Natural killer (NK) cell differentiation from pluripotent CD34+ human hematopoietic stem cells or oligopotent lymphoid progenitors has already been reported. In the present study, long-term cultures of the CD56−/CD34−myeloid-like adherent cell fraction (ACF) from umbilical cord blood (UCB), characterized by the expression of CD14+ as well as other myeloid markers, were set up with flt3 ligand (FL) and interleukin-15 (IL-15). The UCB/ACF gradually expressed the CD56 marker, which reached fairly high levels (approximately 90% of the cells were CD56+) by day 15. FL plus IL-15–driven ACF/CD56+ cells progressively expressed a mature NK functional program lysing both NK- and lymphokine-activate killer (LAK)–sensitive tumor targets and producing high levels of interferon-γ (IFN-γ), granulocyte-macrophage colony-stimulating factor, tumor necrosis factor α, and IL-10 upon stimulation with IL-12 and IL-18. Similar results were obtained when highly purified CD14+ cells from UCB were cultured with FL and IL-15. In contrast, UCB/CD34+ cells cultured under the same conditions showed a delayed expression of CD56 and behaved functionally differently in that they exhibited NK but not LAK cytotoxicity and produced significantly fewer cytokines. Kinetic studies on the phenotype of UCB/ACF or UCB/CD14+ cells cultured in the presence of FL and IL-15 showed a rapid decrease in CD14 expression after day 5, which reached levels of zero by day 20. Approximately 60% of the CD56+ derived from the UCB/ACF or the UCB/CD14+ cells coexpressed CD14 by day 5. Taken together, our data support the role of CD14+ myeloid-like cells within UCB as a novel progenitor for lymphoid NK cells.
Introduction
Natural killer (NK) cells are large granular lymphocytes with innate immune function, playing a critical role in the early host defense against viral, bacterial, and other infections, and against cancer. They exert their effector function, without prior sensitization, by killing virally infected cells and tumor cells and by producing immunoregulatory cytokines and chemokines.1Mature NK cells express CD56 alone or in combination with CD16. The majority of adult peripheral blood (PB) NK cells are CD56+16+, with a minor population of CD56+16− cells.2 There have been 2 functionally distinct subsets of mature human NK cells described: CD56dim/CD16bright, representing the majority (approximately 90%) of the mature NK population, whereas the minority (< 10%) consists of CD56bright/CD16dim/−.3,4CD56bright NK cells are the primary population responsible for cytokine production in response to monokines, whereas CD56dim NK cells are more cytotoxic. The majority of NK cells are characterized by the absence of CD3 expression, although a small subpopulation of these cells express surface CD3 and are designated as NKT cells.5 Other mature NK cell markers include CD161 (NKR-P1A),6 CD94/NKG2,7 and killer immunoglobulin–like receptors (KIRs).8
Although there is considerable information about the phenotype, function, and activation of mature human NK cells, comparatively less information exists about their ontogenic development and differentiation/maturation. NK cells are known to develop from common T/NK bipotent progenitors.9 The most primitive human NK cell progenitors have been identified in the CD34+HSC compartment, which gives rise to all hematopoietic lineages. Purified CD34+ cells from human adult bone marrow (BM),10 umbilical cord blood (UCB), mobilized PB,11 and fetal liver,12 cultured in the presence of flt 3 ligand (FL) or stem cell factor (SCF) and interleukin-15 (IL-15), in the presence or absence of stromal cells, differentiated into CD56+CD3−NK cells. UCB-derived CD34+CD38−CD7+ cells have been found to be the most primitive human lymphoid progenitors, which could generate NK cells as well as B and dendritic cells, but not myeloid or erythroid cells, under the influence of cytokines.13 The mouse counterparts of these cells have also been described as common lymphoid progenitors (CLPs),14 complementary to the common myeloid progenitors (CMPs), which have the potential of generating megakaryocyte/erythrocyte and granulocyte/macrophage progenitors.15
Intermediate differentiation stages of NK cells have also been identified. NKR-P1A (CD161)+/CD56− cells, generated from the Lin− fraction of nonadherent UCB cells under the influence of IL-2 and BM stromal cells, develop into functional CD56+ NK cells in the presence of IL-12.2 In another study, Gaddy and Broxmeyer16 identified UCB CD16+CD56− NK cells as possible precursors of mature CD16+CD56+ and CD16−CD56+ cells, which, when cultured in the presence of IL-2, IL-12, or IL-15, acquired adultlike lytic activity and surface antigen phenotype. Moreover, in vitro studies suggested the existence of a distinct IL-15–responsive CD122+intermediate population in both murine and human NK cell development.10,17 Recently, murine BM-derived CD122+ cells, not expressing mature NK cell markers, have also been identified as committed NK cell progenitors.18
Recent reports (for review see Graf19) suggest that the model of gradual restriction in the differentiation potential of hematopoietic cells, does not always follow the pathway from pluripotent (HSCs) to oligopotent (CLPs or CMPs) and finally to monopotent progenitors. For example, CLPs can differentiate into myeloid cells,20 early T progenitors can give rise to myeloid cells,21,22 and B-cell progenitors can generate macrophages.23 Katsura considers that T-cell and B-cell progenitors are generated from a common myelolymphoid progenitor (CMLP), based on his findings from the multilineage progenitor assay of single cells from a progenitor-enriched population of mouse fetal liver.24
In the present study, we demonstrate that a human UCB-derived population with phenotypical features of myeloid cells, characterized by the expression of CD14, CD11b, CD13, and CD33 surface antigens, differentiates into NK cells. FL in combination with IL-15 support differentiation of this myeloid-like cell population into CD56+ functionally mature NK cells, capable of killing NK- and lymphokine-activate killer (LAK)–sensitive targets and producing cytokines. This is the first report demonstrating differentiation of mature NK cells from CD14+CD11b+CD13+CD33+cell precursors and, as a consequence, the possible existence of a human myeloid-lymphoid progenitor.
Materials and methods
UCB samples and mononuclear cell purification
Samples of human UCB were obtained from umbilical veins of healthy full-term infants after informed consent of donors (N = 10). The cells were processed within 4 hours of collection. UCB samples were diluted 1:2 in Hanks balanced salt solution (HBSS; Life Technologies, Paisley, Scotland). Mononuclear cells were isolated by Ficoll-Hypaque centrifugation using standard procedures. The remaining red blood cells were eliminated by negative selection with anti–glycophorin A microbeads (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's instructions. CD34+ cells were isolated with a CD34-selection kit (Miltenyi Biotec). The CD34− fraction was then serially depleted of CD56+, CD19+, and CD3+ using microbeads conjugated with the respective monoclonal antibodies (mAbs) and passed through depletion columns (all purchased by Miltenyi Biotec). CD34−CD56−CD3−CD19−cells were plated onto 24-well plates (Costar, Corning, NY) at 2 × 106 cells/mL in AIM V medium (Life Technologies) and left to adhere for 2 hours at 37°C in a humidified incubator with 5% CO2. Following several washes to remove nonadherent cells, the adherent cell fraction (ACF) was cultured as described in “Cell cultures.” CD14+ cells were isolated from the CD34−CD56−CD3−CD19−population using anti-CD14–coated microbeads and were passed through 2 sequential LS columns (Miltenyi Biotec). CD14+cells obtained in this way were more than 99.5% pure. UCB/CD56+ and CD56+ cells recovered from cultures with FL and IL-15 were also isolated using Miltenyi microbeads.
Cell lines
The human cell lines K562 (erythroleukemia), Daudi (Burkitt lymphoma), and the mouse myeloma SP2/0-Ag14 were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI-1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS; Life Technologies), 2 mMl-glutamine (Life Technologies), and 50 μg/mL gentamicin (Life Technologies), at 37°C in a CO2 incubator.
Cell cultures
Depending on the cell number of the starting population (5 × 104 cells or 5 × 105 cells per culture), cultures were grown in 0.1 or 1.0 mL medium in 96- or 24-well culture plates, respectively. MyeloCult (Stem Cell Technologies, Vancouver, Canada) was used as a culture medium, supplemented with 10−6 M freshly dissolved hydrocortizone (Sigma, St Louis, MO) and 50 μg/mL gentamicin. FL (R&D Systems, Abington, United Kingdom) was added at 25 ng/mL and IL-15 (R&D Systems) at 20 ng/mL. Every 3 to 4 days, half of the medium was discarded and replenished by fresh medium containing freshly added cytokines, whereby cell density was adjusted to 0.5 × 106 cells/mL.
Limiting dilution analysis (LDA)
For the LDA assay, highly purified UCB/CD14+ cells (> 99.5% purity) were cultured in U-bottomed 96-well plates (96 replicates per cell concentration), either alone at dilutions ranging from 105 to 102 cells/well or at 20, 50, and 100 cells/well on 5 × 104 irradiated (2000 cGy) CD14+ cells from the same UCB as feeders, in the presence of FL and IL-15. UCB/CD34+ cells were tested on only CD14+ feeders. Half of the medium was renewed every 3 to 4 days. After 30 days the wells containing viable cells were tested for CD56 expression by flow cytometry. All the wells containing viable cells were found positive for CD56 expression. For both conditions, the NK progenitor frequency was calculated as the reciprocal of the concentration of cells that resulted in 37% negative wells using Poisson statistics and the weighted mean method.25 26 No proliferation was observed in irradiated CD14+ feeders cultured as a control in parallel with the experimental groups under the same conditions (5 × 104 cells/well; 96 replicates for 30 days). Moreover, the same cultures did not give rise to any CD56+ cells.
Monoclonal Abs and immunophenotyping
mAb anti-CD34 conjugated with phycoerythrin (PE), anti-CD56, and anti-CD33 conjugated with fluorescein isothiocyanate (FITC) were obtained from Becton Dickinson (Mountain View, CA). Anti-CD8, -CD14, -CD56, and -CD161 mAbs conjugated with PE and anti-CD2, -CD3, -CD7, -CD19, -CD14, -CD16, -CD25, -CD158a, and -CD158b mAbs conjugated with FITC were purchased from PharMingen (San Diego, CA). FITC, PE-, or PE–cytochrome 5 (cy5)–conjugated anti-CD11b, -CD15, -CD56, -CD94, -CD117, -CD122 mAbs were obtained from Immunotech (Marseille, France). Anti-CD13/PE was purchased from DAKO A/S (Glostrup, Denmark). Cells were washed twice with ice-cold phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA), followed by incubation with saturating concentrations of the appropriate mAbs for 15 minutes at room temperature. Thereafter, cells were washed twice in ice-cold PBS/1% BSA and fixed with 1% paraformaldehyde in PBS. Samples were analyzed using FACSCalibur (Becton Dickinson) and CellQuest analysis software (Becton Dickinson, Mountain View, CA).
Cluster determinant (CD) detection by reverse transcriptase–polymerase chain reaction (RT-PCR)
To detect whether the purified CD14+ cells were contaminated by any NK/NK-progenitor cells, total RNA was extracted from cells isolated from samples of human UCB using the SV Total RNA Isolation System (Promega, Lyon, France), according to the manufacturer's protocol. First-strand cDNA synthesis was performed using approximately 1 μg total RNA, oligodT primers, and the SuperScript II RNase H (-) reverse transcriptase (Invitrogen, Paisley, Scotland). This cDNA material (2 μL) was used for PCR amplification with Taq Platinum (Invitrogen), using the appropriate set of primers and PCR conditions for each cluster determinant (CD56, CD161, CD7, and CD34).
To determine the sensitivity of the applied technique for detecting the mRNAs mentioned in the previous paragraph, we prepared dilutions of purified CD56+ or CD34+ cells from UCB that were more than 98% positive for the expression of CD56, CD7, and CD161 or CD34, respectively, in the SP2/0-Ag14 myeloma mouse cell line (ATCC). We then proceeded to total RNA extraction and RT-PCR analysis in these samples, using the same sets of primers and PCR conditions.
The sequences of the primers we used were as follows: CD56sense, 5′-GCTGGGACTTCAGGAGACTG; CD56antisense, 5′-CCCCTCAGCCTCTTCTTTCT; CD7sense, 5′-TTTACTACGAGGACGGGGTG; CD7antisense, 5′-AGGTGTAGGTGCCAGTGTCC; CD161sense, 5′-AGAATCCAGCCTGCTGCTTA; CD161antisense, 5′-GAGCCGTTTATCCACTTCCA; CD34sense, 5′-CTTGGGCATCACTGGCTATT; CD34antisense1, 5′-AATTCGGTATCAGCCACCAC; CD34antisense2, 5′-CGTGTTGTCTTGCTGAATGG; β-ACTINsense, 5′-TGCTATCCCTGTACGCCTCT; β-ACTINantisense, 5′-TCGTTAATGTCACGCACGAT. The last set of primers was used to monitor the integrity of the mRNA for each sample by amplifying a conservative region of this mRNA that presents complete homology between human and mouse species.
The samples were denatured at 94°C for 5 minutes, followed by amplification rounds consisting of 94°C for 1 minute (denaturing), 60°C for 1 minute (annealing), and 72°C for 2 minutes (extension), for 39 cycles. Specifically for the detection of CD34 mRNA, seminested PCR was applied. In the first round of amplification CD34sense and antisense1 primers were used, and, subsequently, 2 μL of the products were used as template for the second round using the sense and antisense2 primers in the same conditions.
All the amplified products were subjected to 2% agarose gel electrophoresis containing GelStar dye (FMC BioProducts, Rockland, ME) and visualized by ultraviolet (UV) light.
Cytotoxicity assay
Cytotoxic activity of cultured cells was determined in a standard 4-hour chromium 51 (51Cr)–release assay against the NK-sensitive cell line K562 and the NK-resistant cell line Daudi, as previously described.27 In brief, target cells were labeled with 100 μCi (3.7 MBq) sodium [51Cr] chromate (Radiochemical Centre, Amersham, Cardiff, United Kingdom) per 106 target cells for one hour. Effector cells were incubated with target cells at the indicated ratios. Spontaneous 51Cr release was measured by incubating target cells in the absence of effector cells. Maximum51Cr release was determined by adding 1% Triton X-100 (Sigma). Spontaneous lysis did not exceed 10% of the maximum release. The amount of 51Cr released was measured in a γ-counter (Packard, Downers Grove, IL), and the percent lysis was calculated as follows: % specific lysis = [(experimental 51Cr release − spontaneous 51Cr release)/(maximum51Cr release − spontaneous 51Cr release)] ×100.
Quantitation of cytokines in culture supernatants
For cytokine production determinations, cells recovered from cultures with FL and IL-15 were washed twice with HBSS and incubated for 72 hours in fresh medium containing FL (25 ng/mL), IL-15 (20 ng/mL), IL-12 (2 ng/mL; R&D Systems), and IL-18 (100 ng/mL; R&D Systems). Supernatants were collected by centrifugation and stored at −70°C until use. Cytokines (interferon-γ [IFN-γ], granulocyte-macrophage colony-stimulating factor [GM-CSF], tumor necrosis factor α [TNFα], and IL-10) were quantitated using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Diaclone Research, Besançon, France) according to the manufacturer's instructions.
Results
CD56+ cell differentiation from UCB-adherent CD34−CD56− cells
In a recent publication, Huss28 reported that CD34+ HSCs possibly arise from mesenchymal, fibroblast-like CD34− adherent progenitor cells under the influence of the appropriate cytokine microenvironment. Based on this, we initially asked whether such adherent CD34− progenitor cells could give rise to mature NK cells when cultured in the presence of FL and IL-15, which are known to promote NK differentiation from CD34+ HSCs.10 If so, this would enable us to make assumptions toward the identification of a new NK progenitor and thus to better understand NK cell ontogeny.
At the initiation of our experiments, we studied the cell populations included in our total UCB preparations by forward scatter (FSC) and side scatter (SSC) analysis. In this way, we could distinguish 3 cell populations: a typical low SSC lymphoid population and 2 others scattered above this one, commonly characterized as myeloid (monocytic and polymorphonuclear) in adult PB (data not shown). We next attempted to study more closely the high SSC UCB populations by depleting lymphoid cells via a 2-step procedure: first, we sequentially eliminated CD34+, CD56+, CD3+, and CD19+ cells using immunomagnetic procedures, and then we allowed the recovered cells to adhere in order to remove residual lymphocytes included in the nonadherent cell fraction. The resulting ACF was devoid of cells expressing any of the lymphoid markers mentioned in this paragraph with a purity more than 99.5%, and when subjected to SSC/FSC analysis it showed a myeloid profile (data not shown).
Phenotypic analysis of the ACF population revealed that 77.1 ± 11.4% of the cells expressed CD14; 76.4 ± 8.9%, CD11b; 70.6 ± 6.3%, CD13; 68.8 ± 4.2%, CD33; 100%, CD11c; and 87.8 ± 9.9%, CD4low (mean values ± SDs from 10 UCB samples). The ACF was overpopulated in cells coexpressing all of the cell markers mentioned in this paragraph (66.7 ± 4.6%, mean values ± SDs from the same UCB samples). The remaining cells were positive for at least one of the above-mentioned myeloid cell markers, although they did not coexpress all of them.
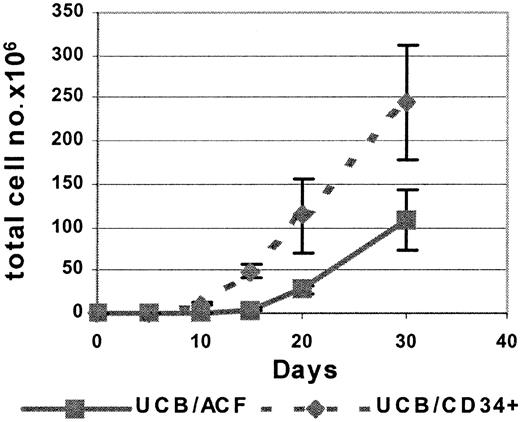
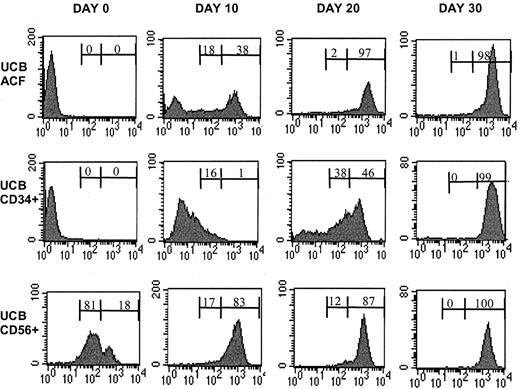
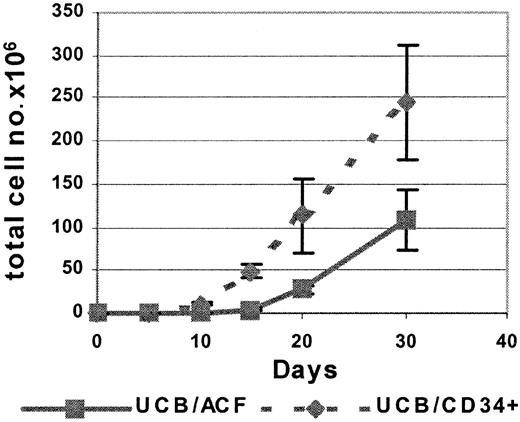
During culture in the presence of FL and IL-15, there was a steady increase in both the percentage and the absolute number of CD56+ cells within the ACF (ACF/CD56+) (Figure1; Table 1). The absolute number of ACF/CD56+ cells increased significantly by day 10 of culture, and by day 30, 100% of the ACF expressed the CD56 marker. It is important to note that already 15 days after culture initiation 81 ± 7% of the ACF cell fraction was CD56+ (Table 1), at which time point only 38 ± 5% of UCB-derived CD34+ cells had developed into CD56+ cells (Table 1).
Kinetics of CD56+ cell differentiation from UCB/ACF and from purified UCB/CD34+cells in cultures with FL and IL-15.
Total cell number as a function of time of culture in the presence of FL and IL-15. Each point represents the mean ± SD of 5 different UCB samples.
Kinetics of CD56+ cell differentiation from UCB/ACF and from purified UCB/CD34+cells in cultures with FL and IL-15.
Total cell number as a function of time of culture in the presence of FL and IL-15. Each point represents the mean ± SD of 5 different UCB samples.
Cytotoxic potential of ACF/CD56+ cells
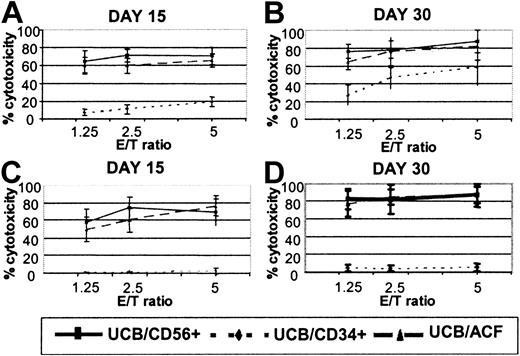
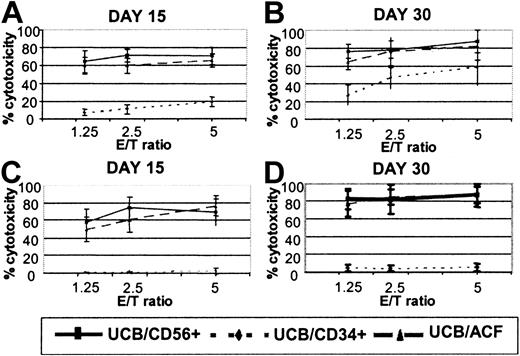
CD56+ cells, differentiated from UCB/ACF after culture with FL and IL-15, were tested as effectors against NK- and LAK-sensitive targets, in parallel with CD56+ effectors differentiated from UCB-derived CD34+ cells and cultured under the same conditions. As shown in Figure2, ACF/CD56+ cells, recovered from 15-day and 30-day cultures, exhibited significantly higher cytotoxicity against K562 (NK) targets than did autologous CD34-derived CD56+ effectors (60% and 76.3% compared with 10.9% and 46.3% at effector-target [E/T] ratio 2.5:1). An even more pronounced effect was observed when the same cells were tested for LAK cytotoxicity. As also shown in Figure 2, only marginal levels of killing against Daudi targets were observed when CD34-derived CD56+ cells were used as cytotoxic effectors. Even after 2 months in culture, CD34-derived CD56+ cells did not acquire significant LAK activity (data not shown). In marked contrast, ACF/CD56+ effectors efficiently lysed the Daudi targets (up to 80% cytotoxicity at E/T ratio 2.5:1; Figure 2). The levels of LAK cytotoxicity observed with ACF/CD56+ cells were comparable with those achieved with highly purified CD56+ effectors isolated from UCB and cultured under the same conditions (Figure2).
Cytotoxic responses of FL and IL-15 differentiated UCB effectors.
(A-B) NK and (C-D) LAK activity of CD56+ cells derived from UCB/ACF (▴), UCB/CD34+ (♦), and UCB/CD56+(▪) after culture with FL and IL-15. Values represent the means ± SDs from 8 UCB samples.
Cytotoxic responses of FL and IL-15 differentiated UCB effectors.
(A-B) NK and (C-D) LAK activity of CD56+ cells derived from UCB/ACF (▴), UCB/CD34+ (♦), and UCB/CD56+(▪) after culture with FL and IL-15. Values represent the means ± SDs from 8 UCB samples.
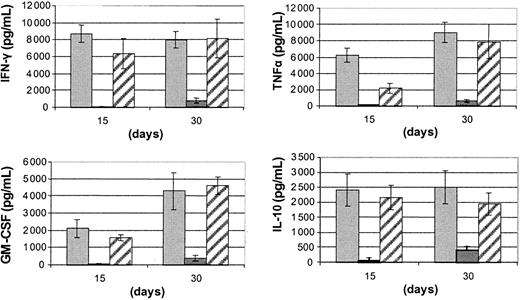
Cytokine production by ACF/CD56+ cells
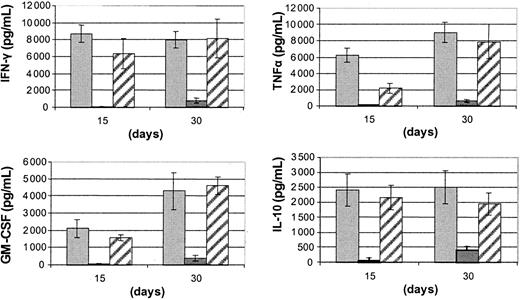
We then examined cytokine production by the IL-15 plus FL-driven ACF/CD56+ cells, upon stimulation with IL-12 and IL-18. As shown in Figure 3, ACF/CD56+ cells were able to produce equally high levels of IFN-γ, GM-CSF, TNFα, and IL-10 comparable with those achieved with autologous UCB-isolated CD56+ cells. As with cytotoxic responses, cytokine production by CD56+ cells differentiated from UCB-isolated CD34+ cells was at significantly lower levels compared with ACF/CD56+cells.
Cytokine production of FL and IL-15 differentiated UCB effectors.
Quantitation of cytokine levels produced by IL-12 (2 ng/mL) and IL-18 (100 ng/mL) stimulated CD56+ cells recovered from long-term cultures of UCB/ACF (▨), UCB/CD34+ (▪), and UCB/CD56+ (░) cells with FL and IL-15. Bars represent the mean values ± SDs from 5 experiments.
Cytokine production of FL and IL-15 differentiated UCB effectors.
Quantitation of cytokine levels produced by IL-12 (2 ng/mL) and IL-18 (100 ng/mL) stimulated CD56+ cells recovered from long-term cultures of UCB/ACF (▨), UCB/CD34+ (▪), and UCB/CD56+ (░) cells with FL and IL-15. Bars represent the mean values ± SDs from 5 experiments.
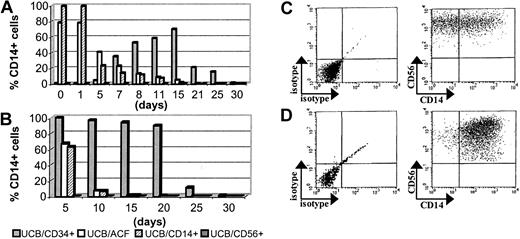
Kinetics of CD14 expression in UCB-derived cell populations
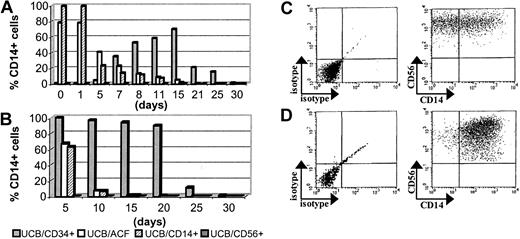
The UCB/ACF was cultured, as in Table2, with FL and IL-15 to generate CD56+ NK cells. The CD56+ NK cells generated in vitro under these conditions (by day 15 almost 90% of the cells were CD56+; Table 2) expressed little (5% by day 15) or no (day 30) CD14 (Figure 4). As also mentioned in “Results,” a fairly high percentage, up to 80% of freshly isolated UCB/ACF, expressed CD14 (day 0), which persisted at these levels until day 5 and thereafter declined rapidly (Figure 4A). Similar phenotypic profiles were obtained from UCB/CD14+ cells cultured under the same conditions (Figure 4A).
Kinetics of CD14 expression on UCB-derived populations.
(A) CD14 expression in total UCB/ACF (■), UCB/CD14+(▨), UCB/CD34+ (░), and UCB/CD56+ (▪) populations and (B) CD14 expression on the CD56+ cells derived from these populations. The percentages in panel B have been obtained by gating on CD56+ cells. (C) Dot plots of UCB/CD14+ cells after 5 days and (D) of UCB/CD34+ cells after 15 days of culture in the presence of FL and IL-15. Gating on CD56+ cells. Of the 3 experiments conducted, 1 representative example is shown.
Kinetics of CD14 expression on UCB-derived populations.
(A) CD14 expression in total UCB/ACF (■), UCB/CD14+(▨), UCB/CD34+ (░), and UCB/CD56+ (▪) populations and (B) CD14 expression on the CD56+ cells derived from these populations. The percentages in panel B have been obtained by gating on CD56+ cells. (C) Dot plots of UCB/CD14+ cells after 5 days and (D) of UCB/CD34+ cells after 15 days of culture in the presence of FL and IL-15. Gating on CD56+ cells. Of the 3 experiments conducted, 1 representative example is shown.
In contrast to ACF, UCB/CD34+ cells expressed no CD14 at culture initiation. More than half (65%) of the cells were found to express CD14 by day 15 and thereafter this percentage progressively declined (Figure 4A). As also shown in Figure 4, UCB/CD56+cells remained CD14− throughout the culture period.
By gating on CD56+ cells, we could demonstrate that the vast majority of CD14+ cells were included in the CD56+ population that was generated from UCB/CD34+ cells. The CD14 marker was maintained on these cells until day 20 and thereafter declined sharply (Figure 4B,D). Approximately 60% of the CD56+ cells derived from the ACF or CD14+ cells also expressed CD14 by day 5 (Figure 4B-C). This percentage, in both cases, was strongly reduced within the next 5 days of culture (down to 5%).
The similarities in the kinetics profile of CD14 expression on (1) the total ACF or the UCB/CD14+ cells (Figure 4A) and (2) the CD56+ cells differentiated from the total ACF or the UCB/CD14+ cells (Figure 4B) strongly suggested that CD14+ cells, representing the most dominant population among the UCB/ACF, are actually the cells that differentiate into CD56+ cells when cultured with FL and IL-15. To test this directly, we assessed the kinetics of FL- and IL-15–driven generation of CD56+ cells in parallel cultures with total ACF or highly purified CD14+ cells from UCB. There was an absolute overlap in the kinetics of appearance of CD56+ cells throughout the entire culture period (data not shown).
To ensure that CD56+ cells generated from either total ACF or UCB/CD14+ cells do not represent functionally distinct populations reflecting different stages of maturity, we also assessed kinetics of NK cytotoxicity and IFN-γ production in parallel with CD56 phenotyping. The kinetics of cytotoxicity against NK-sensitive targets and of IFN-γ production paralleled that of CD56+cell generation (data not shown).
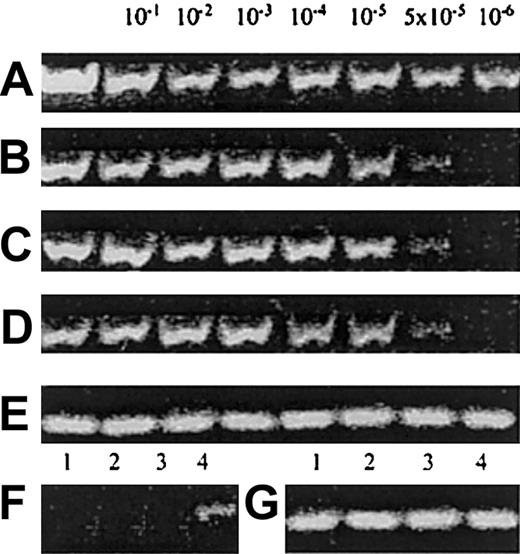
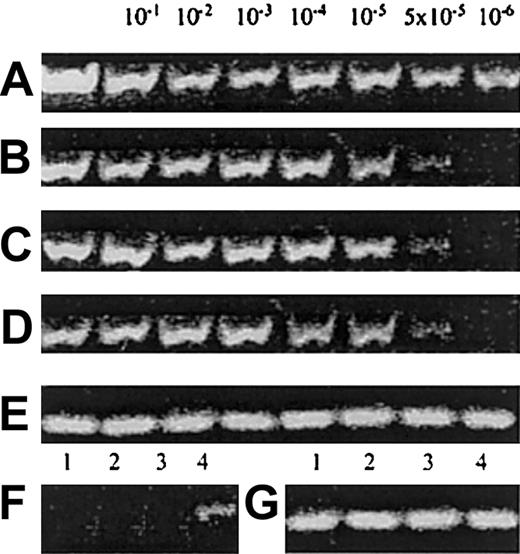
To exclude the possibility that UCB/CD14+ cell cultures contain any NK/NK-progenitor contaminants that could give rise to mature CD56+ cells, we tested UCB/CD14+ cell preparations by RT-PCR for the presence of CD56-, CD161-, CD34-, or CD7-specific transcripts, which characterize such cells. The sensitivities of the applied technique were more than 10−6for CD56 and 5 × 10−5 or less for CD161, CD7, and CD34. Highly purified UCB/CD14+ cells were found negative for the expression of CD56, CD161, and CD34 but were found to express very low levels of CD7 (Figure 5), as expected, because human myeloid progenitors are known to express low amounts of CD7.29
CD detection by RT-PCR.
(A) Amplification of human CD56 in the prepared dilutions of CD56+ cells in SP2/0-Ag14 myeloma mouse cell line. (B) Amplification of human CD161 in the same prepared dilutions. (C) Amplification of human CD34 in the prepared dilutions of CD34+ in SP2/0-Ag14 myeloma mouse cell line. (D) Amplification of human CD7 in the prepared dilutions of CD56+ cells. (E) Amplification of β-actin to monitor the integrity of the mRNA of the samples A-D. (F) Detection of CD mRNA in UCB/CD14+ cells: lane 1, CD56; lane 2, CD161; lane 3, CD34; and lane 4, CD7. Identical results from 4 conducted experiments. (G) Amplification of β-actin of the samples in panel F.
CD detection by RT-PCR.
(A) Amplification of human CD56 in the prepared dilutions of CD56+ cells in SP2/0-Ag14 myeloma mouse cell line. (B) Amplification of human CD161 in the same prepared dilutions. (C) Amplification of human CD34 in the prepared dilutions of CD34+ in SP2/0-Ag14 myeloma mouse cell line. (D) Amplification of human CD7 in the prepared dilutions of CD56+ cells. (E) Amplification of β-actin to monitor the integrity of the mRNA of the samples A-D. (F) Detection of CD mRNA in UCB/CD14+ cells: lane 1, CD56; lane 2, CD161; lane 3, CD34; and lane 4, CD7. Identical results from 4 conducted experiments. (G) Amplification of β-actin of the samples in panel F.
Frequency of NK progenitors within the UCB/CD14+cell fraction
LDA of highly purified UCB/CD14+ cells, cultured alone for 30 days in the presence of FL and IL-15 at cell numbers ranging from 105 to 102, revealed an NK precursor frequency of 1 in 11 426 ± 992 cells (0.009%, n = 3). However, when highly purified UCB/CD14+ cells were cultured on feeders of CD14+ cells isolated from the same UCB sample, at cell numbers 100, 50, and 20 per culture, NK precursor frequency was significantly increased to 1 in 50.5 ± 1.8 cells (1.98%, n = 3). UCB/CD34+ cells cultured on CD14+ feeders, as UCB/CD14+ cells, revealed an NK precursor frequency of 1 in 35 ± 6 cells (2.86%, n = 3).
Phenotypic characterization of CD56+ cells generated from UCB/ACF or UCB/CD14+ cells
UCB/ACF or UCB/CD14+ cells were cultured in the presence of FL and IL-15 to generate CD56+ cells for phenotypic analysis (Table 2). CD56+ cells generated from UCB/ACF or UCB/CD14+ cells expressed CD2, CD7, CD8, CD16, and CD25 at moderate to high levels. The vast majority also expressed the C-type lectin-like receptor CD94/NKG2. The KIRs CD158α and/or CD158b and the c-kit receptor (CD117) were expressed at low levels. Early in culture (5-15 days) UCB/ACF stimulated with FL and IL-15 expressed CD56 at low density, whereas at later stages (> 15-day cultures) these cells became CD56bright (Figure6). Similar results were obtained also with UCB/CD14+ cells (Figure 6). Finally, CD161 expression was evident at culture initiation but thereafter was gradually lost (Table 2). The phenotypic profile of ACF/CD56+ and of CD56+ cells differentiated from UCB/CD14+ cells was similar to that of UCB/CD56+ cells cultured under the same conditions (Table 1). CD56+ cells differentiated from UCB/CD34+ cells exhibited a more immature phenotype (Table2). CD34-derived CD56+ cells expressed significantly lower levels of CD94 and KIRs (CD158a and CD158b), as well as of CD2, CD7, and CD8.
Kinetics of CD56 antigen expression in various UCB-derived cell populations.
The expression of CD56 in UCB/ACF, UCB/CD34+, and UCB/CD56+ cells during culture with FL and IL-15 was examined by flow cytometry. The numbers indicate the percentages of CD56dim and CD56bright cells for each time point. Of the 5 experiments conducted, 1 representative example is shown.
Kinetics of CD56 antigen expression in various UCB-derived cell populations.
The expression of CD56 in UCB/ACF, UCB/CD34+, and UCB/CD56+ cells during culture with FL and IL-15 was examined by flow cytometry. The numbers indicate the percentages of CD56dim and CD56bright cells for each time point. Of the 5 experiments conducted, 1 representative example is shown.
Discussion
In the present study we identify for the first time, in human UCB, a nonlymphoid adherent CD34− population, with phenotypical characteristics of myeloid cells, which has the potential to differentiate into NK cells. CD56+ cells could be detected early in culture of CD34− UCB/ACF, progressively became functional and expressed a series of mature NK cell markers. These in vitro data demonstrate that myeloid-like cells contain novel progenitor cells, which, under the influence of FL and IL-15, differentiate into mature NK cells capable of lysing NK- as well as LAK-sensitive targets and producing high levels of IFN-γ, GM-CSF, TNFα, and IL-10.
The studies presented here show that the CD34−UCB/ACF was mostly composed of CD14+ cells, coexpressing also the myeloid-cell markers CD11b, CD11c, CD13, CD33, and CD4low. Highly purified UCB/CD14+ cells, when cultured with FL and IL-15, showed similar kinetics in terms of growth, CD56 expression, and function as total UCB/ACF cells. By using 2 independent methodologies we could demonstrate that our UCB/CD14+ cell preparations did not contain any NK/NK-precursor contaminants, which by themselves could give rise to CD56+ cells. By applying RT-RCR for CD detection, we found that CD56+, CD161+, CD34+, or CD7+ potential NK/NK-precursor contaminants represented less than 1 in 105 cells and thus the 1 to 50 cells NK-precursor frequency revealed by LDA analysis of cultured UCB/CD14+ cells could not be attributed to any contaminant. Thus, these experimental data provide the first demonstration of human mature NK cell induction in vitro from UCB CD34−CD14+ progenitor cells. It is well established that mature NK cells express CD16, a marker expressed also by myeloid cells, and that lymphoid CD16+/CD56− cells can develop into functional CD56+ cells.2 It has also been reported that a subset of UCB-derived CD56+ cells, which have morphologic features of agranular large lymphocytes, express the CD33 myeloid marker.30 This NK subset, when cultured in the presence of IL-2, showed only low cytolytic activity and probably represented an immature NK cell. Because it has been recently reported in mice that T-cell and B-cell progenitors are generated from a common myelolymphoid progenitor,24 we could possibly suggest that NK cells also develop from a common myelolymphoid progenitor, as it is well established that they arise from a common T/NK progenitor.9 Moreover, the existence of (1) a common bipotent progenitor for NK and dendritic cells,31which have functional similarity to monocytes/macrophages, (2) myeloid leukemias expressing CD56,32-34 and (3) CD14+CD56+ cells in normal human PB (considered a subpopulation of monocytes)35 36 constitute further evidence of the existence of a common myelolymphoid progenitor in humans. Whether UCB/CD14+ cells represent such a progenitor, having the capacity to differentiate into other myeloid or lymphoid lineages as well, remains to be investigated.
So far, the most primitive human hematopoietic progenitors shown to differentiate into NK cells are CD34+Lin−HSCs,10-12 which also give rise to all hematopoietic lineages. It has also been reported, by several groups, that CD34+ HSCs arise from CD34−Lin−precursors.28,37 The fact that we could not detect any CD34+ cells within the UCB/ACF or UCB/CD14+cell populations during the whole process of differentiation to mature CD56+ NK cells under the influence of FL and IL-15 (data not shown) could possibly mean that the acquisition of CD34 expression is not a prerequisite for human pluripotent cells, similarly to what has been described in mice, in which pluripotent progenitors exist mostly in the CD34low/− fraction.38,39Furthermore, some of these CD34low/− cells also express low levels of the macrophage/monocyte marker Mac-1.40Alternatively, the population we describe may represent an intermediate progenitor, appearing later in the differentiation pathway of CD34+ cells.
Another interesting finding in this study is that mature NK cells differentiated from UCB/CD14+ adherent cells behave functionally differently from those differentiated from UCB/CD34+ nonadherent cells, although the latter transiently expressed CD14. UCB/CD14+-derived NK cells produced high levels of cytokines (ie, IFN-γ, GM-CSF, TNFα, and IL-10) and, in addition to NK cytotoxicity, they also lysed LAK-sensitive targets, whereas the UCB/CD34+-derived NK cells produced significantly fewer cytokines and lysed to a lesser extent only NK-sensitive targets. This functional dichotomy was also associated with quantitative phenotypic differences, which included much higher expression of CD7, CD16, the KIRs CD158α and CD158b, and the killer cell lectin-like receptor CD94/NKG2, all of which represent mature NK cell markers in UCB/CD14+-derived CD56+ cells. These differences could be attributed to the need for additional signals, besides FL and IL-15, so that CD34+ cells can fully mature into NK cells. Such signals could be supplied by adherent cells, such as CD14+ cells. This holds true for the differentiation of UCB/CD14+ cells into mature CD56+ NK cells, which was convincingly shown in LDA cultures supported with irradiated UCB/CD14+ cells. By setting up such cultures we could observe high frequencies (1 in 50 cells) of NK precursors as opposed to the significantly lower frequencies (1 in 11 500 cells) achieved solely in the presence of FL and IL-15.
Taken together, our data presented herein identify a new myeloid-like CD14+ NK cell progenitor fraction of UCB. When cultured in the presence of FL and IL-15, this progenitor, without acquiring the CD34+ marker, follows a differentiation pathway that leads to the generation of NK cells with enhanced maturational state compared with those derived from similarly activated UCB/CD34+cells. This novel lineage for NK cell maturation, observed in vitro, points to the fact that NK cell development, and consequently lymphoid cell development, is likely to be substantially more complex in vivo.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-05-1501.
Supported by a grant from the Regional Operational Program Attika no. 20, MIS code 59605GR (M.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sonia A. Perez, Cancer Immunology Immunotherapy Center, Saint Savas Hospital, 171 Alexandras Ave, Athens 115 22, Greece; e-mail: cacenter@otenet.gr.