Abstract
Mobilized peripheral blood stem cells (PBSCs) are widely used for transplantation, but mechanisms mediating their release from marrow are poorly understood. We previously demonstrated that the chemokines GROβ/CXCL2 and GROβT/CXCL2Δ4 rapidly mobilize PBSC equivalent to granulocyte colony-stimulating factor (G-CSF) and are synergistic with G-CSF. We now show that mobilization by GROβ/GROβT and G-CSF, alone or in combination, requires polymorphonuclear neutrophil (PMN)–derived proteases. Mobilization induced by GROβ/GROβT is associated with elevated levels of plasma and marrow matrix metalloproteinase 9 (MMP-9) and mobilization and MMP-9 are absent in neutrophil-depleted mice. G-CSF mobilization correlates with elevated neutrophil elastase (NE), cathepsin G (CG), and MMP-9 levels within marrow and is partially blocked by either anti–MMP-9 or the NE inhibitor MeOSuc-Ala-Ala-Pro-Val-CMK. Mobilization and protease accumulation are absent in neutrophil-depleted mice. Synergistic PBSC mobilization observed when G-CSF and GROβ/GROβT are combined correlates with a synergistic rise in the level of plasma MMP-9, reduction in marrow NE, CG, and MMP-9 levels, and a coincident increase in peripheral blood PMNs but decrease in marrow PMNs compared to G-CSF. Synergistic mobilization is completely blocked by anti–MMP-9 but not MeOSuc-Ala-Ala-Pro-Val-CMK and absent in MMP-9–deficient or PMN-depleted mice. Our results indicate that PMNs are a common target for G-CSF and GROβ/GROβT-mediated PBSC mobilization and, importantly, that synergistic mobilization by G-CSF plus GROβ/GROβT is mediated by PMN-derived plasma MMP-9.
Introduction
Mobilized peripheral blood stem cells (PBSCs) are the primary source of hematopoietic stem cells (HSCs) for autologous and allogeneic transplantation.1-3 A number of agents mobilize PBSCs, including chemotherapy,4,5 hematopoietic growth factors, particularly granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF),6 interleukins,7,8 chemokines,9-12 chimeric growth factor fusion proteins,13,14 sulfated glycans,15,16 and antibodies against β1-integrin (very late activation antigen 4 [VLA-4]) or its receptor (vascular cell adhesion molecule 1 [VCAM-1]).17,18 Mobilization kinetics are variable, requiring multiday dosing with G-CSF, GM-CSF, interleukin 3 (IL-3), chimeric growth factors, and anti-integrin/receptor antibodies or continuous adenoviral production in the case of IL-17 and stromal cell–derived factor-1α (SDF-1α/CXCL12). In contrast, PBSC mobilization occurs within hours with sulfated glycans or a matter of minutes with the CXC chemokines IL-8/CXCL8, GROβ/CXCL2 or GROβT/CXCL2Δ4. Differing kinetics by agents with distinct structures, specificities, and half-lives suggest that multiple mechanisms may be responsible for mobilization or alternatively the agents act indirectly, triggering common events.
Clinically, G-CSF is the predominant PBSC mobilizer based on potency, predictability, and safety.1,19-22 However, poor mobilization is observed in 25% of patients and 10% to 20% of healthy donors.23-25 Mobilization induced by G-CSF can be augmented to various degrees by IL-3,8 stem cell factor (SCF),26,27 Flt3 ligand,28 chemotherapy,29,30 and anti–VLA-4/intercellular adhesion molecule 1 (ICAM-1) antibodies,16,17 but requires multiday administration of both agents. We previously demonstrated that a single dose of the CXCR2-selective chemokines GROβ and GROβT synergistically enhance HSC mobilization when used in combination with various G-CSF dosing regimens.9,31 The addition of a mobilizer with rapid kinetics to standard or reduced-dose G-CSF regimens is clinically attractive.
Recent studies have begun to identify mechanisms associated with PBSC mobilization. We previously described a transient increase in plasma matrix metalloproteinase 9 (MMP-9) that precedes GROβT-induced mobilization and that administration of anti–MMP-9 blocks mobilization.9,31 Similarly, anti–MMP-9 blocks mobilization induced by IL-8.32 Sulfated glycans elevate levels of plasma IL-8, MMP-9, and SDF-1α that are only partially blocked by anti–SDF-1,33 suggesting that IL-8 or MMP-9 or both are involved.16 In addition, HSC recruitment by chemotherapy or G-CSF is impaired in MMP-9–/– mice.34 G-CSF administration results in elevated levels of neutrophil elastase (NE) and cathepsin G (CG) in the bone marrow extravascular compartment of mice that correlates with reduction in VCAM-1 and granulocyte-macrophage colony-forming unit (CFU-GM) mobilization,35,36 which may be mediated by NE degradation of marrow SDF-1α.37 In this report we investigated molecular and cellular mechanisms responsible for synergistic PBSC mobilization by G-CSF in combination with GROβ and GROβT. Our results indicate that mobilization by GROβ, GROβT, and G-CSF is dependent on proteases derived from mature neutrophils and that the synergistic PBSC mobilization response observed when GROβ or GROβT are used with G-CSF is mediated by a synergistic increase in polymorphonuclear neutrophil (PMN)–derived plasma MMP-9.
Materials and methods
Animals
SPF BALB/c, B6D2F1, and C57Bl/6 mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and housed in microisolators for 3 weeks or more before use. Homozygous MMP-9–deficient C57Bl/6 mice38 were obtained from Dr Zena Werb (University of California at San Francisco) and bred in our facility. Maintenance of the MMP-9–null phenotype was monitored by zymography. The Indiana University School of Medicine Animal Care and Use Committee (ICUC) approved all protocols.
Reagents
Lyophilized, carrier-free, endotoxin-free GROβ derived from Escherichia coli was purchased from R&D Systems (Minneapolis, MN). Recombinant GROβT was produced as previously described.39 G-CSF (filgrastim) was purchased from Amgen (Thousand Oaks, CA). Purified carrier and preservative-free, endotoxin-free, rat-antimouse PMN monoclonal antibody (mAb; anti–GR-1, clone RB6-8C5) and rat IgG2b and mouse IgG1κ isotype control mAbs were from eBiosciences (San Diego, CA). NE and CG were obtained from Elastin Products (Owensville, MO). Mouse anti–MMP-9 mAb (clone 6-6B),40 which blocks MMP-9 activation, was from Oncogene Research Products (Cambridge, MA). Murine GM-CSF was purchased from Biovision (Mountain View, CA). Recombinant murine IL-1α (rmIL-1α) and SDF-1α were from R&D Systems. Recombinant murine SCF (rmSCF) was a gift from Dr Karl Nocka (UCB, Cambridge, MA).
Peripheral blood mobilization and preparation of cell suspensions
PBSC mobilization was quantitated 15 minutes after a single subcutaneous injection of 2.5 mg/kg GROβ or GROβT, after 4 days of administration of G-CSF (50 μg/kg/d, twice a day, subcutaneously), or 15 minutes after administration of chemokine to mice mobilized with G-CSF, about 16 hours after the last dose of G-CSF. Injections were scheduled so that control and mobilized mice were evaluated at the same time in every experiment. Mice were killed by CO2 asphyxiation and blood was obtained by cardiac puncture using syringes coated with EDTA (ethylenediaminetetraacetic acid). Plasma was isolated from aliquots of blood for each animal and stored at –20°C. PBMCs were obtained by separation on Lympholyte-M (Cedarlane Labs, Hornby, ON, Canada) as previously described.9 Marrow cells were harvested by flushing femurs with phosphate-buffered saline (PBS). Spleen cells were prepared by pressing between microscope slides as previously described.41 Complete blood counts (CBCs) were performed on a Hemavet Mascot (CDC Technologies, Oxford, CT). Manual differentials were performed on Wright-Giemsa–stained (Hema-Tek 1000, Bayer, Elkhart, IN) blood smears or spleen and bone marrow cell cytospin preparations (Shandon, Pittsburgh, PA).
Preparation of marrow extracellular extracts
Immediately following CO2 asphyxiation and cardiac puncture, femurs were removed and the contents of one femur from each mouse flushed with 1.0 mL ice-cold PBS. The marrow plug was dispersed, centrifuged at 1700g for 4 minutes, and supernates frozen at –20°C.
In vivo depletion of PMNs
To evaluate neutrophil contribution to PBSC mobilization, mice were treated with anti–GR-1 before mobilization. Mice mobilized by GROβ or GROβT received 150 μg/mouse of anti–GR-1 either 1 or 4 days before administration of chemokine. For G-CSF or G-CSF plus GROβ/GROβT mobilization studies, mice received anti–GR-1 prior to the first dose of G-CSF and again midway through the G-CSF regimen. In every experiment, control animals received equivalent amounts of rat antimouse IgG2b isotype mAb (eBiosciences). As described,42 we observed a selective depletion of circulating PMNs between 24 and 96 hours after a single administration of anti–GR-1, with rebound neutrophilia observed at 120 hours after administration.
Gelatin zymography
Gelatinolytic activity was measured by zymography. Plasma and marrow supernates were diluted in Novex Tris (tris(hydroxymethyl)aminomethane)–glycine sodium dodecyl sulfate (SDS) sample buffer (Invitrogen, Carlsbad, CA), loaded on 10% Tris-glycine acrylamide gels containing 0.1% gelatin and electrophoresed for 4 hours at 4°C. Gels were renatured in Novex zymogram renaturing buffer (Invitrogen) containing 2.5% Triton X-100, developed in Novex zymogram developing buffer (Invitrogen) with shaking for 30 minutes, resuspended in fresh developing buffer, and incubated overnight at 37°C. Gelatinolytic activity appeared as colorless bands against a blue background after staining with colloidal blue (Invitrogen). MMP-9 and MMP-2 bands were confirmed by comparison with molecular weight (MW) standards and recombinant enzymes (R&D Systems). Band intensities on digitized gels were quantified in Adobe Photoshop (Adobe Systems, San Jose, CA).
NE and CG protease activities
Proteolytically active NE and CG were quantified using the respective specific NE and CG chromogenic substrates MeOSuc-Ala-Ala-Pro-Val-pNa and Suc-Ala-Ala-Pro-Phe-pNa43 (Calbiochem, San Diego, CA). Purified NE was diluted in 0.1 mL/L Tris-HCl, pH 7.5, 0.5 M NaCl, and 0.01% NaN3 creating standards spanning 0.25 to 2.0 μg/mL. CG was diluted in 0.1 mL/L Tris-HCl, pH 8.3, with 0.01% NaN3 to create a reference range from 2 to 50 μg/mL. Standards and plasma or bone marrow extract diluted in substrate buffer were added to duplicate wells containing substrate, incubated at 37°C for 3 hours and ØD405 nm determined. Sample concentrations were calculated from the linear regression equation of the standard curve.
Mouse SCF and SDF-1α measurement
Mouse SCF was quantitated using the R&D Systems mSCF Quantikine enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions. Mouse SDF-1α was measured by ELISA. Plates were coated with 100 μL SDF-1 mAb79018 (R&D Systems) at 2 μg/mL in PBS and incubated overnight at room temperature. After incubation, wells were washed with 0.05% Tween 20 in PBS, pH 7.4, and blocked with 300 μL PBS containing 1% bovine serum albumin (BSA), 5% sucrose, and 0.05% NaN3 for 60 minutes. The wells were washed, duplicate 100-μL aliquots of standard or sample added, sealed, and incubated for 2 hours. After incubation, the wells were washed; 100 μL biotinylated goat antihuman SDF-1 (R&D Systems) at 200 ng/mL was added and incubated for 2 hours. The wells were washed and 100 μL streptavidin–horseradish peroxidase (HRP; R&D Systems) added for 20 minutes, washed again, and 100 μL tetramethylbenzidine substrate solution (R&D Systems) added and incubated for 30 minutes. The reaction was stopped with 50 μL1 M H2SO4 and ØD determined at 450 nm with correction set to 570. rmSDF-1α was used to generate a standard curve and sample concentrations were calculated from the linear regression equation.
CFU-GM assay
PBMCs and unseparated marrow or spleen cells were assayed for CFU-GM in McCoy 5A media with 15% heat-inactivated fetal bovine serum (Hyclone Sterile Systems, Logan, UT) and 0.3% agar (Difco Laboratories, Detroit, MI).9 PBMCs were cultured at 2 × 105/mL, bone marrow cells at 5 × 104/mL, and spleen cells at 5 × 105/mL. CFU-GM were stimulated with 10 ng/mL recombinant murine GM-CSF (rmGM-CSF), 10 ng/mL rmIL-1α, and 50 ng/mL rmSCF. Triplicate cultures from individual animals were incubated at 37°C, 5% CO2, 5% O2 in air for 7 days. Total CFU-GM/mL blood was determined by multiplying CFU frequencies by PBMC/mL blood corrected for white blood cell (WBC) recovery after Lympholyte-M separation. Total CFU/spleen and femur were determined by multiplying CFU frequencies by total spleen and femur nucleated cells.
PBSC transplantation in mice
BALB/c mice received 875 cGy total body irradiation (lethal dose [LD]100) from a Gammacel-40 irradiator (Nordon International, Kanata, ON, Canada) in 2 sessions 6 hours apart. Irradiated mice received mobilized PBMCs via the tail vein in 0.2 mL saline.
In vivo MMP-9 and NE neutralization
To block MMP-9 activation, mice received either 3 mg/kg anti–MMP-9 antibody or isotype mAb, intravenously, either 2 and 4 days or 2 hours before GROβ or GROβT. For G-CSF mobilization, animals received 3 mg/kg anti–MMP-9, intravenously, 2 hours before the first injection of G-CSF and again midway through the regimen. To block NE, mice received 1 mg of the selective NE inhibitor MeOSuc-Ala-Ala-Pro-Val-CMK (NE-I) in 10% dimethyl sulfoxide (DMSO; Calbiochem, LaJolla, CA), intraperitoneally, every day they received G-CSF. DMSO at 10% (vol/vol) had no effect on PBSC mobilization. NE-I significantly inhibits elastase-induced paw edema in mice at 10 mg/kg with a median effective dose (ED50) for inhibiting elastase-induced lung hemorrhage and pulmonary emphysema of about 8 mg/kg.44 In preliminary studies, we observed 57% to 78% inhibition of NE in plasma and marrow between 1 and 8 hours after administration of G-CSF, and 78% to 85% inhibition of marrow NE on day 5, after 4 days of dosing with NE-I and our standard 4-day G-CSF–mobilizing regimen. This dose of NE-I has also been shown to block CFU-GM mobilization and SDF-1 degradation in marrow in G-CSF–mobilized mice.37
Statistical analysis
Differences were evaluated using the 2-tailed t test function in Microsoft Excel (Microsoft, Seattle, WA).
Results
Enhanced protease release/activation correlates with both GROβ and G-CSF mobilization
We and others have implicated MMP-9 in PBSC mobilization by GROβ and GROβT9,31 and IL-832 used as single agents. Recently, MMP-9–mediated release of SCF was implicated in mobilization by G-CSF.34 Accumulation of NE and CG within marrow35,36 and degradation of SDF-1α by NE, altering SDF-1α gradients,37 have been shown to be associated with G-CSF mobilization. Because we routinely observe elevated plasma MMP-9 levels in mice mobilized by the GROβ chemokines and MMP-9, NE, and CG can degrade SDF-1α,37,45,46 it is possible that either or both of these mechanisms could be relevant to mobilization by GROβ or GROβT, alone or in combination with G-CSF. We quantitated MMP-9, NE, and CG enzyme activities and SDF-1α and SCF protein in plasma and marrow extravascular fluid, to evaluate molecular mechanisms associated with mobilization by GROβT, G-CSF, and, in particular, the combination of G-CSF plus GROβ (Figure 1). As we described previously, GROβ and GROβT mobilize PBSCs within 15 minutes, which is equivalent to a multiday regimen of G-CSF, and synergize when used with G-CSF.9,31 GROβT elevated CFU-GM/mL blood by more than 30-fold compared to PBS-treated controls (Figure 1A) and was associated with a 6-fold elevation of both plasma and marrow MMP-9 levels (Figure 1B). Plasma (not shown) and marrow NE and CG levels were unchanged (Figure 1C), as were SDF-1α (Figure 1D) and SCF protein levels (Figure 1E). G-CSF elevated blood CFU-GM levels by more than 40-fold after 4 days and was associated with a 4- to 7-fold elevation of plasma and marrow MMP-9 levels, respectively (Figure 1B), with corresponding 8-fold and 2-fold increases in marrow NE and CG (Figure 1C). Plasma levels of NE and CG remained unchanged (not shown). G-CSF administration was associated with a 60% ± 0.03% reduction in plasma and 84% ± 0.1% reduction in marrow SDF-1α concentration (Figure 1D). No significant changes in plasma SCF concentration were observed (Figure 1E). In mice receiving G-CSF plus GROβT, a highly synergistic, more than 200-fold, mobilization of CFU-GM was observed (Figure 1A) coincident with a synergistic 22-fold increase in plasma MMP-9 levels, which was 4- to 5-fold higher than observed with either GROβT or G-CSF used alone (Figure 1B). Marrow MMP-9 and NE levels were elevated compared to PBS controls (4.8- and 3.8-fold, respectively), but were significantly lower than observed when either compound was used alone (Figure 1B-C). G-CSF–mediated enhancement of marrow CG was not observed in mice undergoing combination mobilization (Figure 1C). SDF-1α protein levels in plasma and marrow were equivalent to those observed with mobilization by G-CSF alone and did not correlate with synergistic mobilization. SCF levels were unchanged in plasma and marrow (Figure 1E).
CFU-GM mobilization, protease activities, and SDF-1 concentrations in mice mobilized by GROβT, G-CSF, or the combination of G-CSF plus GROβT. Cohorts of 3 B6D2F1 mice/group/experiment were mobilized by a single injection of GROβT (2.5 mg/kg), a multiday regimen of G-CSF (50 μg/kg, twice a day for 4 days), or administration of GROβT on day 5 following the G-CSF regimen. All injections were scheduled so that all control and mobilized mice were evaluated at the same time in every experiment. Combined data from 5 to 7 experiments are shown. (A) Mean ± SEM fold increase in CFU-GM/mL blood. (B) Upper portion is a representative zymogram; lower potion, mean ± SEM fold change in MMP-9 gelatinolytic activity in plasma and marrow extracts determined by zymography. (C) Mean ± SEM fold change in marrow NE and CG activity. (D) SDF-1 protein concentration in plasma and marrow extracts determined by ELISA. (E) SCF protein concentration in plasma and marrow extracts determined by ELISA. Error bars indicate mean ± SEM.
CFU-GM mobilization, protease activities, and SDF-1 concentrations in mice mobilized by GROβT, G-CSF, or the combination of G-CSF plus GROβT. Cohorts of 3 B6D2F1 mice/group/experiment were mobilized by a single injection of GROβT (2.5 mg/kg), a multiday regimen of G-CSF (50 μg/kg, twice a day for 4 days), or administration of GROβT on day 5 following the G-CSF regimen. All injections were scheduled so that all control and mobilized mice were evaluated at the same time in every experiment. Combined data from 5 to 7 experiments are shown. (A) Mean ± SEM fold increase in CFU-GM/mL blood. (B) Upper portion is a representative zymogram; lower potion, mean ± SEM fold change in MMP-9 gelatinolytic activity in plasma and marrow extracts determined by zymography. (C) Mean ± SEM fold change in marrow NE and CG activity. (D) SDF-1 protein concentration in plasma and marrow extracts determined by ELISA. (E) SCF protein concentration in plasma and marrow extracts determined by ELISA. Error bars indicate mean ± SEM.
Synergistic mobilization of long-term repopulating cells (LTRCs) was also observed. Eighty percent (8 of 10 mice) of lethally irradiated BALB/c mice survived more than 180 days after receiving grafts of 2 × 106 PBMCs mobilized by either GROβT or G-CSF alone, whereas no mice (0 of 10) survived after receiving grafts of 2 × 105 PBMCs from either group. In contrast, 10 of 10 mice and 7 of 10 mice survived more than 180 days after receiving 2 × 106 or 2 × 105 PBMCs, respectively, from mice mobilized with GROβT plus G-CSF.
MMP-9 plays a role in GROβ and G-CSF mobilization and is essential for synergistic mobilization by GROβ plus G-CSF
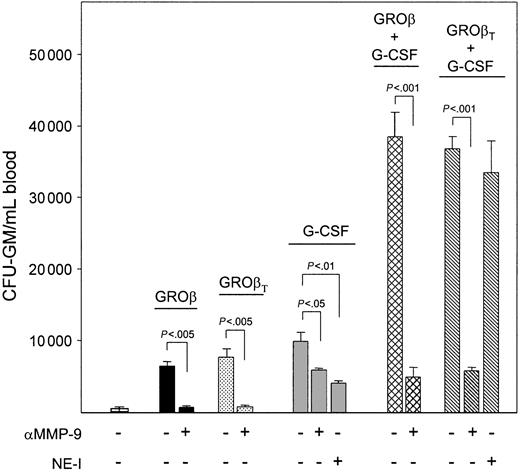
Elevation of MMP-9 activity in both GROβ and G-CSF mobilized mice but elevation of NE and CG only in G-CSF mobilized mice suggest that MMP-9 may represent a common mediator, whereas NE and CG are involved only in G-CSF–induced mobilization. To determine the importance of MMP-9 and NE in combination mobilization, mice were treated with anti–MMP-9 or the NE inhibitor MeOSuc-Ala-Ala-Pro-Val-CMK prior to or during (or both) mobilization. Anti–MMP-9 blocked GROβ- or GROβT-induced CFU-GM mobilization by 84% ± 2% and 92% ± 3%, respectively (Figure 2). Administration of anti–MMP-9 as a single dose 2 hours before administration of GROβ or GROβT resulted in more than 97% inhibition of CFU-GM mobilization (not shown).9,31 MeOSuc-Ala-Ala-Pro-Val-CMK given for 1 or 4 days had no effect on GROβ-induced CFU-GM mobilization (not shown). In mice receiving anti–MMP-9 or MeOSuc-Ala-Ala-Pro-Val-CMK plus G-CSF, CFU-GM mobilization was reduced by 42% ± 4% and 62% ± 3%, respectively, indicating that both MMP-9 and NE contribute to G-CSF–induced mobilization but neither alone is fully effective. Mobilization by G-CSF plus GROβ or GROβT was highly synergistic and completely abrogated in mice treated with anti–MMP-9, with levels of CFU-GM/mL blood equivalent to anti–MMP-9–treated mice mobilized with G-CSF alone. MeOSuc-Ala-Ala-Pro-Val-CMK had no effect on synergistic mobilization by G-CSF plus GROβT. This suggests that MMP-9 is the sole protease involved in the synergistic mobilization response.
Effects of in vivo neutralization of MMP-9 and NE on CFU-GM mobilization in mice mobilized by GROβ or GROβT, G-CSF, or the combination of G-CSF plus GROβ or GROβT. Cohorts of 3 B6D2F1 mice/group received 3 mg/kg anti–MMP-9 on days 0 and 2.5 and were mobilized by a single injection of GROβ or GROβT (2.5 mg/kg) on day 5. Mice being mobilized by G-CSF or G-CSF plus GROβ or GROβT received 3 mg/kg anti–MMP-9 2 hours before and halfway through (day 2.5) the G-CSF regimen (50 μg/kg, twice daily for 4 days) or daily injections of 1 mg/mouse of the NE selective inhibitor MeOSuc-Ala-Ala-Pro-Val-CMK on each day of G-CSF administration. Mice were analyzed on day 5, 15 minutes after administration of PBS or GROβ. Combined data from 2 experiments are shown. Data are expressed as means ± SEM.
Effects of in vivo neutralization of MMP-9 and NE on CFU-GM mobilization in mice mobilized by GROβ or GROβT, G-CSF, or the combination of G-CSF plus GROβ or GROβT. Cohorts of 3 B6D2F1 mice/group received 3 mg/kg anti–MMP-9 on days 0 and 2.5 and were mobilized by a single injection of GROβ or GROβT (2.5 mg/kg) on day 5. Mice being mobilized by G-CSF or G-CSF plus GROβ or GROβT received 3 mg/kg anti–MMP-9 2 hours before and halfway through (day 2.5) the G-CSF regimen (50 μg/kg, twice daily for 4 days) or daily injections of 1 mg/mouse of the NE selective inhibitor MeOSuc-Ala-Ala-Pro-Val-CMK on each day of G-CSF administration. Mice were analyzed on day 5, 15 minutes after administration of PBS or GROβ. Combined data from 2 experiments are shown. Data are expressed as means ± SEM.
PBSC mobilization by GROβ and G-CSF depends on peripheral PMNs
Because mobilization by G-CSF plus GROβ is associated with synergistic elevation in plasma but not marrow MMP-9 (Figure 1B) and MMP-9, NE, and CG involved in G-CSF mobilization are stored in PMN granules,47,48 we investigated the requirement for peripheral versus marrow neutrophils for mobilization by GROβ or GROβT and G-CSF, alone and in combination. Anti–GR-1 administration results in a selective time-dependent depletion of PMNs.42,49 At 96 hours, a more than 95% depletion of circulating PMNs and a more than 97% reduction in marrow PMNs were observed (Table 1; PBS). Immature marrow neutrophilic cells (INCs) were reduced by 63%, but substantial INCs remained (Table 1). Because the kinetics of GROβ and G-CSF mobilization differ, we used 2 protocols to evaluate mobilization in anti–GR-1 or isotype-treated mice. Animals were mobilized with a single dose of chemokine 24 or 96 hours after administration of anti–GR-1 or IgG2b mAbs. For G-CSF mobilization, mice received anti–GR-1 or IgG2b mAbs 2 hours before initiation of G-CSF dosing and again halfway through the mobilizing regimen. Mice mobilized with G-CSF plus GROβ were treated as described for G-CSF. In control mAb-treated mice, GROβ, GROβT, and G-CSF used as single agents mobilized CFU-GMs by 19- to 27-fold (Table 1). The combination of GROβ or GROβT with G-CSF demonstrated synergy, elevating CFU-GM levels by 135- to 147-fold. In contrast, no mobilization by GROβ, GROβT, G-CSF, or their combination was observed in mice treated with anti–GR-1. Peripheral blood and marrow PMNs were reduced by more than 98% in anti–GR-1–treated mice compared to isotype mAb-treated animals; however, INCs still comprised about 12% of total marrow nucleated cells (Tables 1-2).
Levels of plasma and marrow MMP-9, NE, and CG were barely detectable in anti–GR-1–treated mice, in the absence of mature neutrophils, and no elevations in plasma or marrow were observed following GROβ/GROβT or G-CSF administration, alone or in combination (not shown). At the time that G-CSF–induced and GROβ-or GROβT plus G-CSF–induced CFU-GM mobilization was quantitated, circulating PMNs were less than 0.04 × 106/mL blood (< 1.2% PMNs) but with 0.27 to 0.44 × 106 segmented neutrophils/femur (15%-24% PMNs). These results suggest that MMP-9, NE, and CG accumulating following G-CSF or GROβ/GROβT plus G-CSF administration are PMN derived and that a threshold effect is involved, because mature PMN/femur were still within about 20% of normal. It was also noted that the increase in peripheral PMNs (2.3 ± 0.2-fold) observed after G-CSF treatment in control mice was significantly increased by administration of GROβ (4.0 ± 0.2-fold; P < .001) or GROβT (4.1 ± 0.3-fold; P < .001). The increase in marrow PMNs (2.0 ± 0.2-fold) observed after G-CSF treatment was absent in mice mobilized by G-CSF plus GROβ or GROβT; in fact, marrow PMN levels in these mice were about 25% lower than in controls. These data in conjunction with the observation of lower marrow protease levels in mice receiving combination mobilization (Figures 1B-C) support a role for peripheral neutrophil-derived proteases, particularly MMP-9, in synergistic mobilization.
The lack of CFU-GM mobilization by GROβ or GROβT and G-CSF in anti–GR-1–treated mice was not due to anti–GR-1 toxicity, because anti–GR-1 treatment was without significant effect on CFU-GM/femur (Table 2), consistent with the fact that more than 90% of CFU-GM reside in the GR-1neg population.49 Nucleated marrow cells were reduced by about 25%, resulting from selective depletion of neutrophils. Anti–GR-1 did not affect spleen cellularity. G-CSF or G-CSF plus GROβ/GROβT mobilized substantial CFU-GM to the spleen that was also blocked by anti–GR-1 (Table 2). Unlike G-CSF, GROβ and GROβT do not mobilize CFU-GM to the spleen. Mobilization of CFU-GM by GROβ or GROβT and G-CSF, alone and in combination, and the absence of mobilization after treatment with anti–GR-1 in splenectomized mice were equivalent to those observed in normal mice (not shown).
Transplantation of 2 × 106 G-CSF–, GROβ–,orGROβT-mobilized PBMCs from isotype mAb-treated mice into lethally irradiated mice resulted in 8 of 10, 7 of 10, and 8 of 10 mice/group, respectively, surviving more than 180 days, with 0 of 10 mice surviving if receiving transplants with 2 × 105 mobilized PBMCs from each group. Transplantation of 2 × 106 and 2 × 105 PBMCs from mice mobilized by G-CSF plus GROβ or GROβT resulted in 10 of 10 and 8 of 10 mice/group surviving more than 180 days, respectively. Transplantation of 2 × 106 PBMCs from GROβ-, G-CSF–, or G-CSF plus GROβ-mobilized anti–GR-1–treated mice or nonmobilized PBMCs did not protect recipients (0 of 10 mice/group surviving > 16 days) indicating that like CFU-GM, LTRC mobilization by GROβ, GROβT, and G-CSF and the synergistic mobilization by either chemokine with G-CSF are absent in neutropenic mice.
Attenuated mobilization by GROβT and absence of synergistic mobilization by GROβT plus G-CSF in MMP-9–/– mice
We investigated the requirement of MMP-9 for synergistic mobilization by G-CSF plus GROβT in MMP-9–/– mice (Figure 3). Mobilization of CFU-GM by GROβT was significantly lower but not absent in MMP-9–/– mice (Figure 3A) despite the absence of detectable plasma or marrow MMP-9 (Figure 3B). A 2.4 ± 0.6-fold (P < .05) greater mobilization by GROβT was observed in male mice. Mobilization by GROβT in female and male knockout mice was 27% and 42% of that observed in normal female and male mice, respectively. G-CSF–induced mobilization was equivalent in male and female control and MMP-9–/– mice. Equivalent mobilization by G-CSF in control and anti–MMP-9–treated male and female MMP-9–/– mice supports the specificity of the anti–MMP-9 antibody (Figure 3C). Absolute PMN counts between control and knockout mice did not differ significantly; however, the percentage of PMNs in male knockout mice was significantly higher than in female knockout mice or control mice of either sex (Figure 3D). Synergistic mobilization of CFU-GMs by G-CSF plus GROβT was observed in control mice as expected (Figure 3A), coincident with a synergistic increase in plasma MMP-9 levels (Figure 3B), but was completely absent in MMP-9–/– mice, with CFU-GM/mL blood equivalent to that observed with G-CSF alone. No difference in marrow NE and CG accumulation was observed between male and female control mice (not shown). G-CSF–induced mobilization was associated with equivalent elevation of both enzymes in control and knockout mice in agreement with equivalent CFU-GM mobilization, although a trend toward lower CG levels was observed in male and female MMP-9–/– mice (Figure 3C). As described, NE and CG levels were lower in mice mobilized by G-CSF plus GROβT compared to mice mobilized by G-CSF, and no significant differences were noted between control and knockout mice.
CFU-GM mobilization, proteases, and PMNs in MMP-9–/– mice. Cohorts of 3 C57Bl/6 male and female mice/group were mobilized by a single injection of GROβT (2.5 mg/kg), a multiday regimen of G-CSF (50 μg/kg, twice daily for 4 days) or administration of GROβT on day 5 following the G-CSF regimen. (A) Mean ± SEM increase in CFU-GM/mL blood from 2 experiments. (B) Representative zymograms of plasma and marrow extracts from female MMP-9–/– and MMP-9+/+ mice. (C) Mean ± SEM increase in CFU-GM/mL blood in male and female MMP-9–/– mice receiving anti–MMP-9 antibody during the mobilization regimen. Right panel insert confirms activity of anti–MMP-9 in blocking mobilization by GROβT and reducing mobilization by G-CSF in normal mice as described in Figure 2. (D) Mean ± SEM NE and CG in the marrow extravascular compartment. Data from 2 experiments are shown. (E) Mean ± SEM absolute neutrophil count (ANC) and percent PMN/mL blood from 2 experiments.
CFU-GM mobilization, proteases, and PMNs in MMP-9–/– mice. Cohorts of 3 C57Bl/6 male and female mice/group were mobilized by a single injection of GROβT (2.5 mg/kg), a multiday regimen of G-CSF (50 μg/kg, twice daily for 4 days) or administration of GROβT on day 5 following the G-CSF regimen. (A) Mean ± SEM increase in CFU-GM/mL blood from 2 experiments. (B) Representative zymograms of plasma and marrow extracts from female MMP-9–/– and MMP-9+/+ mice. (C) Mean ± SEM increase in CFU-GM/mL blood in male and female MMP-9–/– mice receiving anti–MMP-9 antibody during the mobilization regimen. Right panel insert confirms activity of anti–MMP-9 in blocking mobilization by GROβT and reducing mobilization by G-CSF in normal mice as described in Figure 2. (D) Mean ± SEM NE and CG in the marrow extravascular compartment. Data from 2 experiments are shown. (E) Mean ± SEM absolute neutrophil count (ANC) and percent PMN/mL blood from 2 experiments.
Discussion
Combination mobilization by G-CSF plus the rapid-acting chemokines GROβ and GROβT represents a means to enhance PBSC mobilization by G-CSF, particularly in some patients and healthy donors who respond poorly. We demonstrate unequivocally and for the first time that mobilization by GROβ or GROβT and G-CSF, used alone or in combination, depends on PMN-derived proteases. In addition, synergistic PBSC mobilization observed when these chemokines and G-CSF are used in combination is dependent on PMN-derived plasma MMP-9.
We previously reported that a rapid and transient up-regulation of plasma MMP-9 precedes PBSC mobilization by GROβT.9,31 We now extend these observations by demonstrating that both GROβ and GROβT also elevate marrow MMP-9 levels. Furthermore, the accumulation of both plasma and marrow MMP-9 associated with GROβ-induced PBSC mobilization is absent in mice depleted of mature PMNs despite the presence of appreciable numbers of immature marrow neutrophilic cells, and absent when MMP-9 activation is blocked in vivo, strongly suggesting that the peripheral neutrophil is the source of MMP-9. We have also observed that the return of PBSC mobilization by GROβ following PMN depletion coincides exactly with the endogenous return of peripheral blood PMNs and plasma MMP-9 activity, but not marrow PMNs or the accumulation of marrow MMP-9 (L.M.P., unpublished observations, January 2003). These results are consistent with a neutrophil requirement for IL-8–induced CFU-GM mobilization.50
A positive correlation between neutrophils and NE and CG accumulating in marrow during G-CSF administration and CFU-GM mobilization resulting from proteolytic cleavage of VCAM-135,36 has been described. However, although highly correlative and consistent with the protease repertoire of neutrophils, a requirement for either PMNs or their proteases for mobilization in G-CSF–treated mice was not demonstrated. In addition, administration of NE-I was shown to block G-CSF–induced mobilization37 ; however, because macrophages,51,52 lymphocytes,53 and fibroblasts,54 as well as PMNs, release elastases, one cannot conclude selective involvement of PMNs. We now show that selective PMN depletion blocks G-CSF–induced stem and progenitor cell mobilization and accumulation of marrow NE and CG and plasma and marrow MMP-9 but not MMP-2, clearly demonstrating that the marrow proteases implicated in G-CSF mobilization are in fact derived from PMNs. Furthermore, because plasma NE and CG activity does not change appreciably during G-CSF–induced mobilization, a preferential role for marrow PMN-derived proteases is suggested, which contrasts with GROβ. In vivo neutralization with anti–MMP-9 or NE inhibitor also demonstrates that G-CSF–mediated mobilization requires PMN-derived proteases. The fact that NE and CG accumulation was blocked in anti–GR-1–treated mice, despite the appreciable presence of immature marrow neutrophilic cells, suggests that these residual neutrophilic cells are insufficient to mediate/contribute to mobilization or may not contain sufficient proteases. However, primary granules that contain NE and CG and specific granules containing gelatinases are present by the promyelocyte stage of neutrophil maturation48 and these and more mature neutrophilic cells are present in marrow after anti–GR-1 treatment.
A critical role for PMNs in PBSC mobilization is supported by studies in gene-deficient mice. IL-8, which binds the same receptor as GROβ/GROβT in mice, and G-CSF do not mobilize in G-CSF receptor (G-CSFR)–deficient mice.55,56 Similarly, we have shown that GROβT and G-CSF do not mobilize in CXCR2-deficient mice.31 These studies suggest possible cross-talk between these receptors expressed on PMNs. Alternatively, lack of mobilization by IL-8 in G-CSFR–deficient mice may result from the fact that these mice have few circulating PMNs and protease release in response to IL-8 may not reach threshold levels. In CXCR2-deficeint mice, peripheral PMN levels are normal or elevated57 and granulopoiesis is enhanced58 ; however, impaired PMN migration and chemoattraction59,60 and increased susceptibility to infection60 suggest defects in responses downstream of G-CSFR.
Important novel mechanistic information was identified by analyzing PBSC mobilization by G-CSF plus GROβ or GROβT.A summary of the changes in protease and cytokine levels in plasma and marrow are shown in Table 3. The synergistic mobilization response was mediated solely by a synergistic rise in plasma MMP-9. This was demonstrated by loss of the synergistic response on MMP-9 neutralization but not NE inhibition in vivo and the lack of synergistic mobilization in MMP-9 knockout mice. The importance of plasma MMP-9 was also reflected in the reduction of marrow MMP-9, NE, and CG compared to mice mobilized with G-CSF alone. This can potentially be explained by the shift in neutrophil populations to the periphery. Peripheral blood levels PMNs increased 4-fold (2-fold above G-CSF alone), whereas marrow PMN levels were reduced by 2-fold compared to G-CSF alone and were about 25% lower than controls.
The ability of plasma MMP-9 to mediate HSC release from marrow is not completely understood. MMP-9,61-64 NE, and CG35,36 can degrade extracellular matrix and adhesion molecule/receptor interactions within marrow. In addition, PMN adhesion and release of proteases causes disorganization of endothelial junctions,64-66 facilitating HSC transmigration and egress. In this regard, we observe degradation of recombinant VCAM-1 by activated MMP-9 or marrow supernates from GROβT-mobilized mice and 11% and 31% reduction in CXCR4 expression levels on mobilized c-kit+, lin– cells from GROβT-treated and G-CSF plus GROβT-treated mice, respectively, compared to G-CSF alone (L.M.P. and S.F., unpublished observations, January 2003). Furthermore, administration of recombinant MMP-9 to rabbits induces a rapid peripheral leukocytosis,67 although HSCs were not quantitated. It is likely that in GROβ/GROβT-mobilized mice, elevation of marrow MMP-9 that degrades matrix and releases HSCs, plus disruption of endothelial junctions by plasma MMP-9, coordinately result in HSC transmigration. In mice mobilized by G-CSF plus GROβ/GROβT, release of HSCs within marrow as a consequence of the proteolytic environment created by G-CSF plus the peripheral effect of augmented PMNs and synergistic elevation of plasma MMP-9 levels results in greatly facilitated HSC egress. Although we do not observe an increase in marrow MMP-9 on combination mobilization, we cannot exclude efflux of plasma MMP-9 into marrow that participates in matrix degradation, which is inactivated or complexes with substrate and cannot be detected.
The GROβ chemokines and G-CSF were without effects on plasma MMP-2 levels and we did not detect MMP-2 in marrow after mobilization, suggesting that MMP-2 does not play a role in mobilization by these cytokines. Detection of elevated levels of plasma MMP-9 but not MMP-2 after 4 to 5 days in patients mobilized with G-CSF68 supports our results. Although surprising, because both are stored in neutrophil granules and share substrate specificity, MMP-2 and MMP-9 are structurally divergent in the hemopexin domain and are differentially regulated by the tissue inhibitor of metalloproteinases (TIMP) family of specific inhibitors.69 Because it is the stoichiometry between MMP and TIMP that determines MMP activation, differential activation is possible. G-CSF stimulation of the release of MMP-2 alone or with MMP-9 from nonhematopoietic G-CSFR+ cancer cells has been reported70,71 and also supports differential regulation of MMP-2 versus MMP-9 production from the same receptor.
We did not observe absence of GROβ/GROβT-induced mobilization in MMP-9–/– mice despite the absence of proMMP-9 protein and MMP-9 activity. No compensatory rise in MMP-2 or other gelatinolytic activity or NE or CG was seen, and neutrophil transmigration is normal in MMP-9–/– mice.72 Mobilization was, however, significantly attenuated. This contrasts with the report of equivalent mobilization results by IL-8 in normal and MMP-9–/– mice.50 However, in this report, attenuation of mobilization in response to IL-8 is actually observed in MMP-9–/– mice if total CFU-GM mobilized above control is quantitated rather than fold change, to normalize for different backgrounds in normal versus knockout mice, which is consistent with our results. It is well known that compensatory mechanisms can counteract lifelong gene disruption73 and compensation to development of autoimmune encephalomyelitis in MMP-9–/– mice has been reported.74 The MMPs are a large family of endopeptidases,38, 61,75 with each MMP having preferred as well as overlapping substrate specificity.61,76 The ability of other MMPs to participate in GROβ/GROβT mobilization in the absence of MMP-9 is unknown. The fact that anti–MMP-9 blocked virtually all GROβ/GROβT-induced mobilization in normal mice, but MMP-9 gene deletion only partially attenuated the response, argues that the acute MMP-9 block induced by antibody occurs too rapidly to permit compensation. In contrast to mobilization by GROβ/GROβT alone, synergistic mobilization was completely absent in MMP-9–/– mice, indicating that the potential compensatory mechanisms that allow some mobilization in response to chemokines cannot substitute for plasma MMP-9, supporting the specificity of the plasma MMP-9–mediated synergistic response.
MMP-9–mediated elevation of plasma SCF levels that facilitates HSC mobilization by G-CSF has been described,34 which could explain the lack of GROβ/GROβT mobilization by anti–MMP-9.9,31 However, we were unable to detect elevated plasma or marrow SCF after mobilization by GROβ/GROβT, G-CSF, or their combination and did not observe reduced G-CSF–induced CFU-GM mobilization in MMP-9–/– mice.34 The reasons for these differences are not clear; however, our MMP-9–/– mice are on the C57Bl/6 background, whereas theirs are on the CD1 background.34 Genetic differences in mobilization response to G-CSF77 and GROβ (L.M.P., unpublished observations, January 1997) are known and may be involved. Consistent with our results, 2 recent reports also demonstrate equivalent G-CSF–induced mobilization in normal and MMP-9–/– mice.78,79 In addition, MMP-9 was linked to synergistic mobilization by G-CSF plus pertussis toxin,78 which supports our earlier studies implicating Gαi proteins in MMP-9 production in response to GROβ.31
SDF1-CXCR4 interactions are believed to be involved in HSC homing and mobilization.12,80-82 A correlation between reduction in marrow SDF-1α by NE and PBSC mobilization by G-CSF was reported, suggesting that altered blood/marrow SDF-1 gradients might be responsible.37 This attractive potential mechanism is supported by the fact that SDF-1α can be processed and inactivated by NE,37 CG,46 and MMP-9.45 However, we observed no effect of GROβ or GROβT on plasma or marrow SDF-1, making it unlikely that it plays a role in GROβ mobilization. We did observe preferential reduction in marrow SDF-1 in G-CSF–mobilized mice consistent with this report.37 However, comparison of absolute protein in plasma versus marrow extract does not allow comparison of gradient at the marrow/plasma interface. When expressed as molarity, assuming a 10-μL femoral extravascular compartment volume,35 we observed 0.51 ± 0.03 nM in plasma versus16 ± 0.4 nM/femur, that is, a 31:1 gradient favoring marrow (n = 15 mice). However, in G-CSF–treated mice, despite a 90% reduction of marrow SDF-1, a plasma-marrow gradient of 0.2 ± 0.01 nM versus 3.0 ± 0.01 nM was observed, still favoring marrow by 15:1. It is unlikely that a 2-fold change in SDF-1α gradient alone is sufficient to mediate HSC egress. In checkerboard assays in vitro, 10- to 100-fold differences in SDF-1 gradient were required for migration of MO7e cells83 and more than 10-fold differences were required for maximal migration of primary CFU-GM.84
In summary, we demonstrate that PBSC mobilization by the GROβ chemokines and G-CSF requires PMN-derived proteases. Differential but overlapping protease utilization and preferential sites of release of these proteases are involved, consistent with the different yet synergistic activities of these 2 mobilizers. Lastly and most importantly, we demonstrate that the synergistic PBSC mobilization in response to G-CSF plus GROβ is mediated by a synergistic increase in peripheral PMN-derived plasma MMP-9, strongly suggesting that the peripheral proteolytic environment is responsible for the synergistic mobilization response. Our data also suggest that MMP-9 release or activation may be a common mechanism downstream of both the CXCR2 and G-CSF receptors that can be manipulated for therapeutic utility.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2003-04-1115.
A.G.K. is employed by GlaxoSmithKline, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Supported by grant HL69669 from the National Institutes of Health.