In acute-type leukemia, no method for the prediction of relapse following allogeneic stem cell transplantation based on minimal residual disease (MRD) levels is established yet. In the present study, MRD in 72 cases of allogeneic transplantation for acute myeloid leukemia, acute lymphoid leukemia, and chronic myeloid leukemia (accelerated phase or blast crisis) was monitored frequently by quantitating the transcript of WT1 gene, a “panleukemic MRD marker,” using reverse transcriptase–polymerase chain reaction. Based on the negativity of expression of chimeric genes, the background level of WT1 transcripts in bone marrow following allogeneic transplantation was significantly decreased compared with the level in healthy volunteers. The probability of relapse occurring within 40 days significantly increased step-by-step according to the increase in WT1 expression level (100% for 1.0 × 10−2-5.0 × 10−2, 44.4% for 4.0 × 10−3-1.0 × 10−2, 10.2% for 4.0 × 10−4-4.0 × 10−3, and 0.8% for < 4.0 × 10−4) when WT1 level in K562 was defined as 1.0). WT1 levels in patients having relapse increased exponentially with a constant doubling time. The doubling time of theWT1 level in patients for whom the discontinuation of immunosuppressive agents or donor leukocyte infusion was effective was significantly longer than that for patients in whom it was not (P < .05). No patients with a short doubling time of WT1 transcripts (< 13 days) responded to these immunomodulation therapies. These findings strongly suggest that the WT1 assay is very useful for the prediction and management of relapse following allogeneic stem cell transplantation regardless of the presence of chimeric gene markers.
Introduction
Relapse still remains an obstacle to successful allogeneic stem cell transplantation (SCT) for patients with acute leukemia. Early recognition of relapse at the molecular level provides a window for therapeutic intervention while the burden of disease is still relatively low.1,2 For chronic myeloid leukemia (CML), several reports have shown that serial quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of the chimeric bcr-abl gene after allogeneic SCT can effectively distinguish those patients who will remain in remission from those patients who are destined to have a relapse.3,4 However, for acute types of leukemia, there have been few studies on the association of minimal residual disease (MRD) levels with the occurrence of leukemic relapse after allogeneic SCT. In addition to the heterogeneity of the diseases other than CML, only 40% of patients with acute leukemia have chimeric tumor markers that make it possible to design PCR primers for quantitating MRD.5 Furthermore, the period in which patients can be diagnosed with molecular relapse is short, because the growth speed of blasts in acute leukemia is usually much faster than that of leukemia cells in CML in the chronic phase (CP). To overcome this difficulty, we performed frequent monitoring of MRD based on the expression levels of WT1, which has been reported as a “panleukemic marker” for evaluating MRD in leukemia.
WT1 was isolated as a gene responsible for Wilms tumor, a childhood kidney neoplasm, and categorized as a tumor suppressor gene.6,7 We8,9 and others10-13have recently shown that the WT1 gene is highly expressed in various types of leukemia (acute myeloid leukemia [AML], acute lymphoid leukemia [ALL], and CML) and that the expression of theWT1 gene is thus a tumor marker for leukemic blast cells of almost all leukemias. The WT1 expression level, measured by quantitative RT-PCR, significantly increases at relapse compared with that at the time of diagnosis.14 For CML, theWT1 expression level increases by one log as the clinical stage progresses from CP to the accelerated phase (AP) and also increases by one log from AP to blast crisis (BC).8Furthermore, the WT1 expression level in bone marrow (BM) or peripheral blood (PB) accurately reflects the extent of MRD of 3 types of leukemia (AML, ALL, and CML).15
In our previous paper, using a quantitative RT-PCR method, we showed that either a rapid or a gradual increase in the WT1expression level occurred in BM and PB before the occurrence of hematologic relapse (HR) in all of 3 patients treated by allogeneic transplantation.15 However, the precise relationship between the WT1 expression level and the risk of relapse after allogeneic transplantation still remains to be determined. In addition, one of major problems with the measurement of MRD based onWT1 transcripts is the background expression ofWT1 in BM that stems from normal hematopoietic stem cells. In the present study, we first determined the background level following allogeneic transplantation by quantitating WT1transcript levels in samples from patients who were diagnosed as negative for MRD based on the expression of chimeric genes. After having clearly determined the MRD+ range of WT1transcripts in BM samples, we analyzed the association of theWT1 transcript levels obtained by frequent WT1testing with the probability of relapse occurring within a short period of time (within 40 days), in a retrospective study based onWT1 data in 72 transplantations performed for acute types of leukemia (AML, ALL, CML in AP or BC). Furthermore, we studied the kinetics of the increase in WT1 transcripts in 15 relapsed cases, and the relationship between the rate of increase ofWT1 expression and the efficacy of immunologic therapeutic interventions including the discontinuation of immunosuppressive agents or donor leukocyte infusion (DLI).
Patients, materials, and methods
Patients
Between July 1992 and November 2001, 75 patients with AML (n = 48), ALL (n = 18), or CML (n = 9) in AP or BC underwent allogeneic SCT at Osaka University Hospital. Because 13 of these patients underwent a second transplantation, all together 88 transplantations were performed for patients with these cancers. When the expression level of WT1 in K562, a WT1high-expressing cell line, was defined as 1.0, the WT1 gene expression levels in 4 patients had never increased to more than 1 × 10−2 from diagnosis until transplantation. Because leukemic blasts in these patients were considered not to express high enough levels of WT1 transcripts to assess MRD based on theWT1 assay, these 4 transplantations were removed from the data for WT1 analysis. Furthermore, the data of 12 transplantations were also removed from the WT1 analysis for the following reasons: 3 patients had BM rejection, 8 patients died soon after transplantation (range, days −2 to 46), and 1 patient continued to have leukemic blasts in the PB. Therefore, the time course of WT1 expression levels in BM was analyzed in 72 transplantations (50 AMLs, 15 ALLs, and 7 CMLs), including 18 transplantations using BM and 9 using peripheral blood stem cells (PBSCs) from HLA-identical sibling donors, and 16 using BM from phenotypically HLA-identical unrelated donors. Seventeen and 11 transplantations used BM and PBSCs, respectively, from HLA-mismatched related donors. One transplantation used unrelated cord blood stem cells from HLA2-antigen mismatched donors. Twenty-eight transplantations were performed by the standard preconditioning regimen including CyTBI (cyclophosphamide 120 mg/kg and total body irradiation 12 Gy) and BuCy (busulfan 16 mg/kg and cyclophosphamide 120 mg/kg). Thirty transplantations were performed by intensified preconditioning regimens including CACyTBI (cytosine arabinoside 2 g/m2× 4 + CyTBI), BuCyTBI (busulfan 8 mg/kg + CyTBI), Bul-PAMTBI (busulfan 8 mg/kg, l-PAM [l-phenylalanine mustard] 180 mg/m2, and TBI 12 Gy), and BuCyCA (busulfan 8 mg/kg, cyclophosphamide 120 mg/kg, and cytosine arabinoside 2 g/m2 × 4). Fourteen transplantations were performed by reduced preconditioning regimens including FluBuATG (fludarabine 30 mg/m2 × 6, busulfan 8 mg/kg, and anti–T-lymphocyte globulin [thymoglobulin; IMTIX, Lyon, France] 2.0-5.0 mg/kg). Among the patients with AML and ALL, 24 patients underwent transplantation in the first complete remission (CR), 4 in the second CR, 1 in a later CR, and 36 in non-CR. Among the patients with CML, 3 patients received transplants in AP and 4 in BC. As graft-versus-host disease (GVHD) prophylaxis, patients receiving transplants from HLA-identical related donors received a combination of cyclosporine (CSP) and a short course of methotrexate, and patients receiving transplants from unrelated donors or HLA-mismatched related donors received tacrolimus (FK506), methotrexate, and/or methylprednisolone. Patients who were treated by reduced preconditioning regimens received CSP only or FK506 only.
Discontinuation of immunosuppression was performed for patients who had a relapse while receiving immunosuppressive agents. Infusion of donor leukocytes containing at least 1.0 × 108 CD3 cells/kg was performed for patients who had a relapse while off therapy for GVHD prophylaxis. These immunomodulation therapies were diagnosed as effective when patients achieved CR or partial remission without the occurrence of fatal GVHD. Institutional review board approval by the Osaka University Medical School was obtained for the treatment protocol, and informed consents were obtained from patients and their families.
Sample preparation and quantitation of transcripts ofWT1, major bcr-abl, minor bcr-abl, and AML1-MTG8
WT1 transcript levels in BM of patients after transplantation were monitored as follows: until 6 months after transplantation, once every 1 to 3 weeks for patients who were in non-CR, and at least once every 4 weeks for patients who were in CR at the time of transplantation; and thereafter once every 1 to 6 months until 2 years after transplantation, depending on patients' MRD levels.
Mononuclear cells from samples were obtained by Ficoll-Hypaque density gradient centrifugation and were stored at −80°C in guanidinium thiocyanate. Extraction of total cellular RNA and cDNA synthesis was performed as previously described.15
Until January 1998, quantitative RT-PCR for WT1 transcripts was performed as previously described.16 Because the measurement of WT1 transcripts in the exponential amplification phase was required in this procedure, PCR was performed for various numbers of cycles according to the WT1expression levels in samples. Namely, to quantitate WT1transcript levels that were 10−5 to 10−3, 2 × 10−4 to 10−2, 2 × 10−3to 10−1, and 2 × 10−2 to 100relative to the WT1 transcript level in K562 cells taken as 1.0, PCR was terminated at 36, 33, 30, and 27 cycles, respectively. Serial 1:10 dilutions of standard K562 cDNAs were always amplified simultaneously with leukemic samples. All experiments were performed in duplicate. WT1 levels of samples were adjusted according to the level of β-actin transcripts.
Since February 1998, we have used a real-time PCR method forWT1. For real-time PCR, we designed all primers and probe combinations using Primer-Express software (PE Applied Biosystems, Foster City, CA). WT1 and β-actin probes were labeled with 6-carboxy fluorescein (FAM) at the 5′ end and with 6-carboxy tetramethyl rhodamine (TAMRA) at the 3′ end. The following sequences were used as probes: WT1 probe 5′ FAM-ACACCGTGCGTGTGTATTCTGTATTGG-TAMRA 3′ and β-actin probe 5′ FAM-TGAGCGCAAGTACTCCGTGTGGATCGGCG-TAMRA 3′. The WT1 reverse primer and β-actin forward primer were located on exon-exon junctions to avoid genomic amplification, and the following sequences were used as primers: WT1 forward and reverse primers 5′-GATAACCACACAACGCCCATC-3′ (exon 6) and 5′-CACACGTCGCACATCCTGAAT-3′ (exon 6/7); β-actin forward and reverse primers 5′-CCCAGCACAATGAAGATCAAGATCAT-3′ and 5′-ATCTGCTGGAAGGTGGACAGCGA-3′, respectively. The PCR mixture contained 1 × PCR buffer, 3 mM MgCl2, 250 μM deoxyribonucleoside triphosphates (dNTPs), 1.25 U AmpliTaq gold, 500 nM forward and reverse primers, and 200 nM TaqMan probe in a total volume of 50 μL. After 10 minutes at 95°C to activate AmpliTaq gold, the amplification was carried out by 40 cycles at 95°C for 15 minutes and at 63°C for 60 seconds in the ABI Prism 7700 Sequence Detector System (PE Applied Biosystems). We used 2.5 μL and 1.0 μL of the total 30 μL of the cDNA mixture (corresponding to 100 ng RNA) thus synthesized for the amplification of WT1 and β-actin, respectively. Specific PCR products were quantitated with the fluorescence detector.
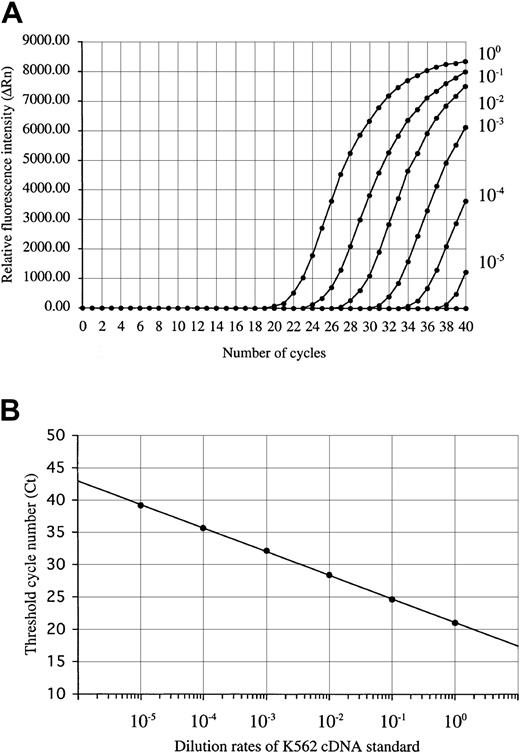
For the construction of standard curves of positive control, RNA of K562 cells was reverse-transcribed into cDNA and serially diluted in 5 log steps. This standard cDNA serial dilution was prepared in large amounts and stored at −20°C. On the WT1 amplification study using the standard dilution of K562 cDNA, the fluorescence intensity (ΔRn) increased with the number of amplification cycles in proportion to the accumulation of PCR products (Figure1A). The standard curve that was constructed from the results of the dilution experiments showed a strong linear correlation between the threshold cycle (Ct: defined as the number of cycles producing at least 10 times the SD of the baseline fluorescence signal) and the logarithm of the initial template concentration of the standard K562 cDNA (Figure 1B). The dynamic range spanned to at least 5 orders of magnitude. A standard curve for the quantitation of β-actin was also produced by performing real-time PCR using serially diluted K562 cDNA.
WT1 amplification plot and standard curve.
(A) Amplification plot of a 10-fold serial dilution of the standard K562 cDNA (ranging from 10−5 to 100) and (B) standard curve of K562 cDNA dilution for real-time RT-PCR. The amplification plot shows the increase of reporter fluorescence (ΔRn) during amplification. The Ct value decrease was proportional to the increase of the target molecules. The standard curve shows the linear correlation between the Ct value and the logarithm of the initial concentration of the standard K562 cDNA and can then be used to calculate the relative WT1 gene expression levels in unknown patient samples.
WT1 amplification plot and standard curve.
(A) Amplification plot of a 10-fold serial dilution of the standard K562 cDNA (ranging from 10−5 to 100) and (B) standard curve of K562 cDNA dilution for real-time RT-PCR. The amplification plot shows the increase of reporter fluorescence (ΔRn) during amplification. The Ct value decrease was proportional to the increase of the target molecules. The standard curve shows the linear correlation between the Ct value and the logarithm of the initial concentration of the standard K562 cDNA and can then be used to calculate the relative WT1 gene expression levels in unknown patient samples.
For quantification of WT1 transcripts of patient samples, samples were always amplified simultaneously with the standard dilution of K562 cDNA. The WT1 transcript level in samples was determined by reference to the corresponding transcript level of K562 cells on the standard curve. Samples were analyzed in duplicate, and the average value was calculated and adjusted according to the level of β-actin transcripts present. The adjustment was performed by dividing the measured WT1 gene expression level of a given patient's sample by the ratio of the β-actin expression level of the sample to that of K562 cells. Interassay variation was less than 5% for standards and less than 10% for unknown samples.
Quantification of transcripts of chimeric genes was performed by real-time PCR. Real-time PCR for AML1-MTG8 was performed using the primers and protocol described by Marcucci et al.17 Kasumi-1, a myeloid leukemia cell line containing t(8;21) was used as a positive control, and the level ofAML1-MTG8 gene transcripts in Kasumi-1 cells was defined as 1.0. Real-time PCR for major bcr-abl was performed using the primers and protocol described by Mensink et al.18 K562 cells were used as a positive control, and the level of majorbcr-abl transcripts in K562 cells was defined as 1.0. Real-time PCR for minor bcr-abl was performed using the same protocol and primers as for major bcr-abl except for the use of the sequence 5′-ACCATCGTGGGCGTCCGCAAGA-3′ as the forward primer. L2,19 a Ph1+ acute lymphoblastic leukemia–derived cell line, was used as a positive control, and the level of transcripts in L2 was defined as 1.0. Real-time PCR was performed using basically the same methods as for WT1. The sensitivity of real-time PCR for these chimeric genes was at least 10−5. A standard curve constructed using serial 10-fold dilutions of cDNA from these control cell lines resulted in correlation coefficients of more than 0.98 in a range of over 5 orders of magnitude (data not shown). Samples were analyzed in duplicate, and the expression level of the chimeric genes was adjusted according to the level of β-actin transcripts present in the same manner asWT1.
Analysis of the relationship between the WT1 level and the risk of relapse after transplantation
Twenty of 72 transplantations were ultimately followed by an HR. Ten, 8, and 2 patients had relapse within 150 days, between 150 and 400 days, and 400 days or more after transplantation, respectively. Of the patients who received the remaining 52 transplants, 23 had transplantation-related mortality in CR at a median of 286 days after transplantation (range, 52-1244 days), and 29 are alive in CR at a median follow-up of 592 days (range, 106-2755 days).
The relationship between the WT1 transcript level in BM after transplantation and the occurrence of relapse until day 400 was analyzed. For patients who had a relapse or developed transplantation-related mortality, WT1 transcript levels were analyzed until these events occurred. A way of standardizing the data was needed because the interval of monitoring ofWT1 was different in each patient. The duration was divided into periods of 20 days each: from day 11 to day 30, from day 31 to 50, from day 51 to 70, and so on. When the WT1 test was performed more than once in a given 20-day period, the mean value of the data was used as the representative value of the period. The mean value was calculated after conversion of the WT1 value into a logarithm. Consequently, each transplantation had 5.1 standardizedWT1 points on average (range, 1-20). Furthermore, there were 3.35 and 5.50 standardized WT1 points per transplantation on average in the periods until 100 days and until 180 days, respectively. For the analysis of time course of WT1 gene expression in relapsed patients, raw data of WT1 were used.
Statistical methods
Statistical analyses were performed with SPSS software (Version 7.5; SPSS, Chicago, IL). For the analysis of the background level of transcripts in BM in patients who underwent allogeneic transplantation, when the expression of chimeric genes was less than 10−5or undetectable (the sensitivity was at least 10−5), MRD was defined as negative. The comparison of WT1 transcript levels of healthy volunteer donors and MRD− BM transplantation (BMT) patients was analyzed by the Mann-WhitneyU test. The relationship between WT1 transcript levels and the probability of relapse occurring within 40 days was analyzed by the Fisher exact test. The relationship between the doubling time of WT1 transcripts in relapsed patients and the efficacy of the immunomodulation therapy or the disease status at transplantation was analyzed by the Mann-Whitney Utest.
The data were “locked ” for analysis on January 29, 2002.
Results
The background level of WT1 gene expression in patients undergoing BMT
We changed the method by which we quantitated the WT1gene expression from the method we used previously16 to a real-time quantitative PCR method in January 1998, because the real-time PCR method was shown to be simple, rapid, and reliable.17,18,20 21 We compared the 2 quantitative RT-PCR methods by analyzing 50 RNA samples that were obtained before January 1998 and stored at −80°C. WT1 transcript levels obtained by the previous method and by the real-time PCR method were found to show a good correlation (r = .998, P < .001). Therefore, we considered that data obtained by the 2 methods could be handled equivalently in the subsequent WT1analysis.
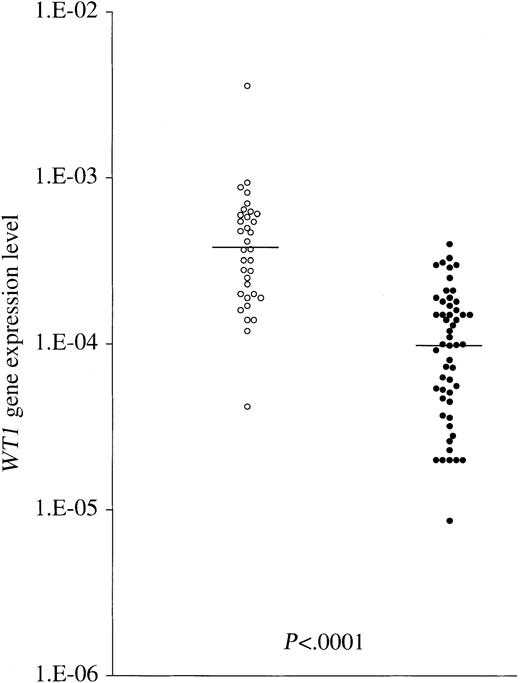
In acute leukemia, the usefulness of high WT1 mRNA expression during follow-up for detection of MRD is still the subject of discussion because normal CD34+ progenitors have also been found to express detectable levels of WT1mRNA.22 To clarify the usefulness of WT1expression as an MRD marker in patients undergoing BMT, the background levels of WT1 transcripts in BM were examined in detail. First, we examined the WT1 gene expression levels in 34 BM samples from healthy donors for related transplantation. As shown in Figure 2, the WT1 gene expression levels in 32 of 34 samples were in the range of 1.0 × 10−4 to 1.0 × 10−3 with a median level of 3.8 × 10−4, which is comparable to our previously reported data.8WT1 expression in healthy donors was shown to be due to normal hematopoietic stem cells, as described. However, alterations in the progenitor cell population during hematopoietic recovery from BMT have been reported.23 Therefore, the background levels ofWT1 expression in BM after BMT may be different from those of healthy donors. To address the issue, in 11 patients with AML, ALL, or CML (BC and AP) who had chimeric DNA markers, including majorbcr-abl, minor bcr-abl, and AML1-MTG8, we quantitated the expression levels of the WT1 gene and chimeric genes simultaneously using the quantitative RT-PCR method in BM samples obtained following transplantation. When the expression of chimeric genes was less than 10−5 or undetectable, MRD was defined as negative. WT1 gene expression levels in MRD− samples that were obtained up to day 400 were found to be significantly lower (median, 1.0 × 10−4; range, 8.6 × 10−6-4.0 × 10−4) than those of healthy volunteer donors (P < .0001; Mann-WhitneyU test; Figure 2). Regarding the WT1 gene expression level of MRD− BM samples, there was no significant difference between samples obtained up to day 150 (n = 38; median, 9.95 × 10−5; range, 2.0 × 10−5-3.0 × 10−4) and those obtained between day 150 and day 400 (n = 15; median, 1.5 × 10−4; range, 8.6 × 10−6-4.0 × 10−4;P = .07), indicating that no recovery of WT1gene expression levels in BM samples occurred up to day 400. These results indicate that the background levels of WT1expression in the BM decrease after BMT, resulting in an increase in the sensitivity of the detection of MRD.
Comparison between WT1 transcript levels of healthy volunteer donors and MRD− BMT patients.
The ○ and ● indicate WT1 gene expression levels of BM samples of 34 healthy volunteer donors and of 53 BM samples from 11 BMT patients, respectively, in the period up to day 400. In patients with AML, ALL, or CML (BC and AP) who had chimeric DNA markers (majorbcr-abl, minor bcr-abl, andAML1-MTG8), when the expression of these chimeric genes was less than 10−5 or undetectable, MRD was defined as negative. WT1 gene expression levels in MRD−BMT patients were found to be significantly lower than those of healthy volunteer donors. Horizontal bars indicate the median values in the 2 groups.
Comparison between WT1 transcript levels of healthy volunteer donors and MRD− BMT patients.
The ○ and ● indicate WT1 gene expression levels of BM samples of 34 healthy volunteer donors and of 53 BM samples from 11 BMT patients, respectively, in the period up to day 400. In patients with AML, ALL, or CML (BC and AP) who had chimeric DNA markers (majorbcr-abl, minor bcr-abl, andAML1-MTG8), when the expression of these chimeric genes was less than 10−5 or undetectable, MRD was defined as negative. WT1 gene expression levels in MRD−BMT patients were found to be significantly lower than those of healthy volunteer donors. Horizontal bars indicate the median values in the 2 groups.
Relationship of expression levels of WT1 gene and chimeric genes as determined by quantitative RT-PCR analysis
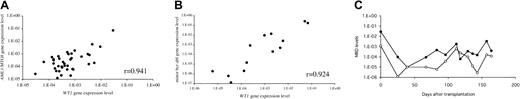
To further investigate the reliability of WT1expression as an MRD marker, we analyzed the correlation in the expression levels between WT1 and chimeric genes in MRD+ samples from patients who had AML1-MTG8 or minor bcr-abl. MRD in BM samples at different time points after transplantation was analyzed by using the real-time PCR methods for WT1 and these chimeric genes (see “Patients, materials, and methods”). The results showed a strong positive correlation between the levels of gene expression of WT1 andAML1/MTG8 (r = 0.941; Figure3A), and between those of WT1and minor bcr/abl (r = 0.924; Figure 3B). The time courses of the gene expression levels of WT1 andAML1-MTG8 in a patient with AML are shown in Figure 3C. The 2 MRD markers generally changed in parallel.
Correlation between WT1 and chimeric gene expression levels in samples of BMT patients.
(A) Relationship between WT1 and AML1-MTG8. MRD levels in MRD+ samples obtained at different time points from 2 patients with AML1-MTG8 chimeric gene were analyzed by quantification of the gene expression levels of WT1 andAML1-MTG8. (B) Relationship between WT1 and minorbcr/abl. MRD levels in MRD+ samples from 2 patients with minor bcr-abl chimeric gene were analyzed as well. (C) The time courses of gene expression levels of WT1and AML1-MTG8 in a patient with AML. The expression level ofAML1/MTG8 in Kasumi-1 cells was defined as 1.0. The 2 MRD markers generally changed in parallel. ● indicates WT1gene expression levels; and ○, AML1-MTG8 gene expression levels.
Correlation between WT1 and chimeric gene expression levels in samples of BMT patients.
(A) Relationship between WT1 and AML1-MTG8. MRD levels in MRD+ samples obtained at different time points from 2 patients with AML1-MTG8 chimeric gene were analyzed by quantification of the gene expression levels of WT1 andAML1-MTG8. (B) Relationship between WT1 and minorbcr/abl. MRD levels in MRD+ samples from 2 patients with minor bcr-abl chimeric gene were analyzed as well. (C) The time courses of gene expression levels of WT1and AML1-MTG8 in a patient with AML. The expression level ofAML1/MTG8 in Kasumi-1 cells was defined as 1.0. The 2 MRD markers generally changed in parallel. ● indicates WT1gene expression levels; and ○, AML1-MTG8 gene expression levels.
Relationship between WT1 level after transplantation and risk of relapse
All of 20 patients who had HR showed WT1 gene expression levels of more than 1.0 × 10−2 at the time of relapse. We analyzed whether HR could be predicted based onWT1 assay. Considering that blasts in acute type of leukemia expand much more rapidly at relapse after transplantation than leukemia cells in CML-CP, we focused on the occurrence of HR within a short period of time after sampling for WT1 testing. Because the interval of monitoring of WT1 was different for each patient, standardization of the WT1 data was performed (see “Patients, materials, and methods”). We then analyzed the association of the standardized WT1 levels with the occurrence of HR within 40 days, based on 367 WT1 values obtained from BMT patients with AML, ALL, and CML-AP or CML-BC (Table1). HR was observed only in patients with a WT1 expression level over 1.0 × 10−2. All patients with WT1 expression levels of 5.0 × 10−2 and over (level A) had full relapse. Of 13 instances of WT1 expression levels between 1.0 × 10−2 and 5.0 × 10−2 (level B), 6 were in patients in HR. However, most BM samples of patients in HR contained less than 20% blasts, which indicates an early phase of HR. The remaining 7 instances were in patients in CR, but all of them were followed by HR within 20 days. Therefore, level B was considered to be the WT1 expression level at which HR had begun to occur. All patients with WT1 expression levels less than 1.0 × 10−2 had morphologic CR. However, the percentage of patients who had HR within 40 days abruptly increased atWT1 expression levels over 4.0 × 10−3. In fact, of 9 patients with WT1 expression levels between 4.0 × 10−3 and 1.0 × 10−2 (level C), 2 and 2 had HR within 20 and 40 days, respectively. The remaining 5 did not have HR within 40 days, but 3 of them ultimately had HR. Therefore, level C corresponds to molecular relapse. Of 78 instances ofWT1 levels between 4.0 × 10−4 and 4.0 × 10−3 (level D), values still considered MRD+ (the background level of WT1 after transplantation was < 4.0 × 10−4; Figure 2), only 8 instances (10.3%) were followed by HR within 40 days. Of 255 instances of WT1 levels less than 4.0 × 10−4 (level E), values considered MRD−, only 2 instances (0.8%) were followed by relapse within 40 days, suggesting that level E had almost no risk of relapse occurring within a short time period. Regarding the incidence of HR within 20 days in cases of CR, there was a significant difference between level B and level C (P < .01; Fisher exact test). Furthermore, regarding the incidence of HR within 40 days in cases of CR, there were significant differences between level B and level C (P = .03), between level C and level D (P = .01), and between level D and level E (P < .001). The ultimate relapse rates of patients in whom WT1 levels showed level C and level D even once after transplantation were 77.8% and 29.7%, respectively (Table1). The ultimate relapse rate of patients in whom WT1 levels showed level E twice in succession after transplantation was 17.1%. Furthermore, in patients having a relapse, the median lengths of time from the first increase of WT1 expression to level B, level C, and level D until a diagnosis of HR were 11, 43, and 92 days, respectively (Table 1).
Analysis of time course of WT1 gene expression in patients having a relapse
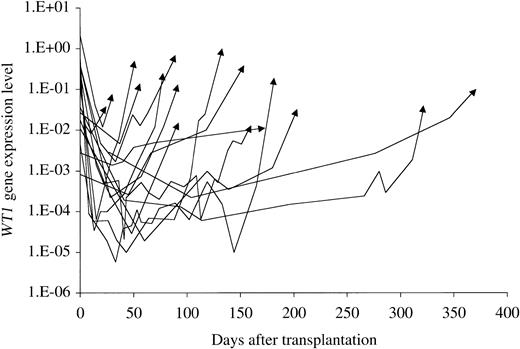
Twenty transplantations ultimately resulted in HR at a median of 139 days after transplantation (range, 14-1386 days). The time course of WT1 gene expression in 16 of 18 patients who had HR within 400 days after transplantation is shown in Figure4. After rapidly decreasing after transplantation in most patients and reaching trough levels between day 13 and day 104, WT1 levels began to increase exponentially with a constant doubling time that was different in each patient, and the patients went on to HR. In some patients, after reaching trough levels, WT1 levels fluctuated slightly and began to increase exponentially at some points after transplantation, and the patients also went on to HR. The doubling times of WT1 in 15 transplantations in which WT1 levels were monitored at more than 3 points in the increasing phase are listed in Table2. No association was found between doubling time and type of donor (sibling versus unrelated or HLA match versus HLA mismatch), or presence of GVHD. Similarly, no relationship was found between doubling time and time of relapse after transplantation. Patients who underwent transplantation in CR had a significantly longer doubling time of WT1 compared with those who were in non-CR at transplantation (P = .04; Mann-Whitney U test). Immunomodulation therapy, consisting of the discontinuation of immunosuppressive agents or DLI, was performed for relapsed patients. The doubling time for patients in whom the immunomodulation therapy was effective was significantly longer than that for patients in whom it was not (P = .014; Mann-Whitney U test). No patients with a WT1doubling time of less than 13 days responded to the immunomodulation therapy. In contrast, 5 of 7 patients with a WT1 doubling time of 13 days and over responded to the therapy.
Time course of WT1 gene expression level in patients with relapse.
The time courses of WT1 gene expression in 16 of 18 patients who had HR within 400 days after transplantation are shown. Arrows indicate WT1 levels at the time of relapse. WT1levels began to increase exponentially with a constant doubling time that was different in each patient.
Time course of WT1 gene expression level in patients with relapse.
The time courses of WT1 gene expression in 16 of 18 patients who had HR within 400 days after transplantation are shown. Arrows indicate WT1 levels at the time of relapse. WT1levels began to increase exponentially with a constant doubling time that was different in each patient.
Discussion
Because the WT1 gene is expressed at low levels even in normal hematopoietic stem cells, most studies using qualitative or semiquantitative analyses of WT1 transcripts have produced negative results with regard to the prediction of relapse.24,25 To accurately determine the background level of WT1 gene expression in BM following allogeneic SCT, we examined WT1 transcript levels in BM samples that could be considered as MRD− based on the gene expression of chimeric tumor markers, including major bcr-abl, minorbcr-abl, and AML1-MTG8. The background level ofWT1 gene expression in BM was thereby found to decrease to less than 4.0 × 10−4 after allogeneic transplantation and to be considerably lower than that of healthy volunteer donors. Together with the observation that WT1 expression levels increased significantly at relapse compared with those at the time of diagnosis,14 the decrease in background levels ofWT1 expression following allogeneic transplantation indicates the usefulness of the WT1 assay for the early diagnosis of relapse. To further ensure the reliability ofWT1 expression as a marker of MRD, we quantitatedWT1 transcripts in samples that expressed chimeric gene markers. A strong correlation in the levels of gene expression betweenWT1 and AML1-MTG8 or between WT1 and minor bcr-abl was thereby observed, again indicating the reliability and validity of the present technique. Considering that BM samples that contained 100% blasts show WT1expression levels of 5.0 × 10−1 to 1.0 × 100, the fact that the background level ofWT1 expression is less than 4.0 × 10−4indicates that the WT1 assay has a sensitivity of at least 10−3 to 10−4.
By sequential measurement of WT1 transcripts using the quantitative RT-PCR methods, we attempted to elucidate the kinetics of MRD following allogeneic SCT in patients with acute-type leukemia. Lin et al reported that 21 (72%) of 29 patients with CML who had increasing or persistently high bcr-abl expression (> 50 transcripts/μg) following allogeneic transplantation ultimately had relapse.3 However, for acute leukemia, the length of time from the initiation of the increase in MRD to a diagnosis of HR must be short, because the growth speed of leukemic blasts at relapse may be much more rapid in acute leukemia than in CML-CP. Thus, an early diagnosis of relapse based on the MRD level is much more difficult in acute leukemia. In accordance with the kinetics of leukemic blasts, we analyzed the probability of relapse occurring within a short period of time (within 40 days) by frequent testing of WT1 expression. The results clearly showed that the probability of relapse was significantly increased according to the increase in the WT1expression level (Table 1). In fact, the analysis of CR samples showed that there were significant differences in the relapse rate within 40 days among 4 MRD levels: level B (1.0 × 10−2 ≤WT1 < 5.0 × 10−2, 100%); level C (4.0 × 10−3 ≤ < 1.0 × 10−2, 44.4%); level D (4.0 × 10−4 ≤ WT1< 4.0 × 10−3, 10.2%); and level E (WT1 < 4.0 × 10−4, 0.8%). Level A (5.0 × 10−2 ≤ WT1) corresponds to full-blown relapse. Level B corresponds to an early phase of HR or an imminent relapse. Patients at level B may still have a chance of successful immunomodulation therapy such as the discontinuation of immunosuppressive agents or DLI. Level C corresponds to molecular relapse. Patients at level C are the best candidates for performing immunomodulation therapy. Level D is still MRD+. Whereas patients at level D have a low risk of relapse within 40 days,WT1 expression levels should be monitored frequently in cases in which they continue to increase exponentially in the range of level D. Level E corresponds to the background level in BM. Patients at level E have almost no risk of relapse occurring within a short time period.
Next, we elucidated the kinetics of WT1 transcripts in patients having a relapse. After rapidly decreasing following transplantation and reaching the trough levels between day 13 and day 104, WT1 levels began to increase exponentially with a constant doubling time that was different for each patient, and eventually the patients underwent HR. Despite the fact that the number of patients who had relapses was relatively small, we found that patients in whom immunomodulation therapy was effective had a significantly longer doubling time of WT1 transcripts (median, 26 days) than patients in whom the therapy was not effective (median, 5.8 days). Lin et al reported similar results for patients with CML, among whom patients with rapidly doubling numbers ofbcr-abl transcripts were less likely to respond to DLI than those with a long doubling time.3 We consider that patients with longer doubling times have some residual graft-versus-leukemia (GVL) effect that slows the rate of relapse and that these patients respond to reinforcement of GVL effects by the withdrawal of immunosuppressive agents or DLI.
By measuring WT1 expression levels at least once every 2 weeks, we were able to diagnose relapse at the molecular level in patients in whom the WT1 expression levels decreased to as low as less than 1.0 × 10−4 in the nadir following transplantation, although leukemia cells have a doubling time of as few as 4 days. However, when WT1 expression levels do not sufficiently decrease after transplantation, more frequentWT1 expression testing may be needed. In this regard, because the MRD detected in PB was approximately 10 times lower than that in BM, as previously described,15 and because obtaining PB samples can be easily accomplished in a routine outpatient follow-up setting after transplantation, monitoring of WT1levels in PB may be more useful, although we have not analyzed PB samples in detail yet. Because the overall relapse rate of patients in whom WT1 levels were less than 1.0 × 10−3(the levels in healthy volunteers), at the time of transplantation was 11.1% (2 of 18), frequent monitoring of WT1 may not be necessary in these patients.
In conclusion, molecular monitoring of WT1 transcripts by using quantitative RT-PCR accurately and reproducibly informs us in real time of the kinetics of MRD after allogeneic SCT, regardless of the presence of chimeric DNA markers. As far as we know, this is the first demonstration in acute-type leukemia that the relapse probability following allogeneic SCT significantly increased step-by-step according to the MRD level, and that a rapid doubling time of WT1transcripts predicted the ineffectiveness of therapeutic interventions including the discontinuation of immunosuppressive agents or DLI. Although our results need to be confirmed in a large-scale, prospective study, we consider that the WT1 expression assay is an essential test for the prevention and management of relapse in allogeneic transplantation. To keep the relapse rate to a minimum, the optimized adjustment of the dose of immunosuppressive agents in real time depending on individual patients' MRD levels may be made possible by use of the WT1 assay in the near future.
We thank Dr Honma and Dr Hayashi for generous gifts of L2 cells, which we used as a positive control in minor bcr-ablPCR analyses.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-06-1831.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroyasu Ogawa, Department of Molecular Medicine, Osaka University Graduate School of Medicine, 2-2, Yamada-Oka, Suita City, Osaka, 565-0871, Japan; e-mail:ogachan@ceres.ocn.ne.jp.