Abstract
Here we demonstrate that keratinocyte growth factor (KGF) and FGFR2IIIb signaling can affect development and function of thymic epithelium (TE) and that αβ-lineage thymocytes contribute to intrathymic levels of KGF. Thymocyte expression of KGF is developmentally regulated, being undetectable in CD3−4−8− thymocytes and expressed at highest levels by mature CD4 or CD8 thymocytes. Exposure of thymocyte-depleted fetal thymic lobes to KGF resulted in reduced thymic epithelial expression of class II major histocompatibility complex (MHC), invariant chain (Ii), and cathepsin L (CatL) molecules involved in thymocyte-positive selection and also stimulated expression of the cytokines interleukin 6 (IL-6) and thymic stromal-derived lymphopoietin (TSLP), while having little effect on IL-7 or stem cell factor expression. Within intact fetal thymic organ culture (FTOC), exogenous KGF impairs the generation of CD4 thymocytes. Two lines of evidence point to responsiveness of the medullary TE compartment to KGF and FGFR2IIIb signaling. First, the medullary compartment is expanded in intact FTOC exposed to KGF in vitro. Second, in the RAG-deficient thymus, where the thymocytes do not express detectable levels of KGF message, the hypoplastic medullary TE compartment can be expanded by administration of recombinant KGF in vivo. This expansion is accompanied by restoration of the normal profile of medullary TE–associated chemokine expression in the RAG2−/−thymus. Collectively, these findings point to a role for KGF and FGFR signaling in the development and function of thymic epithelium.
Introduction
The thymus is a heterogeneous epithelial environment where morphologically and phenotypically distinct epithelial compartments support thymocyte development. Contributions of thymic epithelium (TE) to this process include the elaboration of cytokines affecting thymocyte development, the role of major histocompatibility complex (MHC)–peptide complexes expressed by cortical TE in positive selection, and the participation of medullary TE in some models of negative selection.1Differential expression of chemokines by TE subsets and a developmentally regulated pattern of chemokine receptor expression by thymocytes have been proposed as mechanisms to effect a serial exposure of developing thymocytes to distinct epithelial compartments and may underlie the centripetal movement of thymocytes within the thymus.2 This association of thymocytes at different stages of maturation with distinct epithelial compartments and functional studies of transgenic and mutant mice have led to the notion that these different epithelial compartments contribute sequentially to the intrathymic phase of T-cell development.
There is accumulating evidence that the functional integrity and developmental potential of thymic epithelium is not autonomous and is dependent on signals derived from nonepithelial sources. Thymocytes themselves contribute to the growth and differentiation of TE,3 although the mediators responsible remain largely undefined. Fibroblasts have also been implicated in the development of the thymic environment. Enzymatic removal of connective tissue from fetal thymic lobes prevented their subsequent development when engrafted,4 and a requirement for mesenchyme in the successful reconstitution of functional thymic tissue has also been demonstrated in vitro.5 Other work indicates that this mesenchymal contribution is not restricted to the induction of TE differentiation but is also required for maintenance of TE function.6
Little is known regarding the processes that underlie mesenchymal contribution to TE growth/differentiation. Epidermal growth factor (EGF) is a potential mesenchymally derived mediator of thymus organogenesis,7 and fibroblast growth factor (FGF) family members are also candidate effector molecules. Members of this complex family of polypeptides serve as ligands for cell surface receptors with tyrosine kinase activity and have been implicated in embryonic development and patterning.8,9 We focused on keratinocyte growth factor (KGF, FGF-7) because this member of the FGF family typically exhibits a paracrine mode of action, being produced by mesenchymal cells and acting on a wide range of epithelial-derived cells that express a unique splice variant of the FGFR2 receptor (FGFR2IIIb).10-12 Indications that KGF or other FGF family members that signal through this receptor could play a role in thymic organogenesis include the in situ demonstration of KGF message in the fetal thymus10 and thymic dysgenesis in transgenic mice expressing soluble dominant-negative FGFR2IIIb receptor.13Furthermore, mice lacking either FGF-10 or the IIIb form of FGFR2 display hypoplastic thymic tissue.14 Here we show that αβ-lineage thymocytes are an additional source of KGF in the thymus and demonstrate that KGF can alter important parameters of TE function in vitro and in vivo. The actions of KGF affected both cortical and medullary TE compartments.
Materials and methods
Reagents
Tissue culture media was HL-1 supplemented with 1% nonessential amino acids, 2 mM l-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 M 2-mercaptoethanol (all from Sigma Chemical, St Louis, MO). Recombinant human KGF was a generous gift from Amgen (Thousand Oaks, CA). Immunoblot detection of murine KGF employed polyclonal affinity-purified goat anti–human KGF antibodies (R&D Systems, Minneapolis, MN). Sense and antisense digoxigenin-modified probes were generated with reagents and protocols obtained from Boehringer Mannheim (Indianapolis, IN). Alkaline-phosphatase–conjugated antidigoxigenin antibodies also were purchased from Boehringer Mannheim. The 3G10 monoclonal antibody (Mab) detects an intracellular constituent of medullary TE cell lines that has an electrophoretic mobility pattern similar to keratin 14 in 2D gels (A.G.F., unpublished observations, 1997). Other Mabs used for immunohistochemistry included NLDC-145,1510.1.1,16 anti–E-cadherin (ECCD-217) and anti–B7-1 (1610A118). Antibodies detecting FGFR2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Animals and cell preparation
Timed pregnant female BALB/c and C57Bl/6 mice (National Cancer Institute, Bethesda, MD) were killed on day 16 of gestation (counting the day of the appearance of the vaginal plug as day 0). Dr M Bevan (University of Washington, Seattle, WA) kindly provided RAG2-deficient mice. All procedures involving animals followed institutional guidelines established by the Department of Comparative Medicine of the University of Washington.
Fetal thymic organ culture
Fetal thymic organ culture (FTOC) was performed as described19 with the filter membranes supported on stainless steel screens in 2.5 mL of media. Culture in deoxyguanosine (DOG) to deplete hematogenous cells was done as described.20 DOG-treated lobes were dissociated at 37°C with a mixture of dispase (0.8 U/mL), collagenase (0.1 U/mL), and DNAse (150 U/m) (Boehringer Mannheim) in Hanks balanced salt solution (HBSS) for biochemical and flow cytometric analyses or homogenized in TRIzol (Gibco BRL, Grand Island, NY) for subsequent reverse transcription–polymerase chain reaction (RT-PCR) analyses.
Flow cytometry
Fetal thymic lobes were mechanically dispersed in cold medium, passed through nylon mesh, and washed with additional medium. To minimize Fc receptor–mediated labeling, cells were incubated with anti–FcγRII Mab 2.4G2 (42; 50% hybridoma supernatant in HBSS supplemented with 0.1% NaN3), 1% fetal bovine serum, 10% rat serum, and 10% goat serum prior to labeling with fluorochrome-conjugated Mabs. Cells were stained with either anti–CD3ε-FITC (clone 500A221), anti–CD4-FITC (clone RM4-5; Pharmingen, San Jose, CA), and/or anti–CD8-PE (clone 3B5; Caltag, Burlingame, CA) and analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). All data shown are with gates set on living cells, as assessed by their exclusion of the fluorescent dye 7-amino-actinomycin D (Molecular Probes, Eugene, OR).22 Data were analyzed with CellQuest software (Becton Dickinson).
Thymocyte fractionation
Thymocytes were sorted on the basis of their CD4 and CD8 expression using a Vantage cell sorter (Becton Dickinson). CD4 and CD8 single-positive thymocytes were obtained by sequential enrichment; first depletion with biotinylated anti-CD8 monoclonal antibodies and streptavidin-conjugated magnetic beads (PerSeptive Biosystems, Farmingham, MA), then fluorescence-activated cell sorting of the recovered cells with a combination of directly labeled anti-CD4 and anti-CD8 antibodies. Triple negative (CD3, CD4, and CD8) were obtained by magnetic depletion of CD4 or CD8+ cells, followed by sorting on the basis of CD3 expression.
RNA purification and cDNA synthesis
Total RNA was recovered by phenol/chloroform extraction, treated with DNAse, and then quantitated spectrophotometrically. Synthesis of cDNA was performed with avian myeloblastosis virus (AMV) reverse transcriptase according to the manufacturer's recommendations (Promega, Madison, WI).
Conventional PCR
Sequences for the PCR primers and real-time PCR probes used are given in Table 1. Primers were purchased from Genosys (The Woodlands, TX), and fluorescent probes were purchased from Biosearch Technologies (Novato, CA). For conventional PCR, normalization was done by performing hypoxanthine-guanine phosphoribosyl transferase (HPRT) PCR analysis, separating the reaction products by electrophoresis through a 1.8% agarose gel stained with ethidium bromide and generating negative images of the gel. Negatives were scanned and processed with Image software (public domain; http://rsb.info.nih.gov/nih-image/download.html) to obtain densitometry values for individual bands. Volumes of cDNA samples were adjusted to give equivalent densitometric values. Normalization with a HPRT competitor construct23 also was employed in some studies with equivalent results.
For real-time PCR, KGF and HPRT reactions used an antibody-bound hot start Thermus aquaticus (TAQ) polymerase (Qiagen, Valencia, CA) and the same thermal cycling (50°C hold, 2 minutes; 95°C hold, 10 minutes; cycle 95°C, 20 seconds; 60°C, 1 minute). For both reactions, 50 cycles were carried out using an ABI Prism 7700 real-time thermocycler (PE Biosystems, Norwalk, CT).
A comparative CT method was used to determine relative gene expression (Table 2).24 Threshold cycles (Ct) reflected the cycle number when the fluorescence generated by cleavage of the fluorescent probe passed a predetermined threshold above baseline. The threshold was adjusted above the baseline values and to the start of the logarithmic curve of the plots, as described by the manufacturer. There was an excellent correlation coefficient (HPRT, 0.998; KGF, 1.000) over a 4-log dilution range, with 3.939 and 3.936 cycles representing a 10-fold change in KGF or HPRT cDNA levels, respectively.
In situ hybridization
Analyses of thymus tissue were done according to published protocols25 using digoxigenin-modified sense and antisense probes.
Immunodetection of KGF in thymocyte-conditioned medium
Unfractionated thymocytes were cultured at 106cells/mL in HL-1 medium for 48 hours. After low-speed centrifugation (300g × 10 minutes), the medium was concentrated 10-fold with Centricon devices (3K cutoff, Millipore, Bedford, MA). Aliquots of the concentrated media samples were added to equal volumes of 2 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, heated to 100°C for 5 minutes, and then processed for SDS-PAGE and electrophoretic transfer to nitrocellulose membranes and processed for immunoblot analyses as previously described.16 Peroxidase activity was detected with chemiluminescence (New England Nuclear, Wellesley, MA.)
Immunodetection of invariant chain and cathepsin L
TE cells (2 × 105/sample) were lysed on ice for 40 minutes in 20 μL of cell lysis buffer (0.5% Nonidet P-40 (Sigma), 0.15 M NaCl, 5 mM EDTA (ethylenediaminetetraacetic acid), 50 mM Tris-HCl, pH 7.2) supplemented with a protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN). Following centrifugation at 8000 rpm for 10 minutes, the lysate supernatants were normalized for protein concentration using the Bradford reagent (Pierce Chemical, Rockford, IL). Samples were boiled for 5 minutes in SDS-reducing buffer, separated by SDS-PAGE (12% acrylamide, wt/vol), and then electrophoretically transferred onto nitrocellulose membrane. Membranes were probed for invariant chain with the IN-1 Mabs26 as described.27 Cat L was detected with polyclonal rabbit antisera to mouse Cat L (a gift of A. Erickson, University of North Carolina, Chapel Hill, NC), which has been described previously.28 Affinity-purified rabbit antiactin antibody was purchased from Sigma. Binding was detected using a horseradish peroxidase–conjugated anti–rat or anti–rabbit IgG (Amersham Pharmacia Biotech, Piscataway, NJ) diluted 1:1500 and visualized by chemiluminescence.
Active site labeling
Equal numbers of TE cells per sample (2 × 105) were incubated for 2 hours at 37°C in the presence of 0.25 μM cysteine protease inhibitor Cbz125I-Tyr-Ala-CN2. This radiolabeled inhibitor binds irreversibly to the active site cysteine via a thioester bond.29 Cells lysates were processed for SDS-PAGE and electrophoretic transfer as described above, and the labeled proteins visualized by exposure to Kodak BioMax MR film (Kodak, Rochester, NY).
Immunohistochemistry
Phenotypic analyses of cultured thymic lobes used indirect enzyme immunohistochemical procedures30 Briefly, frozen sections of tissue were serially incubated with optimal dilutions of antigen-specific or control primary antibodies. Some of the primary antibodies had been modified withN-hydroxysuccinimidyl-digoxigenin (Boehringer Mannheim) according to the manufacturer's instructions and detected with horseradish peroxidase–conjugated Fab fragments of sheep antidigoxigenin antibodies (Boehringer-Mannheim). The 3G10 Mab was detected with a peroxidase-conjugated goat anti–rat μ chain–specific antibody (Pierce Chemical). Binding of unconjugated rat IgG monoclonal antibodies to tissue sections was detected with a 3-step procedure, where unmodified primary antibodies were detected by sequential exposure to digoxigenin-conjugated goat anti–rat IgG antibodies and peroxidase-conjugated Fab fragments of goat antidigoxigenin antibodies. Peroxidase activity was revealed with 3,3′-diaminobenzidine in the presence of hydrogen peroxide.
In vivo administration of KGF
Recombinant KGF (500 ug/mL) dissolved in HBSS or vehicle alone was administered intraperitoneally to 4- to 6-week-old RAG2−/−-deficient mice (2.5 μg/g body weight) every other day for 9 days. This dose and administration schedule was based on the work of Danilenko et al.31 Ten days after initiation of treatment, thymic lobes were processed for immunohistochemistry, flow cytometry, or obtention of thymic RNA.
Results
Thymic epithelial stromal cells but not thymocytes express the FGFR2IIIb receptor
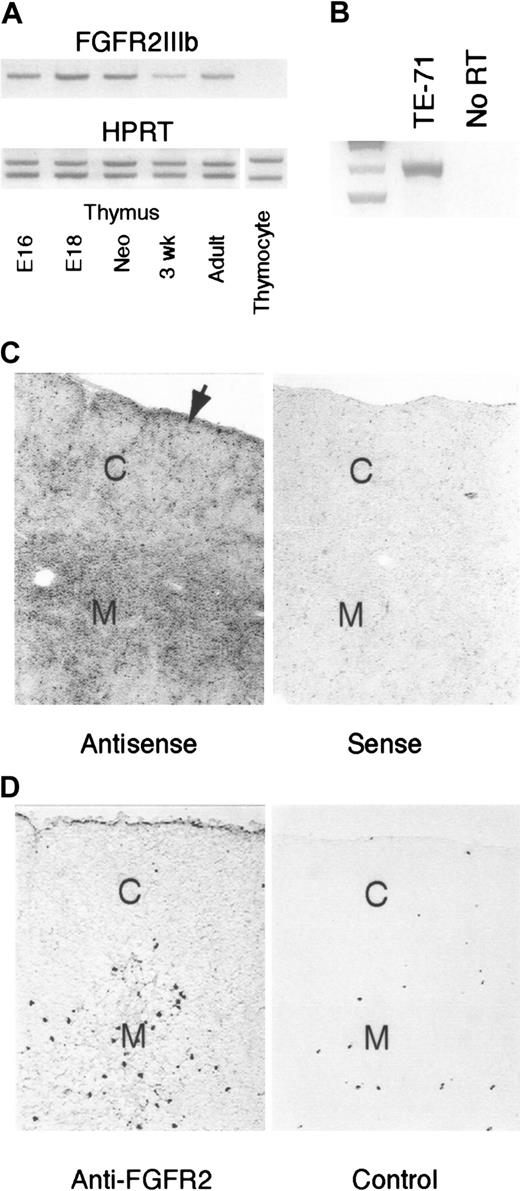
RT-PCR analysis of RNA samples from thymi of different ages indicated that the FGFR2IIIb receptor isoform was present within the thymus at all ages examined (Figure 1A). This mRNA expression was restricted to the predominantly epithelial stromal elements, as no signal was detected in RT-cDNA samples of thymocytes from day-16 embryos (Figure 1A) or 3-week-old postnatal mice (data not shown) but was evident in samples from a thymic epithelial cell line, TE-71 (Figure 1B). Hybridization of a FGFR2IIIb RNA probe to thymic tissue sections from 4- to 6-week-old mice indicated receptor expression throughout cortical and medullary regions, with preferential labeling of the medullary regions and under the capsule, with cortical areas exhibiting lower levels of reaction (Figure 1C). A similar localization pattern was observed with an antibody that recognizes both b and c isoforms of the FGFR2 (Figure 1D).
Thymic expression of FGFR2IIIb.
(A) RT-PCR analysis of cDNA prepared from whole thymi of indicated ages. Samples were normalized for HPRT expression using a constant amount of HPRT competitor cDNA (lower panel). Normalized samples were then analyzed with FGFR2IIIb primers (upper panel). Thymocyte sample was prepared from a pool of day-16 fetal thymi. Representative of 4 independent experiments. (B) Detection of FGFR2IIIb in RT-PCR analysis of the TE-71 thymic epithelial cell line. Representative of 2 independent experiments. (C) In situ hybridization localization of FGFR2IIIb message in young adult thymus. Arrow denotes rim of labeling associated with the subcapsular epithelium. C indicates cortex; M, medulla. Magnification × 40. Representative of 3 independent experiments. (D) Immunohistochemical localization of FGFR2 protein in young adult thymus. C indicates cortex; M, medulla. Magnification × 40. Representative of 2 independent experiments.
Thymic expression of FGFR2IIIb.
(A) RT-PCR analysis of cDNA prepared from whole thymi of indicated ages. Samples were normalized for HPRT expression using a constant amount of HPRT competitor cDNA (lower panel). Normalized samples were then analyzed with FGFR2IIIb primers (upper panel). Thymocyte sample was prepared from a pool of day-16 fetal thymi. Representative of 4 independent experiments. (B) Detection of FGFR2IIIb in RT-PCR analysis of the TE-71 thymic epithelial cell line. Representative of 2 independent experiments. (C) In situ hybridization localization of FGFR2IIIb message in young adult thymus. Arrow denotes rim of labeling associated with the subcapsular epithelium. C indicates cortex; M, medulla. Magnification × 40. Representative of 3 independent experiments. (D) Immunohistochemical localization of FGFR2 protein in young adult thymus. C indicates cortex; M, medulla. Magnification × 40. Representative of 2 independent experiments.
Thymocytes are a source of KGF
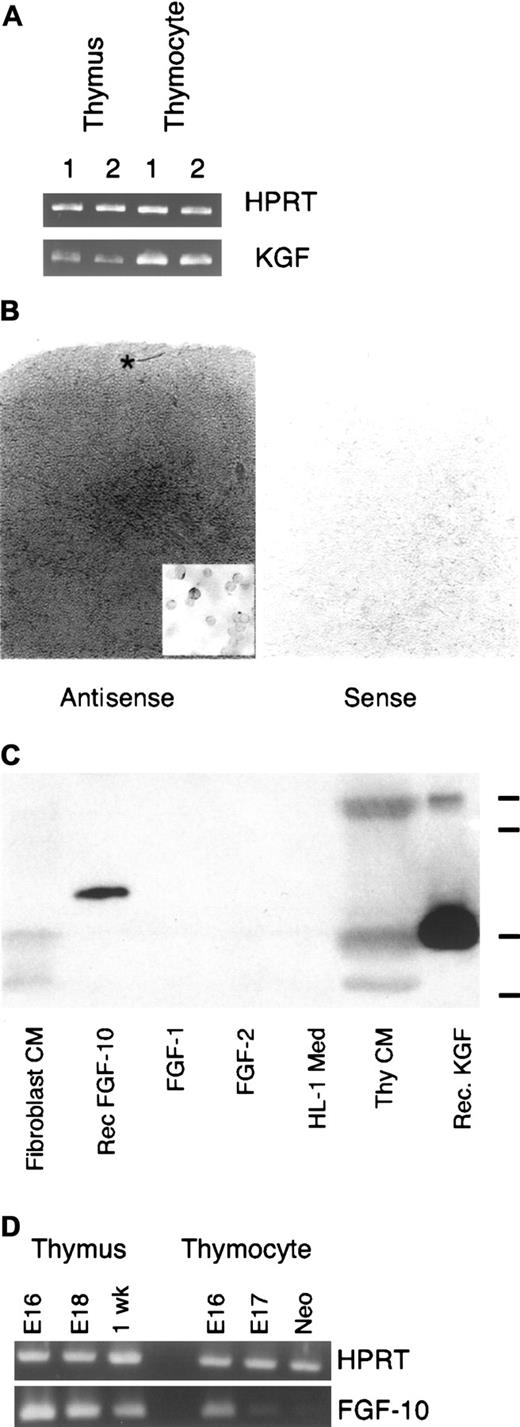
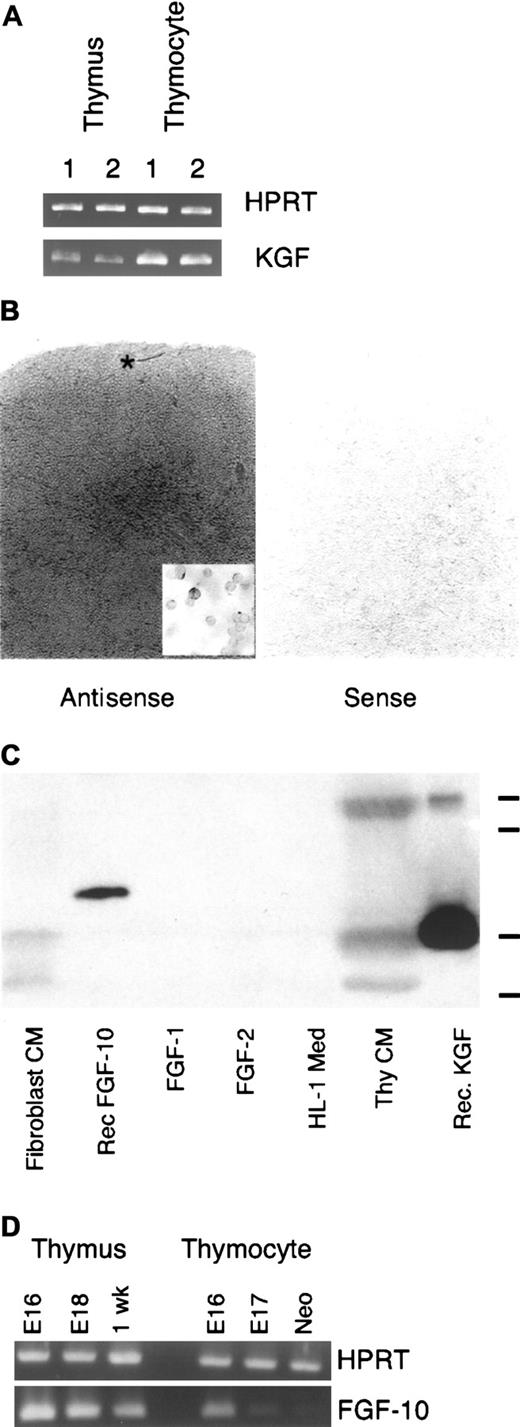
Analysis of cDNA samples prepared from fetal and postnatal thymi with PCR primers specific for KGF revealed the presence of KGF message (Figure 2A). The pattern of KGF mRNA expression by thymocytes at different stages of development was determined by quantitative real-time PCR of thymocyte cDNA. Analysis of cDNA prepared from thymocyte subsets sorted on the basis of CD3, CD4, and CD8 expression is shown in Table 1. KGF RT-PCR product was undetectable in the CD3−4−8−thymocyte population (with 50 cycles of PCR) and highest in the mature thymocyte subsets, with the CD4+8+ population showing intermediate values. Similar to the CD3−4−8− thymocytes in normal mice, RAG2−/− thymocytes also failed to express detectable levels of KGF signal. Results from in situ hybridization studies were consistent with the RT-PCR analysis (Figure 2B). We routinely observed a lightly labeled region under the capsule, intermediate labeling throughout the cortex, and the most intense labeling in medullary areas. That this labeling was associated with thymocytes was demonstrated by hybridization of the KGF probe with cytospot preparations of unfractionated thymocytes, where the heterogeneity of labeling was evident (inset, Figure 2B).
Thymic expression of KGF.
(A) RT-PCR analysis of cDNA prepared from replicate samples of whole thymi or unfractionated thymocytes from young adult mice. Samples were normalized for HPRT. (B) In situ hybridization signal for KGF message preferentially localizes in the thymic medullary compartment in young adult thymus. Note the increasing intensity of signal moving from subcapsular to medullary areas. Asterisk indicates lighter region of labeling in the outer cortex/subcapsular region. Inset: In situ hybridization of thymocyte cytospot preparation. Representative of 3 independent experiments. Magnification × 40; inset × 95. (C) Thymocyte-conditioned medium contains immunoreactive KGF. Serum-free conditioned medium or recombinant FGFs were separated on a polyacrylamide gel, transferred to nitrocellulose membrane, and probed with a polyclonal anti–human KGF antibody. Marks at right indicate relative mobility of molecular weight standards (from top: 29, 24, 20, and 14.2 kDa). Representative of 4 independent experiments. (D) RT-PCR analysis of FGF10 expression by whole thymus and unfractionated thymocytes. Samples were normalized for expression of HPRT. Representative of 2 experiments
Thymic expression of KGF.
(A) RT-PCR analysis of cDNA prepared from replicate samples of whole thymi or unfractionated thymocytes from young adult mice. Samples were normalized for HPRT. (B) In situ hybridization signal for KGF message preferentially localizes in the thymic medullary compartment in young adult thymus. Note the increasing intensity of signal moving from subcapsular to medullary areas. Asterisk indicates lighter region of labeling in the outer cortex/subcapsular region. Inset: In situ hybridization of thymocyte cytospot preparation. Representative of 3 independent experiments. Magnification × 40; inset × 95. (C) Thymocyte-conditioned medium contains immunoreactive KGF. Serum-free conditioned medium or recombinant FGFs were separated on a polyacrylamide gel, transferred to nitrocellulose membrane, and probed with a polyclonal anti–human KGF antibody. Marks at right indicate relative mobility of molecular weight standards (from top: 29, 24, 20, and 14.2 kDa). Representative of 4 independent experiments. (D) RT-PCR analysis of FGF10 expression by whole thymus and unfractionated thymocytes. Samples were normalized for expression of HPRT. Representative of 2 experiments
To assess KGF expression by thymocytes at the protein level, serum-free medium conditioned by thymocytes was subjected to immunoblot analysis with polyclonal anti–human KGF antibodies. As shown in Figure 2C, thymocyte-conditioned medium contained proteins of ∼27, ∼20, and ∼16 kDa detected with this antibody. The 20-kDa species has an apparent molecular weight similar to that of bacterially expressed recombinant human KGF, and the largest band had a mobility similar to that reported for KGF expressed by mammalian cells. KGF fragments with apparent molecular weights in the 16-18 kDa range have been observed before.10,32 Interestingly, processed forms in this size range are several-fold more active than the unprocessed form in keratinocyte mitogenic assays (J.S.R., unpublished observations, 1995, and Ron et al33). The lack of reactivity displayed by unconditioned medium demonstrated the thymocyte origin of the immunoreactive material. The anti-KGF antibody failed to react with recombinant FGF-1 or FGF-2, but did exhibit some cross-reactivity with recombinant FGF-10, which had an electrophoretic mobility distinct from KGF. Probing a replicate blot with polyclonal anti–FGF-1 antibodies detected recombinant FGF-1, but showed no reactivity with thymocyte-conditioned medium (data not shown). In contrast to KGF, intrathymic expression of FGF-10, a closely related FGF family member, was not detectable by RT-PCR analysis of either unfractionated neonatal, 1-week postnatal thymocytes (Figure 2D) or sorted adult thymocyte populations (no signal at 50 cycles in real-time PCR assay; data not shown). RT-PCR analysis of whole thymus consistently generated a signal in the fetal and postnatal thymus, indicating that the FGF-10 expression exhibited in whole thymus is a contribution of stromal cells, presumably mesodermally derived, or hematogenous cells that are not liberated by mechanical dissociation of the thymus. We also noted an FGF-10 signal in unfractionated fetal thymocytes that declined with increasing gestational age. The source of this signal, either fetal thymocytes or contaminating stromal cells, remains to be determined.
Exogenous KGF perturbs several functional characteristics of thymic epithelium in vitro
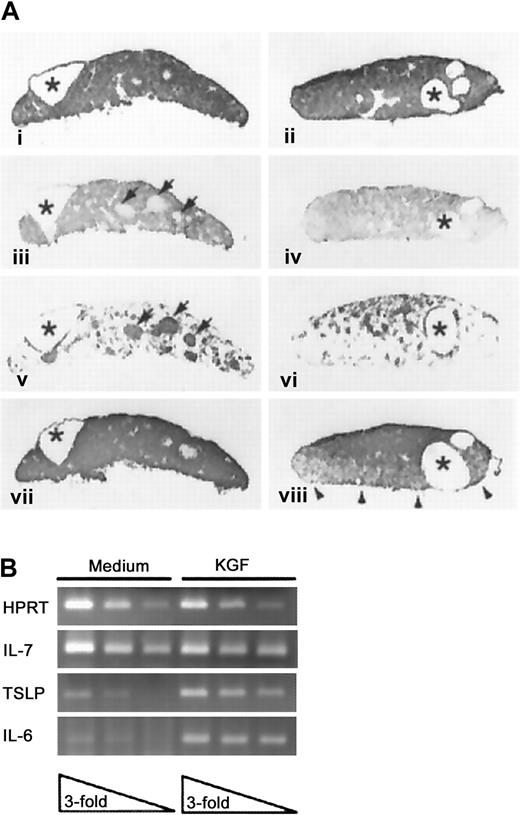
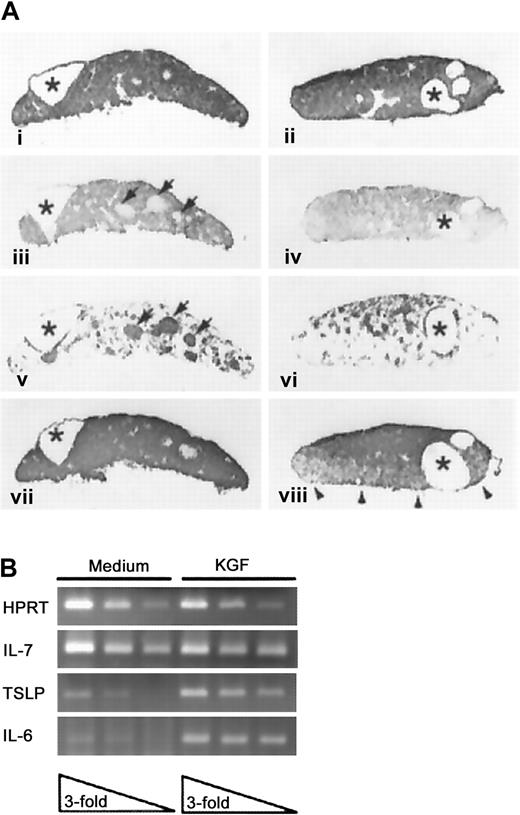
To assess the direct effects of KGF on thymic epithelium without the confounding activity of thymocytes, fetal thymic lobes were cultured in medium containing deoxyguanosine to deplete hematogenous elements, and then cultured an additional 48 hours with or without recombinant KGF prior to analysis. As a consequence of DOG treatment and depletion of hematogenous elements, the cultured lobes consisted of compact epithelium, as reflected by E-cadherin expression (Figure3Ai-ii) that displayed prominent epithelial cysts irrespective of the presence of KGF (asterisks). Such structures have been described in the normal thymus and are more prominent in mice that display an early arrest in thymocyte development.34 The majority of the TE in both the KGF-treated and control thymic lobes displayed a cortical phenotype (Figure 3Aiii,iv). The medullary TE compartment in the control lobes was organized predominately in discrete foci, with surrounding scattered cells. In KGF-treated lobes, these discrete foci of medullary TE failed to develop, and the medullar compartment was more diffusely organized (compare panels iii and iv of Figure 3A). Additional comparison of the organization of the medullary compartment in DOG-treated lobes and intact thymic lobes revealed that KGF exposure partially restored the medullary organization to more closely resemble that of intact lobes cultured in medium where thymocyte–stromal cell cross-talk could occur (compare Figure 3Aiii and 3Aiv with Figure5C).
KGF effects on thymic lobes depleted of hematogenous elements.
(A) DOG-treated thymic lobes were cultured in medium (i, iii, v, vii) or KGF (ii, iv, vi, viii) and then processed to demonstrate expression of E-cadherin (i, ii), cortical epithelium (iii, iv), medullary epithelium (v, vi), or MHC class II antigens (vii, viii). Cystic structures are indicated by asterisks, and the medullary TE foci in the medium-treated FTOC are indicated by arrowheads. Representative of 2 separate experiments, each with 5-7 lobes per condition. Panels i-viii, magnification × 45. (B) RT-PCR analysis of cytokine expression in medium and KGF-treated DOG lobes. Analysis of serial 3-fold dilutions of cDNA samples prepared from medium or KGF-treated DOG lobes (previously normalized for HPRT expression).
KGF effects on thymic lobes depleted of hematogenous elements.
(A) DOG-treated thymic lobes were cultured in medium (i, iii, v, vii) or KGF (ii, iv, vi, viii) and then processed to demonstrate expression of E-cadherin (i, ii), cortical epithelium (iii, iv), medullary epithelium (v, vi), or MHC class II antigens (vii, viii). Cystic structures are indicated by asterisks, and the medullary TE foci in the medium-treated FTOC are indicated by arrowheads. Representative of 2 separate experiments, each with 5-7 lobes per condition. Panels i-viii, magnification × 45. (B) RT-PCR analysis of cytokine expression in medium and KGF-treated DOG lobes. Analysis of serial 3-fold dilutions of cDNA samples prepared from medium or KGF-treated DOG lobes (previously normalized for HPRT expression).
We also assessed the impact of exogenous KGF on the production of cytokines by the DOG-treated thymic lobes by RT-PCR analysis. As shown in Figure 3B, treatment with KGF resulted in elevated expression of interleukin 6 (IL-6) and thymic stromal-derived lymphopoietin (TSLP),35 while having no effect on levels of IL-7 or stem cell factor (data not shown).
Exogenous KGF perturbs thymocyte development and cortical TE function in vitro
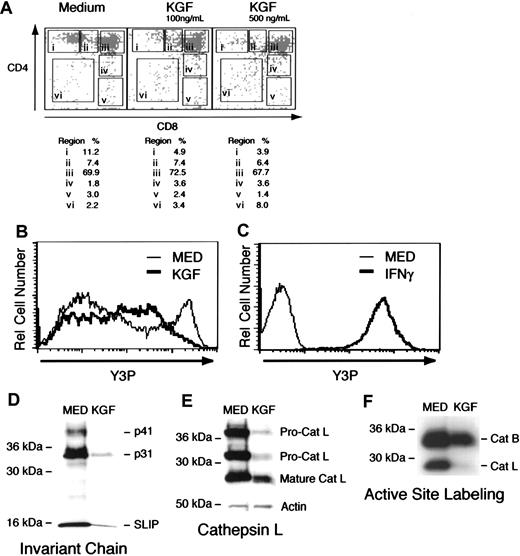
To evaluate the effect of KGF on the ability of the thymus to support thymocyte development in vitro, intact fetal thymic lobes were cultured in the presence or absence of exogenous KGF. In the presence of exogenous KGF, the lobes displayed slightly reduced cellularity (∼80% of control lobes cultured in medium alone) accompanied by a consistent decrease in the representation of CD4 SP thymocyte in these cultures, ranging from 50% to 75%. The CD8 SP population was variably affected (Figure4A), but due to the meager representation of mature CD8 SP thymocytes in FTOC, the impact of KGF on their development could not be reliably assessed. Other members of the FGF family (FGF1, FGF2, FGF4, or FGF10) did not have this activity (data not shown). This effect of KGF was dose-dependent; with modest effects evident at concentrations of 10 ng/mL (data not shown). Prolonged culture in the presence of KGF (15 days) resulted in more profound reductions in the CD4+8− thymocyte subset and reduced cellularity, indicating that exogenous KGF was not simply slowing the tempo of their development (data not shown). These alterations in the representation of CD4+8−thymocytes were not accompanied by detectable alteration of CD69 or CD5 expression (data not shown). Such a phenotype is consistent with a decreased efficiency of positive selection of CD4 thymocytes.
Exogenous KGF thymocyte development in fetal thymus organ culture.
(A) Representation of CD4/CD8 expression by thymocytes from thymic lobes cultured in medium or medium containing KGF. Dead cells were excluded from the analysis on the basis of staining with 7AAD and forward/side scatter profiles. Values represent percentage of cells within each region of the plot. These results are representative of 8 independent experiments. (B) Flow cytometric analysis of MHC class II expression by TE dissociated from DOG-treated lobes. Heavy line depicts lobes cultured in KGF, and the light line represents DOG-treated lobes cultured in the absence of KGF. (C) Flow cytometric analysis of MHC class II expression by AND41.2 TE cell line cultured in medium or 40 U/mL of IFNγ. (D) Immunoblot detection of invariant chain (Ii) by TE cells recovered from DOG-treated lobes cultured in the absence or presence of KGF. (E) Upper panel depicts detection of cathepsin L (Cat L) with a specific polyclonal rabbit antiserum in TE cultured in medium or KGF. Lower panel demonstrates that comparable levels of actin were detected in the same control and KGF-treated samples. (F) Active site labeling of cathepsins present in lysates of 2 × 105 TE cells recovered from DOG-treated lobes cultured in the absence or presence of recombinant KGF. Cathepsin B and cathepsin L are indicated. These results are representative of 5 independent experiments.
Exogenous KGF thymocyte development in fetal thymus organ culture.
(A) Representation of CD4/CD8 expression by thymocytes from thymic lobes cultured in medium or medium containing KGF. Dead cells were excluded from the analysis on the basis of staining with 7AAD and forward/side scatter profiles. Values represent percentage of cells within each region of the plot. These results are representative of 8 independent experiments. (B) Flow cytometric analysis of MHC class II expression by TE dissociated from DOG-treated lobes. Heavy line depicts lobes cultured in KGF, and the light line represents DOG-treated lobes cultured in the absence of KGF. (C) Flow cytometric analysis of MHC class II expression by AND41.2 TE cell line cultured in medium or 40 U/mL of IFNγ. (D) Immunoblot detection of invariant chain (Ii) by TE cells recovered from DOG-treated lobes cultured in the absence or presence of KGF. (E) Upper panel depicts detection of cathepsin L (Cat L) with a specific polyclonal rabbit antiserum in TE cultured in medium or KGF. Lower panel demonstrates that comparable levels of actin were detected in the same control and KGF-treated samples. (F) Active site labeling of cathepsins present in lysates of 2 × 105 TE cells recovered from DOG-treated lobes cultured in the absence or presence of recombinant KGF. Cathepsin B and cathepsin L are indicated. These results are representative of 5 independent experiments.
Efficient positive selection of CD4 thymocytes is dependent on presentation of class II MHC-peptide complexes by cortical TE and is impaired when thymic cortical epithelial cells lack Ii or Cat L.36-39 Cat L previously has been shown to play a critical role in generation of peptide–MHC class II complexes in thymic cortical epithelial cells.39 There was a modest reduction in the intensity of anti–MHC class II labeling in the KGF-treated lobes (compare Figure 3Avii,viii). The in situ demonstration of reduced MHC class II expression by thymic stromal cells was confirmed with flow cytometric analysis of enzymatically dissociated thymic lobes. Figure4B shows that exposure to KGF reduced MHC class II expression, where few cells expressing high levels of MHC class II+ were detected. (For comparison, the expression of MHC class II expression by a thymic stromal cell line in the presence or absence of prior interferon γ [IFNγ] exposure is shown in Figure 4C.) Furthermore, immunoblot analysis revealed that KGF treatment significantly decreased expression of Ii (Figure 4C) or Cat L, particularly immature pro-Cat L forms (Figure 3D), compared to control thymic epithelium. The effect of KGF on these proteins seems to be selective, because the level of a control protein, actin, was not changed upon KGF treatment (Figure 3D). To directly assess Cat L activity, thymic lobes treated with deoxyguanosine in the presence or absence of KGF were dissociated with collagenase/dispase, and lysosomal cysteine proteinases were labeled upon incubation of dissociated TE with Cbz-125I-Tyr-Ala-CN2, an irreversible inhibitor of Cat L, B, and S.29 This active site labeling of lysosomal proteinases in TE cells revealed a substantial decrease in Cat L activity when exposed to KGF (Figure 4E). Thus, in addition to reduced expression of MHC class II and Ii, Cat L activity also is sharply diminished in the presence of KGF. The observed decreases in MHC class II and Ii expression in combination with lowered Cat L activity is likely to account for the impaired development of CD4 thymocytes in KGF-treated FTOC.
Exogenous KGF expands the medullary compartment in vitro and in vivo and restores medullary-type chemokine message levels in RAG2−/− mice
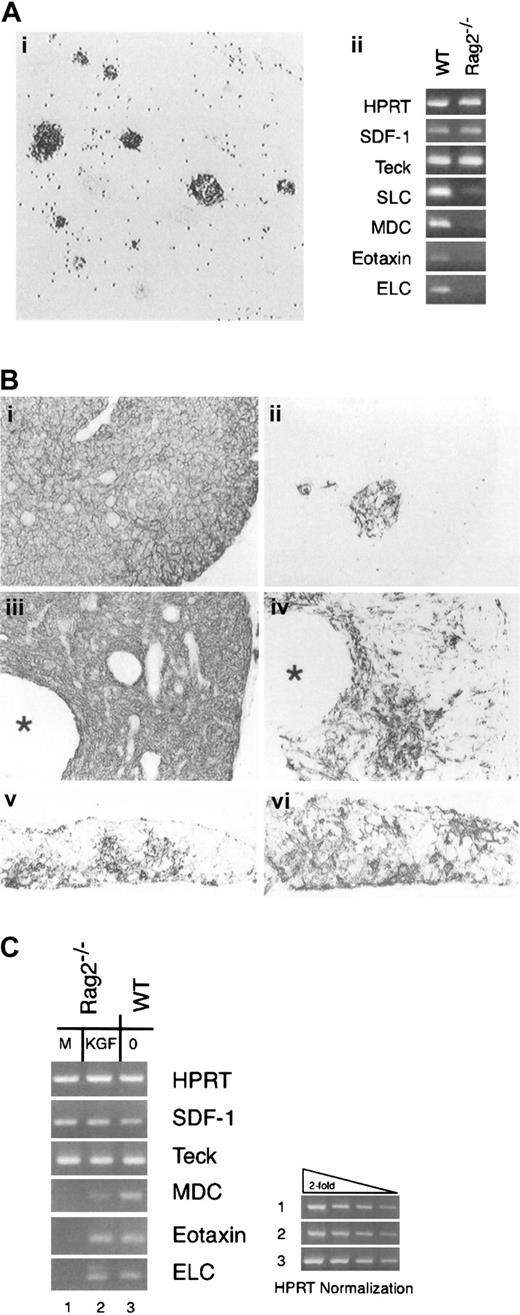
Given the lack of detectable KGF message in RAG2−/−thymocytes, we hypothesized that reduced FGFR2IIIb signaling might be a factor contributing to the hypoplastic medullary compartment in the RAG2−/− thymus. As shown here by the expression pattern of 3G10 staining as a marker of medullary TE, the medullary compartment in the RAG2−/− thymus consisted of isolated cords of cells previously demonstrated in association with vascular elements (Figure 5Ai).40 We also assessed the expression of several chemokines considered to be selectively produced by medullary epithelium in the normal thymus2,41,42 as another parameter of the epithelial environment of the RAG2−/− thymus and found that message levels for macrophage-derived chemokine (MDC), EB-11 ligand (ELC), secondary lymphoid tissue chemokine (SLC), and eotaxin were dramatically reduced in the RAG2−/− thymus, while message levels for thymus-derived chemokine (TECK) and stromal derived factor-1 (SDF-1), 2 chemokines without preferential medullary expression,43 44 were comparable in wild-type and RAG2−/− thymus samples (Figure 5Aii).
KGF and the RAG2−/− thymic environment.
(A) The medullary compartment of the RAG2-deficient thymus. (i) The medullary compartment of RAG2-deficent mice defined by the 3G10 reactivity. Scattered dots represent endogenous peroxidase activity. Magnification × 35. (ii) Analysis of thymus chemokine expression. RNA samples from wild-type (WT) and RAG2−/− thymi were treated with DNAse and then subjected to RT-PCR using a panel of chemokine primers. (B) Thymus tissue from RAG2−/− mice receiving repeated intra-peritoneal administration of vehicle (i, ii) or KGF (iii, iv) were processed for immunohistochemistry. The distribution of all epithelial cells was detected by an antibody against E-cadherin (i, iii), and the distribution of medullary epithelium with 3G10 antibody (ii, iv). Asterisks indicate cystic structures in the KGF-treated RAG2−/− thymus. Intact day-16 FTOC from C57Bl/6 mice cultured for 7 days in the absence (v) or presence (vi) of KGF at 100 ng/mL and then stained with 3G10 to demonstrate the medullary TE compartment. Panels i-iv, magnification × 40; panels v, vi, × 50. (C) RT-PCR analysis of chemokine mRNA expression in RAG2−/− thymi treated with vehicle or vehicle containing KGF. Representative of 2 independent experiments with groups of 4 mice.
KGF and the RAG2−/− thymic environment.
(A) The medullary compartment of the RAG2-deficient thymus. (i) The medullary compartment of RAG2-deficent mice defined by the 3G10 reactivity. Scattered dots represent endogenous peroxidase activity. Magnification × 35. (ii) Analysis of thymus chemokine expression. RNA samples from wild-type (WT) and RAG2−/− thymi were treated with DNAse and then subjected to RT-PCR using a panel of chemokine primers. (B) Thymus tissue from RAG2−/− mice receiving repeated intra-peritoneal administration of vehicle (i, ii) or KGF (iii, iv) were processed for immunohistochemistry. The distribution of all epithelial cells was detected by an antibody against E-cadherin (i, iii), and the distribution of medullary epithelium with 3G10 antibody (ii, iv). Asterisks indicate cystic structures in the KGF-treated RAG2−/− thymus. Intact day-16 FTOC from C57Bl/6 mice cultured for 7 days in the absence (v) or presence (vi) of KGF at 100 ng/mL and then stained with 3G10 to demonstrate the medullary TE compartment. Panels i-iv, magnification × 40; panels v, vi, × 50. (C) RT-PCR analysis of chemokine mRNA expression in RAG2−/− thymi treated with vehicle or vehicle containing KGF. Representative of 2 independent experiments with groups of 4 mice.
To test the hypothesis that low intrathymic levels of KGF contribute to the medullary phenotype in RAG2−/− thymi, we administered recombinant KGF to RAG2−/− mice and assessed the impact on thymic architecture and thymic expression of chemokine message. As shown in Figure 5B, E-cadherin expressing epithelial cells formed a dense stromal framework in the RAG2−/− thymus, reflecting the paucity of thymocytes (panel i), and the representation of 3G10+ medullary TE was scanty (panel ii). Following administration of KGF, although the epithelial component remained densely packed (panel iii), large cystic structures (indicated by asterisks) were commonly observed, and the medullary epithelial compartment was dramatically expanded. In addition to expansion of focal accumulations of 3G10+ cells, the KGF-treated RAG2−/− thymus also displayed scattered 3G10+cells throughout cortical areas as well (panel iv). The focal expansions of 3G10+ TE displayed other markers associated with medullary TE, including B7-1,45 CD40,46and 10.1.116 (data not shown). Importantly, this effect of KGF was not associated with any significant progression of thymocyte development past the CD4−8− stage in response to KGF treatment (data not shown). Analysis of intact FTOC cultured in the presence or absence of KGF revealed a similar expansion of medullary TE compartment (compare panels v and vi of Figure 5B).
This KGF-mediated expansion of the medullary epithelial compartment in RAG2−/− mice was accompanied by alterations in the pattern of chemokine mRNA expression (Figure 5C). Treatment with KGF resulted in dramatically up-regulated expression of mRNA for medullary-type chemokines (MDC, ELC, and eotaxin), while having no discernible effect on thymic levels of mRNA for SDF or TECK. Thus, thymi from KGF-treated RAG2−/− mice displayed an altered pattern of chemokine message expression consistent with expansion/maturation of the medullary TE compartment.
Discussion
Evidence that FGFR2IIIb signaling influences thymic development has come largely from disruption of this signaling pathway by targeted deletion of this receptor isoform or one of the primary ligands, FGF10.14 Here we analyze the reciprocal situation, where stimulation of thymic FGFR2IIIb signaling by administration of recombinant KGF affects both the developmental and functional activity of TE. Given unremarkable thymic phenotype of KGF-deficient mice,47 the response of thymic epithelium to exogenous KGF described here indicates that KGF probably plays a redundant role in thymic development/function and that the level of FGFR2IIIb signaling may be more important than the levels of an individual ligand for these receptors. The basis for the nonequivalent actions of KGF and FGF-10 observed in organ cultures is unknown. Since KGF and FGF10 differ in their dependence on low-affinity interactions with proteoglycans to signal via FGFR2IIIb,48 the differential effects of KGF and FGF10 reported here may reflect differences in presentation of these mediators by extracellular matrix components within the thymus.
One consequence of elevated KGF signaling through the FGFR2IIIb receptor in thymic epithelial cells is the reduced expression of class II MHC, invariant chain, and cathepsin L. Given the dramatic reduction of class II-MHC–mediated positive selection observed in mice bearing targeted deletions of these molecules,36,39,49 this effect of KGF on TE presents a potential explanation for the impaired generation of CD4+8− thymocytes in FTOC cultured with exogenous KGF. We speculate that the reduced levels of surface class II expression in response to elevated levels of KGF may reflect limiting amounts of invariant chain, based on the important role of invariant chain in the regulation of class II MHC transport.37Furthermore, reduced levels of cathepsin L might impair the proteolytic processing of invariant chain, thus leading to less efficient peptide loading of class II MHC molecules. While the mechanism whereby FGFR2IIIb signaling impacts the expression of cathepsin L is presently not clear, KGF has been shown to be a potent inhibitor of collagenase-1 expression by keratinocytes.50 It is presently not clear if these alterations of TE phenotype and function reflect a response to supra-physiological levels of KGF or if levels of endogenous KGF produced by thymocytes and other mesodermally derived cells could exert a similar regulatory effect on MHC class II expression in vivo.
The medullary TE compartment is also a target of FGFR2IIIb signaling activity. The KGF-mediated expansion of the medullary compartment in intact FTOC but not DOG-treated lobes may indicate a requirement for additional thymocyte-derived signals for expansion of the medullary TE compartment, or may reflect the kinetics of medullary expansion, since the intact lobes were cultured for longer periods of time. In the RAG2−/− thymus, the expanded medullary TE compartment also expresses 10.1.1, B7-1, and CD40, molecules that are expressed by normal medullary TE,16,45 46 indicating that the medullary expansion does not reflect the aberrant expansion of an epithelial type not normally found in the thymus. Interestingly, KGF administration also dramatically increased the representation of TE with a medullary phenotype in the cortical compartment. This observation suggests that levels of KGF may affect the fate choices made by progenitor TE cells with cortical and medullary potential or may alter the differentiation program of cortical epithelium. Based on the affinity of FGFs for charged extracellular matrix components and the preferential production of KGF by mature thymocytes, we suggest that there is normally a gradient of KGF within the thymus that is associated with extracellular matrix components, such that KGF levels would be maximal in medullary and cortico-medullary compartments and lower in the cortical TE compartment. The functional impact of such a gradient might be magnified by the different levels of FGFR2IIIb expression within cortical and medullary compartments. Systemic administration of exogenous KGF would eliminate any concentration gradient and promote elevated levels of FGFR2-mediated signaling in the cortical TE compartment.
Chemokine expression, as a functional parameter of TE, was also found to be a target of KGF activity. In the hypoplastic medullary TE compartment in the RAG2−/− thymus, undetectable levels of KGF message expression by RAG2−/− thymocytes is correlated with a dramatic reduction in the expression levels of medullary-type chemokines. It is presently not clear if this elevation of medullary-type chemokines in the RAG2−/− thymus is secondary to KGF-mediated expansion of the medullary TE or reflects direct activation of chemokine gene expression. These chemokines, produced by medullary TE and chemotactic for more mature thymocyte subsets, are thought to play an important role in directing thymocyte navigation within the thymic environment.2 41-44 Thus, these data suggest a possible role of FGFR2IIIb signaling in establishing chemokine gradients that could influence the directed migration of thymocytes within the thymic environment.
The finding here of expression of KGF by αβ-lineage thymocytes and in in vitro propagated helper T-cell lines (A.G.F. and Bix, unpublished observations, 2001) was unexpected in light of an earlier report that KGF production was restricted to γδ-lineage T cells51 but is in agreement with the work of others.52 We feel this apparent discrepancy may reflect somewhat lower levels of KGF expression by αβ-lineage thymocytes relative to γδ-lineage T cells. Detection of KGF message in αβ thymocytes cDNA required several more PCR cycles than were used previously51 to detect a positive signal in unfractionated thymocytes using the same KGF primers. Elevated KGF expression by γδ-lineage T cells compared to αβ-lineage cells could provide an explanation for the medullary TE expansion reported in mice expressing a T-cell receptor gamma chain transgene53and the preferential association of γδ thymocytes with emerging medullary TE foci in the fetal thymus.52
While thymocytes contribute to intrathymic levels of KGF, they are not the sole source of KGF within the thymus. Although not demonstrated here, fibroblasts also are a source of KGF in the thymus as they are in other tissues (Revest et al14 and vida infra). The well-established requirement for fibroblasts in thymic organogenesis and thymic function in vitro5 54 likely reflects their elaboration of mediators of epithelial/mesenchymal interaction, including KGF. Thymocyte production of KGF may provide a feedback mechanism to more precisely regulate levels of this factor within the intrathymic milieu.
Considering thymocytes as a mesenchymal derivative, their ability to affect TE growth and/or differentiation through the elaboration of KGF has ample precedent in the literature. In a number of tissues, including accessory male reproductive organs,55lung,56 and epidermal epithelium,31,57 KGF elaborated by mesenchymal cells in connective tissue has been implicated in the regulation of epithelial cell growth and/or differentiation (reviewed in Mason et al11 and Rubin et al58). There are probably redundant mechanisms to control these processes in vivo, and the relatively mild thymic and systemic phenotype exhibited by KGF−/− mice may reflect the contributions of other members of the FGF family, principally FGF1, 3, and 10, that also can transduce signals through the FGFR2IIIb receptor.48,59 By RT-PCR, we have detected messages for these FGF family members in the adult thymus (A.G.F., unpublished observations, 1999) and are presently determining the cell type(s) responsible for these signals. The recent report that targeted disruption of the FGFR2IIIb receptor results in impaired thymic development that is more severe than that observed in FGF-10 deficient mice14 would also be consistent with the notion of functional redundancy of FGFs in thymic development.
The findings here, that exogenous KGF can affect several parameters of the thymic environment, are relevant to studies investigating the use of KGF to enhance alloengraftment efficiency in bone marrow transplantation models. In addition to blunting epithelial damage from conditioning regimens,60,61 exogenous KGF also appears to enhance allogeneic engraftment through other mechanisms.62The data presented here suggest that KGF may do so by altering the functional activity of the thymic epithelium. From a clinical standpoint, these findings also raise a cautionary note by demonstrating that exogenous KGF may affect T-cell receptor repertoire selection.
We thank Dr Bevan for comments on the manuscript, Amgen for generously providing recombinant human KGF, and Ms Dawna Dennis for expert technical assistance.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-04-1036.
Supported by National Institutes of Health (grant numbers AI24137, AG04360, and AI24206) and the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew G. Farr, University of Washington, School of Medicine, Department of Biological Structure, Box 357420, Seattle, WA 98195-7420; e-mail: farr@u.washington.edu.