Abstract
Gene transfer into T lymphocytes is currently being tested for the treatment of lymphohematologic disorders. We previously showed that suicide gene transfer into donor lymphocytes infused to treat leukemic relapse after allogeneic hematopoietic stem cell transplantation allowed control of graft-versus-host disease. However, the T-cell receptor (TCR) activation and sustained proliferation required for retroviral vector transduction may impair the half-life and immune competence of transduced cells and reduce graft-versus-leukemia activity. Thus, we tested lentiviral vectors (LVs) and stimulation with cytokines involved in antigen-independent T-cell homeostasis, such as interleukin 7 (IL-7), IL-2, and IL-15. Late-generation LVs transduced efficiently nonproliferating T cells that had progressed from G0 to the G1 phase of the cell cycle on cytokine treatment. Importantly, IL-2 and IL-7, but not IL-15, stimulation preserved physiologic CD4/CD8 and naive-memory ratios in transduced cells with only minor induction of some activation markers. Functional analysis of immune response to cytomegalovirus (CMV) showed that, although CMV-specific T cells were preserved by all conditions of transduction, proliferation and specific killing of autologous cells presenting a CMV epitope were higher for IL-2 and IL-7 than for IL-15. Thus, LV transduction of IL-2 or IL-7 prestimulated cells overcomes the limitations of retroviral vectors and may significantly improve the efficacy of T-cell–based gene therapy.
Introduction
Genetic modification of primary human T lymphocytes is currently tested for the genetic treatment of inherited and acquired diseases.1-4 We and others previously showed that the infusion of donor lymphocytes transduced by a retroviral vector (RV) to express a suicide gene to patients with disease relapse occurring after hematopoietic stem cell transplantation (HSCT) results in graft-versus-leukemia activity and graft-versus-host disease efficiently controlled by the suicide gene/prodrug system.5,6 Although appropriate ex vivo manipulation results in a polyclonal population of transduced T lymphocytes, able to mediate antitumor and antiviral responses in vivo,7 several observations suggest that the T-cell receptor (TCR) activation and sustained proliferation of activated T cells required for efficient transduction by RV impair the half-life, repertoire, and immune competence of transduced cells.8 In particular, the variability in response to activation stimuli, activation-induced cell death (AICD), exposure to high doses of human recombinant interleukin-2 (hu-r-IL-2), and proliferation-induced T-cell exhaustion alter the immune competence and repertoire of ex vivo–manipulated T cells. RV transduction often results in an inversion of the physiologic CD4/CD8 ratio, enrichment in memory cells associated with loss of the naive T-cell subsets,7 a skew in the TCR repertoire,9 and a reduced alloreactivity.10,11 Therefore, to better exploit genetically modified T lymphocytes, we must develop protocols of ex vivo manipulation that fully preserve the T-cell immunologic competence. To this aim, we tested vesicular stomatitis virus glycoprotein (VSV-G)–pseudotyped late-generation lentiviral vectors12 for T-cell transduction in the absence of TCR triggering. Although lentiviral vectors (LVs) transduce efficiently several types of nondividing cells13,14 through the active transport of the viral preintegration complex into the nucleus,15 quiescent T cells are not permissive to HIV replication16-19 or to HIV-vector transduction.20-23 However, it was shown that cytokines, such as IL-7, IL-2, and IL-15, able to promote long-term survival in vitro of memory and naive T lymphocytes,24,25 can render T cells susceptible to LV transduction in the absence of TCR activation.22,23,26 In this study, we confirmed and extended these findings using late-generation LVs with advanced performance and safety profiles and showing efficient transduction of T cells that have progressed from G0 into the G1 phase of the cell cycle on cytokine treatment without becoming committed to proliferation. Most importantly, we showed that culture conditions allowing for LV transduction of T cells in the absence of TCR triggering result in preservation of full immune competence, thus allowing to overcome the intrinsic functional limitations of retroviral-mediated transduction.
Materials and methods
Cell culture
HeLa and 293T human embryonic kidney cell lines were cultured in Iscove modified Dulbecco medium (IMDM; Sigma Chemical, Milan, Italy) supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Inchinnam, Scotland) and a combination of penicillin (100 IU/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM).
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors by leukapheresis and Ficoll-Hypaque gradient separation (Pharmacia, Uppsala, Sweden). This study was approved by the H.S. Raffaele institutional ethics committee, and informed consent was obtained from all healthy subjects before blood sampling. Cells were cultured in IMDM supplemented with 10% fetal calf serum (FCS; Gibco BRL) at a density of 1 × 106 cells/mL in the presence of different cytokines: human recombinant IL-2 10 U/mL (EuroCetus Italia S.r.l., Milan, Italy), human recombinant IL-7 5 ng/mL (Boehringer Mannheim-Roche GmbH, Mannheim, Germany), human recombinant IL-15 10 ng/mL (R&D System or PeproTech, Rocky Hill, NH). TCR-mediated activation was performed with anti-CD3 antibody (OKT3) 30 ng/mL (Orthoclone, Milan, Italy) plus anti-CD28 monoclonal antibody (mAb) 1 μg/mL (PharMingen, San Diego, CA) followed by culture in the presence of human recombinant IL-2 50 U/mL. Culture medium was changed every 3 to 4 days. As indicated, some experiments were also performed with T cells purified from contaminating monocytes by 2 steps of adherence to plastic that resulted in more than 96% purity of peripheral blood lymphocytes (PBLs).
Resting T lymphocytes were isolated from PBMCs by negative selection using the Miltenyi magnetically activated cell sorter (MACS) pan T isolation kit (Miltenyi Biotec, Gladbach, Germany) as instructed by the manufacturer. The kit contains a cocktail of antibodies specific for a panel of surface markers (CD11b, CD16, CD19, CD36, CD56). To deplete all activated T lymphocytes, cells were further purified using MACS CD25 and HLA-DR microbeads. Culture conditions were the same described for PBMCs.
Vector production
Vector stocks were produced by calcium-phosphate transient transfection of 293T cells using a third-generation lentiviral vector system.27,28 The transfer vector, containing the cDNA for the truncated form of low affinity nerve growth factor receptor (ΔLNGFr) deleted of most of the intracytoplasmic tail, was obtained from pRRLsin.cPPT.hPGKeGFP.Wpre12 by replacing the transgene, the green fluorescent protein (GFP), and the RRL sequences with the CCL sequence to improve production by the third-generation packaging construct.27 The following amount of DNA was used per subconfluent 10-cm dish: 11 μg pCCLsin.PPT.hPGK.ΔLNGFr.Wpre vector, 3.5 μg VSV-G envelope encoding pMD.G plasmid,13 5 μg packaging pMDLg/p plasmid,27 and 2.5 μg Rev-expressing plasmid.27 Sixteen hours after transfection medium was replaced, and 2 collections of viral supernatant were made 24 and 48 hours after transfection. After filtering through a 0.2-μm filter (Millipore, Bedford, MA), the vector was purified and concentrated by supernatant ultracentrifugation as described.12 Viral p24 antigen concentration was determined by HIV-1 p24 core profile enzyme-linked immunosorbent assay (ELISA; NEN Life Science Products, Boston, MA). The average titer was evaluated on HeLa cells by adding serial dilutions of concentrated vector to 105 cells in a 6-well plate (Costar, Corning, NY) in the presence of polybrene (8 μg/mL). Cells were analyzed 96 hours after transduction for ΔLNGFr expression by flow cytometry. Transduction activity was expressed in transduction units (TUs). Multiply of infection (MOI) was calculated dividing the vector titer for the number of cells transduced. Some experiments were confirmed using a lentiviral vector coding for GFP, already described.12
Transduction and selection of human lymphocytes
Transduction of T cells was performed by one cycle of 3-hour exposure to the viral supernatant in the presence of polybrene (8 μg/mL) at 37°C and 5% CO2. Resting T cells were transduced by 1 or 2 cycles of 8-hour exposure to the supernatant performed at a 12-hour interval. Cells were then washed and plated at the density of 1 × 106/mL in the presence of different cytokines. Transduced cells were analyzed by flow cytometry for ΔLNGFr expression 96 hours after transduction as described.7,29 Briefly, cells were washed twice with phosphate-buffered saline (PBS) and incubated on ice for 30 minutes with mouse-antihuman LNGFr 20.4 (American Type Culture Collection [ATCC], Manassas, VA) mAb at a density of 3 × 105 cells for 0.1 mL mAb. Cells were then washed and incubated with fluorescein isothiocyanate (FITC)–conjugated or phycoerythrin (PE)–conjugated goat-antimouse antibodies (DAKO, Glostrup, Denmark). Untransduced T cells stained as described and transduced cells stained only with the secondary antibody were used as negative controls.
To analyze T cells, viability cells were stained with propidium iodide 100 ng/mL for 1 minute on ice. Cells were then vortexed, washed with PBS, resuspended in PBS–2% FCS, and analyzed on a FACSCalibur (Becton Dickinson, Milan, Italy) using Cell Quest software (BD Biosciences, Milan, Italy). Approximately 10 000 events were analyzed.
For immunoselection, cells were transduced with LV and immuno-selected on the same day of fluorescence-activated cell sorter (FACS) analysis as previously described.5,29 Briefly, cells were incubated with a mouse-antihuman LNGFr mAb and goat antimouse immunoglobulin G1 (IgG1)–coated magnetic beads (Dynabeads M-450; Dynal AS, Oslo, Norway) to obtain an enriched population of transduced cells.
Flow cytometric analysis of genetically engineered T cells
Transduced T cells and unmodified PBMCs from the same donors were characterized by cell surface staining with FITC-, PE-, and PE-Cy5–conjugated antibodies to CD3, CD4, CD8, CD56, CD16, CD45RA, CD45RO (Becton Dickinson, Mountain View, CA), CD62L, and CD95 (Pharmigen, San Diego, CA); and FITC-, PE-, and PE-Cy5–conjugated isotype controls. Activation of T cells was evaluated by staining with FITC- and PE-conjugated antibodies to CD25 and HLA-DR (Becton Dickinson).
For LNGFr triple fluorescence, cells were stained with a biotinylated anti-LNGFr antibody (Boehringer Mannheim-Roche) followed by streptavidin conjugated to tricolor (CALTAG, Burlingame, CA), FITC-conjugated, and PE-conjugated antibodies. The samples were analyzed by flow cytometry.
Intracytoplasmic staining for cytokine production
To determine the cytokine production profile of genetically modified T cells, intracellular cytokines were detected by flow cytometry as published,30 with slight modifications. Pairs of transduced and unmodified T cells (1 × 106/mL) were stimulated with immobilized 10 μg/mL OKT3 and 10 ng/mL TPA (12-O-tetradecanoylphorbol 13-acetate tannic acid). After 3 hours of activation, 1 ng/mL brefeldin A (Sigma, Milan, Italy) was added. After a total of 6 hours of activation, T cells were collected, washed in PBS, and fixed overnight in 2% formaldehyde. After fixation, T cells were permeabilized by incubation in PBS supplemented with 2% FCS and 0.5% saponin (Sigma).
Permeabilized T cells were incubated with PE-labeled anti–IL-4 or anti–IL-10, and FITC-coupled anti–γ-IFN (interferon) mAbs (Pharmingen). After washing, cells were analyzed by flow cytometry. Quadrant markers were positioned to include 95% of stained, unstimulated cells in the lower left square.
Analysis of cell division by CFSE dilution
The proliferative status of T cells at the day of transduction was analyzed by staining with carboxy-fluorescein diacetate succinimidyl ester (CFSE) as previously described.31 In brief, T cells were washed with PBS and resuspended at a density of 2 × 107 cells/mL in PBS. An equal volume of 5 μM CFSE (CFDASE; Molecular Probes, Milan, Italy) in PBS was added, and cells were gently mixed for 8 minutes at 20°C. Unbound CFDASE, or the deacetylated form, CFSE, was quenched by the addition of an equal volume of FCS. Labeled cells were washed twice in IMDM supplemented with 10% FCS and then stimulated. At the day of transduction CFSE-labeled cells were collected and washed in PBS. Cell division analysis was performed by flow cytometry.
Cell cycle analysis
Cells were washed with PBS and resuspended in 0.5 mL nucleic acid staining buffer composed of 0.15 M NaCl in 0.1 M phospate citrate buffer (Sigma), containing 5 mM EDTA (ethylenediaminetetraacetic acid) and 0.5% bovine serum albumin (BSA Fraction V; Sigma), pH 4.8, supplemented with 0.02% saponin (Sigma). A volume of 10 μL of 10 μg/mL 7-aminoactinomycin D (7AAD; Sigma) was added, and cells were incubated at 20°C for 20 minutes. Cells were then incubated for 10 minutes on ice, and 0.5 μL of 1 mg/mL Pyronin Y (Sigma) was added. Cells were incubated on ice for an additional 10 minutes and then analyzed by flow cytometry for DNA and RNA staining. Cell aggregates and dead cells were eliminated from further analysis by gating individual cells in the FL3-area versus FL3-width plot.
Analysis of antigen-specific T-cell frequency on transduced and unmanipulated lymphocytes using peptide-MHC (major histocompatibly complex) tetramers
The nonamer pp65495-503 peptide derived from the structural protein pp65 of cytomegalovirus (CMV), which binds HLA-A020132 was synthesized. Tetramers were made according to the protocol of Altman et al.33 In brief, human β2-microglobulin and the soluble domain of the HLA-A2 heavy chain (residues 1-276) linked at its COOH terminus to BirA substrate peptide (BSP) and β2-microglobulin subunits were solubilized and refolded together in vitro in the presence of the peptide. Folded material was biotinylated by BirA enzyme. HLA-A2 peptide complexes were purified on gel filtration and ion exchange columns. Tetrameric complexes of biotinylated HLA-A2 peptide were produced by mixing purified, biotinylated heterodimer with NeutrAvidin-PE (Molecular Probes) at a molar ratio of 4:1. To compare the frequency of antigen-specific T cells on transduced and unmanipulated T cells, 1 × 106 cells were stained with anti-LNGFr antibody followed by staining with FITC-conjugated goat-antimouse antibody (DAKO) (1/60) and with PE-conjugated pp65-tetramer, anti-CD8–PE-Cy5 antibody (1/60). The frequency of pp65-specific cytotoxic T lymphocytes (CTLs) is presented as a fraction of tetramer+, CD8+ lymphocytes over the total number of LNGFr+ T lymphocytes.
Analysis of CMV-specific cytotoxic T-lymphocyte precursors (CTLp) frequency
To establish the frequency of anti-CMV CTLp, T lymphocytes previously cultured as described, were plated in limiting dilutions at 100 × 103, 30 × 103, 10 × 103, 3 × 103, and 1 × 103 cells per well (96 wells/plates) and stimulated with 60 Gy irradiated autologous PBMCs at the concentration of 40 × 103 cells/well pulsed with the pp65495-503 CMV peptide. Unpulsed stimulators were used as negative controls. Twenty wells were plated for each dilution of responders. IL-2 10 U/mL was added on days 3 and 6, and cells were tested in a cold inhibition 51Cr release assay on day 10. Statistical analysis was performed with the chi-square Poisson distribution method.
Results
Human lymphocytes are efficiently transduced by lentiviral vectors in the absence of TCR triggering
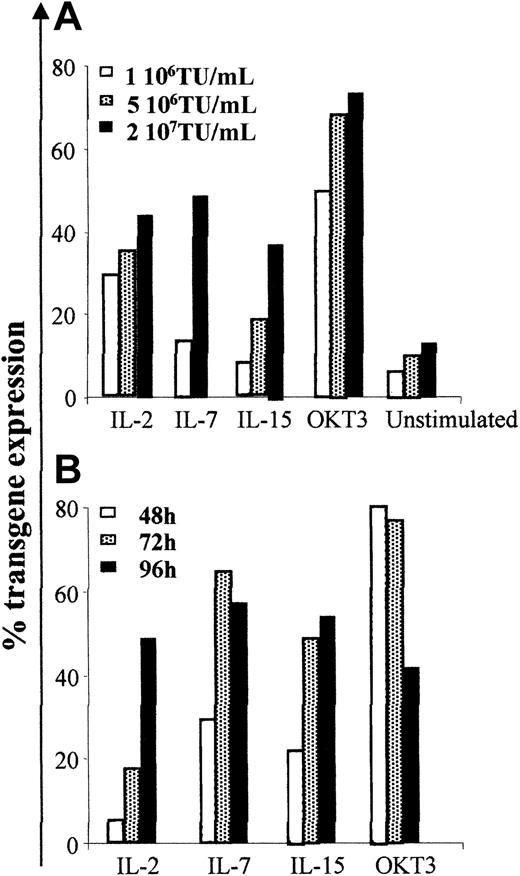
To transduce human T cells in the absence of TCR triggering, we cultured PBMCs for 96 hours in the presence of different cytokines (IL-2, IL-7, or IL-15) known to promote long-term survival of resting T cells in vitro, while maintaining their maturational status,24,34 and exposed them to increasing concentrations of third-generation lentiviral vectors encoding the ΔLNGFr marker gene under the control of the human phosphoglycerate kinase (PGK) promoter. We determined transduction efficiency by the proportion of viable cells expressing ΔLNGFr 96 hours after transduction by immunostaining and FACS analysis. We used T cells activated for 48 hours by the combination of anti-CD3 mAb and anti-CD28 mAb, and T cells cultured for the same time in the absence of mitogenic or cytokine stimulation as positive and negative controls, respectively. As shown in Figure 1A, TCR triggering resulted in the highest transduction efficiency. However, a 96-hour prestimulation with IL-2, IL-7, or IL-15 in the absence of TCR triggering was sufficient for efficient LV transduction. A low level of LV transduction was observed in unstimulated cells, most likely because of the low proportion of activated cells present in these nonpurified cultures (described in “LV transduction of human lymphocytes after cytokine stimulation does not require proliferation”). No significant differences in transduction efficiency were observed after stimulation with IL-2, IL-7, or IL-15. Transduction efficiency increased with LV concentration, reaching a plateau (ranging between 35% and 60%) at 2 × 107 TU/mL (MOI of 20:1) in all culture conditions. Therefore, this vector concentration was used for further experiments.
Human lymphocytes cultured in the presence of IL-2, IL-7, or IL-15 in the absence of TCR triggering are efficiently transduced by lentiviral vectors. PBMCs isolated from healthy donors were cultured in media alone or stimulated with 10 U/mL IL-2, 5 ng/mL IL-7, 10 ng/mL IL-15, or activated with 30 ng/mL OKT3 and 1 μg/mL anti-CD28, and cultured with 50 U/mL IL-2. (A) Cells were incubated for 3 hours with LV at different MOI: 1 × 106, 5 × 106, 2 × 107 TU/mL 96 hours after culture was started. (B) Cells were transduced with the highest vector dose (2 × 107 TU/mL) after 48, 72, or 96 hours of culture. Data shown are from 1 representative experiment of 3.
Human lymphocytes cultured in the presence of IL-2, IL-7, or IL-15 in the absence of TCR triggering are efficiently transduced by lentiviral vectors. PBMCs isolated from healthy donors were cultured in media alone or stimulated with 10 U/mL IL-2, 5 ng/mL IL-7, 10 ng/mL IL-15, or activated with 30 ng/mL OKT3 and 1 μg/mL anti-CD28, and cultured with 50 U/mL IL-2. (A) Cells were incubated for 3 hours with LV at different MOI: 1 × 106, 5 × 106, 2 × 107 TU/mL 96 hours after culture was started. (B) Cells were transduced with the highest vector dose (2 × 107 TU/mL) after 48, 72, or 96 hours of culture. Data shown are from 1 representative experiment of 3.
To identify the optimal time of transduction, cells were transduced at different times after stimulation. Although mitogen-activated cells could be efficiently transduced as early as 12 hours after activation, 96 hours of cytokine prestimulation were required to obtain the highest transduction efficiency in the absence of TCR triggering (Figure 1B; other data not shown). Similar results were obtained using LV coding for the GFP transgene. On the contrary, Moloney leukemia virus (MLV) retroviral vectors, used at optimized culture conditions for transduction of human lymphocytes, resulted reproducibly inefficient in transducing cells in the absence of TCR triggering (data not shown).
LV transduction of human lymphocytes after cytokine stimulation does not require proliferation
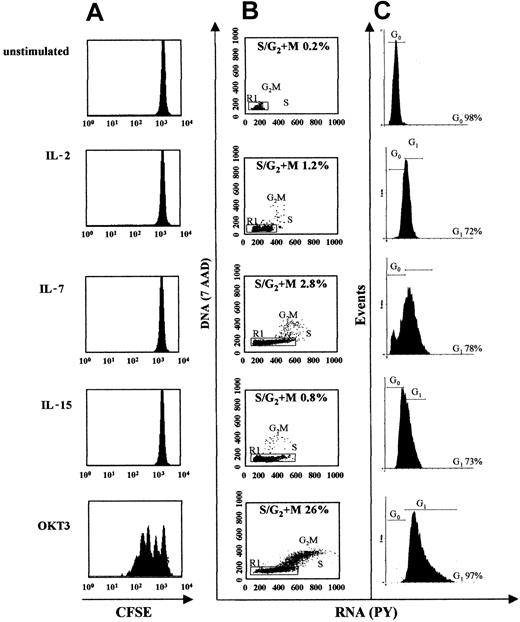
It has been proposed that the homeostatic expansion of T cells, responsible for the maintenance of a constant number of naive and memory T cells in individuals, is an antigen-independent event, regulated by cytokines binding γc-containing receptors.35 To determine whether prestimulation with cytokines was permitting LV transduction by promoting TCR-independent T-cell proliferation, PBMCs were stained with CFSE, a fluorescent dye that binds irreversibly to cellular components and becomes diluted at each subsequent cell division. Cells were stained with CFSE at the beginning of the culture and analyzed on the day of transduction. Cells activated with anti-CD3 plus anti-CD28 antibodies, and cells cultured in the absence of cytokines, were used as positive and negative controls. Although TCR-mediated activation induced a strong T-cell proliferation, no proliferation was observed after cytokine stimulation (Figure 2A), documenting that in these culture conditions cells become permissive to transduction by HIV-based vectors in the absence of proliferation, and suggesting that the LV susceptibility of human lymphocytes is dependent on cellular conditions other than proliferation.
LV transduction of human lymphocytes does not require cell proliferation and correlates with cell cycle progression from G0 to G1b (A) PBLs labeled with CFSE were cultured in vitro as described in Figure 1. Cells were analyzed 96 hours later for CFSE intensity by flow cytometry. (B) DNA and RNA contents were measured by 7AAD and pyronin Y (PY) staining, respectively. Analysis was performed after 96 hours of culture. The percentage of cells in S/G2/M is indicated. The R1 gate, encompassing the G0/G1 population, was used for further analysis. (C) Histogram of RNA staining of R1-gated cells plotted for PY staining to distinguish G0 from G1 cells. The percentage of cells in G0 or in G1 is indicated. Data are representative of 3 experiments.
LV transduction of human lymphocytes does not require cell proliferation and correlates with cell cycle progression from G0 to G1b (A) PBLs labeled with CFSE were cultured in vitro as described in Figure 1. Cells were analyzed 96 hours later for CFSE intensity by flow cytometry. (B) DNA and RNA contents were measured by 7AAD and pyronin Y (PY) staining, respectively. Analysis was performed after 96 hours of culture. The percentage of cells in S/G2/M is indicated. The R1 gate, encompassing the G0/G1 population, was used for further analysis. (C) Histogram of RNA staining of R1-gated cells plotted for PY staining to distinguish G0 from G1 cells. The percentage of cells in G0 or in G1 is indicated. Data are representative of 3 experiments.
Progression from G0 to G1 phase of the cell cycle correlates with LV susceptibility
We then performed cell cycle analysis on PBLs obtained from PBMCs by depletion of adherent cells and cultured in different conditions by measuring the cellular DNA and RNA content as described in “Materials and methods.” As shown by the DNA staining in Figure 2B, although 26% of lymphocytes were in the S/G2/M phases of the cell cycle 96 hours after TCR-mediated activation, less than 3% of cells prestimulated with cytokines entered S/G2/M phases 96 hours after cytokine prestimulation, at the time of efficient LV transduction. However, as shown by the RNA staining, most of the cytokine-treated cells moved out of the G0 phase, as observed in resting cells, into the G1 phase. The extent of progression into the G1 phase varied with the cytokine treatment, as most IL-2–treated cells remained in G1a/early G1b and a good fraction of IL-15– and most IL-7–treated cells further progress into late G1b.We obtained the same results when we repeated the analysis after transduction and 4 additional days of culture, suggesting that neither the cell culture nor the transduction procedures induced further progression of T cells through the cell cycle (Figure 2C). These results suggested that lymphocyte transition from G0 to the G1 phase without commitment to proliferation correlated with the acquisition of susceptibility to LV transduction.
To avoid any potential interference by the small fraction of activated cells in the PBL samples, we purified resting T cells from PBMCs isolated by Ficoll gradient separation as described earlier, by 3 subsequent rounds of negative selection as described in “Materials and methods.” We reproducibly obtained 98% pure fractions of resting T cells (CD3+, CD25–, HLA-DR–) and cultured them for 96 hours in the presence of IL-2, IL-7, or IL-15, respectively, before transduction at an MOI of 20:1. Transduction efficiency was 22% on average (ranging between 13% and 33%). A representative experiment of transduction of resting T cells is shown in Figure 3A. By a second vector incubation we could reach but not overcome an average level of transduction of 30%. As previously observed with total T cells, 96 hours of prestimulation were required to obtain the maximal transduction efficiency (data not shown). Nonstimulated T cells, transduced 24 hours after purification, were reproducibly and completely negative for transgene expression. The exposure to cytokines did not induce cell proliferation, as documented by CFSE staining (Figure 3B), whereas TCR-mediated activation of resting T cells induced multiple cell divisions during the time of analysis (data not shown). In accordance with the results obtained with unselected lymphocytes, cell cycle analysis revealed that the 3 cytokines tested induced a progression of resting T cells into the G1 phase but not into the S/G2/M phases (Figure 3C-D). Again, IL-2–treated cells remained in G1a/early G1b, whereas IL-15–treated and even more pronouncedly IL-7–treated cells progressed into late G1b.The viability of transduced lymphocytes ranged between 65% and 68% after 9 days of culture. We did not observe significant modifications in the level of transgene expression or in the expression of activation markers during culture (data not shown), suggesting that cytokine prestimulation allows stable transduction of resting T cells without inducing full T-cell activation.
Resting lymphocytes stimulated with IL-2, IL-7, or IL-15 in the absence of TCR triggering are efficiently transduced by lentiviral vectors. Resting T lymphocytes were isolated and purified from healthy donors as described in “Materials and methods.” Cells were cultured in the presence of 10 U/mL IL-2, 5 ng/mL IL-7, or 10 ng/mL IL-15. After 96 hours of stimulation resting T lymphocytes were transduced by LV. (A) Expression of ΔLNGFr 96 hours after transduction. The percentage of positive cells is shown. Only viable cells, as assessed by propidium iodide (PI) exclusion, were analyzed. (B) Cells were labeled with CFSE before stimulation with cytokines and analyzed at the day of transduction for CFSE intensity by flow cytometry. (C) DNA and RNA contents at the day of transduction were measured by 7AAD and PY staining, respectively. The percentage of cells in S/G2/M phases is indicated. The R2 gate, encompassing the G0/G1 population was used for further analysis. (D) Histogram of RNA staining of R2-gated cells plotted for PY staining to distinguish G0 from G1 cells. The percentage of cells in G1 is indicated. Data are representative of 4 experiments.
Resting lymphocytes stimulated with IL-2, IL-7, or IL-15 in the absence of TCR triggering are efficiently transduced by lentiviral vectors. Resting T lymphocytes were isolated and purified from healthy donors as described in “Materials and methods.” Cells were cultured in the presence of 10 U/mL IL-2, 5 ng/mL IL-7, or 10 ng/mL IL-15. After 96 hours of stimulation resting T lymphocytes were transduced by LV. (A) Expression of ΔLNGFr 96 hours after transduction. The percentage of positive cells is shown. Only viable cells, as assessed by propidium iodide (PI) exclusion, were analyzed. (B) Cells were labeled with CFSE before stimulation with cytokines and analyzed at the day of transduction for CFSE intensity by flow cytometry. (C) DNA and RNA contents at the day of transduction were measured by 7AAD and PY staining, respectively. The percentage of cells in S/G2/M phases is indicated. The R2 gate, encompassing the G0/G1 population was used for further analysis. (D) Histogram of RNA staining of R2-gated cells plotted for PY staining to distinguish G0 from G1 cells. The percentage of cells in G1 is indicated. Data are representative of 4 experiments.
To further investigate the relationship between progression from G0 phase into G1 phase and efficient transduction of resting naive and memory T cells by LV, we performed cell cycle analysis on transduced cells after immune selection (Figure 4).
Immunoselected LV-transduced resting T cells are mainly in G1 phase. Resting T lymphocytes were cultured with 5 ng/mL IL-7 or 10 ng/mL IL-15 and transduced 96 hours after stimulation. After an additional 96 hours, cells were analyzed for ΔLNGFr expression and immunoselected, as described in “Materials and methods.” Expression of ΔLNGFr after transduction (A) and after immunoselection (B) is shown. The percentage of ΔLNGFr-positive cells is indicated. Only viable cells, as assessed by PI exclusion, were analyzed. (C) DNA and RNA contents of immunoselected cells were measured by 7AAD and PY staining, respectively. The percentage of cells in S/G2/M phases is indicated. The R2 gate, encompassing the G0/G1 population, was used for further analysis. (D) Histogram of RNA staining of R2-gated cells plotted for PY staining to distinguish G0 from G1 cells. The percentage of cells in G1 is indicated. One of 2 similar experiments is shown.
Immunoselected LV-transduced resting T cells are mainly in G1 phase. Resting T lymphocytes were cultured with 5 ng/mL IL-7 or 10 ng/mL IL-15 and transduced 96 hours after stimulation. After an additional 96 hours, cells were analyzed for ΔLNGFr expression and immunoselected, as described in “Materials and methods.” Expression of ΔLNGFr after transduction (A) and after immunoselection (B) is shown. The percentage of ΔLNGFr-positive cells is indicated. Only viable cells, as assessed by PI exclusion, were analyzed. (C) DNA and RNA contents of immunoselected cells were measured by 7AAD and PY staining, respectively. The percentage of cells in S/G2/M phases is indicated. The R2 gate, encompassing the G0/G1 population, was used for further analysis. (D) Histogram of RNA staining of R2-gated cells plotted for PY staining to distinguish G0 from G1 cells. The percentage of cells in G1 is indicated. One of 2 similar experiments is shown.
Because IL-7 is more specifically active on naive T cells than IL-15, which exerts its activity mainly on memory cells,25,35-39 and both T-cell subsets are crucial for efficient T-cell–mediated gene therapy, we prestimulated resting T cells with IL-7 or IL-15 and subsequently transduced them twice with LV as described in “Materials and methods.” Transduced cells were immunoselected to enrich in ΔLNGFr-expressing cells as previously described5,29 and cell cycle was analyzed. All immunoselected cells remained in the G1 phase, confirming that the fraction of resting T lymphocytes transduced by LV was not committed to proliferation by IL-7 or by IL-15. Furthermore, immunoselection did not appear to enrich for cells in late G1b as compared with the starting population. Thus, progression from G0 to G1 phase correlates with efficient transduction of resting cells.
Cytokine stimulation does not affect the phenotype of human lymphocytes
To analyze the effects of cytokine prestimulation on different cell subsets, we analyzed the expression of cell surface molecules in unmanipulated (PBMCs) and genetically modified T cells from the same donor at day 9 of culture.
As shown in Table 1, in all tested cultures most of the transduced cells were represented by T lymphocytes (CD3+ cells), whereas a variable number of cells (ranging from 2% to 7%) showed a natural killer (NK) phenotype (CD3–/CD16+). As previously reported with RV-mediated transduction,7 among TCR-activated T cells, we usually observed an inversion of CD4+/CD8+ ratio. On the contrary, LV transduction after IL-2 and IL-7 but not IL-15 stimulation resulted in a physiologic CD4+/CD8+ ratio. To determine if cytokine-permitting LV transduction was acting by inducing a T-cell activation, or by promoting the selective survival of previously activated cells, we analyzed transduced lymphocytes for the expression of CD25, CD56. The analysis revealed that, although most of the transduced TCR-triggered cells were activated, more than 75% of cells transduced after IL-2 or IL-7 prestimulation were CD25–/CD56–, indicating that up-regulation of activation markers is not required for LV transduction. Interestingly, more than 50% of transduced cells stimulated with IL-15 up-regulated CD25, consistently with the ability of IL-15 to promote survival of activated T cells.25 Finally, in all tested conditions a substantial proportion of genetically modified T cells maintained the expression of CD62L, a crucial molecule for lymphocyte migration to lymph nodes,40 suggesting that the in vitro manipulation does not interfere with homing abilities. As already observed by Soares et al,36 both cytokine stimulation and TCR triggering induced an increase in CD95 expression.
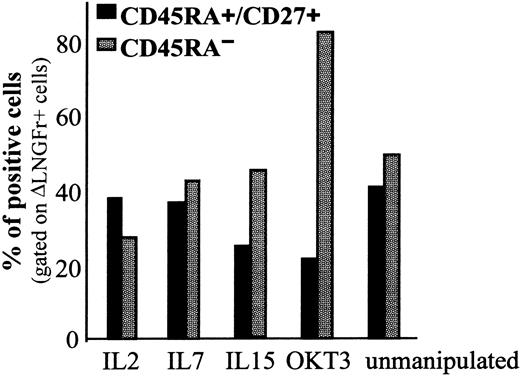
Naive (CD45RA+/CD27+) T cells, able to mount efficient immune responses to novel antigens, represent approximately half of the total T cells circulating in adults. Thus, transduction of naive T cells is a crucial prerequisite for any T-cell–mediated gene therapy trial aimed at providing long-lasting immune reconstitution to patients. As shown in Figure 5, both IL-2 and IL-7 prestimulation resulted in a physiologic memory–naive T cell ratio. On the contrary, IL-15 pretreatment and even more profoundly TCR activation resulted in a significant loss of naive cells.
Naive and memory phenotypes are preserved after cytokine stimulation and LV-mediated transduction. PBMCs were cultured in vitro as described and tested for the coexpression of CD45RA/CD27/ΔLNGFr. Percentage of naive (CD45RA+/CD27+) and memory (CD45RA–) cells was determined on ΔLNGFr+ cells by flow cytometry 96 hours after transduction.
Naive and memory phenotypes are preserved after cytokine stimulation and LV-mediated transduction. PBMCs were cultured in vitro as described and tested for the coexpression of CD45RA/CD27/ΔLNGFr. Percentage of naive (CD45RA+/CD27+) and memory (CD45RA–) cells was determined on ΔLNGFr+ cells by flow cytometry 96 hours after transduction.
Cytokine prestimulation results mainly in γ-IFN–producing cells
To determine the effect of in vitro manipulation on T-cell cytokine production, we analyzed T cells transduced in different conditions for intracytoplasmic cytokine production by stimulation with anti-CD3 and TPA followed by cell fixation, permeabilization, and staining as described in “Materials and methods.” We chose γ-IFN as representative cytokine for the Th1/Tc1 and IL-4 as representative cytokine for the Th2/Tc2 T-cell subsets.30 We used IL-10 as a representative cytokine produced by regulatory T cells.41,42 As shown in Table 2, we detected intracytoplasmic production of all cytokines, although in different amounts. In fact, a high percentage of cells prestimulated with IL-7 or IL-15 and of cells activated with anti-CD3 produced γ-IFN. IL-2–stimulated cells produced only a low level of γ-IFN. A minimal proportion of cells produced IL-4 and IL-10.
Cytotoxic T cells specific for CMV antigens are preserved after gene transfer in different culture conditions
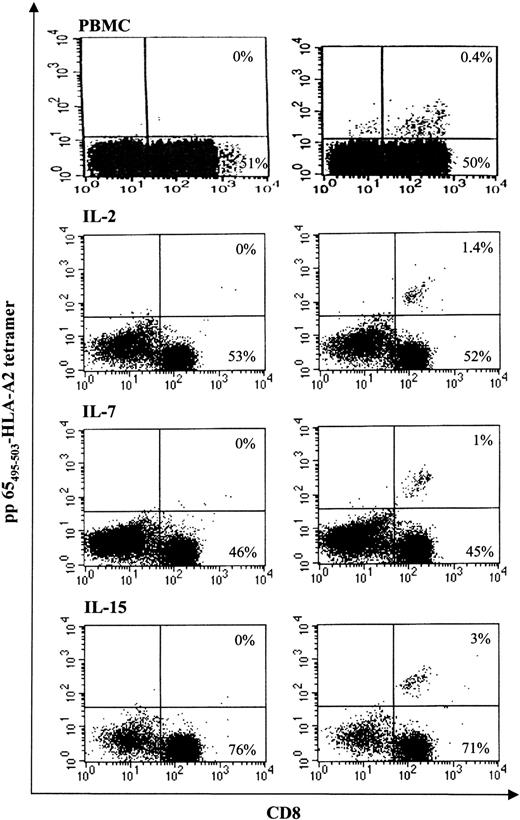
CMV is a significant cause of morbidity and mortality in individuals undergoing stem cell transplantation and represents a useful model antigen to investigate the immune potential of transduced T cells. To determine if cytokine prestimulation and gene transfer resulted in a reduced frequency of CTL specific for CMV, we stained LV-transduced cells and autologous PBMCs from CMV seropositive, HLA-A0201+ donors with a PE-conjugated tetramer specific for the immunodominant HLA-A0201–restricted pp65495-503 peptide derived from the matrix protein pp65 of CMV. As shown in Figure 6, the frequency of CD8+ pp65 tetramer binding cells detected in unmanipulated cells was fully preserved in all populations of transduced T cells cultured for 9 days with IL-2, IL-7, or IL-15. The percentage of pp65 tetramer binding cells was on average similar in PBMC, IL-2–, IL-7–, IL-15–stimulated cells. The same frequency was detected in untransduced T cells cultured in the same conditions (not shown), documenting that neither cytokine treatment nor LV-transduction results in a loss of CMV-specific T-cell precursors.
CTLs specific for pp65 from CMV are preserved in LV-transduced cells. T cells specific for the immunodominant HLA-A0201–restricted pp65495-503 peptide derived from the matrix protein pp65 of CMV were detected by staining with CD8-QR, pp65 tetramer-PE, and LNGFr-FITC on IL-2–, IL-7–, IL-15–stimulated and transduced cells or CD3-FITC on PBMCs followed by flow cytometry analysis. The right panels show the percentage of CD8+/pp65-tetramer+ T cells analyzed on CD3+ cells on PBMCs and on LNGFr+ T cells and IL-2, IL-7, and IL-15 cells. Appropriate isotypic controls are shown in the left panels.
CTLs specific for pp65 from CMV are preserved in LV-transduced cells. T cells specific for the immunodominant HLA-A0201–restricted pp65495-503 peptide derived from the matrix protein pp65 of CMV were detected by staining with CD8-QR, pp65 tetramer-PE, and LNGFr-FITC on IL-2–, IL-7–, IL-15–stimulated and transduced cells or CD3-FITC on PBMCs followed by flow cytometry analysis. The right panels show the percentage of CD8+/pp65-tetramer+ T cells analyzed on CD3+ cells on PBMCs and on LNGFr+ T cells and IL-2, IL-7, and IL-15 cells. Appropriate isotypic controls are shown in the left panels.
Immune competence of cytotoxic T cells specific for CMV antigens is preserved after IL-2 and IL-7 prestimulations
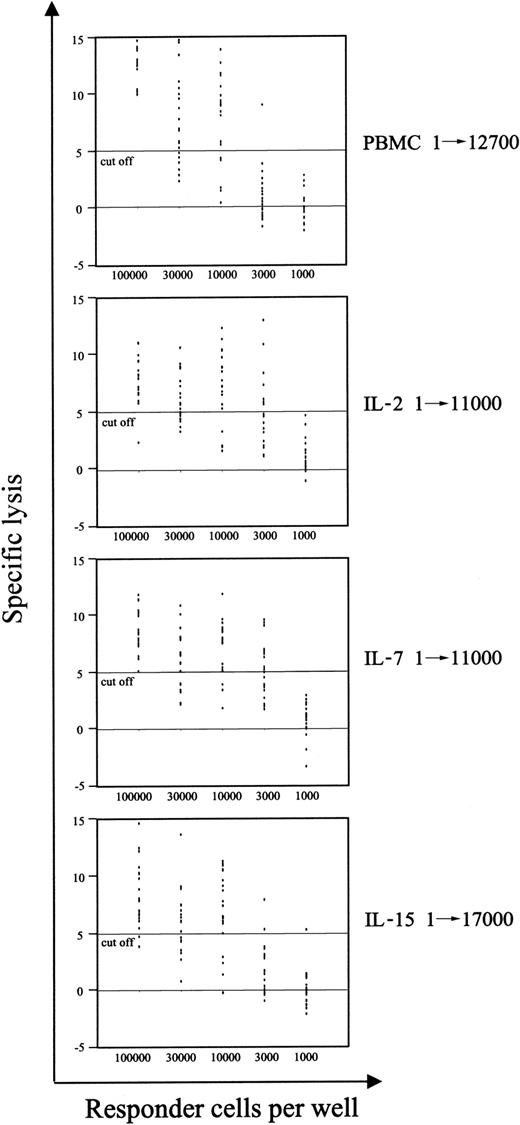
An efficient T-cell–mediated immune response against viral as well as tumor antigens relies not only on the presence of a sufficient number of antigen-specific T cells but also on the ability of T cells to be specifically and properly activated by the exposure to the antigen. In our previous report,7 we showed by limiting dilution analysis (LDA) that TCR activation resulted in a decrease in the frequency of CTLps. In the current work, we analyzed the immune competence resulting by the new conditions of LV transduction, by measuring the frequency of CTLp specific for pp65 of CMV. In Figure 7, we show that stimulation with IL-15 impaired the ability of CTLs specific for CMV to proliferate and specifically kill autologous cells presenting the pp65495-503 peptide. On the contrary, the immune reactivity of such CMV-specific T cells was completely preserved after IL-2 or IL-7 stimulation, as suggested by the frequency of CMV-specific CTLp (1/11 000), which was the same detected in unmanipulated T cells, and was in the normal range observed in CMV+ healthy donors.43 Furthermore, analysis of T-cell proliferation induced by polyclonal, allogeneic, and viral antigens showed that IL-15–stimulated cells have reduced proliferative ability compared with IL-7– and IL-2–stimulated effectors (data not shown), suggesting that protocols of LV transduction based on the use of IL-2 and/or IL-7, but not IL-15, could preserve a functional T-cell repertoire.
The frequency of CMV-specific CTL precursors is preserved after IL-2 and IL-7 prestimulations. The frequency of pp65-specific CD8+ T-cell precursors was measured for each culture condition as described in “Materials and methods.” PBMCs and cells at day 9 of culture were stimulated in limiting dilution numbers with autologous cells pulsed with the pp65495-503 peptide. A 51Cr release assay was performed 10 days later. Regression curves were interpolated, and precursor frequencies were determined according to Poisson statistics. Precursor frequencies are shown.
The frequency of CMV-specific CTL precursors is preserved after IL-2 and IL-7 prestimulations. The frequency of pp65-specific CD8+ T-cell precursors was measured for each culture condition as described in “Materials and methods.” PBMCs and cells at day 9 of culture were stimulated in limiting dilution numbers with autologous cells pulsed with the pp65495-503 peptide. A 51Cr release assay was performed 10 days later. Regression curves were interpolated, and precursor frequencies were determined according to Poisson statistics. Precursor frequencies are shown.
Discussion
Since gene therapy entered the era of clinical testing, a number of issues potentially limiting its efficacy have been raised, such as the immunogenicity of the transgene44,45 or perturbation of cell function8,9 as a result of in vitro manipulation procedures. The use of a cell surface marker, such as the nonimmunogenic human ΔLNGFr to immunoselect transduced cells,29 partially overcame some of these limitations.7,8,46 However, the polyclonal T-cell activation and the high doses of hu-r-IL-2 required for efficient RV-mediated gene transfer into human lymphocytes alter the immune potential of donor T cells, thus reducing the efficacy of retroviral-mediated gene therapy. In this work, we showed that lentiviral vectors allow for efficient gene transfer into human lymphocytes in the absence of TCR triggering, resulting in preservation of an intact T-cell repertoire. T-cell populations are maintained in vivo at constant numbers by a tight homeostatic control,47,48 able to maintain appropriate numbers and proportions of naive and memory CD4+ and CD8+ T cells both in the absence and in the presence of antigenic stimulations.49,50 Although memory T cells are crucial for recall responses to antigens, naive cells play a pivotal role in maintaining the capacity to respond to novel antigens. Therefore, efficient T-cell gene therapy requires the maintenance of the physiologic proportion of all T-cell subsets. Cytokines whose receptors use the common γ-chain have been reported to mediate survival signals for both naive and memory T cells without altering their phenotype.25,48 In this paper we showed that stimulation with low doses of IL-2, IL-7, or IL-15 allows for an efficient LV-mediated gene transfer by late-generation LV, without inducing T-cell proliferation. The third-generation LV, optimized by the inclusion of the central polypurine tract and termination sequences and by the posttranscriptional regulatory element of the woodchuck hepatitis virus,12 packaged by a conditional split-genome system lacking HIV-1 tat and all accessory genes to improve its safety,27 resulted in a transduction efficiency on human lymphocytes significantly higher than the one reported in the literature for earlier vector versions. The cytokine-treated cells moved out of G0 into the G1 phase of the cell cycle but did not become committed to further progression and did not start to proliferate. Our results confirm and extend previously published findings with HIV-1 and second-generation HIV-based vectors that analyzed the relationship between cytokine-induced progression of resting T cells from G0 into the G1b phase of the cell cycle and the acquisition of HIV susceptibility.22,23,26 In accordance with these works, most of the T cells that we analyzed after transduction with third-generation LV and immunoselection for transgene expression had entered the G1 phase of cell cycle but not the S/G2/M phases. Although a small fraction of cells appeared in G0 after immunoselection, it is highly unlikely that these cells represented transduced lymphocytes, because we have been consistently unable to transduce purified resting T cells. Thus, most likely, these cells represented a fraction of untransduced cells contaminating the immunoselected transduced lymphocytes. Although all cytokines tested induced the treated cells to exit from G0, the extent of progression into the G1b phase differed, with IL-2–treated cells remaining in a G1a/earlyG1b phase and most IL-7–treated cells progressing to a late G1b phase. However, we did not detect significant differences in the efficiency of transduction of cells treated with the different cytokines, suggesting that progression to a late G1b phase was not required for LV transduction. As was also observed in the other works, we only reached transduction of a fraction of cytokine-treated cells, even by repeated exposure to the vector, as opposed to almost 100% transduction of TCR-activated lymphocytes. To avoid any interference from the expression of fluorescent marker genes in the cells to be analyzed, we used the ΔLNGFR marker, immunoselected the transduced cells, and analyzed them without costaining. We found that immunoselection did not enrich for cells in late G1b. Thus, the cellular condition(s) induced by cytokine treatment and responsible for LV susceptibility may be distinct from those promoting cell cycle progression and remain to be identified. Their identification may illuminate the nature of a major in vivo target of HIV-1 infection.51,52
However, because we demonstrated efficient transduction without cell proliferation not only in purified resting T cells24 but also in unmanipulated PBMCs, and with the use of advanced generation LV, this approach is particularly attractive and feasible for clinical application. Furthermore, we observed that cytokine-induced G0 to G1 cell cycle progression did not select or modify the phenotype of transduced T cells. However, analysis of T-cell function revealed that, despite the use of a common γ-chain receptor, different cytokines mediate preferential survival of different T-cell subsets. Although cells stimulated with IL-7 or IL-2 remained similar to unmodified cells in terms of CD4+/CD8+ ratio, low expression of activation markers, and ratio of naive to memory T cells, IL-15 consistently proved to enrich the population of gene-modified T cells in CD8+ memory/activated T-cell subsets. This skew of the immune repertoire was almost as profound as the one observed after polyclonal activation, making this cytokine less attractive for gene therapy purposes.
Transduction of resting primary T lymphocytes without phenotypic skewing was recently shown using LVs engineered to express IL-7 on their surface,53 providing an independent confirmation of our results in different experimental settings.
One of the first clinical applications of T-cell–mediated gene therapy relies on the transfer of a suicide gene in allogeneic lymphocytes for immune reconstitution, antitumor activity, and control of graft-versus-host disease occurring after hematopoietic stem cell transplantation. Therefore, in this work, immune competence of genetically modified cells was studied using as a model antigen CMV, a significant cause of morbidity and mortality after SCT.54
As already reported on RV-transduced T cells, LV-mediated gene transfer after cytokine stimulation maintained an intact repertoire of antigen-specific T cells, as measured by staining with a tetramer of the immunodominant pp65495-503–HLA-A2 complex. However, a functional quantitative assay showed that a significant proportion of T cells stimulated with IL-15 failed to mediate a cytotoxic activity against autologous cells presenting the pp65495-503 peptide. This observation, combined with the activated phenotype observed after IL-15 stimulation, is in accordance with our previous work, showing that T-cell activation required for RV-mediated transduction results in loss of antiallogeneic CTL precursors7 and could be explained as a result of a refractory phase of nonresponse to additional stimuli induced by T-cell activation, or by activation-induced T-cell death secondary to TCR religation.55 Accordingly, stimulation with IL-2 or IL-7, able to fully preserve the maturational status of T cells, resulted in a complete preservation of the immune reactivity of CMV-specific T cells. On the basis of these results, we propose a method of lentiviral-mediated transduction of human T cells based on prestimulation with the lowest concentration of IL-2 and Il-7 reported in the literature for in vitro culture of T cells to preserve an intact and functional T-cell repertoire. Possible limitations of this approach are the lower frequency of transduction as compared with TCR-activated cells and the lack of ex vivo expansion of gene-modified cells. Further studies will be carried out to identify whether the survival signal provided by IL-2 and the antiapoptotic signal provided by IL-7 could act synergistically, thus increasing the overall efficacy of the T-cell–mediated gene therapy.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2003-01-0297.
Supported by the European Community (QLK3-1999-00859 and QLK3-CT-2001-01265), the Italian Health Ministry (AIDS Program 40C.66), and the Italian Association for Cancer Research (AIRC).
S. Cavalieri and S. Cazzaniga contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.
We thank Francesca Santoni De Sio and Lucia Sergi Sergi (IRCC) for help with some of the experiments and Catia Traversari and Paolo Dellabona for helpful discussion.