Abstract
Recombinant human interleukin-11 (rhIL-11), a glycoprotein 130 (gp130)–signaling cytokine approved for treatment of thrombocytopenia, also raises von Willebrand factor (VWF) and factor VIII (FVIII) by an unknown mechanism. Desmopressin (1-deamino-8-d-arginine vasopressin [DDAVP]) releases stored VWF and FVIII and is used for treatment of VWF and FVIII deficiencies. To compare the effect of these 2 agents, heterozygous von Willebrand disease (VWD) and normal dogs were treated with either rhIL-11 (50 μg/kg/d subcutaneously × 7 days) or DDAVP (5 μg/kg/d intravenously × 7 days). The rhIL-11 produced a gradual and sustained elevation of VWF and FVIII levels in both heterozygous VWD and normal dogs while DDAVP produced a rapid and unsustained increase. Importantly, rhIL-11 treatment produced a 2.5- to 11-fold increase in VWF mRNA in normal canine heart, aorta, and spleen but not in homozygous VWD dogs, thus identifying a mechanism for elevation of plasma VWF in vivo. Moreover, dogs pretreated with rhIL-11 retain a DDAVP-releasable pool of VWF and FVIII, suggesting that rhIL-11 does not significantly alter trafficking of these proteins to or from storage pools. The half-life of infused VWF is unchanged by rhIL-11 in homozygous VWD dogs. These results show that rhIL-11 and DDAVP raise plasma VWF by different mechanisms. Treatment with rhIL-11 with or without DDAVP may provide an alternative to plasma-derived products for some VWD and hemophilia A patients if it is shown safe in clinical trials.
Introduction
The von Willebrand factor (VWF) is a large multimeric adhesive glycoprotein that performs 2 vital roles in hemostasis: it mediates adhesion of platelets to endothelium at sites of vascular injury, and it serves as a carrier for coagulation factor VIII (FVIII), protecting it against proteolysis and delivering it to sites of vascular injury.1-4 The VWF molecule also contributes to arterial thrombosis in thrombotic thrombocytopenic purpura5 and in experimental models of myocardial infarction.6 VWF is produced by endothelium and megakaryocytes and is stored in endothelial Weibel-Palade bodies and platelet alpha granules. Plasma VWF appears to come largely from the endothelial compartment by constitutive secretion.6,7 Factors that regulate VWF levels in each of these compartments are of criticial importance for maintaining hemostasis and preventing thrombosis.
Lack of the largest VWF multimer forms, mutations in the VWF protein, or decreased levels of either the largest VWF multimer forms or the VWF protein result in von Willebrand disease (VWD), a common hereditary bleeding disorder.8 More than 70% to 80% of VWD patients have mild to moderate disease and are treated with desmopressin (1-deamino-8-d-arginine vasopressin [DDAVP]).9 DDAVP is thought to raise plasma levels of VWF and FVIII through release from Weibel-Palade bodies, as suggested by the rapid effect of the drug (less than 1 hour) and by the appearance of high-molecular-weight VWF multimers.10,11 The release of VWF and FVIII from the Weibel-Palade bodies has recently been demonstrated to take place via an adenosine 3′,5′-cyclic monophosphate (cAMP)–mediated response to DDAVP binding to vasopressin type 2 (V2) receptors located on the surface of endothelial cells.12,13 Most patients treated with DDAVP become progressively less responsive to repeated administration over short intervals (fewer than 24 hours). Patients unresponsive to DDAVP therapy and those with more severe forms of VWD (type III VWD) are currently treated with plasma concentrates containing FVIII and VWF.9
Interleukin-11 is a glycoprotein 130 (gp130)–signaling cytokine with both hematopoietic and anti-inflammatory activities. Recombinant human interleukin 11 (rhIL-11) (Neumega; Wyeth BioPharma [Andover, MA]) has been approved for treatment of thrombocytopenia following high-dose chemotherapy.14 In addition to increased platelet counts, an increase in plasma levels of VWF and fibrinogen was detected in preliminary clinical trials with rhIL-11.15 Likewise, rhIL-11 was recently reported to increase VWF and fibrinogen and also FVIII levels in mice.7 These reports suggest that rhIL-11 might have potential as a treatment of VWD and hemophilia A patients with quantitative factor deficiencies.
In contrast to mice, dogs respond to DDAVP therapy,12,16 thus allowing us to perform experiments that compare the effects of rhIL-11 and DDAVP therapies, and to further characterize potential mechanisms of rhIL-11–induced changes in levels of hemostasis factors. The results presented here show that treatment with rhIL-11 leads to a gradual and sustained elevation of VWF and FVIII levels in both heterozygous VWD and normal dogs different from the rapid and unsustained increase observed with DDAVP. Furthermore, dogs pretreated with rhIL-11 still exhibit a rapid and transient additional rise in VWF and FVIII levels when given DDAVP. This documents the persistence of a DDAVP-releasable VWF pool in rhIL-11–treated dogs and suggests that VWF trafficking to Weibel-Palade bodies is not significantly altered in vivo. Finally, rhIL-11 treatment did not prolong the half-life of infused VWF in homozygous VWD dogs. These data suggest that rhIL-11 alone or in combination with DDAVP may provide an alternative to plasma-derived products for some VWD and hemophilia A patients if it is shown to be safe in clinical trials. Preliminary reports of parts of this study have been presented in abstract form.17,18
Materials and methods
Experimental animals
Normal, heterozygous VWD and homozygous VWD dogs came from the closed colony at the Francis Owen Blood Research Laboratory at the University of North Carolina, Chapel Hill.19 All animals were treated in accordance with the standards set in Guide for the Care and Use of Laboratory Animals (National Institutes of Health no. 85-23). The Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill approved all experiments. No animals had received blood products within 2 weeks of these studies.
VWD in the Chapel Hill strain of dogs is inherited in an autosomal recessive manner.19 The underlying molecular defect in these VWD dogs is currently unknown. The dogs used in this study were classified on the basis of phenotype and parental background. Homozygous VWD dogs had less than 1% VWF activity and VWF antigen (VWF:Ag) levels, variably depressed levels of FVIII activity, and saline bleeding times longer than 15 minutes. Dogs were defined as phenotypically normal if they had VWF activity and VWF:Ag levels at least 60% of that found in normal canine pooled plasma and had normal bleeding times. Heterozygous VWD dogs had VWF:Ag levels lower than 60% and one parent known to be homozygous VWD.
VWF antigen, activity, and multimer analysis
VWF:Ag level was determined by “sandwich” enzyme-linked immunosorbent assay (ELISA) as described.19,20 The multimer distribution of VWF:Ag levels was analyzed by sodium dodecyl sulfate (SDS) gel electrophoresis with the use of 1.5% or 0.65% agarose gels.21 The antihuman VWF antibody used for VWF:Ag ELISA and multimer gels was purchased from Dako (A082) (Carpinteria, CA). Plasma was assayed for VWF activity by means of a macroscopic agglutination test procedure with botrocetin and paraformaldehyde-fixed, lyophilized human platelets.19,22,23 The VWF:Ag content and activity values are expressed as a percentage of normal reference dog plasma pool (4 to 5 animals) that was assigned a value of 100%.
FVIII activity
FVIII activity was determined by chromogenic substrate assay (Coamatic; DiaPharma Group, Westchester, OH), as specified by the manufacturer. FVIII activity is expressed as a percentage of normal canine reference plasma.
Plasma and platelet sampling
Platelet-poor citrated plasma samples were prepared prior to, and at, 0.5, 1, 2, 3, and 4 hours after administration of either rhIL-11 or DDAVP.19 Plasma samples were stored at –80°C until analyzed. Platelet counts were determined daily in a cell counter (either Baker [Serono Baker Diagnostics, Allentown, PA] or Heska [Heska, Fort Collins, CO]) calibrated for canine platelets. Serum electrolytes and liver enzymes were assayed by Antech, a commercial veterinary clinical laboratory (Farmingdale, NY). The fibrinogen content (milligrams per deciliter) was determined by clotting assay and fibrinogen degradation products were determined by ELISA (Esoterix, Aurora, CO).
Protocols for comparing VWF and FVIII response to rhIL-11 and DDAVP in dogs
Endotoxin-free human rhIL-11 (lot TOC411) was prepared at Wyeth BioPharma.24 The rhIL-11 treatments were administered as daily subcutaneous injections (50 μg/kg). Treatment with DDAVP (lot ZE963; Aventis Behring, King of Prussia, PA) was administered daily by intravenous infusion (5 μg/kg). Four different treatment protocols were used in this study. In each case, the product was administered in dosages as described above. Complete blood counts, serum electrolytes, liver enzymes, and fibrinogen degradation products were assayed during treatment to monitor for potential adverse effects.
Protocol 1: rhIL-11 versus DDAVP
To compare the effects of rhIL-11 and DDAVP on plasma VWF, 1 heterozygous VWD dog and 2 normal dogs were treated with rhIL-11 daily for 7 consecutive days. Plasma and platelet samples were drawn during the 7 treatment days and for an additional 7 days after treatment. Following a washout period, the same 3 dogs were treated with DDAVP daily for 7 consecutive days and had identical plasma and platelet samples prepared on the 7 treatment and 7 posttreatment days.
Protocol 2: rhIL-11 and VWF mRNA levels
To determine if rhIL-11 changed VWF mRNA levels in vivo, normal and homozygous VWD dogs were given rhIL-11 for 7 consecutive days, and a second pair of normal and homozygous VWD dogs received no rhIL-11. Plasma samples and platelet counts were prepared from these dogs to document the effect of rhIL-11 administration. Immediately following humane killing on day 7, tissues (cardiac right and left ventricles and atria, aorta, and spleen) were snap-frozen in liquid nitrogen. Total RNA was extracted from snap-frozen tissues by means of the Trizol reagent method (Invitrogen, Carlsbad, CA) according to manufacturer's specifications. The RNA was separated and analyzed by Northern blot analysis. Canine VWF mRNA was detected by a probe prepared from canine VWF cDNA (3494 bp to 7187 bp, GenBank accession no. U66246). The blots were also probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), to account for variations in RNA loading. Changes in VWF mRNA were normalized to GAPDH mRNA levels by densitometric quantitation of the autoradiographs.
Protocol 3: rhIL-11 plus DDAVP
To determine whether heterozygous VWD and normal dogs treated with rhIL-11 would still respond to DDAVP, 2 heterozygous VWD dogs and 1 normal dog were given rhIL-11 on days 1 through 7 and DDAVP on days 6 through 14. These dogs thus received combined rhIL-11 and DDAVP treatments on days 6 and 7 of this 14-day protocol.
Protocol 4: rhIL-11 and VWF clearance
The influence of rhIL-11 on VWF clearance was examined in 2 homozygous VWD dogs infused with canine cryoprecipitate prepared from normal dog plasma.25 VWF activity, antigen concentration, and multimer distribution were determined for each infusate. Citrated plasma samples were collected at baseline and at 5 minutes, 15 minutes, 30 minutes and 1, 2, 4, 6, 8, 12, 24, 27, 30, 48, and 72 hours following VWF infusion. Each VWD dog was infused with cryoprecipitate twice: either in its naive state or following 7 days of pretreatment with rhIL-11 and with continuing daily rhIL-11 treatments through infusion and sampling (ie, a total of 14 days of rhIL-11 therapy).
Results
Clinical evaluation during rhIL-11 and DDAVP treatment
No obvious clinical sequelae were noted during drug administration (eg, changes in behavior, eating habits). No significant changes were noted in hemoglobin, hematocrit, white blood cell counts, serum electrolytes, liver enzymes, or fibrinogen degradation products (data not shown).
Protocol 1: rhIL-11 versus DDAVP
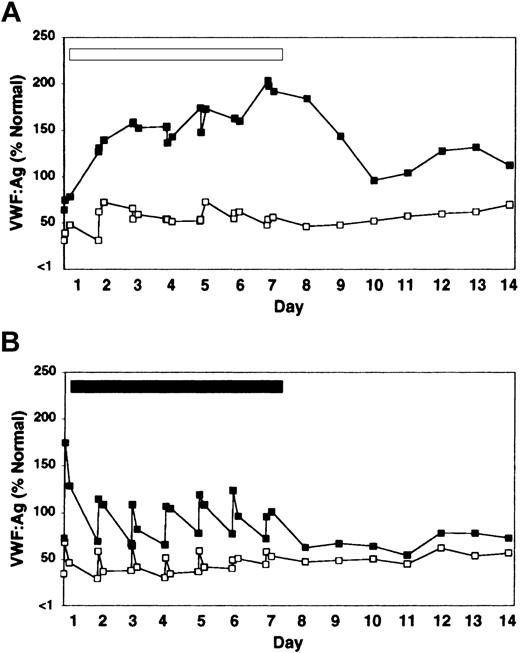
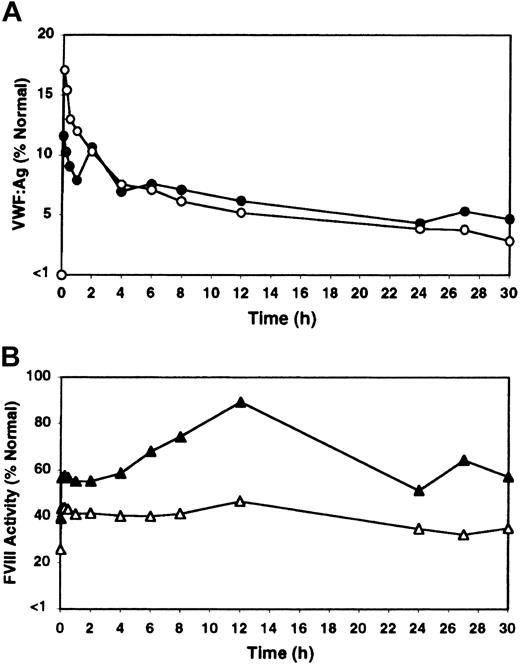
Treatment with rhIL-11 produced a sustained and progressive increase in VWF:Ag level and VWF activity throughout the 7-day treatment period in both heterozygous VWD and normal dogs (Figure 1A; Table 1). FVIII activity followed the same pattern of sustained increase (Table 1). The increases in VWF and FVIII in response to rhIL-11 were continuous during the 7-day treatment period and gradually abated, but were still detectable several days after treatment ended. Platelet counts increased by 78% (264 ± 103 to 468 ± 70 × 106/mL by day 10), and the total fibrinogen increased by 150% (154 ± 21 to 385 ± 40 mg/dL by day 7) during rhIL-11 treatment (complete data not shown).
Effect of rhIL-11 and DDAVP on VWF:Ag levels in heterozygous VWD and normal dogs. One heterozygous VWD dog C107 (open squares) and 2 normal dogs, C80 and C81 (filled squares, average value labeled) were treated daily for 7 days with either rhIL-11 (panel A, 50 μg/kg subcutaneously; white bar), or DDAVP (panel B, 5 μg/kg intravenously; black bar). Results are shown for pretreatment; for 30-minute and 4-hour samples for each of the 7 treatment days; and for the subsequent 7 days after treatment. C80 and C107 developed estrus on day 2 of rhIL-11 therapy.
Effect of rhIL-11 and DDAVP on VWF:Ag levels in heterozygous VWD and normal dogs. One heterozygous VWD dog C107 (open squares) and 2 normal dogs, C80 and C81 (filled squares, average value labeled) were treated daily for 7 days with either rhIL-11 (panel A, 50 μg/kg subcutaneously; white bar), or DDAVP (panel B, 5 μg/kg intravenously; black bar). Results are shown for pretreatment; for 30-minute and 4-hour samples for each of the 7 treatment days; and for the subsequent 7 days after treatment. C80 and C107 developed estrus on day 2 of rhIL-11 therapy.
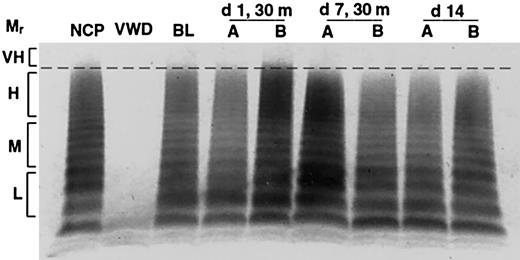
In contrast to the gradual and progressive increase in factor levels observed when dogs were treated with rhIL-11, DDAVP treatment induced rapid but transient and often less pronounced increases in VWF:Ag levels, VWF activity, and FVIII activity, with peak levels observed 30 minutes after treatment (Figure 1B; Table 1). This progressively attenuated response to DDAVP appears consistent with tachyphylaxis. To our knowledge, tachyphylaxis to DDAVP has not been reported in dogs (see “Protocol 3: rhIL-11 plus DDAVP” and Figure 4). Yet another difference between the effects of the 2 treatments becomes apparent when the VWF multimeric composition is analyzed as illustrated in Figure 2. While no obvious change in multimer distribution is observed when the dogs are treated with rhIL-11, DDAVP treatment results in a very clear shift toward release of very-high-molecular-weight VWF multimers. Neither platelet count nor fibrinogen level was significantly changed when the dogs were treated with DDAVP (data not shown).
Effect of combined treatment with rhIL-11 and DDAVP on VWF:Ag levels in heterozygous VWD and normal dogs. Two heterozygous VWD dogs, W68 and D55, and one normal dog, X60, were treated daily with either rhIL-11 (50 μg/kg subcutaneously; white and gray bars) or DDAVP (5 μg/kg intravenously; gray and black bars). The gray bar indicates combined treatment with rhIL-11 and DDAVP on days 6 and 7. The normal dog is represented by (filled squares), and the average value for the 2 heterozygous VWD dogs by (open squares). Daily results from pretreatment and 30-minute and 4-hour samples are shown.
Effect of combined treatment with rhIL-11 and DDAVP on VWF:Ag levels in heterozygous VWD and normal dogs. Two heterozygous VWD dogs, W68 and D55, and one normal dog, X60, were treated daily with either rhIL-11 (50 μg/kg subcutaneously; white and gray bars) or DDAVP (5 μg/kg intravenously; gray and black bars). The gray bar indicates combined treatment with rhIL-11 and DDAVP on days 6 and 7. The normal dog is represented by (filled squares), and the average value for the 2 heterozygous VWD dogs by (open squares). Daily results from pretreatment and 30-minute and 4-hour samples are shown.
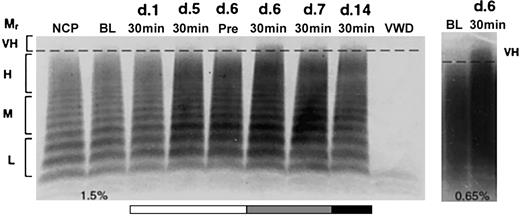
Release of very-high-molecular-weight VWF multimers by DDAVP but not rhIL-11. VWF multimers were separated on agarose gels and detected by immunostaining. Pooled plasma is shown for normal and homozygous VWD dogs in lanes NCP and VWD, respectively. The remaining lanes show samples drawn from C80, 1 of 2 normal dogs treated daily for 7 days with either rhIL-11 (50 μg/kg subcutaneously; lanes marked A), or DDAVP (5 μg/kg intravenously; lanes marked B). The plasma samples include baseline (BL), and days 1 and 7 at 30 minutes following treatment. Day 14 represents samples drawn 7 days after cessation of treatment. The relative multimer weights are indicated to the left of the gel: L, M, H, and VH indicate low, medium, high, and very-high-molecular-weight VWF multimers, respectively. Very-high-molecular-weight ratio (Mr) multimers are seen on day 1 with DDAVP treatment.
Release of very-high-molecular-weight VWF multimers by DDAVP but not rhIL-11. VWF multimers were separated on agarose gels and detected by immunostaining. Pooled plasma is shown for normal and homozygous VWD dogs in lanes NCP and VWD, respectively. The remaining lanes show samples drawn from C80, 1 of 2 normal dogs treated daily for 7 days with either rhIL-11 (50 μg/kg subcutaneously; lanes marked A), or DDAVP (5 μg/kg intravenously; lanes marked B). The plasma samples include baseline (BL), and days 1 and 7 at 30 minutes following treatment. Day 14 represents samples drawn 7 days after cessation of treatment. The relative multimer weights are indicated to the left of the gel: L, M, H, and VH indicate low, medium, high, and very-high-molecular-weight VWF multimers, respectively. Very-high-molecular-weight ratio (Mr) multimers are seen on day 1 with DDAVP treatment.
Protocol 2: rhIL-11 and VWF mRNA levels
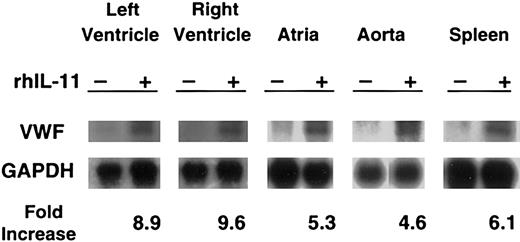
Northern blot analysis showed that rhIL-11 stimulation induced markedly increased levels of VWF mRNA in the cardiac left and right ventricles and atria, aorta, and spleen from the normal dog (Figure 3). At the time the tissues were collected for RNA analysis, rhIL-11 had induced a 92% increase in VWF antigen (88% to 169% by day 7); a 40% increase in platelet counts (206 to 289 × 106/mL by day 7); and a 177% increase in fibrinogen level (179 to 496 mg/dL by day 7). The control normal dog that was sampled in an identical fashion had no significant change in these values (data not shown). The homozygous VWD dog treated with rhIL-11 did not show any changes in VWF mRNA levels but did have a 20% increase in platelet count (268 to 323 × 106/mL) and a 103% increase in fibrinogen level (346 to 703 mg/dL).
In normal dogs, rhIL-11 up-regulates VWF mRNA levels. For 7 consecutive days, rhIL-11 was given to a normal dog. A second normal dog was used as a control. Tissues were harvested, and total RNA was analyzed by Northern blot analysis for the presence of VWF mRNA with the use of canine VWF cDNA as probes. Changes in VWF mRNA were normalized to GAPDH mRNA levels by densitometric quantitation of the autoradiographs. Representative gels are shown for each tissue. Only faint VWF bands appear at baseline, and these increase markedly with rhIL-11 treatment. Results are presented for 5 tissues (left ventricle, right ventricle, atria, aorta, and spleen) as fold increase between treated and untreated animal for the gels shown. No change in VWF mRNA was seen in the homozygous VWD dog treated with rhIL-11 (data not shown).
In normal dogs, rhIL-11 up-regulates VWF mRNA levels. For 7 consecutive days, rhIL-11 was given to a normal dog. A second normal dog was used as a control. Tissues were harvested, and total RNA was analyzed by Northern blot analysis for the presence of VWF mRNA with the use of canine VWF cDNA as probes. Changes in VWF mRNA were normalized to GAPDH mRNA levels by densitometric quantitation of the autoradiographs. Representative gels are shown for each tissue. Only faint VWF bands appear at baseline, and these increase markedly with rhIL-11 treatment. Results are presented for 5 tissues (left ventricle, right ventricle, atria, aorta, and spleen) as fold increase between treated and untreated animal for the gels shown. No change in VWF mRNA was seen in the homozygous VWD dog treated with rhIL-11 (data not shown).
Protocol 3: rhIL-11 plus DDAVP
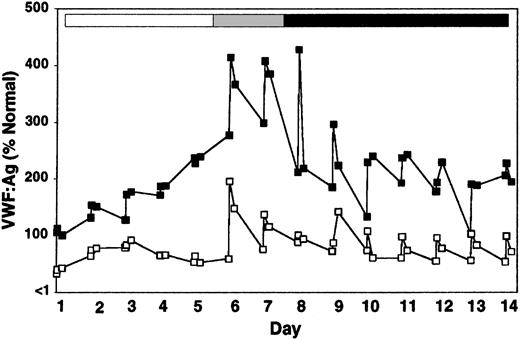
When DDAVP was administered on day 6 to rhIL-11–treated heterozygous VWD and normal dogs, VWF:Ag levels, VWF activity, and FVIII activity increased by an additional 70% to 100% within 30 minutes of treatment (Figure 4; Table 2). As in the naive animal, this DDAVP effect was transient; VWF and FVIII levels returned to predose levels in fewer than 24 hours; and the magnitude of the response to DDAVP appears to diminish with repeated exposure. Multimer analysis showed that DDAVP appears to cause a release of very-high-molecular-weight VWF multimers independently of rhIL-11 treatment (Figure 5). This was especially pronounced on day 6, the first day of DDAVP treatment (compare Figure 5; day 6, before treatment, and day 6, 30 minutes). As expected, the rhIL-11 treatment produced an increase in platelet counts by 66% (282 ± 66 to 469 ± 112 × 106/mL by day 12), and a 154% increase in total fibrinogen (223 ± 9 to 566 ± 110 mg/dL by day 7) (complete data not shown).
Release of very-high-molecular-weight VWF multimers by DDAVP during rhIL-11 treatment. VWF:Ag multimer gel analysis of a normal dog, X60, treated daily for 7 consecutive days with rhIL-11 (50 μg/kg subcutaneously; white and gray bars). This dog then received combined rhIL-11 and DDAVP treatment (5 μg/kg intravenously) on days 6 and 7 (gray bar). The same daily dose of DDAVP was continued on days 8 through 14 (black bar). The multimer composition of X60 is shown at baseline (BL), prior to treatment on day 6 (Pre), and 30 minutes following administration of treatments on days 1, 5, 6, 7, and 14. Lanes and molecular weights are labeled as in Figure 2. Note the presence of very-high-molecular-weight multimers after treatment with DDAVP on day 6 (30 minutes) and to a lesser extent on days 7 and 14. This DDAVP-induced release of very-high-molecular-weight VWF multimers was confirmed on the 0.65% agarose gel shown in the panel to the right.
Release of very-high-molecular-weight VWF multimers by DDAVP during rhIL-11 treatment. VWF:Ag multimer gel analysis of a normal dog, X60, treated daily for 7 consecutive days with rhIL-11 (50 μg/kg subcutaneously; white and gray bars). This dog then received combined rhIL-11 and DDAVP treatment (5 μg/kg intravenously) on days 6 and 7 (gray bar). The same daily dose of DDAVP was continued on days 8 through 14 (black bar). The multimer composition of X60 is shown at baseline (BL), prior to treatment on day 6 (Pre), and 30 minutes following administration of treatments on days 1, 5, 6, 7, and 14. Lanes and molecular weights are labeled as in Figure 2. Note the presence of very-high-molecular-weight multimers after treatment with DDAVP on day 6 (30 minutes) and to a lesser extent on days 7 and 14. This DDAVP-induced release of very-high-molecular-weight VWF multimers was confirmed on the 0.65% agarose gel shown in the panel to the right.
Protocol 4: rhIL-11 and VWF clearance
The possibility that rhIL-11 might alter VWF half-life in circulation was examined in homozygous VWD dogs. No differences were detected in the clearance rate of canine VWF between naive and rhIL-11–treated VWD dogs, with a half-life of approximately 12 to 18 hours observed in both instances (Figure 6A). Platelet counts and fibrinogen content went up as expected with rhIL-11 treatment (30% from 349 to 454 × 106/mL and 107% from 299 to 618 mg/dL by day 7, respectively, with average values listed). Importantly, as in normal and heterozygous VWD dogs, an approximately 50% increase occurred in FVIII activity upon rhIL-11 treatment prior to infusion of cryoprecipitate in the homozygous VWD dogs (from 23% to 32% and from 29% to 47% in the 2 dogs, respectively). It therefore appears that the FVIII increase observed in dogs after rhIL-11 treatment is not simply a consequence of the presence of increased amounts of circulating VWF.
Influence of rhIL-11 on clearance of infused VWF and FVIII in homozygous VWD dogs. Two homozygous VWD dogs, C94 and B80, were infused twice with canine cryoprecipitate either in their naive state (open symbols, average values labeled), or following 7 days of pretreatment with rhIL-11 (filled symbols, average values labeled) and with continuing daily rhIL-11 treatments through infusion and sampling (ie, a total of 14 days of rhIL-11 therapy). (A) The change in VWF:Ag levels expressed as a percentage of normal canine VWF:Ag levels. (Note: both dogs had pretreatment values below 1%.) (B) The increase in FVIII activity with rhIL-11 treatment in the same experiments as in panel A.
Influence of rhIL-11 on clearance of infused VWF and FVIII in homozygous VWD dogs. Two homozygous VWD dogs, C94 and B80, were infused twice with canine cryoprecipitate either in their naive state (open symbols, average values labeled), or following 7 days of pretreatment with rhIL-11 (filled symbols, average values labeled) and with continuing daily rhIL-11 treatments through infusion and sampling (ie, a total of 14 days of rhIL-11 therapy). (A) The change in VWF:Ag levels expressed as a percentage of normal canine VWF:Ag levels. (Note: both dogs had pretreatment values below 1%.) (B) The increase in FVIII activity with rhIL-11 treatment in the same experiments as in panel A.
Discussion
Plasma VWF and FVIII levels increase in heterozygous VWD and normal dogs given rhIL-11 or DDAVP as single or combined therapy. The pattern of increase, however, is markedly different in the 2 agents. When the dogs were treated with rhIL-11, the increases in VWF and FVIII antigen and activity levels were gradual and progressive, with peak factor levels occurring after several consecutive days of rhIL-11 therapy at a time when VWF mRNA was found to be markedly up-regulated in normal dogs. Importantly, rhIL-11 induced no apparent change in VWF multimer composition. Thus, the mechanism by which rhIL-11 raises plasma VWF antigen level and activity appears to be mediated by the up-regulation of VWF mRNA. Similar to the response observed in humans, DDAVP treatment in dogs was followed by an immediate and relatively briefly sustained increase in circulating levels of VWF and FVIII (Figure 1). The immediacy of the response along with the appearance of very-high-molecular-weight VWF multimers is consistent with release from storage granules in Weibel-Palade bodies (Figure 2; day 1, 30 minutes). Considered together, these data support the hypothesis that these 2 agents induce an increase in VWF by different mechanisms.
Dogs challenged with DDAVP after prolonged rhIL-11 therapy exhibited a marked additional increase in VWF:Ag levels and activity in response to DDAVP. Moreover, very-high-molecular-weight multimers appeared not just on the first day but on both days of combined rhIL-11/DDAVP therapy. However, this experiment did not determine if rhIL-11 changed the number of Weibel-Palade bodies, changed the concentration of VWF in individual Weibel-Palade bodies, or prevented the relative loss of response to DDAVP with repeated injections beyond the 2 days of combined therapy. The goal of this protocol was to determine if rhIL-11 directed intracellular trafficking of VWF away from Weibel-Palade bodies to a degree that would reduce the response to DDAVP. Our data suggest that any change in intracellular VWF trafficking to Weibel-Palade bodies induced by rhIL-11 is not detected by these in vivo studies (Figures 4, 5).
The absence of obvious differences in the half-life of infused VWF between rhIL-11–treated and untreated homozygous VWD dogs suggests that rhIL-11 does not alter clearance of the circulating VWF (Figure 6A). Likewise, the increase in plasma VWF in normal and heterozygous VWD dogs treated with rhIL-11 is unlikely to be due to altered clearance. However, we cannot rule out the possibility that rhIL-11 treatment alters posttranslational modification of expressed VWF in a manner that alters its clearance rate, as has been seen in mice.26
An interesting effect of rhIL-11 therapy in the homozygous VWD dogs shown here is the approximately 50% increase of circulating FVIII activity (from 23% to 32% and from 29% to 47%). The mechanism for this increase in FVIII was not determined in this study. These data suggest that the beneficial effect of rhIL-11 on FVIII levels occurs independent of the presence of VWF, and that factors that regulate the levels of FVIII may, at least to some extent, be independently regulated. Of note, DDAVP treatment had no significant effect on FVIII levels in homozygous VWD dogs (data not shown). Whereas dogs with homozygous VWD have a range of FVIII that spans from very low to normal,19 humans with type III VWD have low FVIII activity levels. It therefore remains to be seen if there is any independent rhIL-11 stimulation of FVIII in VWF-deficient humans.
The response to rhIL-11 therapy observed in our dogs is mostly in agreement with recent data obtained in mice by Denis and coworkers.7 In both cases, rhIL-11 treatment of normal and heterozygous animals raised levels of VWF, FVIII, and fibrinogen and increased platelet counts and, as in the VWD dogs, increased FVIII levels when homozygous VWD animals were given rhIL-11 therapy. Moreover, the half-life of infused VWF was not influenced by rhIL-11 in either species. However, our analysis of rhIL-11– treated dogs showed a marked increase in VWF mRNA content in heart, aorta, and spleen by Northern blot (Figure 3). In contrast, no significant change in mouse VWF mRNA was detected by Taqman polymerase chain reaction (PCR) of mouse VWF mRNA prepared from lung, kidney, or spleen. It is possible that the different methods employed for detection of VWF mRNA, differences in length of the rhIL-11 treatment period prior to tissue harvest (4 days in mice versus 7 days in dogs), or the different tissues examined in part explain the different results. Alternatively, various species may respond differently to human rIL-11, as seen with DDAVP among humans, mice, and dogs.
The increase in VWF mRNA detected in vivo varied from 2.5- to 11-fold among several canine tissues (Figure 3). It is likely that these differences are due to varying number of endothelial cells in these tissues as well as differences in endothelial phenotype within specific vascular beds.27-30 Our study did not determine the exact cell of origin of the increased VWF mRNA. Further in vivo studies, possibly with in situ hybridization, will be required to confirm that endothelial cells are the source of increased VWF mRNA. It is also interesting to note that cultured human umbilical vein endothelial cells did not respond to rhIL-11 therapy.7 Cultured cells often respond differently from those in the in vivo setting, underscoring the rationale for additional in vivo studies.
DDAVP is presently one of the most frequently used therapies in the treatment of patients with mild forms of VWD and hemophilia A.9 The benefit of DDAVP is its ability to stimulate release of VWF and FVIII from tissue-storage sites, resulting in a rapid increase in circulating factors. However, the response is transient, and daily administration of DDAVP results in markedly diminishing responses, limiting its usefulness to very brief periods. The present study implies that rhIL-11 can be used to induce progressive increases in plasma levels of VWF and FVIII that are sustained by daily administration in a manner that does not deplete DDAVP-releasable VWF pools. Since rhIL-11 and DDAVP raise VWF levels in different and potentially cumulative ways, rhIL-11, or variants of rhIL-11, could become an alternative to DDAVP and plasma products, or be used in combination with DDAVP. Possible clinical settings would include elective surgery or dental procedures in patients with VWD and hemophilia A. Also, women with VWD who experience menorrhagia might have reduced bleeding with rhIL-11 treatment. The safety and efficacy of these new approaches will need to be established in clinical trials.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2003-01-0290.
Supported in part by research funding from Wyeth Research (T.C.N.).
Several of the authors (J.C.K., R.G.S.) are employed by a company (Wyeth) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge the outstanding care for these animals provided by Ms Melissa Fleishman and her staff at the Francis Owen Blood Research Laboratory.