The developmental origin of dendritic cells (DCs) is controversial. In the mouse CD8α+ and CD8α− DC subsets are often considered to be of lymphoid and myeloid origin respectively, although evidence on this point is conflicting. Very recently a novel CD11c+ B220+ DC subset has been identified that appears to be the murine counterpart to interferon alpha (IFNα)–producing human plasmacytoid DCs (PDCs). We show here that CD11c+ B220+ mouse PDCs, like human PDCs, are present in the thymus and express T lineage markers such as CD8α and CD4. However, the intrathymic development of PDCs can be completely dissociated from immature T lineage cells in mixed chimeras established with bone marrow cells from mice deficient for either Notch-1 or T-cell factor 1, two independent mutations that severely block early T-cell development. Our data indicate that thymic PDCs do not arise from a bipotential T/DC precursor.
Introduction
Dendritic cells (DCs) comprise a heterogeneous population of antigen presenting cells (APCs) that can be classified into subsets based on their surface phenotype and functional characteristics.1-4 In the mouse, 2 major subsets of DCs have been identified. Both DC subsets express CD11c and major histocompatibility complex (MHC) class II, but they differ in their expression of other markers such as CD8α, DEC-205, and CD11b (Mac-1). The CD8α+, DEC-205+ CD11b−subset of DCs is preferentially localized in the thymus, while the reciprocal CD8α− DEC-205−CD11b+ DC subset is mainly found in peripheral lymphoid tissues.
The developmental origin of CD8α+ and CD8α− DC subsets remains controversial. Whereas thymic CD8α+ DCs were initially reported to be of lymphoid origin since they developed from an immature T-cell progenitor,5 more recent studies suggest that CD8α+ and CD8α− DCs can also develop from a common myeloid precursor.6 7 Moreover, analysis of mutant mice deficient for either Notch-18 or the growth factor receptors CD117 and CD1329 has revealed that thymic CD8α+ DCs can develop in the complete absence of detectable immature T-cell progenitors. Despite these reports, CD8α+ and CD8α− DC subsets are still frequently referred to in the literature as lymphoid and myeloid DCs, respectively.
Very recently, a novel subset of murine CD11c+ cells has been independently identified by 3 groups.10-12 These cells differ from other mouse DCs in that they express high levels of B220 (CD45R) and Gr-1 (Ly-49G/C), markers usually associated with the B-cell and granulocyte lineages, respectively. Interestingly, CD11c+ B220+ Gr1+ cells are a unique subset that produces type I interferon (IFN) in response to challenge with viruses or CpG oligonucleotides and has a distinct immature plasmacytoid morphology. Based on these functional and morphologic similarities, CD11c+ B220+Gr1+ cells have been proposed to be the murine equivalent of human plasmacytoid dendritic cells (PDCs), a population that previously had no known mouse counterpart.10-12
The developmental origin of human PDCs is particularly controversial, which is perhaps not surprising given the difficulty in performing precursor-to-product analysis in humans. Part of this confusion probably stems from the unusual phenotype of human PDCs, which lack the prototypic mouse DC marker CD11c but express detectable transcripts for pTα13 as well as other T-cell markers such as CD4 and CD7.14,15 On the other hand, human PDCs also express myeloid markers such as CD31, CD36, CD68, and CD123 (IL-3Rα).16,17 Although there is some evidence that human PDCs can differentiate into mature T cells in fetal thymic organ culture (FTOC),18 other in vitro studies suggest that PDCs are precursors of conventional DCs.19 20
In the present study we have taken advantage of the recent identification of CD11c+ B220+ Gr1+cells as the mouse counterpart of human PDCs in order to investigate in more detail the possible lineage relationship between PDCs and T cells. Using a mixed bone marrow (BM) chimera approach, we found that mouse PDCs (but not immature T lineage cells) can develop normally in the thymus from precursors that are deficient in either Notch-1 or T-cell factor 1 (Tcf-1). Our data indicate that PDCs (like other DCs) do not develop from intrathymic bipotential T/DC precursors.
Materials and methods
Mice
C57BL/6 mice were purchased from Harlan Netherlands (Zeist, The Netherlands). CD45.1 C57BL/6 and RAG1−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). Induced Notch-1–deficient mice have been described.21Tcf-1–deficient mice (targeting of exon VII)22 were kindly provided by Dr Hans Clevers (University Hospital, Utrecht, The Netherlands). All mice were used at 4 to 6 weeks of age.
Preparation of low density fraction
To enrich for DCs, a low-density fraction (LDF) was obtained from thymus and spleen after collagenase digestion and subsequent gradient in optiprep solution as described in detail elsewhere.23 In the case of BM, the LDF was obtained without collagenase digestion.
Generation of mixed BM chimeras
Mixed BM chimeras were generated using a 1:2 mixture (10 × 106 and 20 × 106) of T-cell–depleted BM cells from polyI-polyC–treated CD45.1+wild-type (wt) and CD45.2+–induced Notch-1−/− mice.7 17 Additional mixed BM chimeras were generated using a 1:1 mixture (3 × 106 and 3 × 106) of T-cell–depleted BM cells from CD45.1+ wt and CD45.2+ Tcf-1−/−mice. The 10-week-old CD45.1+ hosts were given 1000 rads γ irradiation 24 hours prior to receiving the BM transplant. Radiation chimeras were maintained on antibiotic water and analyzed after 3 months.
Antibodies
The following monoclonal antibody (mAb) conjugates were used: anti-B220–Cychrome (clone RA3.6B2), anti-F4/80–Cy5 (clone F4/80), anti-CD45.2–Cy5 (clone 104), anti-CD11c–fluorescein isothiocyanate (FITC) (clone HL3), anti-DEC205–biotin (clone NLDC-145), anti-MHCII–phycoerythrin (PE) (clone 2G9), anti-CD40–biotin (clone 3/23), anti-CD86–biotin (clone GL-1), anti-CD8α–PE (clone 53.6.7), anti-CD4–PE (clone RM4-5), anti-Gr1–PE (clone RB6-8C5), anti-CD11b–PE (clone M1/70), anti-CD19–PE (clone 1D3), anti-NK1.1–PE (clone PK136). Anti-CD45.2–Cy5 was conjugated in this laboratory from protein purchased from PharMingen (San Diego, CA). DEC-205–biotin and F4/80-Cy5 were purified and conjugated in this laboratory. The rest of the mAb conjugates were purchased from PharMingen.
Flow cytometry
Cells were preincubated with 2.4G2 culture supernatant to block Fcγ receptors, then washed and incubated with the indicated mAb conjugates for 30 minutes at 4°C in a final volume of 100 μL phosphate buffered saline (PBS) containing 2% fetal calf serum (FCS). Cells were washed and analyzed on a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson, San Jose, CA). Dead cells were gated out by their forward scatter (FSC) and side scatter (SSC) profiles.
Morphologic analysis
Cytospins were prepared from sorted thymic CD11c+B220− or CD11c+ B220+ cells (FACStar flow cytometer; Becton Dickinson), fixed in acetone, and stained with hematoxylin-eosin.
Results
Tissue distribution of mouse PDCs
Several recent studies10-12 have identified a novel subset of CD11c+ cells present in lymph node (LN), BM, and spleen that differ from conventional DCs in that they coexpress B220 (CD45R) and Gr-1 (Ly-6G/C). These CD11c+B220+ Gr1+ cells exhibit an immature plasmacytoid morphology and have been shown to be the major producers of type I IFN in the mouse.10-12 Based on these criteria, CD11c+ B220+ Gr1+ cells are presumed to be the murine equivalent of human PDCs.
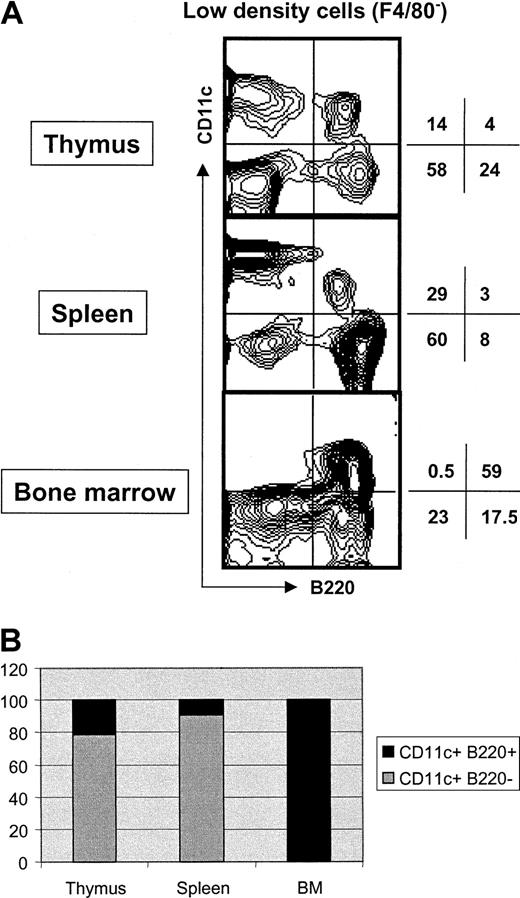
As shown in Figure 1A, CD11c+B220+ mouse PDCs are found in the LDF of thymus, spleen, and BM. Interestingly, the relative proportion of PDCs and conventional DCs varies considerably between tissues (Figure 1B). Whereas PDCs represent only 20% to 25% and 10% of total CD11c+ cells in thymus and spleen, respectively, they account for approximately 99% of CD11c+ cells in BM.
Analysis of CD11c+ B220+ cells in different lymphoid tissues.
(A) LDF was prepared from the indicated tissues. Four-color staining was performed with anti-F4/80–Cy5, anti-CD11c–FITC, anti-B220–CyChrome, and anti- MHCII–PE. Contour plots correspond to CD11c versus B220 expression after gating out F4/80+ cells. MHCII expression by CD11c+ B220+ cells was very low compared with CD11c+ B220− or CD11c− B220+ cells (data not shown). Numbers indicate percentage of cells in the respective quadrant. The data are representative of 3 independent experiments. (B) Bar diagram showing the relative proportion of DCs and PDCs among CD11c+ cells in the indicated lymphoid tissue. The data are representative of 3 independent experiments.
Analysis of CD11c+ B220+ cells in different lymphoid tissues.
(A) LDF was prepared from the indicated tissues. Four-color staining was performed with anti-F4/80–Cy5, anti-CD11c–FITC, anti-B220–CyChrome, and anti- MHCII–PE. Contour plots correspond to CD11c versus B220 expression after gating out F4/80+ cells. MHCII expression by CD11c+ B220+ cells was very low compared with CD11c+ B220− or CD11c− B220+ cells (data not shown). Numbers indicate percentage of cells in the respective quadrant. The data are representative of 3 independent experiments. (B) Bar diagram showing the relative proportion of DCs and PDCs among CD11c+ cells in the indicated lymphoid tissue. The data are representative of 3 independent experiments.
Phenotype of thymic PDCs
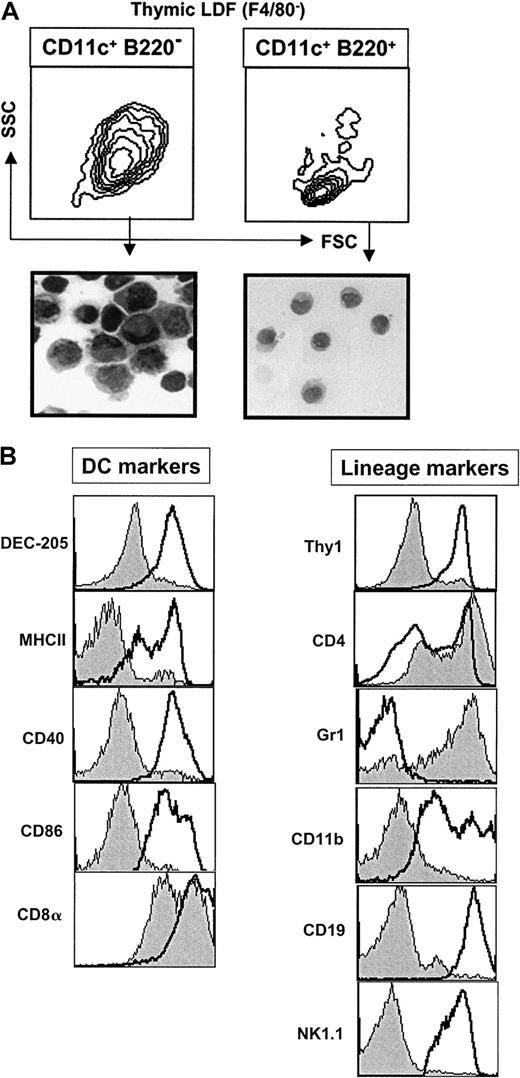
The presence of significant numbers of CD11c+B220+ PDCs in the thymus is of considerable interest in view of the controversy surrounding the myeloid versus lymphoid origin of human PDCs. We therefore analyzed the phenotype of thymic PDCs in greater detail. As shown in Figure 2A, CD11c+ B220+ thymic PDCs differed from conventional DCs (CD11c+ B220−) in that they were smaller (as defined by FSC and SSC) and had a typical immature plasmacytoid morphology. Moreover, in contrast to conventional thymic DCs, PDCs lacked expression of DEC-205 as well as several molecules associated with APC function including MHC class II, CD40, and CD86 (Figure 2B).
Phenotype of thymic CD11c+ B220+cells.
(A) LDF was prepared from thymus. Contour plots show the FSC versus SSC profile of CD11c+ B220+ F4/80− or CD11c+ B220− F4/80− cells. These 2 populations were sorted and stained with hematoxylin and eosin (magnification ×100). (B) Histograms show the expression of the indicated markers by thymic CD11c+ B220+ cells (gray profile). For DEC-205, MHCII, CD40, CD86, CDα, Thy1, CD4, and GR1 empty profiles correspond to CD11c+ B220−thymic DCs. The empty profiles for CD11b, CD19, and NK1.1 histograms correspond to thymic macrophages (F4/80+), thymic B cells (CD11c− B220+ F4/80−) and thymic NK cells (NK1.1+), respectively. Data are representative of 3 experiments.
Phenotype of thymic CD11c+ B220+cells.
(A) LDF was prepared from thymus. Contour plots show the FSC versus SSC profile of CD11c+ B220+ F4/80− or CD11c+ B220− F4/80− cells. These 2 populations were sorted and stained with hematoxylin and eosin (magnification ×100). (B) Histograms show the expression of the indicated markers by thymic CD11c+ B220+ cells (gray profile). For DEC-205, MHCII, CD40, CD86, CDα, Thy1, CD4, and GR1 empty profiles correspond to CD11c+ B220−thymic DCs. The empty profiles for CD11b, CD19, and NK1.1 histograms correspond to thymic macrophages (F4/80+), thymic B cells (CD11c− B220+ F4/80−) and thymic NK cells (NK1.1+), respectively. Data are representative of 3 experiments.
As is the case for human PDCs, mouse thymic CD11c+B220+ PDCs expressed several markers normally associated with the T-cell lineage. For example, thymic PDCs expressed CD8α (although at slightly lower levels than conventional thymic DCs). Interestingly, most thymic PDCs (but not conventional DCs) also expressed high levels of CD4 (Figure 2B), similar to human PDCs.14 15
Although thymic PDCs expressed B220 (which is an epitope present on the B-cell isoform of CD45) they did not express the pan–B-cell marker CD19 (Figure 2B), making it highly unlikely that PDCs are B cells. Thymic PDCs also did not express the natural killer (NK) cell marker NK1.1 or CD11b, which is normally expressed on macrophages and the CD8α− subset of conventional DCs (Figure 2B). Consistent with earlier studies of PDCs in peripheral tissues,10,11most thymic PDCs expressed Ly-6G, as indicated by high-level staining with the anti-GR1 mAb RB6-8C524 (Figure 2B).
Thymic PDCs are present in RAG1−/− mice
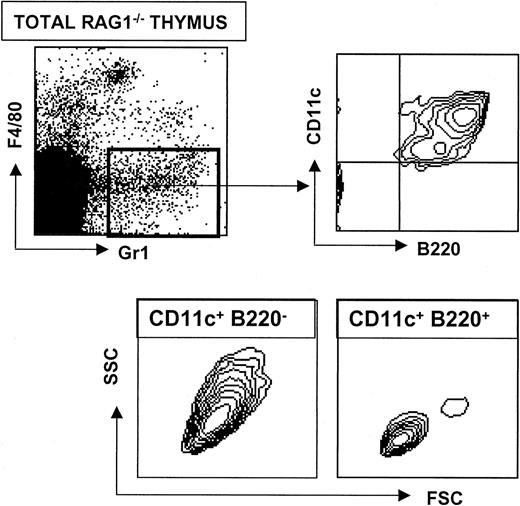
RAG1−/− mice are deficient in recombination at the T-cell receptor (TCR) and immunoglobulin (Ig) loci. As a consequence, mature T and B cells are absent in RAG1−/− mice but immature T- and B-cell precursors remain present in the thymus and bone marrow, respectively. In agreement with a recent study of LN,10 CD11c+ B220+Gr1+ PDCs were present in normal numbers in the thymus of RAG1−/− mice (Figure 3). These results demonstrate that PDCs arise independently of mature T and B cells but do not exclude the possibility that they diverge from the T- or B-cell lineage at an earlier developmental stage (prior to recombination of TCR or Ig genes).
Analysis of CD11c+ B220+ cells in RAG1−/− mice.
A RAG1−/− thymic suspension was obtained after collagenase digestion. Four-color staining was performed with anti-F4/80–Cy5, anti-Gr1–PE, anti-CD11c–FITC and anti-B220–CyChrome. Upper plots show that a typical thymic population of CD11c+ B220+ Gr1+F4/80− cells develop in RAG1−/− mice. Lower contour plots show the characteristic FSC versus SSC profiles of CD11c+ B220− and CD11c+B220− cells.
Analysis of CD11c+ B220+ cells in RAG1−/− mice.
A RAG1−/− thymic suspension was obtained after collagenase digestion. Four-color staining was performed with anti-F4/80–Cy5, anti-Gr1–PE, anti-CD11c–FITC and anti-B220–CyChrome. Upper plots show that a typical thymic population of CD11c+ B220+ Gr1+F4/80− cells develop in RAG1−/− mice. Lower contour plots show the characteristic FSC versus SSC profiles of CD11c+ B220− and CD11c+B220− cells.
Both Notch-1−/− and Tcf-1−/− BM precursors generate normal numbers of PDCs in the absence of T lineage cells
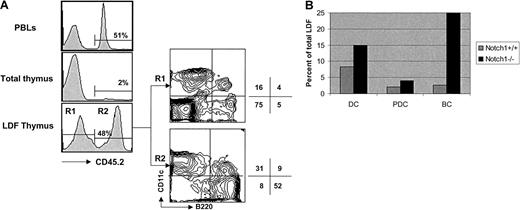
In order to further study the lineage relationship between PDCs and T cells we investigated the ability of hematopoietic stem cells deficient in either Notch-1 or Tcf-1 to give rise to DCs and T cells in the thymus. We have shown previously that Notch-1 is essential for T-cell fate specification at an early developmental stage II1 and that Notch-1–deficient common lymphoid precursors (CLPs) adopt an alternative (B cell) fate in the thymus.25We have also demonstrated that conventional thymic DCs develop normally from Notch-1−/− BM precursors,8 suggesting that thymic DCs and T cells arise from independent lineages. To extend this analysis to thymic PDCs we constructed BM chimeras in which CD45.2+ BM from Notch-1−/− mice was mixed with CD45.1+ wt BM and injected into lethally irradiated CD45.1 recipients. After reconstitution thymi were prepared and the LDF was analyzed for CD11c+ B220+ and CD11c+ B220− DCs. In agreement with previous results8 only a small fraction (∼2%) of total thymocytes developed from CD45.2+ Notch-1−/−BM precursors; however, approximately 50% of the LDF was of Notch-1−/− origin (Figure4A). Moreover, staining of the LDF with CD11c and B220 revealed that both thymic PDCs (CD11c+B220+) and conventional DCs (CD11c+B220−) were present in the CD45.2+(Notch-1−/−) and CD45.1+ (wt) fractions (Figure 4A). Additional staining with MHC class II confirmed that thymic PDCs in the chimeras expressed low levels of MHC class II compared with conventional DCs (data not shown). In terms of absolute numbers, PDCs and DCs were slightly (∼2-fold) increased in the CD45.2+ LDF (Figure 4B), likely reflecting the fact that these chimeras were set up with a 2:1 (CD45.2/CD45.1) BM ratio. As expected from earlier studies8 25 the proportion (Figure 4A) and absolute number (Figure 4B) of thymic B cells (CD11c−B220+) was dramatically (10-fold) increased in the CD45.2+ (Notch-1−/−) LDF, whereas T cells (CD11c− B220−) were absent (Figure 4A). These data demonstrate that thymic PDCs, like conventional thymic DCs, develop normally from Notch-1−/− BM precursors in a situation where immature cells of the T lineage are not detectable.
Analysis of CD11c+ B220+ cells in (wt + Notch-1−/−) mixed BM chimeras.
PBLs and thymi of Notch-1+/+ chimeric mice were pooled and stained with anti-CD45.2–Cy5. LDF was also obtained from a pool of thymi that was 4-color stained with anti-CD45.2–Cy5, anti-CD11c–FITC, anti-B200–CyChrome, and anti-F4/80–biotin AvPE. Results are representative of 2 independent experiments. (A) Histograms show the reconstitution with Notch-1−/− BM precursors in PBLs, total thymus, and thymic LDF. Contour plots show CD11c versus B220 expression (after gating out F4/80+ cells), by thymic LDF derived from wt BM precursors (CD45.2−, R1) or from induced Notch-1−/− BM precursors (CD45.2+, R2). Numbers indicate the percentage of cells in the respective quadrant. (B) Bar diagram shows the relative proportion of DCs (CD11c+ B220−) and PDCs (CD11c+B220+) derived from wt BM precursors (gray bar) or induced Notch-1−/− BM precursors (black bar) in the thymic LDF. Numbers were obtained by multiplying the percentage of wt BM-derived cells (CD45.2−, 52%) or induced Notch-1−/−BM-derived cells (CD45.2+, 48%) by the percentage of DCs or PDCs indicated in the respective quadrants.
Analysis of CD11c+ B220+ cells in (wt + Notch-1−/−) mixed BM chimeras.
PBLs and thymi of Notch-1+/+ chimeric mice were pooled and stained with anti-CD45.2–Cy5. LDF was also obtained from a pool of thymi that was 4-color stained with anti-CD45.2–Cy5, anti-CD11c–FITC, anti-B200–CyChrome, and anti-F4/80–biotin AvPE. Results are representative of 2 independent experiments. (A) Histograms show the reconstitution with Notch-1−/− BM precursors in PBLs, total thymus, and thymic LDF. Contour plots show CD11c versus B220 expression (after gating out F4/80+ cells), by thymic LDF derived from wt BM precursors (CD45.2−, R1) or from induced Notch-1−/− BM precursors (CD45.2+, R2). Numbers indicate the percentage of cells in the respective quadrant. (B) Bar diagram shows the relative proportion of DCs (CD11c+ B220−) and PDCs (CD11c+B220+) derived from wt BM precursors (gray bar) or induced Notch-1−/− BM precursors (black bar) in the thymic LDF. Numbers were obtained by multiplying the percentage of wt BM-derived cells (CD45.2−, 52%) or induced Notch-1−/−BM-derived cells (CD45.2+, 48%) by the percentage of DCs or PDCs indicated in the respective quadrants.
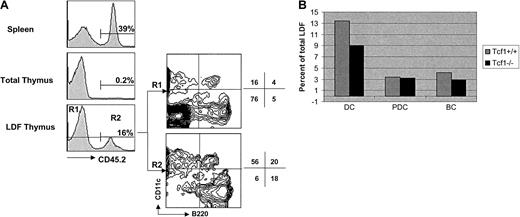
Although the mixed (Notch-1−/−: wild-type) chimeras strongly suggest that thymic PDCs and DCs are not of T lymphoid origin, it could still be argued that PDCs and DCs develop from CLPs that are diverted to an ectopic B-cell fate in the thymus of Notch-1–deficient mice. To further address this issue we constructed similar mixed chimeras using a mixture of CD45.2+ Tcf-1−/−BM and CD45.1+ wt BM to reconstitute lethally irradiated CD45.1 recipients. Although T cells develop to some extent in the thymus of young Tcf-1−/− mice,22 they disappear with age and adult Tcf-1−/− BM is unable to significantly reconstitute the thymus of irradiated syngeneic recepients.26 As shown in Figure5A, CD45.2+ thymocytes of Tcf-1−/− origin were almost undetectable (0.2% of total) in the mixed chimeras. In the LDF, however, Tcf-1−/−CD45.2+ cells were readily detectable and this subset contained both CD11c+ B220+ PDCs and CD11c+ B220− DCs in normal proportions and absolute numbers (Figure 5). In contrast to Notch-1−/− BM precursors (Figure 4) Tcf-1−/− BM precursors did not give rise to increased numbers of immature B cells (CD11c−B220+) in the thymus (Figure 5), although T cells (CD11c− B220−) were again absent. These results thus demonstrate that both PDCs and DCs develop normally in the thymus from Tcf-1−/− BM precursors, despite the absence of immature progenitor cells of either the T- or B-cell lineage.
Analysis of CD11c+ B220+ cells in (wt + Tcf1−/−) mixed BM chimeras.
Spleen and thymus cell suspension of chimeric mice were stained with anti-CD45.2–Cy5. LDF obtained from the thymus was 4-color stained with anti-CD45.2–Cy5, anti-CD11c–FITC, anti-B220–CyChrome, and anti-F4/80–biotin AvPE. Results shown here are representative of 2 independent experiments. (A) Histograms show the reconstitution with Notch-1−/− BM precursors in spleen, total thymus, and thymic LDF. Contour plots show CD11c versus B220 expression (after gating out F4/80+ cells) by thymic LDF derived from wt BM precursors (CD45.2−, R1) or from Tcf-1−/− BM precursors (CD45.2+, R2). Numbers indicate the percentage of cells in the respective quadrant. (B) Bar diagram shows the relative proportion of DCs (CD11c+ B220−) and PDCs (CD11c+ B220+) derived from Tcf1+/+BM precursors (gray bar) or Tcf-1−/− BM precursors (black bar) in the thymic LDF. Numbers were obtained by multiplying the percentage of wt BM-derived cells (CD45.2−, 84%) or Tcf1−/− BM-derived cells (CD45.2+, 16%) by the percentage of DCs or PDCs indicated in the respective quadrants.
Analysis of CD11c+ B220+ cells in (wt + Tcf1−/−) mixed BM chimeras.
Spleen and thymus cell suspension of chimeric mice were stained with anti-CD45.2–Cy5. LDF obtained from the thymus was 4-color stained with anti-CD45.2–Cy5, anti-CD11c–FITC, anti-B220–CyChrome, and anti-F4/80–biotin AvPE. Results shown here are representative of 2 independent experiments. (A) Histograms show the reconstitution with Notch-1−/− BM precursors in spleen, total thymus, and thymic LDF. Contour plots show CD11c versus B220 expression (after gating out F4/80+ cells) by thymic LDF derived from wt BM precursors (CD45.2−, R1) or from Tcf-1−/− BM precursors (CD45.2+, R2). Numbers indicate the percentage of cells in the respective quadrant. (B) Bar diagram shows the relative proportion of DCs (CD11c+ B220−) and PDCs (CD11c+ B220+) derived from Tcf1+/+BM precursors (gray bar) or Tcf-1−/− BM precursors (black bar) in the thymic LDF. Numbers were obtained by multiplying the percentage of wt BM-derived cells (CD45.2−, 84%) or Tcf1−/− BM-derived cells (CD45.2+, 16%) by the percentage of DCs or PDCs indicated in the respective quadrants.
Discussion
In this report we have investigated the possible lineage relationship between T cells and the recently described CD11c+ B220+ PDC population in the mouse. Like human PDCs, CD11c+ B220+ PDCs are present in significant numbers in the mouse thymus and express several T-cell markers including CD8α and high levels of CD4, consistent with a common origin of PDCs and T cells. Nevertheless, PDCs develop normally from Notch-1−/− or Tcf-1−/− precursors in the thymus of mixed BM chimeras, despite the fact that both of these genetic defects result in a severe and selective block of intrathymic T-cell development at a very early stage (prior to the expression of T lineage–specific markers). Thus it seems unlikely that PDCs develop from a common intrathymic T/PDC precursor.
Recent evidence indicates that Notch-1 controls a binary T/B cell fate decision of a CLP.25 According to this model, CLPs entering the thymus are instructed by Notch-1 signaling to adopt a T-cell fate. In the absence of Notch-1, CLPs adopt a B-cell fate in the thymus, presumably as a default pathway. If PDCs (or DCs in general) also derive from an intrathymic CLP, it remains possible that, in the absence of Notch-1 CLPs developing along the ectopic B-cell pathway may give rise to PDCs. However, this explanation seems very unlikely since normal numbers of PDCs (and DCs) also develop in the thymus from Tcf-1−/− precursors, despite the absence of the ectopic B-cell developmental pathway.
If PDCs do not arise from an intrathymic T/PDC precursor, what is their developmental origin? One possibility would be that PDCs are still of lymphoid origin, but more closely related to B cells or NK cells than to T cells. In this context the expression of the B-cell marker B220 per se cannot be used as an argument favoring a common PDC/B cell precursor, since B220 is also expressed on preapoptotic T cells27 and abnormal peripheral T cells that accumulate in Fas-deficient lpr mice and FasL-deficient gldmice.28 Moreover, the fact that there is no increase in the number of thymic PDCs derived from Notch-1−/− BM precursors (despite a marked increase in ectopic B-cell development) is inconsistent with a shared PDC/B cell precursor. With regard to the possibility of a bipotential PDC/NK cell precursor, it is noteworthy that Tcf-1−/− BM precursors did not give rise to thymic NK cells (defined as NK1.1+ CD3−) (W.H. and L. Scarpellino, manuscript in preparation), whereas both wild-type and Notch-1−/− BM precursors did so.8 Thus, intrathymic PDCs and NK cell development can also be dissociated in Tcf-1−/− mice. Nevertheless, PDCs are particularly abundant in the BM where both B and NK cell lymphopoiesis normally occurs. Further analysis of PDCs in mutant mice with early blocks in B- or NK cell development should help to clarify this issue.
Finally (although perhaps less interesting to developmental immunologists), the possibility must be considered that most PDCs (and other DCs) are of myeloid origin under physiologic conditions,6 despite the fact that some DCs can apparently be generated from lymphoid precursors following in vitro culture or in vivo transfer.
We thank Céline Marechal for technical help, Pierre Zaech for his assistance with cell sorting, Jeannine Bamat for her help for the morphologic analysis, and Audrey Poncelet for helping with the manuscript. We thank also Hans Clevers for providing the Tcf-1–deficient mice.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-01-0214.
Supported in part by a grant from HFSP (RG0168/2000) to H.R.M., and a grant from Swiss Cancer Ligue (KFS-0094509) to F.R. W.H. is the recipient of a Swiss Talents for Academic Research and Training (START) fellowship from the Swiss National Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Robson MacDonald, Ludwig Institute for Cancer Research, Lausanne Branch, University of Lausanne, CH-1066 Epalinges, Switzerland; e-mail: hughrobson.macdonald@isrec.unil.ch.