Abstract
Induction of transplantation tolerance to alloantigens without general immunosuppression remains an enduring challenge. Injecting a donor-specific transfusion (DST) of spleen cells together with blocking αCD154 antibody prior to graft transplantation is an effective way to induce long-lived graft acceptance. Using a novel T-cell receptor (TCR) transgenic (Tg) model of CD4+ T-cell–mediated rejection, this study sheds new insights into the cellular basis for enhanced graft survival induced by DST and αCD154. The study shows that DST and αCD154 induce an early, robust, abortive expansion of the Tg T cells that results in profound anergy. This is contrasted with the more delayed, regional, productive response elicited by an allogeneic graft. Studies show that the induction of tolerance to the allograft induced by DST is mediated by indirect presentation by host antigen-presenting cells. Based on these observations, we conclude that DST and αCD154 preemptively tolerize the alloreactive T-cell compartment to prohibit subsequent responses to the immunogenic allograft.
Introduction
For more than 2 decades, it has been recognized that the infusion of whole blood from donors (donor-specific transfusion [DST]) into recipients can prolong allograft survival in humans, as reviewed in humans1 and mice.2 More recently, it has been shown that the prolonged survival of allografts induced by DST is synergistically enhanced by the coadministration of αCD154 (αCD40L).3 In some cases, permanent graft survival of allogeneic grafts can be observed with αCD154 and DST. While some insights into the underlying mechanisms of graft survival have been gained, our understanding of the cellular basis for allograft tolerance is incomplete. A number of recent studies using this means to induce graft survival have shown that active suppression plays an essential role in silencing the effector function of graft-rejecting T cells. While a role of regulatory T cells (Treg's) in this process has been tentatively identified, little is known about the fate and function of alloreactive effector T cells upon exposure to DST and αCD154.
It is hypothesized that one cellular mechanism of DST/αCD154-induced graft tolerance is the inactivation of alloreactive CD4+ and CD8+ effector T cells.2 Based on studies of Buhlmann et al,4 it was speculated that direct recognition of the infused allogeneic B cells (DST) by alloreactive T cells in the αCD154-suppressed environment resulted in inadequate DST activation, with reduced up-regulation of costimulatory molecules, and cytokine production by the DST. Under these conditions, it was hypothesized that clonal tolerance of the alloreactive T-cell population was rendered. Whether this tolerance was the result of clonal ignorance, anergy, or apoptosis has not been resolved because of the inherent constraints of the systems used. The use of TCR transgenic (Tg) models to study the basis for T-cell tolerance has permitted great insights into the spectrum of possible defects that can account for the tolerant state. Alloreactive T-cell fate and function have been directly assessed in the studies presented herein through the use of a novel CD4+ TCR Tg model wherein alloantigens expressed by the DST, together with αCD154 blockade, induce profound unresponsiveness. As such, questions as to the fate and function of normal and tolerant alloreactive T cells have been addressed.
Many hypotheses of DST-induced tolerance are based on the proposition that host alloreactive T cells directly recognize alloantigen on the DST. However, recent studies have shed doubt on this premise. Using a spectrum of DST allotypes, matched or mismatched with the host, studies by Niimi et al5 suggested that presentation of alloantigen-derived peptides in the context of self–major histocompatibility complex (MHC) was essential for the beneficial effect of haplotype-shared blood transfusions. If this is true, then processing and presentation of DST-derived allopeptides by the host antigen-presenting cells (APCs) is critical in alloantigen-induced tolerance. Accepting this proposition, one must presume that the synergy observed with αCD154 is due to its impact on the host APC machinery. Blockade of CD154 exerts profound effects on the function, longevity, and differentiation of dendritic cells (DCs).6 As such, the effect of CD154 blockade is to shorten the duration of antigen presentation by the DCs and to limit their capacity to be immunogenic. Preventing DC maturation, and at the same time delivering DST, may be a superlative means for inducing tolerance to alloantigen-derived epitopes that are presented by host DCs. It is thought that autologous apoptotic cells that are processed and presented by nonmatured host DCs are critical to maintain peripheral tolerance.7-9 DST and αCD154 may, in fact, take advantage of similar mechanisms to those used in maintaining peripheral self-tolerance in order to induce allotolerance. Data presented herein directly address and measure the contribution of indirect presentation to DST-induced graft survival.
Using the CD4+ TCR Tg system (TEa), in which receptor specificity is directed to an allopeptide derived from I-Eα and presented in the context of class II MHC (H-2b), we have tracked the behavior of CD4+ alloreactive T cells in response to DST, αCD154, and an allograft. Data show that DST induces a rapid, robust expansion of alloreactive T cells that is abortive and results in profound T-cell anergy. Blocking of CD154 does not qualitatively change the response to DST, but serves to magnify the intensity of the unresponsiveness. In contrast to the alloresponse to DST, the Tg T-cell response to the allograft is regional, robust, and productive, giving rise to highly responsive alloreactive T cells that ultimately infiltrate the graft and mediate rejection. Therefore, the preemptive and near complete unresponsiveness induced by DST/αCD154 serves to extinguish the subsequent response to the allograft. Furthermore, the data clearly show that DST does not directly present alloantigen to the host, and serves only as an alloantigen depot, providing allopeptides to be presented by host APCs. In light of these findings, a novel and cohesive model of DST/αCD154-induced allotolerance is presented.
Materials and methods
Mice
C57BL/6 MHC class II–deficient mice and recombination activating gene (RAG) knock-out (KO) mice were purchased from Taconic (Germantown, NY). CB6F1 mice (hybrid of C57BL/6 and Balb/c), C57BL/6, C57BL/6 CD45.1, and Balb/c mice were purchased from the National Cancer Institute (Frederick, MD). The TEa CD4+ TCR Tg mice10 were kindly provided by Dr Alexander D. Rudensky (University of Washington, Seattle). CD4+ Tg cells express a TCR that recognizes the peptide ASFEAQGLANIAVDKA in the context of I-Ab. This peptide corresponds to the positions 52 to 68 from the α-chain of I-E class II molecules and is expressed in all APCs from H-2b/I-E+ strains (CB6F1). C57BL/6 mice are H-2b but I-E–, whereas BALB/c are H-2d and I-E+, and therefore their F1 hybrids are H-2b/I-E+ and able to directly present antigen. The TEa Tg mice were bred to C57BL/6, congenic C57BL/6 CD45.1 (Ly5.2) mice at the animal facility at Dartmouth Medical School. All mice were bred and housed in microisolator cages in a pathogen-free facility.
Skin grafting
Skin grafting was performed as a modification of the technique used by Markees et al.11 Briefly, age-matched male CB6F1 mice were used as donors of both spleen cells (DST) and skin grafts. In some groups, age-matched C57BL/6 grafts were used as negative control skin donors. CD45.1 and CD45.2 were used as additional congenic markers for tracking the TEa Tg T cells. Age-matched CD45.1+ TEa Tg mice were used as donors of alloreactive T cells. Quantification of the TEa Tg T cells was done by staining with anti–Vα2-TCR–phycoerythrin (PE) and anti–CD4–fluorescein isothiocyanate (FITC). Recipients were age-matched RAG KO mice. On day –1, donor CB6F1 mice were killed and tail-skin grafts (0.5 cm × 0.5 cm)12 or mechanically disaggregated spleen cell suspensions were prepared from them. Recipient mice were injected with or without 5 × 107 DST cells and 1 × 106 TEa Tg T cells in 500 μL Hanks balanced salt solution by tail vein injection (intravenously) and 500 μg of αCD154 (clone MR-1) or control hamster immunoglobulin (H-Ig) in phosphate-buffered saline (PBS) intraperitoneally. Mice were injected with αCD154 or H-Ig 3 times per week for the duration of the experiment. On day 0, recipient mice were anesthetized with 50 μg per gram body weight of each of ketamine and xylazine injected intraperitoneally (15 mg/mL in PBS), and CB6F1 or C57BL/6 skin grafts were prepared using established methods.12 Rejection was defined as the day on which less than 20% of the skin graft remained.
All antibodies were obtained from Pharmingen (San Diego, CA).
Statistical analysis
Survival data were analyzed using the Kaplan-Meier method with the Wilcoxon rank test and the log-rank test used to verify the significance of the difference in survival between groups. P values less than .05 were considered statistically significant.
Fluorescence-activated cell analysis
Lymph node (LN) cell suspensions were stained to analyze expansion and purity of TEa Tg T cells in all different groups. This was assessed by staining with anti–CD45.1-FITC and anti–Vα2-TCR-PE. All antibodies were obtained from Pharmingen.
Purification and adoptive transfer of in vivo–stimulated TEa Tg T cells
Age-matched RAG KO mice were injected intravenously with 5 × 107 DST cells and 1 × 106 CD45.1+ TEa Tg T cells, and intraperitoneally with 500 μg of either αCD154 or H-Ig 3 times per week. On day 7, recipients or naive TEa mice were killed, and their LNs were harvested and mechanically disaggregated. Cells were positively selected for CD45.1 using anti–CD45.1-biotin and streptavidin magnetic beads according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA), stained with anti–Vα2-TCR-PE and anti–CD45.1-FITC to determine percentage of selected T cells (recipients, > 70%; naive TEa, > 90%), and counted. Age-matched male or female RAG KO recipients were injected with 1 × 105 TEa Tg T cells harvested from DST+/– αCD154–treated RAG KO mice. Skin grafting of CB6F1 grafts was performed, and grafts were monitored as described in “Skin grafting.” Antibodies were obtained from Pharmingen.
In vivo expansion of TEa Tg T cells
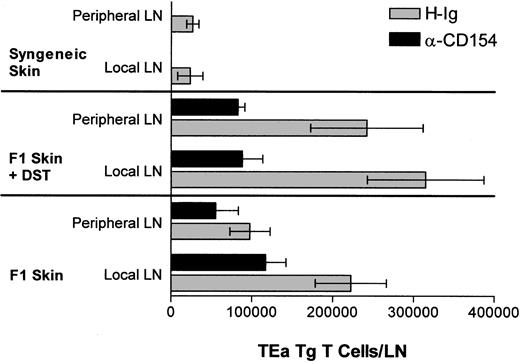
On day 0, recipient RAG KO mice received 1 × 106 CD45.1+ TEa Tg T cells and either (1) 5 × 107 DST cells (from CB6F1 or Balb/c donors) with or without αCD154, (2) a CB6F1 skin graft with or without αCD154, (3) 5 × 107 DST cells (CB6F1 or Balb/c) and a CB6F1 skin graft with or without αCD154, or (4) a C57BL/6 skin graft. Mice treated with αCD154 received 500 μg 3 times per week, as described in “Skin grafting,” and were compared with mice receiving the same dose of H-Ig. Mice were killed either at day 7 for in vitro recall assays or at day 9 for determination of in vivo expansion. For comparison of local versus nonlocal expansion, lymph node cells were harvested, and mechanically disaggregated. Total number of cells per lymph node was determined and standardized to the percentage of TEa Tg–positive cells determined by flow cytometry.
In vitro recall responses
Media used were RPMI (Bio-Whitaker, Walkersville, MD) containing 10% fetal bovine serum, 2 mM l-glutamine, 5 × 10–5 M 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin. Splenocytes from CB6F1 or C57BL/6 mice were irradiated with 30 Gy (3000 rad), and 5 × 105 cells per well were added to separate wells of a 96-well plate in 100 μL media. At day 7, LN cells from the different groups were harvested and selected for CD45.1 expression as described in “Purification and adoptive transfer of in vivo–stimulated TEa Tg T cells,” stained with anti–CD45.1-FITC and anti–Vα2-TCR-PE, counted, and plated in 96-well plates. TEa Tg T cells (20 000) in 100 μL media were added to wells containing 100 μL irradiated CB6F1 cells, 100 μL irradiated C57BL/6 cells, or 100 μL media. Some wells containing irradiated CB6F1 cells or irradiated C57BL/6 cells received 100 μL media and no TEa Tg T cells. Replicate plates were incubated to assess cytokines and proliferation. Proliferation and cytokines were assessed as previously described.13
Assessment of cell proliferation by measuring cytoplasmic dye dilution
To follow in vivo kinetics of division, TEa Tg T cells were labeled with the intracellular fluorescent dye 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE) obtained from Molecular Probes (Eugene, OR) prior to adoptive transfer into naive RAG KO recipients. At days 4 and 8, local and nonlocal LN cells were recovered and assayed by multicolor flow cytometry, gating in the TEa Tg cell population (as previously described) to detect the dilution of the dye caused by cell proliferation. Each successive cellular generation exhibits half of the intensity of CFSE fluorescence of its parental population.
Assessment of graft infiltration
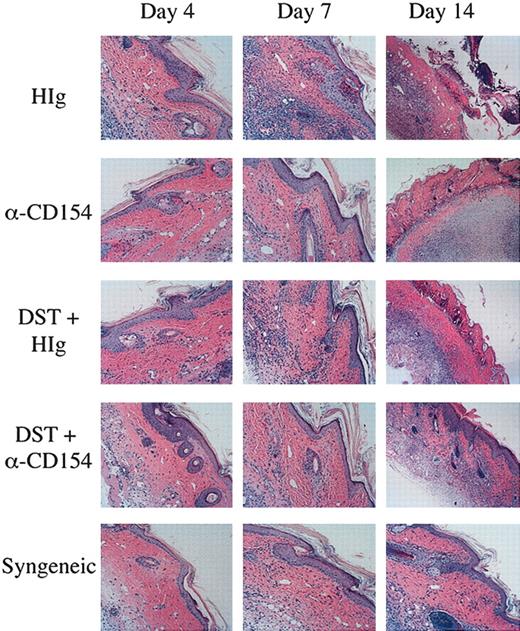
On day 0, recipient RAG KO mice received 1 × 106 CD45.1+ TEa Tg T cells and either (1) 5 × 107 DST cells with or without αCD154, (2) a CB6F1 skin graft with or without αCD154, (3) 5 × 107 DST cells and a CB6F1 skin graft with or without αCD154, or (4) a C57BL/6 skin graft. Mice treated with αCD154 received 500 μg 3 times per week, as described throughout “Materials and methods,” and were compared with mice receiving the same dose of H-Ig. On days 4, 7, and 14 skin grafts were removed, formalin fixed, and processed for hematoxylin and eosin (H&E) staining by established protocols.
Results
Skin graft rejection and prolonged graft acceptance induced by DST and CD154 blockade in a CD4+ TCR transgenic model
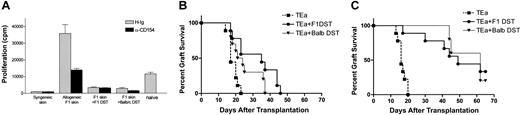
Initial experiments demonstrated that TEa Tg T cells could mount an effective graft rejection response in vivo. As shown in Figure 1, transfer of CD4+ TEa Tg T cells into RAG–/– C57BL/6 mice alone mediates graft rejection with similar kinetics to those seen in non-Tg rejection systems (mean survival time [MST] = 18.6 days in the Tg system; MST = 10 days in the non-Tg system).12 To evaluate if treatment with αCD154 with or without DST could interfere with TEa Tg T-cell–mediated graft rejection, recipient RAG–/– mice were injected with or without 5 × 107 CB6F1 spleen cells and 500 μg αCD154 or control H-Ig. One day after injection, treated mice received a CB6F1 skin graft. αCD154 or control antibody was subsequently administered 3 times per week. Treatment with DST + αCD154 significantly delayed graft rejection by the CD4+ TEa Tg T cells (MST = 72.4 days, P < .0001), and the degree to which it delayed the rejection is also similar to that seen in polyclonal systems (MST = 51 days).12 DST or αCD154 alone significantly delayed graft rejection (MST = 35.5 days, P < .05; MST = 41.7 days, P < .0002, respectively), but not to as great a degree as with DST and αCD154 together (MST = 72.4 days, P < .0001). All groups treated with αCD154 or DST have some rare long-term surviving grafts (> 100 days), the largest percentage of these being in the DST + αCD154–treated group (DST = 13%, αCD154 = 11%, DST + αCD154 = 24% long-term surviving grafts). Control groups received TEa Tg T cells and a syngeneic graft or no TEa Tg T cells and CB6F1 grafts with or without DST. None of these groups ever rejects its grafts (data not shown).
αCD154 + DST delays skin graft rejection by alloreactive Tg CD4+ T cells. On day –1, C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells followed by a CB6F1 skin graft on day 0. Mice were treated with αCD154 or control H-Ig and scored for graft rejection 3 times per week. Grafts were considered rejected when completely necrotic, or when less than 20% of the graft remained. Data were pooled from 3 separate experiments. (▾ represents H-Ig [n = 13]; ▪, DST [n = 16]; ♦, αCD154 [n = 18]; and •, DST +αCD154 [n = 17].)
αCD154 + DST delays skin graft rejection by alloreactive Tg CD4+ T cells. On day –1, C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells followed by a CB6F1 skin graft on day 0. Mice were treated with αCD154 or control H-Ig and scored for graft rejection 3 times per week. Grafts were considered rejected when completely necrotic, or when less than 20% of the graft remained. Data were pooled from 3 separate experiments. (▾ represents H-Ig [n = 13]; ▪, DST [n = 16]; ♦, αCD154 [n = 18]; and •, DST +αCD154 [n = 17].)
αCD154 reduces the in vivo expansion of alloreactive Tg T cells induced by DST or an allogeneic skin graft in vivo
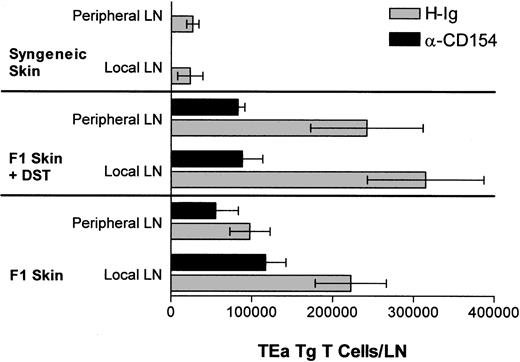
The in vivo response profile of the TEa Tg T cells in response to DST, αCD154, and/or a skin allograft was measured. Briefly, on day 0, mice received 1 × 106 TEa Tg T cells and either (1) 5 × 107 DST cells with or without αCD154, (2) an allogeneic CB6F1 skin graft with or without αCD154, (3) 5 × 107 DST cells and a CB6F1 skin graft with or without αCD154, or (4) a syngeneic C57BL/6 skin graft. αCD154 or H-Ig was administered 3 times per week. As shown in Figure 2, at day 9, CB6F1 skin grafts induced extensive expansion of TEa Tg T cells in draining LNs compared with expansion induced in nondraining lymph nodes. On the other hand, DST induced systemic expansion of TEa Tg T cells with equivalent numbers in all LNs. In all groups, treatment with αCD154 blocked TEa Tg T-cell expansion by approximately 50%.
αCD154 inhibits in vivo expansion of alloreactive Tg CD4+ T cells. C57BL/6 RAG KO mice were injected with TEa Tg T cells in the presence or absence of CB6F1 spleen cells and a CB6F1 skin graft. Mice were injected with αCD154 or control H-Ig 3 times per week. Then, 9 days later, local and nonlocal LNs were harvested and cells were counted and analyzed by fluorescence-activated cell sorter for the percentage of TEa Tg T cells in order to determine the total number of TEa Tg T cells in each group.
αCD154 inhibits in vivo expansion of alloreactive Tg CD4+ T cells. C57BL/6 RAG KO mice were injected with TEa Tg T cells in the presence or absence of CB6F1 spleen cells and a CB6F1 skin graft. Mice were injected with αCD154 or control H-Ig 3 times per week. Then, 9 days later, local and nonlocal LNs were harvested and cells were counted and analyzed by fluorescence-activated cell sorter for the percentage of TEa Tg T cells in order to determine the total number of TEa Tg T cells in each group.
αCD154 accentuates the alloreactive T-cell unresponsiveness induced by DST
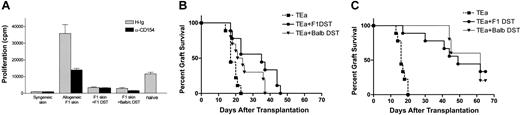
The combined administration of αCD154 and DST enhanced graft survival more effectively than either agent alone. To address the cellular basis for enhanced survival, the functional activity of TEa Tg T cells following in vivo tolerization was measured. Tg T cells were purified from treated and untreated mice and restimulated in vivo and in vitro. Briefly, CD45.1+ TEa Tg T cells were adoptively transferred into RAG–/– recipients together with DST, CB6F1 skin, and with or without αCD154 treatment. On day 7, mice were killed, LN cells were harvested, and the TEa Tg T cells were purified by positive selection for CD45.1 expression. For in vivo restimulation, equivalent numbers of “tolerized” (DST + αCD154–exposed TEa Tg T cells [1 × 105, > 70% pure]) or control (DST + H-Ig–exposed TEa Tg T cells) TEa Tg T cells were transferred into naive RAG–/– recipients. A third group of mice received only naive TEa Tg T cells (1 × 105 cells). A CB6F1 skin graft was placed on each mouse, and rejection was followed over time. As shown in Figure 3A, TEa Tg T cells exposed to DST + αCD154 showed a reduced capacity to reject CB6F1 skin grafts (MST = 94.75 days; P < .0001), compared with naive controls (MST = 25.8 days). TEa Tg T cells exposed to DST + hamster Ig (H-Ig) also showed a reduced capacity to reject CB6F1 skin but to a less significant degree (MST = 41.11 days; P < .0001). Thus, DST and αCD154 treatment induced substantial T-cell unresponsiveness to alloantigen restimulation in vivo.
αCD154/DST treatment reduces the functional activity of TEa Tg T cells in vivo and in vitro. C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells, and in the presence or absence of a CB6F1 skin graft on day 0. Anti-CD154 or control H-Ig was injected 3 times per week. On day 7, LNs were harvested and TEa Tg T cells were positively sorted. (A) Purified TEa Tg T cells were transferred to naive C57BL/6 RAG KO recipients together with a CB6F1 skin graft. Graft rejection was monitored as described previously (▾ represents naive TEa Tg T cells [n = 10]; ▪, DST-exposed TEa Tg T cells [n = 9]; •, DST + αCD154–exposed TEa Tg T cells [n = 8]). Data were pooled from 2 separate experiments. Also, purified effector TEa Tg T cells were plated in vitro with irradiated CB6F1 stimulator cells as described in “Materials and methods.” Then, 72 hours later, cells and supernatants were harvested for quantification of (B) 3H-thymidine incorporation, (C) IL-2 production, and (D) IFN-γ production.
αCD154/DST treatment reduces the functional activity of TEa Tg T cells in vivo and in vitro. C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells, and in the presence or absence of a CB6F1 skin graft on day 0. Anti-CD154 or control H-Ig was injected 3 times per week. On day 7, LNs were harvested and TEa Tg T cells were positively sorted. (A) Purified TEa Tg T cells were transferred to naive C57BL/6 RAG KO recipients together with a CB6F1 skin graft. Graft rejection was monitored as described previously (▾ represents naive TEa Tg T cells [n = 10]; ▪, DST-exposed TEa Tg T cells [n = 9]; •, DST + αCD154–exposed TEa Tg T cells [n = 8]). Data were pooled from 2 separate experiments. Also, purified effector TEa Tg T cells were plated in vitro with irradiated CB6F1 stimulator cells as described in “Materials and methods.” Then, 72 hours later, cells and supernatants were harvested for quantification of (B) 3H-thymidine incorporation, (C) IL-2 production, and (D) IFN-γ production.
Seeking an explanation to the hyporesponsiveness seen in Figure 3A, we assessed the in vitro functional responsiveness of purified TEa Tg T cells. TEa Tg T cells were purified from treated mice (as described in “Materials and methods”), and their proliferative and cytokine production profiles in response to alloantigen were determined. All results presented used purified TEa Tg T cells and represent responses on a per cell basis. As shown in Figure 3B-D, TEa Tg T cells from mice that were grafted with allogeneic skin had markedly higher in vitro proliferative and cytokine production responses (interleukin-2 [IL-2] and interferon γ [IFNγ]) than all other groups. DST, while inducing substantial in vivo expansion (Figure 2), did not appear to prime the TEa Tg T cells to respond upon recall to alloantigen in vitro. Thus, alloantigen provided by allogeneic skin compared with that provided by allogeneic spleen cells produced markedly distinct effects on the recall response of the TEa Tg T cells in vitro. Most interestingly, administration of DST to mice that received skin grafts markedly reduced the recall responses to challenge with alloantigen in vitro. Even more, concomitant administration of αCD154/DST led to a more profound inhibition of recall responses. The recall proliferative response to allogeneic skin (65 000 cpm/culture) was reduced to less than 5% (2000 cpm/culture) by the coadministration of αCD154/DST. Similarly, cytokine production profiles in response to allogeneic skin were greatly reduced by the coadministration of αCD154/DST. In addition, tolerized TEa Tg T cells were incapable of producing the Th2 cytokine IL-4 or to suppress skin rejection by naive TEa Tg T cells (data not shown). Finally, no overt phenotypic differences in cell surface profiles (CD25, CD44, and CD62L) were apparent between the tolerized and immune Tg T cells. In summary, on a per cell basis, DST and, moreover, αCD154/DST induced profound T-cell anergy.
DST induces an early, abortive T-cell response that preempts the productive response to an allograft
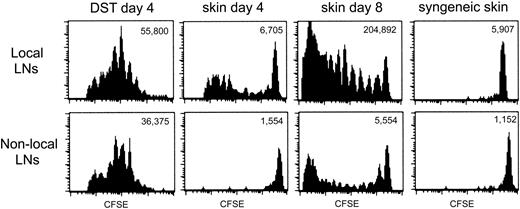
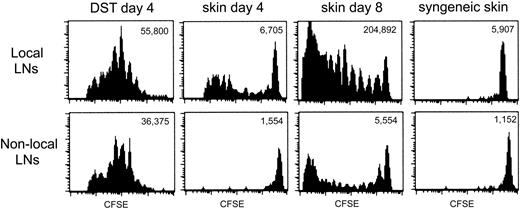
The data thus far indicated that heightened TEa Tg T-cell recall responses were induced by an allograft and that the coadministration of DST prior to an allograft could block allograft-induced T-cell “priming.” Furthermore, the T-cell unresponsiveness elicited by DST was further accentuated by αCD154. One way in which DST could exert a dominant impact over priming by an allograft was if the Tg T cells were anergized by the DST prior to encountering the immunogenic allograft. The tempo of the response of TEa Tg T cells to DST versus an allograft was studied in vivo by evaluating the extent of in vivo Tg T-cell division over time. This was achieved by following the dilution of the intracellular fluorescent dye, CFSE. CFSE-labeled Tg TEa T cells were adoptively transferred into RAG–/– recipients together with CB6F1 DST, CB6F1 skin, or syngeneic skin. At days 4 and 8, cells from draining and nondraining LNs were harvested and CFSE dilution was analyzed by flow cytometry. Figure 4 shows that at day 4, all the TEa Tg T cells in draining or nondraining LNs from the recipient that received DST had divided, with no remaining cells within the high-staining population. The same effect was observed in the presence of DST and αCD154, but with a reduction in overall expansion (data not shown). At the same time point, allogeneic skin induced limited division of the Tg cells in the draining LNs and no division in nondraining LNs. This is demonstrated by a small number of total TEa Tg T cells with reduced dye dilution, yet with a sizable high-staining population still present on day 4. However, after 8 days, skin alone was able to strongly stimulate T-cell division in draining LNs with some evident migration of dividing cells to the nondraining LNs. As a control, syngeneic skin did not induce TEa Tg T-cell division in draining or nondraining LNs. Therefore, DST induces an early, systemic expansion of alloreactive T cells leading to T-cell anergy, which may preempt the ensuing productive response to the allograft.
The allogeneic response of TEa Tg T cells to DST and skin is temporally and spatially separated. CFSE-labeled Tg TEa T cells were adoptively transferred into RAG–/– recipients together with CB6F1 DST, CB6F1 skin, or syngeneic skin as a negative control for proliferation. At days 4 and 8, cells from draining and nondraining LNs were harvested and CFSE dilution of the TEa Tg T cells was analyzed by flow cytometry. Total number of TEa Tg T cells/LNs is shown for each group.
The allogeneic response of TEa Tg T cells to DST and skin is temporally and spatially separated. CFSE-labeled Tg TEa T cells were adoptively transferred into RAG–/– recipients together with CB6F1 DST, CB6F1 skin, or syngeneic skin as a negative control for proliferation. At days 4 and 8, cells from draining and nondraining LNs were harvested and CFSE dilution of the TEa Tg T cells was analyzed by flow cytometry. Total number of TEa Tg T cells/LNs is shown for each group.
DST-induced hyporesponsiveness and increased graft survival is mediated by indirect presentation of alloantigen and CD154 blockade
While the prevailing hypothesis proposes that DST-induced tolerance is due to direct presentation of donor alloantigens to host alloreactive T cells, this has not been rigorously tested. We evaluated the potential contribution of direct versus indirect antigen presentation to T-cell hyporesponsiveness. Cells from Balb/c mice (H-2d) provide the antigen (I-Eα), but not the appropriate MHC-restricting element (I-Ab). Thus Balb/c DST cannot be directly recognized by the TEa Tg T cells. If Balb/c DST results in reduced TEa Tg T-cell response to in vitro restimulation or enhanced graft survival in B6 RAG–/– recipients, it can be due only to the fact that the Balb/c-derived I-Eα was presented indirectly by host (H-2b) APCs. As shown in Figure 5A, the response to an F1 skin graft was reduced equivalently by either Balb/c or CB6F1 DST+/– αCD154. In concordance with this observation, BALB/c, like CB6F1 DST, was also able to induce enhanced graft survival with αCD154 treatment (Figure 5B-C). Thus, in the absence of direct presentation, indirect presentation of alloantigen is capable of inducing Tg TEa systemic expansion (data not shown), unresponsiveness to in vitro restimulation, and enhanced allograft survival.
Indirect presentation of DST antigens by host APCs mediates hyporesponsiveness of TEa Tg T cells and long-term allograft survival. TEa Tg T cells (1 × 106) were adoptively transferred into C57BL/6 RAG–/– recipients (n = 15 per group) in the presence of CB6F1 skin alone or together with CB6F1 DST or T-cell–depleted Balb/c DST to assess indirect presentation. A separate group of mice received TEa cells and syngeneic skin as a negative control. All the groups received injections of αCD154 or control H-Ig 3 times per week. (A) After 7 days, 4 mice per group were killed and cells were harvest from LNs. The same number of TEa Tg T cells were plated with irradiated CB6F1 spleen cells to verify their ability to respond to in vitro restimulation by measuring 3H-thymidine incorporation. The remainder of the mice were followed for transplant rejection kinetics over time in presence of (B) control H-Ig or (C) αCD154.
Indirect presentation of DST antigens by host APCs mediates hyporesponsiveness of TEa Tg T cells and long-term allograft survival. TEa Tg T cells (1 × 106) were adoptively transferred into C57BL/6 RAG–/– recipients (n = 15 per group) in the presence of CB6F1 skin alone or together with CB6F1 DST or T-cell–depleted Balb/c DST to assess indirect presentation. A separate group of mice received TEa cells and syngeneic skin as a negative control. All the groups received injections of αCD154 or control H-Ig 3 times per week. (A) After 7 days, 4 mice per group were killed and cells were harvest from LNs. The same number of TEa Tg T cells were plated with irradiated CB6F1 spleen cells to verify their ability to respond to in vitro restimulation by measuring 3H-thymidine incorporation. The remainder of the mice were followed for transplant rejection kinetics over time in presence of (B) control H-Ig or (C) αCD154.
DST is incapable of inducing TEa Tg T cell systemic expansion via direct presentation of alloantigen
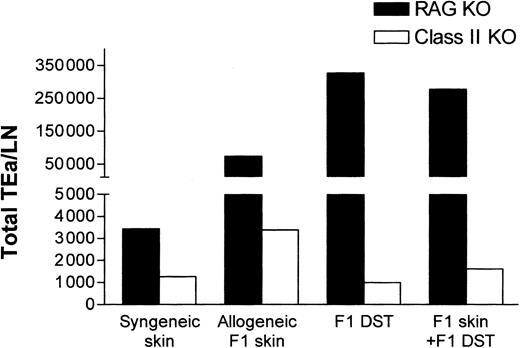
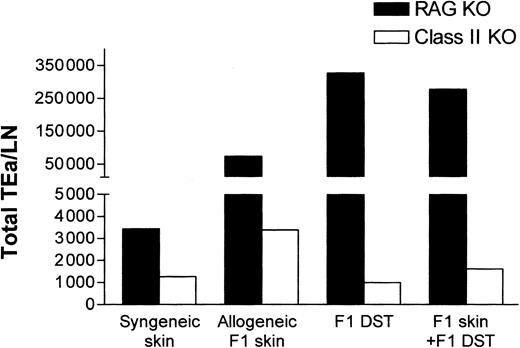
While the aforementioned data demonstrated that indirect presentation of alloantigen was sufficient for Tg T-cell tolerance induction (Figure 5), it did not address the potential contribution of direct alloantigen presentation by DST. To evaluate the contribution of direct presentation, the responsiveness of TEa Tg T cells to CB6F1 DST in class II–/– mice was determined. In contrast to RAG–/– recipients, the class II–/– recipients are unable to indirectly present the Eα peptide provided by the F1 DST. As shown in Figure 6, while F1 DST induced vigorous expansion of Tg cells in RAG–/– mice, in the class II–/– mice there was no expansion of TEa Tg T cells in response to CB6F1 DST. These data strongly indicate that the CB6F1 DST is not directly “seen” by the TEa Tg T cells but provides allopeptides for host APC presentation.
Direct presentation of alloantigens in class II KO recipients is unable to induce systemic expansion of Tg TEa cells. TEa Tg T cells (1 × 106) were adoptively transferred into C57BL/6 RAG–/– or class II KO recipients (n = 3 per group) in the presence of CB6F1 skin alone or together with CB6F1 DST. After 7 days in vivo, lymph nodes were collected and the total number of Tg TEa cells per LN were quantified as previously described.
Direct presentation of alloantigens in class II KO recipients is unable to induce systemic expansion of Tg TEa cells. TEa Tg T cells (1 × 106) were adoptively transferred into C57BL/6 RAG–/– or class II KO recipients (n = 3 per group) in the presence of CB6F1 skin alone or together with CB6F1 DST. After 7 days in vivo, lymph nodes were collected and the total number of Tg TEa cells per LN were quantified as previously described.
Reduced graft infiltration by TEa Tg T cells by DST and αCD154 treatment
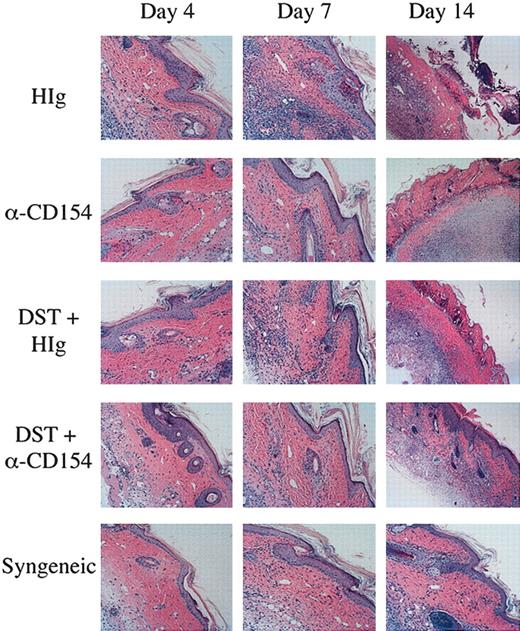
To critically evaluate the impact of αCD154/DST on T-cell infiltration and pathology, skin grafts were histologically evaluated. Skin grafts were removed from the mice on days 4, 7, and 14, and examined by H&E staining for infiltration and inflammation of the grafts. As can be seen in Figure 7, mice receiving a syngeneic graft have no inflammation or infiltration of the graft until day 14, at which point there is very minor spongiosis of the stratum basale of the epidermis and hair follicles, and a very minor lymphocytic infiltrate. This is most likely due to the normal process of wound healing, as these grafts are never rejected. In mice receiving a CB6F1 graft, on day 4 the grafts appear the same as those seen in mice receiving syngeneic grafts, but by day 7 there is well-established interface dermatitis. The interface dermatitis consists of vacuolar changes in the stratum basale of the epidermis, necrotic keratinocytes, and lymphocytic infiltrates apparent in the dermis. By day 14, the grafts on these mice are necrosing. Mice receiving a CB6F1 graft and DST cells show a similar, although not quite as pronounced, pattern of graft infiltration and necrosis as seen in mice receiving CB6F1 grafts alone. Mice receiving a CB6F1 graft together with αCD154 treatment show a different pattern, with no inflammation on day 7 with the exception of rare dead keratinocytes (typically one dead cell per section). By day 14, the grafts on these mice are necrosing. Skin graft sections from mice receiving a CB6F1 graft together with DST/αCD154 treatment demonstrate a few scattered dead keratinocytes on days 4 and 7. On day 14, one can observe the beginnings of an interface reaction, milder than that seen on day 7 of the untreated or DST-treated mice. Thus αCD154 treatment delays skin graft infiltration and together with DST treatment can greatly delay infiltration and prolong graft survival. Further characterization of the infiltrating lymphocytes by confocal microscopy showed clear staining of CD4+CD45.1+ donor TEa Tg T cells correlating to the levels of lymphocyte infiltration observed with H&E staining (data not shown).
Reduced graft infiltration with DST and αCD154 treatment. TEa Tg T cells (1 × 106) were adoptively transferred into C57BL/6 RAG–/– recipients that received transplants of a CB6F1 skin graft and were treated with or without CB6F1 DST and/or αCD154 or H-Ig. Another group of mice received a syngeneic C57Bl/6 skin transplant as a negative control. Skin grafts were removed on days 4, 7, and 14, formalin fixed, and processed for H&E staining. Original magnification, × 100.
Reduced graft infiltration with DST and αCD154 treatment. TEa Tg T cells (1 × 106) were adoptively transferred into C57BL/6 RAG–/– recipients that received transplants of a CB6F1 skin graft and were treated with or without CB6F1 DST and/or αCD154 or H-Ig. Another group of mice received a syngeneic C57Bl/6 skin transplant as a negative control. Skin grafts were removed on days 4, 7, and 14, formalin fixed, and processed for H&E staining. Original magnification, × 100.
Discussion
Insights into the behavior of the CD4+ alloreactive T-cell compartment in graft rejection and acceptance have been hampered by the lack of the appropriate in vivo systems to visualize T-cell responses. Here we present a novel model using alloreactive CD4+ TCR Tg T cells to follow the fate and function of T cells specific to a major donor antigen, and at the same time define the contribution of direct and indirect pathways of antigen presentation to the tolerogenic process. The validity of this model for studying graft rejection and tolerance is shown by the fact that CD4+ T cells from a TCR transgenic mouse (TEa) demonstrate the capacity to reject allogeneic (H-2bxd) but not syngeneic skin at a tempo consistent with that observed with polyclonal, alloreactive T-cell populations. Furthermore, we show that immune intervention (αCD154/DST) can enhance allograft longevity. Unique to this system is the ability to directly measure, on a per cell basis, the response profiles of alloreactive T cells that have been immunized or “tolerized” in vivo. Compared with naive TEa Tg T cells, TEa Tg T cells that have been primed to an allograft in vivo have higher proliferative indices and higher levels of cytokine production on a per cell basis. Other studies of in vivo–primed TCR Tg T cells have also shown that primed or memory CD4+ TCR Tg T cells are hyperresponsive.14 Using this system, we were able to address the cellular basis of the synergy between αCD154 and DST. First, expansion of alloreactive Tg T cells induced by the allograft in the regional nodes is reduced by 60% by αCD154. Hence, the total number of allospecific T cells in the host is reduced. Second, while DST alone induces profound anergy, the anergy induced by DST/αCD154 is more pronounced. That is, the proliferative responsiveness of the residual T cells following DST/αCD154 is about 30% of that observed with DST alone (on a per cell basis). Furthermore, when equivalent numbers of DST-treated versus DST/αCD154-treated TEa Tg T cells are transferred into naive mice (Figure 3A), the latter retain grafts for more than 50 days longer. Thus, the intrinsic functional capacity of the DST/αCD154-treated T cells is impaired compared with DST-treated TEa Tg T cells. Remarkable is the fact that the major qualitative difference between tolerized and nontolerized TEa Tg T cells resides in their capacity to proliferate and produce proinflammatory cytokines upon restimulation; meanwhile other parameters, such as their activation phenotype, remain unchanged.
DST/αCD154 induces a profound systemic T-cell expansion that is rapid and results in the generation of a population of anergic T cells. The finding that indirect presentation mediates DST-induced T-cell anergy alters the prevailing paradigm and strongly suggests that DST-induced tolerance may use mechanisms usually operative in inducing cross-tolerance to self-antigens.15 In models of peripheral T-cell tolerance to self-antigens, it has been shown that specific subsets of host, immature dendritic cells16-18 constitutively present self-antigens to autoreactive T cells resulting in T-cell anergy or deletion.15,19 It is also known that tissue destruction and apoptotic cells are superb sources of self-antigen for delivery to these immature APCs.7,20-22 We would propose that shortly after infusion, cells of the DST apoptose and efficiently deliver alloantigen to host APCs for the indirect presentation of donor allopeptides. The overwhelming tolerogenic impact of alloantigen delivered by DST can be seen by the anergy induced by DST alone. The synergistic actions of αCD154 with DST can be explained by this same model. First, αCD154 is likely proapoptotic for the infused leukocytes, as we know that CD154 expressed by the host would lead to activation and longevity of the donor-derived B cells and DCs in the DST. Second, and more important, blocking CD154 blocks the potential activation of those immature DCs that are presenting newly acquired alloantigen from the DST.6 It has been shown that triggering via CD40 activates DCs and “breaks” peripheral tolerance, and thus in the context of DST, it is important to block endogenous CD154 function.23,24
The contrast in scope and tempo of T-cell responsiveness to DST versus the allograft helps to explain the effectiveness of DST-induced tolerance. Studies have shown that the timing of DST administration relative to allografting is an important parameter in long-lived graft survival. Longer periods of time between DST (within limits) and grafting, and multiple administrations of DST prior to grafting can enhance graft survival.25 All of these parameters likely manifest as more effective measures to preemptively induce anergy in the alloreactive T-cell compartment prior to transferring the immunogenic allograft. The second feature of DST is its systemic impact on alloreactive T-cell responses. As would be predicted, DST administered intravenously systemically anergizes the alloreactive T-cell pool. In contrast, the predominant impact of the allograft is regional, eliciting profound local expansion and T-cell activities.
It is becoming increasingly clear that Treg's play a central role in long-lived graft acceptance induced by DST/αCD154.26-28 However, in this Tg system, there is a lack of CD4+ CD25+ regulatory T cells, allowing us to study the independent impact of DST/αCD154 on the effector T-cell compartment. In the absence of Treg's, it is shown that DST and αCD154 induce hyporesponsiveness of the TEa Tg T effector T cells. The hyporesponsive TEa Tg T cells when cotransferred with naive TEa Tg T cells do not “suppress” graft rejection, establishing that they are not regulatory in nature (data not shown). We have similarly shown this effect of DST and anti-CD154 using purified polyclonal CD4+CD25– T cells transferred into RAG–/– mice (data not shown). Future studies using this transgenic system will investigate the dynamic interactions between regulatory and effector allogeneic T cells in response to the tolerogenic stimuli provided by DST/αCD154 therapy.
Finally, this novel TCR Tg system offers unique insights into the processes governing immunity and tolerance in graft acceptance. While only CD4 responses were studied in this report, the systematic inclusion of CD4+ regulatory T cells and CD8 Tg T-cell populations will allow a more expansive understanding of how these individual alloreactive populations influence the development of graft tolerance. The capacity to track each of these populations independently in vivo will provide a more comprehensive appreciation for how complex interactions between distinct alloreactive T-cell populations result in graft rejection or acceptance.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-02-0586.
Supported by grant A148667 (R.J.N.), CA91436 (R.J.N.), AI 34495 (B.R.B.), 2R37 HL56067 (B.R.B.), HL63452 (B.R.B.), and the Rosaline Borison fellowship (S.A.Q.).
S.A.Q. and B.F. contributed equally to the work presented in this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Anne Perry, MD, for interpretation of H&E sections, Evan F. Lind for help with histology, Kathy Bennett for assistance with animal care, and the Englert Cell Analysis Laboratory.

![Figure 1. αCD154 + DST delays skin graft rejection by alloreactive Tg CD4+ T cells. On day –1, C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells followed by a CB6F1 skin graft on day 0. Mice were treated with αCD154 or control H-Ig and scored for graft rejection 3 times per week. Grafts were considered rejected when completely necrotic, or when less than 20% of the graft remained. Data were pooled from 3 separate experiments. (▾ represents H-Ig [n = 13]; ▪, DST [n = 16]; ♦, αCD154 [n = 18]; and •, DST +αCD154 [n = 17].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-02-0586/6/m_h81734878001.jpeg?Expires=1767756308&Signature=yFSBeAj5W2g1c-dm1lpWhAhmQedD0uoZ9Hkv6Gyo5WtSo0-bnBLXMFL32-y5ap-xr0J51xv8BkbxU6WkVU24P7jyYv7t3IQRxi8L4nfpKONgM46vyJeUfAfyPDHnf7PnfrsRo0umEtBqxHK1CFG-Pe6YEQEHTNvKV7LeF2V5ySVVv6dEX9OhNGCQ2syN1cdMY-stvUNC9krhf6UHDfosA34tTtVYugz94Mmx1rPdR0VTqYilMktwnQWgXt9uSevMOi4sxxRjqbr47-BpDSH8lC8iCRLNBWycI9QCBvScrA08fUIWdkETqto1E58nHqOgUM7w1laffKJlNN~1mUYeqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. αCD154/DST treatment reduces the functional activity of TEa Tg T cells in vivo and in vitro. C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells, and in the presence or absence of a CB6F1 skin graft on day 0. Anti-CD154 or control H-Ig was injected 3 times per week. On day 7, LNs were harvested and TEa Tg T cells were positively sorted. (A) Purified TEa Tg T cells were transferred to naive C57BL/6 RAG KO recipients together with a CB6F1 skin graft. Graft rejection was monitored as described previously (▾ represents naive TEa Tg T cells [n = 10]; ▪, DST-exposed TEa Tg T cells [n = 9]; •, DST + αCD154–exposed TEa Tg T cells [n = 8]). Data were pooled from 2 separate experiments. Also, purified effector TEa Tg T cells were plated in vitro with irradiated CB6F1 stimulator cells as described in “Materials and methods.” Then, 72 hours later, cells and supernatants were harvested for quantification of (B) 3H-thymidine incorporation, (C) IL-2 production, and (D) IFN-γ production.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-02-0586/6/m_h81734878003.jpeg?Expires=1767756308&Signature=RfVPF2OSlduqtjbKtfQNlneq24SpxvD0rndIN6q4jOeoiYq4Er~HUBJKM6R4LNFrsvfv4KPaQFIUS1mlYutfUKVg6Z3BMFpyO841n7D1a~t-O8n~L4oC7GySdhkpKS7821UCJj3drBwi8bqp5mfdUbvm~MbJvfEIiZlaqJrWoPXvYwvnEggdO~AHl4H4FSb8Dlm0BREbwJbINyWhISga7CHzUIMB9NMn1XBJ3PeWqDjMGWE~BslUlXMmIbDLmivdKqu723vchcUE3fRAcY9tF1TvIjL2OwBFfFjOgaXN-og~4CzcngnIsPk-Nxc6r1ykVj4Lm2urfv41pt2qcNRloQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. αCD154 + DST delays skin graft rejection by alloreactive Tg CD4+ T cells. On day –1, C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells followed by a CB6F1 skin graft on day 0. Mice were treated with αCD154 or control H-Ig and scored for graft rejection 3 times per week. Grafts were considered rejected when completely necrotic, or when less than 20% of the graft remained. Data were pooled from 3 separate experiments. (▾ represents H-Ig [n = 13]; ▪, DST [n = 16]; ♦, αCD154 [n = 18]; and •, DST +αCD154 [n = 17].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-02-0586/6/m_h81734878001.jpeg?Expires=1767756309&Signature=X4r1zS39emevBLnhrnP0KRUCpxoWp7j9BsbXQSFd7SOgemJDthcwCSzPAlS0-i73LPQmYvUci0m6emQnvgWKFb2KRm4i9n1dmZym1Zx1RuMPJ58FVbHZZdegAEn-NWSbAdN1iRs3kKjRmsOLRWivZTqbdxpWrVDKOyVZVxG4SfmjIephljBBZaVqj9~btEHutNwlGyoENyIB-w857m0omPb11czYPom5kb0jdl0lVD7GOSlEksUsz1~UdcNUIScir9fQu4aY~xRvCvtT8j42uHbT5MEnD~XNsjPYLEd4UD52ryV-Sm3pA97Hk5t~aHr2fiRRizWV-oRwBrCP3CSvzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. αCD154/DST treatment reduces the functional activity of TEa Tg T cells in vivo and in vitro. C57BL/6 RAG KO mice were injected with TEa Tg T cells with or without CB6F1 spleen cells, and in the presence or absence of a CB6F1 skin graft on day 0. Anti-CD154 or control H-Ig was injected 3 times per week. On day 7, LNs were harvested and TEa Tg T cells were positively sorted. (A) Purified TEa Tg T cells were transferred to naive C57BL/6 RAG KO recipients together with a CB6F1 skin graft. Graft rejection was monitored as described previously (▾ represents naive TEa Tg T cells [n = 10]; ▪, DST-exposed TEa Tg T cells [n = 9]; •, DST + αCD154–exposed TEa Tg T cells [n = 8]). Data were pooled from 2 separate experiments. Also, purified effector TEa Tg T cells were plated in vitro with irradiated CB6F1 stimulator cells as described in “Materials and methods.” Then, 72 hours later, cells and supernatants were harvested for quantification of (B) 3H-thymidine incorporation, (C) IL-2 production, and (D) IFN-γ production.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-02-0586/6/m_h81734878003.jpeg?Expires=1767756309&Signature=BCO7ajFA3WRGqLxImprHIqoZt~370Biv-Jhqpw-kWIa1wcn84dCxF3jrMHEeLXduxDcUyTbe~S0ukrsnpX-6kuQe2KPctyKHzCvyoDgRtioEv36XGBlyDd4XkHQ2WjlFcEZ7MoEl9-~pi259Yg5jv8qsrK3llimwBecnYwE86WXAWEPtxyxtiGtns1TiJ3Ut0z7LAddZ5ith8qPPBc5JltjT0lV~eoWG9vt~EUbHE6yFcA6P5lIflpuF7VPDSldvUAn2vRzwhUP6YpUlKrhMSlFPxIT-k6LgfqCFj8XXcbpP6bJpN5WWSUlyg50tFcktsIHiqUv5WSEX73PIhyxIDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)