−8 M) as a positive control for immature DCs and MIP-3β (10−8 M) as a positive control for mature DCs provoke at optimal concentration an increase of the chemotactic index of 1.85 ± 0.18 and 2.04 ± 0.27, respectively. The chemotactic index is calculated as the ratio between the number of migrated cells in the presence or in the absence of the nucleotide. Data are expressed as means SEM (n = 4).
Nucleotides are chemotactic for immature but not mature DCs.
Immature and mature DCs were exposed for 90 minutes at 37°C in a Transwell chamber to the indicated concentrations of ATP (A), UTP (B), ADP (C), 2-MeSATP (D), αβ-meATP (E), or BzATP (F). C5a (10−8 M) as a positive control for immature DCs and MIP-3β (10−8 M) as a positive control for mature DCs provoke at optimal concentration an increase of the chemotactic index of 1.85 ± 0.18 and 2.04 ± 0.27, respectively. The chemotactic index is calculated as the ratio between the number of migrated cells in the presence or in the absence of the nucleotide. Data are expressed as means SEM (n = 4).
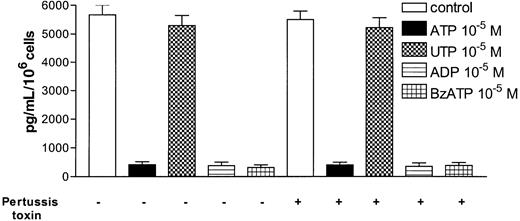
ATP, ADP, and BzATP inhibit TNF-α release from mature DCs through a PT-insensitive mechanism.
DCs were treated with LPS and the indicated nucleotides in the presence or the absence of 4 μg/mL PT, and the TNF-α content was measured in the supernatants 24 hours later. Data are expressed as mean pg/mL/106 cells ± SEM (n = 3).
ATP, ADP, and BzATP inhibit TNF-α release from mature DCs through a PT-insensitive mechanism.
DCs were treated with LPS and the indicated nucleotides in the presence or the absence of 4 μg/mL PT, and the TNF-α content was measured in the supernatants 24 hours later. Data are expressed as mean pg/mL/106 cells ± SEM (n = 3).
Expression of mRNA for P2XR and P2YR subtypes does not change during DC maturation
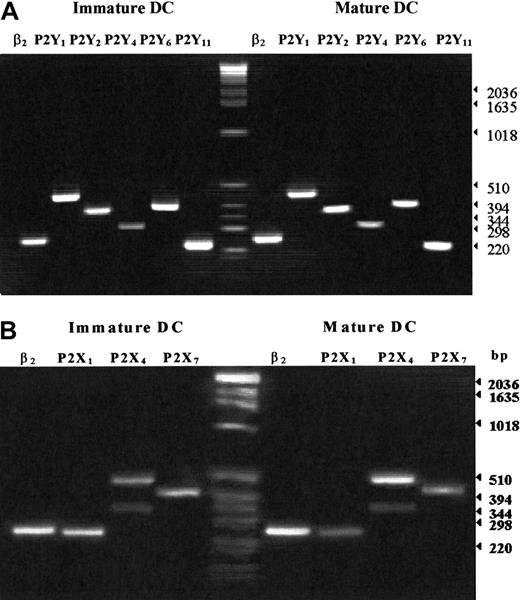
The mRNA expression of different P2R was analyzed by RT-PCR as reported in “Materials and methods.” In agreement with previous data,22,25,26 we found that human DCs express P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2X1R, P2X4R, and P2X7R (Figure6). Amplification of P2X4R yielded 2 products of 319 and 484. Cloning and sequencing revealed that the 484 base product represented the original sequence, whereas the smaller product corresponded to a described splice variant of this receptor.31 We were unable to detect by semiquantitative RT-PCR major differences in mRNA expression of the various receptor subtypes in immature and mature DCs.
Expression of the mRNA for P2XR and P2YR subtypes in immature and mature human DCs.
RT-PCR was performed as detailed in “Materials and methods.” Experiments were repeated 5 times with identical results.
Expression of the mRNA for P2XR and P2YR subtypes in immature and mature human DCs.
RT-PCR was performed as detailed in “Materials and methods.” Experiments were repeated 5 times with identical results.
Discussion
Recent findings indicate that ATP, a well-known extracellular mediator in the nervous and the cardiovascular systems, might also have an important role in the immune system.32-34 All immune and inflammatory cells express plasma membrane receptors of the P2 type, coupled to an array of different responses such as chemotaxis, generation of nitric oxide or superoxide anion, secretion of lysosomal constituents, release of cytokines, cell differentiation, and cytotoxicity.32-34 The extracellular ATP concentration is very low under resting conditions (nanomolar or low micromolar range),35 but there are good reasons to believe that following cell activation or cell injury, it can increase substantially, especially in the pericellular space.9,36,37 Mechanisms that participate in nonlytic ATP release are secretion of granular ATP content (eg, from platelet-dense granules)38,39 and export via gap junctions or ABC transporters.40,41 Furthermore, it appears conceivable that massive amounts of ATP are released following cell injury or acute cell death. In the extracellular space, ATP is subjected to hydrolysis by ubiquitous ecto-ATPases and ecto-nucleotidases.14 Activity of some members of this family, such as ATP diphosphohydrolase, is down-modulated by proinflammatory cytokines (eg, TNF-α),42 thus further increasing the chance that substantial ATP amounts accumulate at inflammatory sites. Recent reports showed a dendrite-redirecting activity by ATP in DCs26 and revealed a chemotactic effect of ATP on other leukocytes.43-45 Therefore, it is not surprising that ATP and different other nucleotides have chemotactic activity and stimulate migration-associated intracellular signaling events such as actin reorganization or mobilization of intracellular Ca++ in DCs, as shown in the present study. The concentrations giving maximal responses in Ca++ transients, actin polymerization, and migration were slightly different, with the most sensitive response in chemotaxis followed by actin polymerization and Ca++ transients. These different sensitivities of function and intracellular signal events to ATP are in accordance with studies in neutrophils, where we could show that migration and actin polymerization required a much lower number of ligand receptor interactions or stimulus concentrations as Ca++transients.55 This different affectivity of signal transmission might serve to regulate in a very sensitive manner different cell functions. Quite intriguingly, however, ATP and other nucleotides had a differential chemotactic activity on immature and mature DCs, an observation that might have far-reaching implications.
There is increasing interest in the role of inflammatory mediators in regulation of immune response.4 ATP in the micromolar range induces phenotypic and functional maturation of DCs, although it suppresses the production of inflammatory cytokines and IL-12 and does not affect IL-10 release.23,25,46,47 DC maturation is a crucial checkpoint in the initiation of the immune response, as it converts antigen-capturing DCs into immunostimulatory antigen-presenting cells. On one hand, DC maturation is powerfully boosted by inflammation,3 and on the other hand, DCs are recruited at inflammatory sites in order to capture antigenic peptides. Thus, it would be logical for a DC to be able to sense the ATP concentration in the environment and direct its movement toward the origin of the ATP gradient. However, it would also be necessary to express a graded response to ATP, in order to be able to fully exploit the information content of this mediator. This means that chemotaxis should be stimulated by low ATP concentrations unable to start other DC functions. On the contrary, differentiation should be stimulated by higher nonchemotactic nucleotide concentrations. This scheme is fulfilled by the experiments reported here and those published previously.23 Optimal ATP concentration for chemotaxis was 10−7, while optimal ATP concentration for expression of differentiation markers was 10−4 or higher.23Furthermore, the chemotactic response was bell-shaped, suggesting that once immature DCs sense a high nucleotide concentration, as can be found at an inflammatory site, they receive a “stop signal” that allows antigen endocytosis and the start of maturation under the effect of bacterial products, cytokines, and ATP itself. Down-modulation of chemotactic responsiveness to ATP in DCs might be a prerequisite for departure on the way to secondary lymphoid organs. However, as previously shown by our laboratories,23 25 mature DCs do not lose their ability to respond to nucleotide stimulation. In mature DCs, nucleotides finely regulate the release of proinflammatory cytokines (eg, TNF-α, IL-12) and other products that affect Th1/Th2 lymphocyte polarization.
According to this theory, mature DCs should lose ATP-dependent chemotactic activity, since these cells must migrate from ATP-rich inflammatory sites to lymph nodes in order to establish contact with T lymphocytes and initiate immune responses. This theory is also supported by these results. Thus, extracellular ATP appears to be a flexible and powerful multitask modulator of DC functions.34,48,49 Down-modulation of ATP-mediated chemotactic responses during maturation is not unprecedented, as it has already been reported that DCs show different reactivity toward chemokines and other proinflammatory agents at different maturation stages.27,48,50,51 In these cases, reduced chemotaxis is caused by transcriptional down-regulation of the respective receptor protein, although signaling via some of these receptors might also be regulated at posttranscriptional levels.52 In agreement with previous results, we found a similar set of P2Y and P2X receptor expression patterns in DCs.22,25 However, we could not find major differences in respect to the mRNA expression of these receptors by semiquantitative reverse transcription and polymerase chain reaction between immature and mature DCs, although this does not exclude differences in plasma-membrane expression of the receptor proteins at different maturation stages. Alternatively, altered P2R coupling to intracellular signal transduction effector systems during maturation could explain this behavior. The P2XR agonists BzATP and αβ-meATP had weak chemotactic activity and marginally stimulated actin reorganization in DCs. In agreement with the results in other leukocytes,53our data with PT implicate a main role of a Gi/o-protein–coupled P2YR in ATP-driven chemotaxis in DCs. Involvement of P2YR in the migration response is further supported by the chemotactic activity of ADP and UTP, which are ligands for P2Y1R and P2Y12R, as well as P2Y2R, P2Y4R, and P2Y6R.19,29 In mast cells and monocyte-derived macrophages, it has been suggested that nucleotide-stimulated chemotaxis is mainly due to P2Y2R, while in J774 macrophages the main receptor involved appears to be P2Y1R.43,44 In rat microglia, a possible role for P2YTAC (now P2Y12R54) has been suggested.45 At present, the abundant expression of multiple P2YR at the mRNA level in DCs and the lack of selective agonists, antagonist, or isotype-specific antibodies limits further identification of the P2YR involved in DC chemotaxis.
In summary, here we describe that ATP has chemotactic activity mediated by Gi/o-protein–coupled P2Y receptors in immature DCs. These receptors might have a prominent role in attracting DCs toward inflammatory sites, whereas loss of this PT-sensitive responsiveness in mature DCs might clear the way for DC migration to secondary lymphoid organs. These data lend further support to the hypothesis that P2R is a novel class of immunomodulatory plasma membrane receptors suitable for pharmacological intervention.
The authors gratefully acknowledge the assistance of A. Komann.
Supported by Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (grant 01 GC 9701); the Italian Association for Cancer Research (AIRC); the National Research Council of Italy (target project on biotechnology); the Italian Ministry for Education (MURST); the Telethon of Italy; and the Italian Space Agency (ASI).
M.I. and S.D. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Johannes Norgauer, Department of Dermatology, University of Freiburg, Hauptstraße 7, D-79104 Freiburg i. Br., Germany; e-mail: norgauer@haut.ukl.uni-freiburg.de.