In a patient who presented with a severe coagulation deficiency in plasma contrasting with a very mild hemorrhagic diathesis a homozygous Arg67His mutation was identified in the prothrombin gene. Wild-type (factor IIa [FIIa]-WT) and mutant Arg67His thrombin (FIIa-MT67) had similar amidolytic activity. By contrast, the kcat/Km value of fibrinopeptide A hydrolysis by FIIa-WT and FIIa-MT67 was equal to 2.1 × 107M−1s−1 and 9 × 105M−1s−1. Decreased activation of protein C (PC) correlated with the 33-fold decreased binding affinity for thrombomodulin (TM; Kd = 65.3 nM vs 2.1 nM, in FIIa-MT67 and in FIIa-WT, respectively). In contrast, hydrolysis of PC in the absence of TM was normal. The Arg67His mutation had a dramatic effect on the cleavage of protease-activated G protein–coupled receptor 1 (PAR-1) 38-60 peptide (kcat/Km = 4 × 107M−1s−1 to 1.2 × 106M−1s−1). FIIa-MT67 showed a weaker platelet activating capacity, attributed to a defective PAR-1 interaction, whereas the interaction with glycoprotein Ib was normal. A drastic decrease (up to 500-fold) of the second-order rate constant pertaining to heparin cofactor II (HCII) interaction, especially in the presence of dermatan sulfate, was found for the FIIa-MT67 compared with FIIa-WT, suggesting a severe impairment of thrombin inhibition by HCII in vivo. Finally, the Arg67His mutation was associated with a 5-fold decrease of prothrombin activation by the factor Xa-factor Va complex, perhaps through impairment of the prothrombin-factor Va interaction. These experiments show that the Arg67His substitution affects drastically both the procoagulant and the anticoagulant functions of thrombin as well as its inhibition by HCII. The mild hemorrhagic phenotype might be explained by abnormalities that ultimately counterbalance each other.
Introduction
Prothrombin deficiency is an autosomal recessive bleeding disorder characterized by 2 phenotypes: hypoprothrombinemia, with concomitantly low levels of coagulant activity and antigen (type I), and dysprothrombinemia, with low activity but borderline or normal antigen levels (type II). These disorders are rare and there is always residual prothrombin procoagulant activity measurable in patients, in agreement with the fact that the phenotype found in prothrombin-deficient mice indicates that complete prothrombin deficiency may be lethal in humans.1,2 Genetic and biochemical analyses show that these disorders are the result of substitution, deletion, or insertion of single nucleotides in the prothrombin gene, resulting in the substitution of an amino acid in the protein or in a premature stop codon. To date, 32 such defects in prothrombin have been identified.3-5 Homozygous hypoprothrombinemia (type I) is always characterized by severe bleeding manifestations, whereas the bleeding tendency in dysprothrombinemia is more variable, even though there is usually a good correlation between the levels of prothrombin activity and clinical severity.3 6
Recently, we identified a homozygous Arg67His missense mutation in the prothrombin gene of an Iranian girl with dysprothrombinemia.3 The only symptoms in this patient were sporadic ecchymosis and one episode of buttock hematoma following a major trauma. Arginine 67 (chymotrypsin numbering) is centrally located on the surface of the thrombin domain referred to as fibrinogen recognition site (FRS). Its guanidyl side chain is engaged in an extended internal salt bridge cluster and interacts with many thrombin macromolecular substrates, modulators, and inhibitors, such as fibrinogen, thrombomodulin, and heparin cofactor II.7Previously, a substitution of Arg67 with cysteine was identified in the compound heterozygous dysprothrombins referred to as Quick I and Corpus Christi.8,9 Moreover, alanine and glutamine scanning mutagenesis studies, in which the highly exposed charged or polar residues on thrombin were individually mutated to neutral residues, demonstrated the relevance of Arg67 for thrombin activity toward various substrates.10-15 The peculiar substitution Arg67His observed in the homozygous state in our patient was never identified and studied before. This prompted us to investigate both in vivo and in vitro the functional properties of this natural thrombin variant, which might be regarded as an FRS-knockout model of thrombin in humans. To this end we investigated the functional properties of the variant, by means of in vitro expression analysis and evaluation of the function of purified recombinant wild-type (WT) and mutant thrombin, tested on different substrates and modulators to verify the change in specificity of the Arg67His mutant.
Patient, materials, and methods
Patient
At the age of 1 year an Iranian girl, born from a consanguineous marriage, developed a buttock hematoma following a fall from a chair. Even though the hematoma was minor and needed no specific treatment, it was judged disproportionate to the trauma by her pediatrician and led to the laboratory evaluation of coagulation. A prolongation of both the prothrombin time (79 seconds; normal, 12-14 seconds) and of the activated partial thromboplastin time (102 seconds; normal, 30-40 seconds) was found with undetectable levels of prothrombin functional activity (< 1%). Since then the girl, who is now 11 years old, has had no spontaneous or posttraumatic bleeding symptoms, with the exception of sporadic bruising after minor traumas. She has had no surgical operations, and the loss of deciduous teeth was uneventful. The parents, who are first cousins, are asymptomatic and have approximately half-normal levels of prothrombin activity (59% and 69%) contrasting with normal levels of prothrombin antigen (103% and 121%). Other relatives are asymptomatic but were not investigated.
Coagulation assays
The level of prothrombin antigen was measured in plasma by quantitative Laurell electroimmunoassay and enzyme-linked immunoabsorbent assay (ELISA) using a polyclonal antihuman prothrombin rabbit antiserum (Asserae II; Diagnostica Stago, Asnieres, France).Thrombin coagulant activity was measured by a 1-stage assay, using Taipan snake venom (Diagnostic Reagents, Thame, Oxon, United Kingdom) as activator and plasma fibrinogen as substrate. Activation and measurement of both control and patient prothrombin in plasma were also carried out using a 2-stage assay withd-Phe-Pip-Arg-pNA as substrate, and either ecarin (Sigma-Aldrich, St Louis, MO) or Taipan snake venom as activating agents. Briefly, 100 μL of either normal control or patient plasma was mixed to an equal volume of 50 mM Tris-HCl (0.15 M NaCl, 0.1% polyethylene glycol [PEG] 6000, pH 7.50) at 37°C (buffer A), and incubated for 5 minutes at 37°C with either 10 U/mL ecarin alone or 0.5 mg/mL Taipan snake venom in buffer A plus 0.1% bovine serum albumin (BSA), 5 mM CaCl2, and diluted (1:5, vol/vol) synthetic phospholipids (SynthAsil, Hemoliance; Instrumentation Laboratory, Milan, Italy). At the end of incubation 100 μL of the solution was added to 1 mL of a solution containing 50 mM Tris-HCl, 0.15 M NaCl, 0.1% PEG 6000, 5 mM EDTA, pH 8.00, and containing 100 μM d-Phe-Pip-Arg-pNA. The formation ofp-nitroaniline was followed at 405 nm in a Varian Cary 2200 spectrophotometer (Mulgrave, Australia). Under these conditions the contribution of ecarin to the synthetic substrate hydrolysis was null, whereas that of the Taipan snake venom was negligible, being the formation of p-nitroaniline less than 0.5 nM/min. A reference curve to convert the rate of pNA formation into active thrombin concentration was obtained using purified WT α-thrombin. Plasma levels of protein C (PC) and antithrombin (AT) were measured by chromogenic assays (COAMATIC Protein C and Electrachrome Antithrombin III; Hemoliance, Instrumentation Laboratory). Free protein S was measured by ELISA (Free Protein S, Chromogenix, Instrumentation Laboratory). The G1691A mutation in the factor V and the G20210A mutation in the prothrombin gene were searched for by specific DNA amplification and digestion, as previously published.16
DNA analysis
Following DNA extraction from leukocytes, the coding region, intron/exon boundaries, and the 5′ and 3′ untranslated regions (UTRs) of the prothrombin gene were amplified by polymerase chain reaction (PCR) and screened for mutations by single-strand conformation polymorphism (SSCP) analysis.3
Site-directed mutagenesis and construction of expression vectors
Full-length complementary DNA (cDNA) of human prothrombin (including 38 base pairs [bp] of 5′-UTR and 97 bp of 3′-UTR) was obtained by PCR amplification of M13mp18 (kindly provided by Dr Barbara C. Furie, Harvard Medical School, Boston, MA). The entire cDNA generated by PCR amplification (2021 bp) was sequenced before transfection. To clone the product of PCR amplification into theSalIPT7EcoRI vector, we designed the forward and reverse primers, with SalI and EcoRI restriction sites, respectively (forward: 5′-ACGCGTCGACGACAGACAATTCCTCAGTGACCCAGGAGCTGACACACTATGGCGCACGTCCGAG-3′ and reverse: 5′-GGAATTCCGCTGAGAGTCACTTTTATTGGGAACCATAGTT TTAGAAACACAAAAATAA-3′). The product of PCR amplification was purified, digested with SalI and EcoRI and ligated into theSalIPT7EcoRI vector that had been digested with the same enzymes, to make the clone pT7-SalIFII-WTEcoRI.
To investigate the influence of the Arg67His substitution on prothrombin activity, mutant factor II (FII) His67 was obtained by site-directed mutagenesis of PT7-SalIFII-WTEcoRI using a commercially available kit (Clontech, Palo Alto, CA). Oligonucleotide (5′-CCGAGAATGACCTTCT GGTACACATTGGCAAGCACT-3′) spanning nucleotides 1301-1336 of the human FII cDNA were used to introduce a G to A at position 1322 (bold letters) coding for His67. This primer also introduced a KpnI restriction site (underlined), arising from a silent GTG-to-GTA mutation at nucleotide 1319, to facilitate screening for clones carrying the mutation. The cloned insert was sequenced and sequencing confirmed that the mutation had been introduced.
SalIFII-WTEcoRI andSalIFII-MT67EcoRI fragments were prepared from the corresponding pT7 vectors (pT7-SalIFII-WTEcoRI and pT7-SalIFII-MT67EcoRI), and ligated separately into the expression plasmid pED-mtxr. The resulting plasmids, pED-FII-WT and pED-FII-MT67, are dicistronic messenger RNA mammalian expression vector carrying the WT or MT67 FII cDNAs at the 5′ open reading frame and the dihydrofolate reductase (DHFR) gene at the 3′ open reading frame.
Cell culture and transfection assays
For transient transfection experiments, African green monkey COS-7 cells (CRL1650; American Type Culture Collection, Manassas, VA) were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 2 mMl-glutamine, 10 mM HEPES, pH 7.2, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 8 μg/mL vitamin K1(phytonadione; Abbott Laboratories, North Chicago, IL) in a 5% CO2 atmosphere at 37°C. The DNA of pED-FII-WT or pED-FII-MT67 (30 ng) was transfected by electroporation into 5 × 106 COS-7 cells according to the manufacturer's instructions. After 72 hours, supernatants and cell lysates were assayed for FII antigen level.
Purification of WT and Arg67His prothrombin
Recombinant human WT and Arg67His mutant prothrombin were isolated from about 100 mL cell supernatant concentrates using an affinity chromatography column containing activated agarose coupled to monoclonal antibody human factor II (HFII) lot 103.55 from Enzyme Research (Indianapolis, IN), followed by activation of prothrombin by ecarin and ion-exchange purification by high-performance liquid chromatography (HPLC), as previously described.15WT and Arg67His were purified and characterized as previously described.17 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 4% to 20% gradient gels showed that both WT and Arg67His thrombins were pure with an apparent molecular weight of 35 kd. The purified enzyme was immediately aliquoted and frozen at −80°C until use. The concentration of recombinant thrombins was measured spectrophotometrically at 280 nm, using an extinction coefficient (0.1%) equal to 1.83.
Activation of purified WT and Arg67His prothrombins by the prothrombinase complex
Initial rates of prothrombin activation by human factor Xa (FXa) both in the presence and absence of factor Va (FVa) were measured by discontinuous method of the formed thrombin, assessed from the increase in the steady-state hydrolysis rate of 100 μMd-Phe-Pip-Pro-Arg-pNA at 405 nm. On the basis of a recent report suggesting the involvement of prothrombin pro-FRS in FVa interaction,18 no phospholipid reagent was used in this assay to investigate the direct prothrombin interaction with either FXa or FVa and unequivocally attribute the observed effects to each of these interactions only. Reaction mixtures contained 50 nM of either purified WT or Arg67His prothrombin, 5 nM FVa (American Diagnostica, Instrumentation Laboratory, Milan, Italy), 10 nM FXa (Calbiochem, Inalco, Milan, Italy) in 50 mM Tris-HCl, 0.15 M NaCl, 5 mM CaCl2, 0.1% PEG 6000, pH 7.50. Reactions were started by the addition of FXa. At various times (5-30 minutes when FXa alone was used; 1-5 minutes when both FXa and FVa were used), 100-μL aliquots of the reaction mixtures were quenched by addition to 0.9 mL 50 mM Tris-HCl, 0.15 M NaCl, 5 mM EDTA, 0.1% PEG 6000, pH 7.50, containing 100 μM d-Phe-Pip-Pro-Arg-pNA. The rate of active thrombin production was measured at 405 nm.
Michaelis constants pertaining to WT and Arg67His thrombins for synthetic substrate hydrolysis
The kcat and Km values for substrate hydrolysis by WT and Arg67His thrombins were measured as previously detailed, in 10 mM Tris-HCl, 0.15 M NaCl, 0.1% PEG 6000, pH 7.50 at 25°C (buffer A).19 The synthetic substrate used was d-Phe-Pip-Arg-pNA. Thrombin was used at a concentration of 1 nM.
Binding affinity of thrombin for thrombomodulin and glycoprotein Ibα by solid-phase assay
Human recombinant thrombomodulin (TM) was purchased from American Diagnostica (Greenwich, CT). Soluble glycoprotein Ibα (GpIbα) 1-282 fragment was purified from outdated platelet concentrates, as previously detailed.15 Binding of thrombin to human TM and purified GpIbα fragment was performed by solid-phase assays, as described.17 20 Detection of thrombin was accomplished by incubating each well with 150 μL 100 μM d-Phe-Pip-Arg-pNA in 50 mM Tris, 0.2 M NaCl, 0.1% PEG 6000, pH 8.00, at 25°C. The absorbance was measured at 405 nm using a microplate reader Auto-Reader III system (Ortho Clinical Diagnostics, Milan, Italy). Each determination (sample and blank) was performed in duplicate.
Measurement of kcat/Km for PC activation by WT and Arg67His thrombin forms
Hydrolysis of PC by free WT and Arg67His thrombin forms was monitored using 50 nM α-thrombin, 0.5 μM PC in buffer A at 25°C. At appropriate time intervals the reaction was stopped with 0.3 M HClO4. The solution was then centrifuged at 14 000 rpm and the supernatant aspirated and filtered through 0.22-μm membranes for reversed-phase high-performance liquid chromatography (RP-HPLC) analysis, using a Bio-Rad C18 column (Bio-Rad Laboratories, Hercules, CA). The eluants were 0.1% trifluoroacetic acid (TFA) and 40% acetonitrile in 0.1% TFA. The gradient was from 0% to 100% of 40% acetonitrile in 30 minutes. The activation peptide was monitored at 205 nm and quantified by a reference curve using the synthetic activation peptide, DTEDQEDQVDPR, purchased from Primm (Milan, Italy). Concentration of the activation peptide at a given time, [aP]t, was fitted to the following equation: [aP]t = [aP]max [1 − exp(−kobs)], where the pseudo first-order rate constant kobs is equal to e0kcat/Km(e0 = thrombin concentration), as the data were obtained at PC concentration less than Km of the hydrolytic reaction. When the kcat/Km value was calculated in the presence of TM, the experimental conditions were the same as indicated above, except that the solution contained 100 nM TM, 2 mM CaCl2 and 10 nM thrombin forms.
Fibrinopeptide release by thrombin
The rate of fibrinogen hydrolysis was studied by monitoring the rate of fibrinopeptide A release at different fibrinogen concentrations ranging from 0.5 to 64 μM, using a RP-HPLC method, previously detailed.21 WT and Arg67His thrombin forms were used in buffer A at 0.2 and 2 nM concentrations, respectively. The Michaelis parameters for steady-state hydrolysis were calculated by the GRAFIT software (Erithacus Software, Staines, United Kingdom).
Inhibition of WT and Arg67His thrombin forms by AT and heparin cofactor II
Pseudo–first-order kinetics formalism was used to investigate the interaction of WT and Arg67His thrombin forms with 2 serpins, that is, AT and heparin cofactor II (HCII) in the absence of GAG. HCII (American Diagnostica) was incubated at 1 μM concentration with 0.1 μg/mL polybrene (Sigma-Aldrich) in 10 mM Tris-HCl, 0.15 M NaCl, 0.1% PEG 6000, pH 7.50 (buffer A) at 37°C. The reaction was started by adding 50 nM wild-type or Arg67His. At various time points, a 25-μL aliquot was removed and added to a solution containing 100 μMd-Phe-Pip-Arg-pNA in buffer A at 37°C to measure the velocity of amidase activity of the residual thrombin in a thermostatted Varian Cary 2200 spectrophotometer. The amount of residual thrombin present in solution was proportional to the initial velocity of the amidase reaction, following the equation12: Vt = V0 exp(-k′t), where Vt and V0 are the velocity at time t and zero, respectively, while k′ is the apparent first-order rate constant of thrombin-HCII interaction. The value of the second-order rate constant, kon, was calculated by dividing k′ by the serpin concentration.
For experiments using AT, progress curve kinetics was used to analyze the experimental data. AT was used between 10 and 15 μM in buffer A at 37°C. The reaction with thrombin was started by adding 0.1 nM WT or Arg67His and the release of p-nitroaniline was measured as a function of time at 405 nm. Progress curves for the thrombin-AT complex formation were analyzed by the following relation12: pNAt = vst + [(v0 – vs)(1 – exp-k′t)/k′, where pNA is the concentration ofp-nitroaniline at time t, k′ has the same meaning as the immediately preceding equation, and v0 and vs are the initial and steady-state velocities of substrate hydrolysis, respectively. Progress curves fitted to this equation provided estimates for k′, v0, and vs. The association second-order rate constant, kon, for thrombin-AT interaction was calculated by using the relation12: kon = k′(1-vs/ v0)(1+[S]/Km)/[AT], where S is the Phe-Pip-Arg-pNA concentration and Km is the Michaelis constant of its hydrolysis by thrombin, calculated in separate experiments. The measurements were also performed in duplicate.
Progress curve kinetics was also used to derive the kon of both AT and HCII interaction with WT and mutant thrombin in the presence of high-molecular-weight heparin from porcine intestinal mucosa (Sigma-Aldrich; sodium salt grade I-A, 170 USP/mg, average molecular weight, 16 500 d) and porcine dermatan sulfate (Sigma-Aldrich; alternating copoly(β-iduronic acid-[1→3]-N-acetyl-β-galactosamine-4-sulfate-[1→4], average molecular weight, 30 000 d), respectively. For thrombin-AT interaction an optimal heparin concentration of 75 nM was previously determined and used in functional experiments using 0.15 μM AT, 0.15 nM WT or mutant thrombin, and 100 μMd-Phe-Pip-Arg-pNA as substrate. Preliminary control experiments showed that for thrombin-HCII interaction the optimal dermatan sulfate concentration was 25 μM. Thus for thrombin-HCII interaction, the experiments were carried out using 25 nM HCII, 25 μM dermatan sulfate, 0.2 to 1 nM WT or mutant thrombin.
Measurement of the PAR1 peptide hydrolysis by WT and mutant Arg67His thrombins
Hydrolysis of the protease-activated thrombin receptor-1 (PAR1) peptide (PAR1P, NH2-LDPRSFLLRNPNDKYEPFWEDEE-COOH; single-letter amino acid codes), purchased from Primm (Milan, Italy) by the different thrombin forms was followed by measuring the release of the peptide LDPR, resulting from the cleavage of the NH2-terminus of PAR-1, according to a previously described method.17 Briefly, 0.5 μM PAR1P peptide was incubated with 50 to 100 pM WT or mutant thrombins (1 nM for the Arg67Ala form) in 10 mM HEPES, 0.15 M NaCl, 0.1% PEG 6000, pH 7.5, at 25°C. At time intervals (1, 2, 3, 4, 8, 12, and 15 minutes) the reaction was stopped with 0.3 M HClO4 and the cleaved peptide was measured by RP-HPLC, using a 250 × 4.6 mm RP-304 column (Bio-Rad), as previously detailed.17
Interaction of WT and Arg67His thrombin forms with platelets
We investigated the effect of Arg67His thrombin on the enzyme capacity to activate gel-filtered platelets. The latter were prepared in 10 mM HEPES, 0.15 M NaCl, 5.5 mM glucose, 0.2% BSA, pH 7.50, at 25°C as previously detailed.15 In these experiments, thrombin concentration ranged from 0.25 to 128 nM. Aggregation of gel-filtered platelets (3 × 105/μL) was studied in a PACKS 4 aggregometer (Helena Laboratories, Milan, Italy), using a final volume of 500 μL. Aggregation response was evaluated by taking into account the maximum velocity of transmittance increase per minute (expressed as percent of transmittance, set by using plain buffer).
Results
Coagulation and genomic studies
Coagulation assays repeated in Italy in the patient's fresh-frozen plasma confirmed a marked prolongation of both the prothrombin time and the activated partial thromboplastin time and severe prothrombin deficiency as measured by a classical coagulant assay (< 1%) contrasting with almost normal levels of prothrombin as antigen (61% ± 7%) and active enzyme (66% ± 8%) as measured by a 2-stage ecarin assay. On the other hand, the prothrombin concentration measured by the Taipan venom amidolytic assay was lower than the control, being 44% ± 5%. The dissociation between these results suggested the presence of a dysfunctional molecule and prompted us to study the activation of purified prothrombin by the prothrombinase complex, as reported below. A thrombophilic condition was ruled out in the patient, who had AT (115%), PC (71%), and free protein S (70%) levels within the normal range. The patient had neither G20210A mutation in the prothrombin gene nor the G1691A mutation in the factor V gene.
To determine the molecular abnormality associated with the deficiency, the entire coding region and 5′-3′UTR of the prothrombin gene were amplified and screened by SSCP analysis. An abnormally migrating fragment was detected in exon 10. Sequence analysis revealed the patient to be homozygous for a nucleotide substitution that resulted in the Arg67His substitution. Both the parents were heterozygous for the same substitution.
Expression studies
To investigate the function of the mutant proteins, the pED-FII-WT and pED-FII-MT67 vectors were expressed in COS-7 cells by transient transfection. Enzyme immunoassay of the cell lysates and conditioned media demonstrated that the prothrombin antigen level of mutant Arg67His was similar to WT prothrombin (data not shown).
Enzymatic activity of the mutant Arg67His thrombin toward synthetic and natural substrates
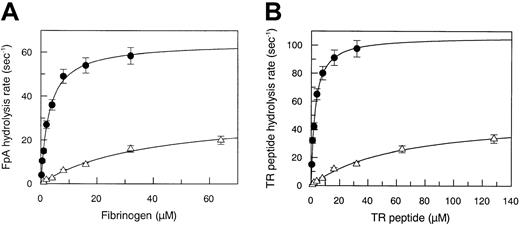
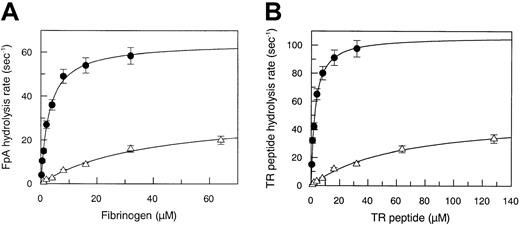
The Michaelis parameters pertaining to the hydrolysis of the synthetic substrate Phe-Pip-Arg-pNA are given in Table1. The Arg67His mutation did not significantly alter the amidase activity of thrombin, suggesting that this substitution does not affect the interaction of the substrate with S1-S4 of the enzyme. By contrast, the interaction of thrombin with natural substrates, such as fibrinogen and thrombin receptor (TR), showed profound changes compared to WT. In fact, fibrinopeptide A hydrolysis was characterized by an approximate13-fold increase of the Km value (38 μM vs 3 μM) and by a 2-fold decrease of the kcat value (32.5 s−1 vs 64 s−1), as shown in Figure 1A. Thus, the kcat/Km value decreased from 2.1 × 107 M−1 s−1 to 9 × 105 M−1 s−1, that is, 22-fold, as shown in Table 1. This finding may explain the loss of the clotting capacity of the mutant thrombin in patient plasma.
Steady-state kinetic parameters.
(A) Measurement of steady-state kinetic parameters concerning fibrinopeptide A hydrolysis by WT (●) and Arg67His mutant (▵) thrombin. Continuous lines were drawn according to the best-fit kcat and Km values listed in Table 1. (B) Measurement of steady-state kinetic parameters concerning hydrolysis of the PAR-1 38-60 peptide by WT (●) and Arg67His mutant (▵) thrombin. Continuous lines were drawn according to the best-fit kcatand Km values listed in Table 1. The SE is expressed by the vertical bars.
Steady-state kinetic parameters.
(A) Measurement of steady-state kinetic parameters concerning fibrinopeptide A hydrolysis by WT (●) and Arg67His mutant (▵) thrombin. Continuous lines were drawn according to the best-fit kcat and Km values listed in Table 1. (B) Measurement of steady-state kinetic parameters concerning hydrolysis of the PAR-1 38-60 peptide by WT (●) and Arg67His mutant (▵) thrombin. Continuous lines were drawn according to the best-fit kcatand Km values listed in Table 1. The SE is expressed by the vertical bars.
Another macromolecular substrate, the TR-derived 38-60 peptide, which interacts with both the catalytic pocket and FRS of thrombin, showed a severe defect in interacting with the enzyme, as shown in Figure 1B. TR 38-60 hydrolysis was characterized by an approximate 23-fold increase of the Km value (62 μM versus 2.7 μM) and by a 2-fold decrease of the kcat value (50 s−1 versus 106 s−1). Thus, the kcat/Km value decreased from 4 × 107 M−1s−1to 1.2 × 106 M−1s−1, that is, 32-fold (Table 1).
Hydrolysis of PC in the absence of TM was not affected by the mutation, because the kcat/Km value was very close to that observed with the WT enzyme, as shown in Table 1. This finding is in substantial agreement with previous data, showing that thrombin does not involve its FRS in its interaction with free PC, except when the enzyme forms a ternary complex with TM and PC, as shown in experiments using TM as cofactor (see below).
Interaction of the mutant Arg67His thrombin with TM
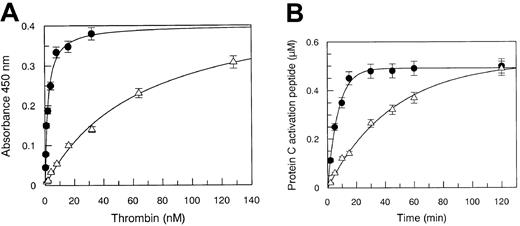
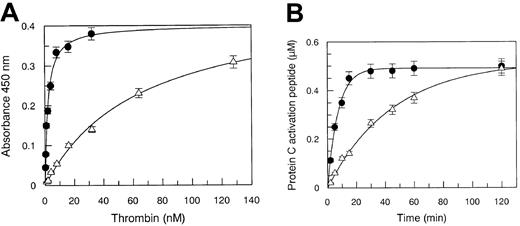
Solid-phase binding experiments showed that Arg67 plays a central role in the interaction with TM, because the Kd decreased 33-fold in the Arg67His mutant, as shown in Figure2A. The defective binding of the mutant thrombin severely affected the catalytic competence of the enzyme in PC hydrolysis. Under experimental conditions using 100 nM TM and either 10 nM WT or mutant thrombin, the apparent kcat/Kmvalue pertaining to PC hydrolysis decreased from 2.3 × 105 M−1 s−1 to 3.7 × 104 M−1 s−1 as shown in Figure 2B. This inhibitory effect was attributed to a defective TM interaction, because PC hydrolysis by the Arg67His mutant enzyme, as reported above, was unchanged compared to the wild-type form.
Solid-phase binding and kinetics of PC hydrolysis.
(A) Solid-phase binding of WT (●) and Arg67His mutant (▵) thrombin to recombinant immobilized TM. The experimental conditions are reported in the text. Continuous lines were drawn according to the best-fit Kd values listed in Table 1, with vertical bars expressing the SE. (B) Kinetics of PC hydrolysis by WT (●) and Arg67His mutant (▵) thrombin, under pseudo–first-order conditions. Continuous lines were drawn with the best-fit parameter values of kcat/Km reported in Table 1. The SEs are expressed by the vertical bars.
Solid-phase binding and kinetics of PC hydrolysis.
(A) Solid-phase binding of WT (●) and Arg67His mutant (▵) thrombin to recombinant immobilized TM. The experimental conditions are reported in the text. Continuous lines were drawn according to the best-fit Kd values listed in Table 1, with vertical bars expressing the SE. (B) Kinetics of PC hydrolysis by WT (●) and Arg67His mutant (▵) thrombin, under pseudo–first-order conditions. Continuous lines were drawn with the best-fit parameter values of kcat/Km reported in Table 1. The SEs are expressed by the vertical bars.
Interaction of WT and Arg67His thrombin with AT and HCII
The best-fit second-order rate constants for both WT and Arg67His interaction with AT and HCII in the presence and absence of heparin and dermatan sulfate, respectively, are listed in Table3. HCII interaction with Arg67His thrombin decreased roughly 3-fold compared to WT, in the absence of dermatan sulfate. This result was in agreement with previous findings that the N terminus of HCII interacts with the thrombin FRS.21 This defect was even more evident in the presence of dermatan sulfate, where Arg67His showed a 500-fold decrease of its kon value compared to WT thrombin. On the contrary, the Arg67His interaction with AT did not show such drastic decrease both in the absence and presence of heparin. This finding is in agreement with previous findings that FRS is not engaged in thrombin interaction with this serpin.12 22
Interaction of WT and mutant Arg67His thrombin with gel-filtered platelets
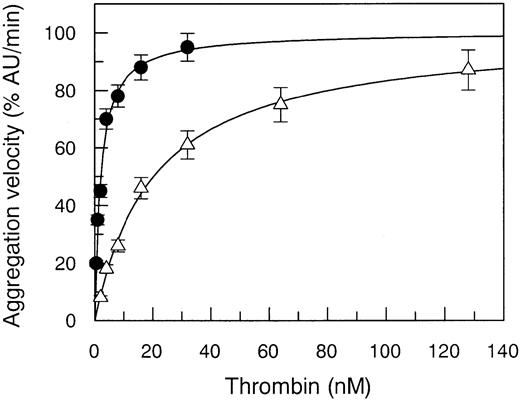
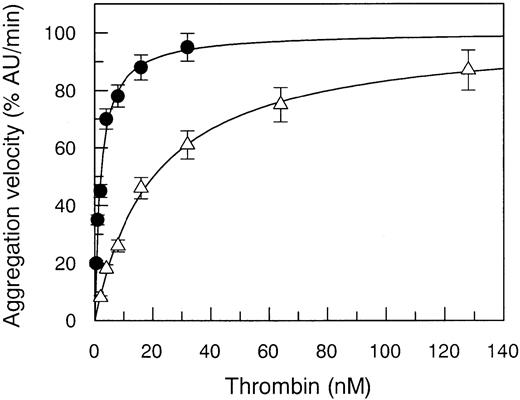
The Arg67His mutant thrombin showed a defect in its capacity to activate gel-filtered platelets, the EC50 value being increased 10-fold compared to WT (Figure3). This biologic effect was attributed to a defective PAR-1 interaction only, because solid-phase binding experiments showed a normal interaction with GpIbα, the other major thrombin receptor involved in the activation of human platelets.23 The Kd value of this binding was equal to 109 ± 12 nM in WT and 123 ± 18 nM in Arg7His. This finding is in agreement with recent data showing that a similar but not isosteric and isoelectric mutation at Arg67, that is Arg67Ala, does not affect thrombin interaction with GpIbα.15
Effect of Arg67His mutation on aggregation of gel-filtered platelets.
Aggregation of gel-filtered platelets (300 000/μL) induced by WT thrombin (●) compared to that of Arg67His (▵) mutant. Continuous lines were drawn using the best-fit EC50 values reported in Table 2.
Effect of Arg67His mutation on aggregation of gel-filtered platelets.
Aggregation of gel-filtered platelets (300 000/μL) induced by WT thrombin (●) compared to that of Arg67His (▵) mutant. Continuous lines were drawn using the best-fit EC50 values reported in Table 2.
Activation of purified WT and Arg67His prothrombin with the FXa-FVa complex
The Arg67His mutation caused a net decrease of the initial rates of prothrombin activation by FXa only when FVa was present in the activation mixture. When FXa alone was used at concentrations of 10 nM in the activation mixture, the initial rates of prothrombin activation were 6.5 ± 0.9 pM/min and 5.7 ± 0.1 pM/min for WT and Arg67His prothrombin, respectively (Table 4). By contrast, when 10 nM FXa plus 5 nM FVa was used, the initial rates of prothrombin activation were 500 ± 90 pM/min and 100 ± 15 pM/min for WT and Arg67His, respectively (Table 4). These experiments showed that Arg67His mutation reduced the cofactor effect of FVa in the FXa-catalyzed prothrombin activation.
Discussion
In a recent study aimed at screening natural variants of prothrombin in a large cohort of families,3 we identified and investigated a woman with undetectable plasma levels of prothrombin activity, borderline levels of prothrombin antigen, and a very mild bleeding diathesis. The latter finding is in contrast with what we found in other patients with hyperprothrombinemia, who usually suffer from more severe spontaneous and posttraumatic bleeding symptoms, despite the fact that, at variance with our patient, they have measurable levels of prothrombin activity.3 A homozygous Arg67His mutation in the prothrombin gene, which results in the loss of a positive charge within the FRS of thrombin, was found. The amino acid substitution involves an arginine and can be hypothesized to alter the enzyme interaction with natural substrates and modulators that bind to FRS, such as fibrinogen, PAR-1, or thrombomodulin. The positively charged FRS residues contribute significantly to enhance the rate of complex formation with thrombin ligands through steering with complementary electrostatic fields and a coordinated combination of molecular contacts within the complex that is peculiar for each macromolecular ligand.
A severe reduction of the catalytic competence of thrombin toward the fibrinogen Aα-chain was observed for the Arg67His mutant. Among the congenitally dysfunctional forms of prothrombin, 2 other compound heterozygous forms, Quick I and Corpus Christi, were found to bear a mutation at Arg67 and to have a reduced interaction with fibrinogen.8,9 Both these variants have a cysteine residue at position 67, besides a Gly→Val mutation at residue 226 and a stop codon at the site of Gln209 in prothrombin Quick I and Corpus Christi, respectively. It is difficult to attribute the functional defect to a specific mutation in compound heterozygous forms, especially in mutants bearing a Gly→Val mutation at residue 226 or a stop codon at the site of Gln209, which may be expected to severely inhibit the thrombin function. In fact, the presence of a stop codon at the position of Gln209 and the Gly226Val mutation lead to the lack of the sodium binding Tyr225-loop, whose integrity is needed for a normal enzyme function,24 or would impede the docking of the bulky P1 residue into the primary catalytic subsite of (chimo)trypsinlike serine proteases,25 respectively. However, in both these thrombin variants the defective enzymatic activity could be attributed also to the common Arg67Cys mutation.8,9 The findings obtained with the homozygous Arg67His variant reported herewith confirm this hypothesis, although the R→H mutation is not isosteric and isoelectric compared to the R→C substitution identified in the Quick I and Corpus Christi prothrombins. The difference in the free activation energy of fibrinogen hydrolysis between the Arg67His mutant and the WT thrombin was equal to 2 kcal/mol, which reflects a 25-fold reduction of the kcat/Km ratio. This parameter is a rough estimate of the kinetic association rate constant of thrombin-fibrinogen interaction,26 reflecting the efficiency of the molecular recognition. This finding may indicate that Arg67 is strongly involved in this process, explaining the severe reduction of the clotting capacity observed both in plasma and with purified proteins.
The Arg67His mutant showed also a severely defective interaction with PAR-1, associated with a net reduction of platelet-activating capacity, that was not due to a defective interaction with GpIbα as demonstrated by solid-phase binding experiments. This finding is consistent with the known involvement of FRS in PAR-1 ligation and with the involvement of a different enzyme exo-domain, referred to as the heparin binding site, in GpIbα binding.15,17 27
Activation of Arg67His prothrombin by the FXa-FVa prothrombinase complex was severely impaired. This finding is in agreement with recent results suggesting a role of pro-FRS in the molecular recognition of prothrombin by the complex, possibly by direct involvement of pro-FRS in FVa binding.18 The 5-fold reduction of the initial rate of the Arg67His prothrombin activation by FXa only in the presence of FVa corroborates the recently proposed role, direct or allosteric, of the pro-FRS domain in prothrombin interaction with FVa.18The present findings would suggest that Arg67 participates indeed in this interaction. It is also of interest that activation of the mutant Arg67His prothrombin by the Taipan snake venom gave a value of plasma prothrombin concentration lower than that obtained with the ecarin assay. These snake venoms belong to different classes of prothrombin activators. Ecarin converts prothrombin into meizothrombin, which autocatalytically produces active thrombin, in absence of FVa or phospholipids.28 By contrast, Taipan snake venom needs Ca++ and phospholipids to activate prothrombin, being associated with a FVa-like cofactor activity.29 The Arg67His mutation could thus inhibit the FVa-like activity of this venom. These findings suggest also that the initial characterization of prothrombin variants should include the use of different classes of venom activators to disclose dysfunctional features of the variants.
Despite the major functional defects discussed above, the Arg67His mutant was associated with very mild bleeding symptoms. This prompted us to investigate other thrombin interactions with modulators and natural inhibitors involved in the regulation of the anticoagulant functions as well as in the inhibition of the enzyme. TM is an integral glycoprotein of the endothelial cell membrane and binds to thrombin, switching the function of this enzyme from procoagulant to anticoagulant through the activation of the PC pathway. Previous nuclear magnetic resonance (NMR) and x-ray diffraction studies showed that the endothelial growth factor (EGF) 5-6 domains of TM make many hydrogen and salt bridges with FRS thrombin residues. Arg67 is involved in this bond network because the hydroxyl group of TM Tyr413 makes a hydrogen bond with the guanidyl side chain of thrombin Arg67.30-32 In our experiments the Arg67His variant showed a reduced interaction with TM, expressed by a Kd value 30-fold higher than in the WT form. This defect caused a defective TM-catalyzed PC hydrolysis. The activation of PC alone, in the absence of TM, did not undergo a reduction of the apparent kcat/Km value, as reported in Table 1. On the other hand, in the presence of a high TM concentration, the apparent kcat/Km value was 6-fold lower for the Arg67His mutant than for the WT. It may be thus hypothesized that in vivo the TM-linked physiologic formation of activated PC undergoes a drastic impairment. Thus, the most relevant TM-linked anticoagulant function of thrombin was as defective as that pertaining to the clotting capacity of the enzyme. This anticoagulant dysfunction might in part counterbalance the procoagulant defect of the mutant, leading to surmise that the formation of active thrombin in the patient is not down-regulated by activated PC. This may help explain the very mild bleeding tendency of the patient carrying this natural variant.
The study of HCII and AT interaction with the Arg67His mutant might further unravel the apparent enigma of the absence of a severe hemorrhagic syndrome in the patient. Kinetic experiments showed for the Arg67His mutant a strong decrease of the second-order rate constant of HCII association both in the absence (3-fold) and especially in the presence of dermatan sulfate (500-fold). In addition, a moderate decrease of the AT association rate constant in the presence of heparin (1.5-fold) was observed. The severely defective interaction of the mutant thrombin with HCII is in agreement with the involvement of the N-terminus of HCII in the ligation of thrombin FRS.12,33The HCII inhibitory activity toward thrombin is strongly enhanced by the presence of glycosaminoglycans (GAGs), such as dermatan sulfate, which allosterically “activates” HCII. A specific dermatan sulfate hexasaccharide sequence composed of repeats of iduronic acid 2–sulfate and N-acetylgalactosamine 4–sulfate has been shown to bind to a cationic domain of HCII.34 This binding disrupts the intramolecular docking, allowing the free N-terminal acidic domain of the inhibitor to bind to the FRS of thrombin.33 Given the selectivity with which dermatan sulfate activates HCII among all serpins interacting with GAGs, and the presence of dermatan sulfate in the extracellular matrix of a wide variety of tissues, HCII has been proposed to inhibit “extravascular” thrombin activity. This inhibitory mechanism has been proposed to oppose the proatherogenic activity of thrombin.35,36 In our patient, the impaired thrombin-HCII interaction might oppose the defective procoagulant capacity of the thrombin mutant, increasing the half-life of free thrombin. The interaction of Arg67Ala thrombin with AT showed only a modest reduction, consistent with the fact that the thrombin-AT interaction does not involve directly the FRS.22 The modest reduction of the association rate of the interaction in the presence of heparin might further contribute to a reduced inhibition in vivo of the thrombin variant.
In conclusion, the experimental data showed that both procoagulant and anticoagulant functions of the Arg67His mutant are impaired. Moreover, the mutation induced a strongly defective inhibition of thrombin by HCII. Altogether, these functional abnormalities might somewhat counterbalance each other so that ultimately the hemostatic equilibrium does not undergo drastic changes, explaining the very mild clinical phenotype associated with this congenital type II prothrombin deficiency.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0243.
Supported by a grant (COFIN 2000) of the Ministry of the University and Scientific and Technological Research of Italy (R.D.C.), IRCCS Maggiore Hospital, Milan, Italy; and Fondazione Italo Monzino (P.M.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sepideh Akhavan, A. Bianchi Bonomi Hemophilia and Thrombosis Center, Via Pace 9, 20122 Milan, Italy; e-mail:sepidehakhavan@yahoo.it or sakhavan@bichat.inserm.fr.