Abstract
We created parabiotic mice, joining ROSA26 and PeP3b animals, to study the trafficking of hematopoietic stem cells (HSCs) from marrow to blood and their return to marrow. The transfer of HSCs was assayed by secondary marrow transplantation and was 1.0% to 2.5% after 3, 6, 8, and 12 weeks. Thus, HSC homeostasis is primarily maintained by the retention of stem cells derived from replication events within the marrow, not the homing and engraftment of HSCs from the circulation. Of interest, the phenotypes of marrow progenitors and granulocytes were similar to those for HSCs, implying that the marrow functions as an intact compartment where differentiating cells derive from endogenous HSC. In contrast, 50% of splenic granulocytes and progenitor cells derived from the parabiotic partner, suggesting splenic progenitor cells were in constant equilibrium with progenitors in blood. In additional studies, animals were exposed to granulocyte–colonystimulating factor (G-CSF) and stem cell factor at days 17 to 20 of parabiosis and were studied 3 weeks later; 10.1% of marrow HSCs derived from the parabiotic partner. These data imply that HSCs, mobilized to the blood in response to cytokine exposure, are destined to later return to marrow, an observation that supports the concept that the mobilized peripheral blood stem cells used in clinical transplantation function physiologically.
Introduction
Although much is known about how hematopoietic stem cells (HSCs) traffic from marrow to blood,1,2 little is understood concerning the physiologic necessity for such trafficking. Is mobilization a mechanism to protect stem cells from toxic injury? If so, stem cells may continuously exit marrow, circulate in blood, then return to marrow. Alternatively, is mobilization a homeostatic mechanism to maintain a fixed stem cell number? If so, stem cells may exit the marrow when the adjacent microenvironment cannot support their survival or persistence. This latter hypothesis is consistent with the concept that stem cell integrity requires an accommodating niche. Understanding the functional significance of stem cell trafficking has major implications for stem cell biology and transplantation.
To investigate this issue, we studied parabiotic mice, adapting the procedures of Bunster and Meyer introduced in 1933.3 Parabiosis has been used to demonstrate the existence of hormones4,5 and to analyze lymphocyte trafficking.6,7-9 In early hematopoietic studies, Warren et al10 linked a healthy rat to a lethally irradiated partner. Hematopoiesis was restored in the irradiated partner, and its marrow showed normal cellularity when studied at 14 weeks. Thus, sufficient numbers of stem cells are present in blood to reconstitute damaged or depleted hematopoiesis, and the protection of HSCs against environmental insult may be a physiologic role for mobilization. Others have studied the transfer of hematopoietic cells between phenotypically distinct healthy (ie, nonirradiated) parabionts. Harris et al9 showed that 5% of mitotic cells in marrow had a partner cytogenetic phenotype after 4 to 5 weeks of parabiosis; Mejino et al7 showed a stable low (5%) level of partner spleen cell colony-forming unit (CFU-S) after 5 and 15 weeks of parabiosis; and Wright et al11 demonstrated that there was little (0%-9%) transfer of Thy1.1low, lineage-negative, Sca-1+, c-kit+ cells, a population inclusive of HSCs, after 7, 16, or 39 weeks of parabiosis. In our studies, ROSA26 and PeP3b mice, congenic on a C57BL6 background, were used to create parabiotic pairs. PeP3b mice express the CD45.1 antigen on their hematopoietic cells, whereas hematopoietic cells in ROSA26 mice express CD45.2, phenotypes readily distinguishable by flow cytometry. In addition, all cells in ROSA26 mice express a transgene encoding β-galactosidase and neomycin resistance. In initial studies, we confirmed that the circulation of parabiotic mice is shared after 14 days by determining the percentage of granulocytes and lymphocytes of partner phenotype in the blood of each parabiont. We then tested the transfer of HSCs from PeP3b to ROSA26 mice and vice versa after 3, 6, 8, and 12 weeks of parabiosis using a functional (transplantation) assay. Marrow cells from the ROSA26 parabiont were transplanted into lethally irradiated ROSA26 (or C57BL6) mice, and marrow cells from the PeP3b parabiont were transplanted into lethally irradiated PeP3b mice. In all transplantations, 5 to 10 million cells were transferred to ensure that studies of recipient mice would reflect the phenotype of partner HSCs, without the variable stochastic contributions that occur when small numbers of stem cells are transplanted. HSC phenotype was determined by assaying blood granulocytes, marrow granulocytes, and marrow granulocyte/macrophage progenitor cells (CFU-GMs) in the recipient mice 3 months after transplantation, using the independent techniques of CD45.1 versus CD45.2 expression on blood and marrow cells and of β-galactosidase staining of GM colonies. The trafficking of HSCs to spleen was also assessed using a comparable experimental design.
Our findings confirm that few HSCs exit marrow, transit blood, and reenter marrow during 3 to 12 weeks of parabiosis. Thus, they suggest that under normal homeostatic conditions, HSCs exit marrow as a 1-way process, potentially a death pathway. In contrast, however, cytokine exposure significantly increased the return of HSCs to marrow, as well as their mobilization to blood.
Materials and methods
Mice
Heterozygous ROSA26 (C57BL/6J-Gtrosa26. Ly5.2), C57BL/6 (B6.Ly5.2), and PeP3b (B6 SJL/.Ly5.1) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were bred at the University of Washington under specific pathogen-free conditions. ROSA26 homozygous offspring of heterozygous matings were identified by polymerase chain reaction (PCR) typing of tail-tip genomic DNA.12 ROSA26 homozygous mice were used in all experiments.
Parabiosis
Pairs of 6- to 10-week-old, sex- and weight-matched ROSA26 and PeP3b mice were housed together in a single cage for 1 to 2 weeks, then were subjected to parabiotic surgery using methods adapted from Bunster and Meyer.3 Mice were anesthetized with 0.020 to 0.026 mL 2.5% tribromoethanol (Avertin; Aldrich, Milwaukee, WI) per gram body weight. Operative sides were shaved and sterilized. Lateral skin was opened from hip to shoulder and freed of attached tissue. Opposing muscle and perineum was sutured with 4-0 chromic gut (Roboz, Rockville, MD), and corresponding skin was joined with 9-mm wound clips (Fisher Scientific, Houston, TX). Parabiotic mice were provided with water bottles with extra-long necks for easy access. Half the wound clips (alternating) were removed after 1 week of parabiosis, and the remaining clips were removed after 2 weeks. One hundred microliters blood was collected from the orbital sinus (after sedation with a 2.5%-4.0% isoflurane inhalant anesthetic; Summit Medical, Portland, OR) to document joint circulation by flow cytometry of granulocytes and lymphocytes. At the time of killing (weeks 3-12), parabiotic mice were anesthetized with tribromoethanol and separated through transection at the anastomosis site. Before separation, 0.6 to 1.0 mL blood was obtained by eye puncture. After separation, 2 to 4 × 107 marrow cells were obtained by dissection of femurs, and 3 to 6 × 107 spleen cells were obtained by the passage of minced spleen through nylon mesh.
Growth factor mobilization
Recombinant human granulocyte colony-stimulating factor (G-CSF) and stem cell factor (SCF) were provided by Amgen (Thousand Oaks, CA). Each day from days 17 to 20 of parabiosis, after light sedation with isoflurane inhalant, each parabiont (average weight, 25 g) was injected subcutaneously with 200 μg/kg recombinant human G-CSF and 25 μg/kg recombinant human SCF. Mice were separated and studied at day 21 (3 weeks of parabiosis) or day 42 (6 weeks of parabiosis).
Transplantation
Before transplantation of 5 to 10 × 106 marrow or blood mononuclear cells or 30 × 106 spleen mononuclear cells, recipient mice received 1100 cGy radiation (from a dual cesium source). Cells from the ROSA26 and PeP3b parabionts were infused into 2 to 3 C57BL6 (or ROSA26) and 2 to 3 PeP3b recipients, respectively. For some experiments, blood cells from 2 parabionts were pooled to allow sufficient numbers of HSCs for transplantation into 1 to 2 irradiated recipients. Three months after transplantation, the percentages of blood and marrow granulocytes of ROSA26 versus PeP3b phenotype were determined by flow cytometry, and the percentage of marrow CFU-GM of ROSA26 versus PeP3b phenotype was determined by analysis of agar cultures.13 The average of these 3 values was considered the percentage of HSCs of partner phenotype in each secondary recipient. For each parabiont, the percentage of partner HSCs was set equal to the average (± SD) of values from all secondary recipients. Transplanting cells from the ROSA26 parabiont into C57BL6 recipients allowed us to quantify any recovery of endogenous hematopoiesis in the secondary host and thus served as an internal methodological control. If inadequate numbers of HSCs were transferred and recipient hematopoiesis recovered, the percentage of PeP3b partner cells, as estimated by the GM colony assay, would have been greater than the value derived by measuring the percentage of granulocytes or marrow cells with a CD45.1 phenotype.
Flow cytometry
Percentages of partner cells were determined by staining with partner-specific antibody (fluorescein isothiocyanate [FITC]–antimouse CD45.1 or CD45.2) coupled with antibodies to Gr1 (granulocytes, phycoerythrin [PE] labeled) and CD3 (lymphocytes, chrome-labeled)6 (PharMingen, San Diego, CA). Gates were set so that less than 0.5% of cells from control mice were considered positive. In early experiments, fluorescein di-β-d galactopyranoside (FDG; Molecular Probes, Eugene, OR) and PE-Gr1 were used to determine β-galactosidase expression in granulocytes. The separation of FDG-positive and -negative cells was not as distinct as for CD45.1- or CD45.2-positive and -negative cells (data not shown).
Progenitor cell culture
Methods for CFU-GM assays and for quantitating the number of GM colonies containing β-galactosidase have been described.13
Results
Parabiont circulations rapidly link after surgery
Anastomosis of the 2 circulations was detected 5 to 7 days after parabiotic surgery, as evidenced by FITC-labeled bovine serum albumin in the circulation of the parabiont opposite the site of intravenous injection. The percentage of partner blood cells was also observed and reached plateau levels between days 10 and 14 in all animals. Mean ± standard deviation of partner lymphocytes at day 14 was 47.9% ± 13.2% (n = 22), demonstrating full patency for cell trafficking. The percentage of partner granulocytes (39.1% ± 6.3%; n = 22) was lower than the percentage of partner lymphocytes (P < .01; 2-tailed Student t test), which could reflect the stress release of host granulocytes from marginal to circulation pools at the time of these analyses because of the lighter (isoflurane) sedation, or it could reflect changes in granulocyte kinetics specific to the postoperative setting. In studies obtained at the time of killing (tribromoethanol anesthesia; 3, 6, and 12 weeks of parabiosis), there was no significant difference between the percentages of lymphocytes and granulocytes of partner phenotypes (P > .3).
Hematopoietic stem cells do not rapidly and continuously exit and reenter marrow
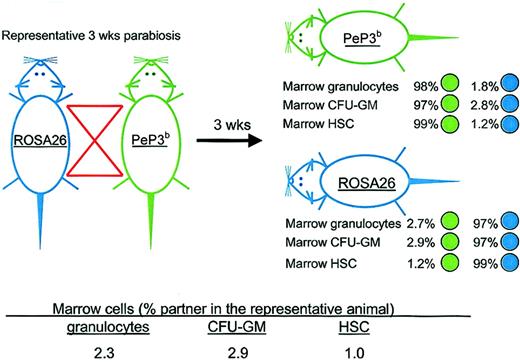
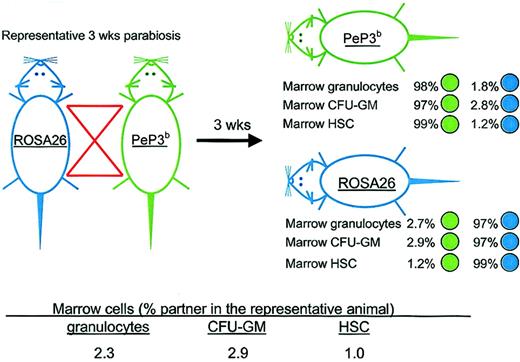
The transfer of HSCs was assayed after 3, 6, 8, and 12 weeks of parabiosis. Figure 1 shows the experimental design and results from a representative pair studied at 3 weeks of parabiosis. After ROSA26 and PeP3b parabionts were separated, the origins of marrow granulocytes, CFU-GMs, and HSCs were assayed by flow cytometry, β-galactosidase staining of GM colonies in culture, and long-term engraftment of marrow cells transplanted into irradiated recipients, respectively. Cumulative data from studies at all time points are shown in Table 1. After 3 weeks of parabiosis, the percentage of partner HSCs in marrow was 1.0% ± 0.8%; after 6 weeks, it was 1.4% ± 0.4%; after 8 weeks, it was 2.5% ± 3.0%; and after 12 weeks, it was 1.2% ± 0.5%. Thus, few HSCs transferred from the marrow of one parabiont to the marrow of its partner during the time of observation. Interestingly, the percentages of marrow granulocytes and CFU-GMs of partner origin at the times of separation of the parabiotic pairs were also low and similar to those of HSCs (Table 1), implying that these cells derived from HSC resident in the marrow, not from the engraftment of progenitors circulating in blood, and that marrow functions as an intact compartment.
Representative parabiotic study. Outline of the experimental approach and results from a representative study in which ROSA26 and PeP3b parabionts were joined for 3 weeks. After separation, the percentages of marrow granulocytes, CFU-GMs, and HSCs of PeP3b (green) versus ROSA26 (blue) phenotype were assessed in each parabiont. To calculate the percentage of marrow granulocytes and of CFU-GMs and HSCs that were exchanged, data from the parabiotic pair were averaged as shown.
Representative parabiotic study. Outline of the experimental approach and results from a representative study in which ROSA26 and PeP3b parabionts were joined for 3 weeks. After separation, the percentages of marrow granulocytes, CFU-GMs, and HSCs of PeP3b (green) versus ROSA26 (blue) phenotype were assessed in each parabiont. To calculate the percentage of marrow granulocytes and of CFU-GMs and HSCs that were exchanged, data from the parabiotic pair were averaged as shown.
HSC trafficking in spleen
Similar studies of HSC trafficking to the spleen show that 38.1% to 55.3% of splenic granulocytes and 46.0% to 49.1% of splenic CFU-GMs were of partner origin, values comparable to those for granulocytes, lymphocytes, and progenitors in blood (Table 1). Thus, it appears that splenic progenitors, and likely mature cell populations, are in continual equilibrium with circulating cells and do not derive exclusively from HSCs resident in spleen. When assessed by secondary transplantation studies, 1.5% to 3.6% of splenic HSCs had a partner phenotype.
Cytokines induce the exit of HSCs to blood from marrow, their transit through blood, and their reentry to marrow
Additional experiments were performed to study the effect of cytokine mobilization on HSC trafficking. For these experiments, each mouse received G-CSF and SCF subcutaneously on days 17, 18, 19, and 20 of parabiosis. Seven pairs of ROSA26/PeP3b mice were studied. Marrow and blood cells from 3 pairs were analyzed on day 21 (ie, 1 day after cytokine administration and 3 weeks after joining). Marrow and blood cells from the other 4 pairs were analyzed on day 42 (ie, 3 weeks after cytokine administration and 6 weeks after joining). As shown in Table 2, when studies were performed at day 21, 2.9% ± 1.1% marrow HSCs were of partner origin. Thus, few HSCs exited marrow and immediately returned.
Circulating blood cells from each parabiont were also studied at day 21. As expected, 40.9% ± 4.3% of blood granulocytes were of partner origin confirming that circulations were indeed shared. Similarly, 46.3% ± 11.1% of blood CFU-GM were of partner origin. Importantly, however, only 25.4% ± 8.6% of blood HSCs were of partner origin (P = .001; 2-tailed Student t test). The range was 15.5% to 30.9% in individual parabionts. This likely implies that HSCs in blood are in rapid flux so that complete mixing does not occur despite the shared circulation. These data also reinforce the concept that marrow is an intact compartment where HSCs engraft. Although 25.4% of blood HSCs were of partner origin, these cells did not freely equilibrate with marrow because 2.9% of marrow HSCs had a partner phenotype.
In the parabiotic animals treated with cytokines on days 17 to 20 and killed on day 42, 10.1% ± 6.2% of marrow stem cells were of partner origin (Table 2), significantly more than observed in baseline experiments (ie, with no cytokine administration) (P = .02). The data thus demonstrate that G-CSF and SCF induce the mobilization of HSCs from marrow to blood and their subsequent return to marrow. It is likely that circulating HSCs returned to marrow during the first week after cytokine exposure because the marrow compartment achieved a new steady state by 3 weeks after cytokine exposure, in which the percentage of partner HSCs (10.1%) was equivalent to the percentage of partner progenitors (10.6%) and the percentage of partner granulocytes (10.9%).
To assess the phenotype of blood HSCs on day 42 of parabiosis, blood cells from 2 ROSA26 (or PeP3b) parabionts were pooled before secondary transplantation into irradiated C57BL6 (or PeP3b) recipients. As shown in Table 2, only 1.5% ± 0.9% of blood HSCs had a partner phenotype. If the percentage of blood HSCs with partner phenotypes in each parabiont mirrored the percentage of its marrow HSCs with partner phenotypes, one would expect that 10% of blood HSCs would have a partner phenotype. Any additional contribution of HSCs from the parabiotic partner would raise this percentage. Thus, our observations argue that mobilized HSCs, after returning to marrow, are relatively unlikely to exit.
Discussion
Our studies of HSC trafficking provide novel insights into stem cell biology and into clinical transplantation. Mobilized peripheral blood stem cells are the preferred cell source for transplantation because large numbers can be collected with minimal morbidity for the donor and because their engraftment in patients is faster, resulting in less morbidity and mortality from cytopenias.14,15 Prior investigations argue that peripheral blood stem cells differ qualitatively from marrow stem cells. These cells have altered cell surface phenotypes, including the decreased expression of adhesion molecules, different cell cycle characteristics, and different mRNA expression patterns.16-19 Whether these are relevant determinants of persistence or function is unclear, but the presumption is that mobilized HSCs not only reconstitute blood cell production after transplantation, they also function to support hematopoiesis throughout the recipient's lifetime. Our parabiosis studies of the physiologic fate of mobilized HSCs strongly support this concept. Three weeks after mobilization with G-CSF and SCF, 10.1% of marrow HSCs were of partner origin. These cells must have exited the marrow, transited the blood, and entered the marrow of the opposite parabiont. Because 10.6% of marrow CFU-GMs and 10.9% of marrow granulocytes were also of partner origin, the HSCs that circulated and re-engrafted, contributed to hematopoiesis comparably to endogenous (nonmigratory) marrow HSCs.
Of note, the cytokine mobilization protocol used for these studies was not optimized to ensure maximal HSC exit or return but instead was adapted from the studies of Bodine et al20 so that the results of our experiments could be discussed in the context of these investigations. In those studies and in our preliminary experiments, maximum numbers of blood granulocytes were seen at 4 to 6 days after cytokine exposure and reached numbers only 2 to 4 times baseline20 (and data not shown), contrasting the more dramatic mobilization induced by other agents21 and in studies of non-C57BL6 strains of mice.22 Bodine et al20 tracked HSC mobilization with competitive transplantation and demonstrated that fewer HSCs were present in marrow immediately after cytokine exposure than at baseline but that high numbers were seen 2 weeks later. Our results prove that the high number of HSCs in marrow after cytokine exposure reflects the engraftment of circulating HSCs and cannot exclusively result from cytokine-induced replication and retention of HSCs resident in marrow. Cytokines indeed mobilize HSCs from marrow to blood, and circulating HSCs later compete for open marrow niches.
Our data also provide unique insights into the trafficking and compartmentalization of HSCs during homeostasis. Perhaps surprisingly, there was little exchange of HSCs from the marrow through the blood and into the marrow under basal conditions (ie, animals not given cytokines). Data from several previous studies provide a context for these observations. 5-Bromodeoxyuridine (BrdU) uptake and limiting-dilution competitive transplantation studies suggest that the median time to murine HSC replication is 1.7 weeks.23-25 To maintain steady state with a constant number of marrow HSCs, this rate must approximate the combined rates of HSC apoptosis, differentiation, and mobilization. Thus, effectively, the HSC replication rate is equivalent to the number of niches available per unit time, and the data imply that approximately 50% of marrow niches would be available during the first 1.7 weeks of shared circulation (or 3.7 weeks of parabiosis). If partner and host HSCs competed equally for these niches, one would anticipate that 25% (not 1.2%-2.5%) of marrow HSCs would have a partner phenotype. Thus, HSCs that exit the marrow of one parabiont do not generally engraft in the marrow of its partner. Rather host HSCs, generated by replication events and transiting the marrow sinus, are preferentially retained.
These kinetics may also reflect the low number and rapid flux of HSCs in blood. In the baseline studies, under steady state conditions, too few HSCs were present in blood pooled from 2 mice to reconstitute hematopoiesis in 1 or 2 irradiated recipients (Table 1). The studies of others demonstrate that early progenitors, CFU-S, disappear quickly from the circulation after infusion.11 Our results also argue that HSCs are in rapid flux as their circulatory lifetime (even immediately after cytokine administration) is sufficiently short that ROSA26-derived HSCs and PeP3b-derived HSCs do not fully mix (Table 2). It appears that HSC trafficking to blood is a death pathway, important for maintaining the steady state number of marrow HSCs.
Our studies further demonstrate that the marrow is a protected environment. Although CFU-GMs circulate (and have a circulatory lifetime that allows full mixing to occur), progenitors in marrow (and mature cells such as granulocytes) derive from marrow HSCs. The marrow should thus be considered an intact structure or compartment. Microenvironmental cells likely define niches that support the persistence (and quiescence) of marrow HSCs.
The spleen does not function in a comparable way because the percentages of partner progenitors and granulocytes in spleen are equivalent to those in blood. Rather, it seems to function as a sieve, a place where progenitors reside or passage in equilibrium with the blood. This finding has major implications for studies in mice that use splenic hematopoiesis as the end point to determine the in vivo response to an experimental manipulation. This is particularly important because the spleen is not a hematopoietic organ for normal adult human blood cell production. Studies in parabiotic mice with transgenes or null mutations affecting the function or number of HSCs or of marrow or spleen microenvironmental cells may provide additional insight into those processes regulating homeostasis.
Although our experiments clarify many issues regarding stem cell kinetics, additional questions remain. Why are the percentages of marrow granulocytes and CFU-GMs of partner origin higher, though minimally, than the percentages of marrow HSCs of partner origin during steady state parabiosis (Table 1) but not at day 42 in the cytokine exposure studies (Table 2)? This likely reflects the relative frequencies of these cells in blood circulating within the marrow sinuses and perhaps small contributions from progenitor cell engraftment. We cannot, however, exclude the possibility that HSCs that have recently transited blood compete less effectively in the reconstitution (transplantation) assay used for their detection because it is possible that the homing defect described in mobilized cells18,26 persists after their return from blood to marrow. Do the number of partner HSCs in marrow plateau or increase slowly over time, as suggested by our studies (Table 1) and by those of Mejino et al7 and Wright et al,11 or is the increment more dramatic (24% at 6-7 months), as recently reported by Wagers et al27 using a related experimental design? Are results strain-specific? Additional studies are needed to clarify this issue. Given that parabiotic experiments are labor-intensive studies and that only 1 to 3 pairs of mice are analyzed for each observation, discrepancies may simply reflect biologic variability.
We interpret the cumulative data as follows. The number of HSCs in marrow is fixed and tightly controlled.28 Should an HSC die, differentiate, or mobilize, circulating HSCs and newly generated HSCs transiting the marrow sinus compete for this open geographic or functional space. Because the HSC replication rate is high,23 the new HSCs outnumber the few HSCs entering the marrow from the peripheral blood. Once HSCs exit marrow, their lifespans in the circulation is extremely short, contributing to the competitive advantage of endogenously generated cells. If the marrow is irradiated, resulting in HSC depletion and damage, circulating HSCs effectively compete, engraft, and reconstitute marrow hematopoiesis.10
After cytokines, additional HSCs exit marrow and circulate in blood. The lifespans of HSCs in blood may also increase, perhaps because of the loss or dysfunction of adhesion molecules.19,26 Circulating (mobilized) HSCs can thus effectively compete with endogenous HSCs for the increased number of open niches. It is likely that circulating HSCs return to marrow within 1 week of the cessation of cytokine exposure because the marrow compartment appears to have achieved a new steady state 3 weeks after cytokine exposure—the percentage of partner HSCs is equivalent to the percentage of partner CFU-GMs and of partner granulocytes. Interestingly, at this time, because the percentage of partner HSCs in blood is significantly lower than the percentage of partner HSCs in marrow (Table 2), HSCs that recently re-engrafted may be least likely to exit. That competition among HSC drives engraftment is consistent with the studies of Stewart et al29 in which marrow cells were able to engraft nonmyeloablated recipients when transplanted repetitively and in large numbers. Numerical analyses of these findings29 in the context of parabiotic experiments may provide further insight into the kinetics of marrow HSC homeostasis.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-01-0318.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.