Abstract
We followed 141 patients treated with imatinib mesylate (> 300 mg) for chronicphase chronic myelogenous leukemia (CML) following failure of treatment with interferon. During 12 months from the start of imatinib mesylate treatment, 96.5% achieved a complete hematologic response, 47.0% achieved a major cytogenetic response, and 32.4% achieved a complete cytogenetic response. The proportion of patients with hematologic relapse was 10.9% at 12 months and 14.6% at 18 months. In a univariate Cox regression analysis, the only pretreatment characteristics that correlated with an increased risk of hematologic relapse were hemoglobin level less than 120 g/L (12 g/dL) (P = .02), increased bands in the peripheral blood (P = .01), and clonal evolution (P < .0001). In a multivariate analysis, an elevated platelet count (P = .03) and clonal evolution (P < .0001) were the only significant factors for hematologic relapse. During treatment, the absence of a major cytogenetic response within the first 6 months also significantly correlated with relapse (P = .03). Notably, patients failing to achieve a major cytogenetic response by 6 months had a significantly higher rate of hematologic relapse (27%) compared with those who achieved a major cytogenetic response by 6 months (3%), and patients with clonal evolution had a significantly higher risk of hematologic relapse (50%) than those without clonal evolution (9%).
Introduction
Chronic myelogenous leukemia (CML) is a clonal hematopoietic stem cell disorder characterized by a specific chromosomal translocation, t(9;22), resulting in a shortened chromosome 22, referred to as the Philadelphia (Ph) chromosome.1,2 The molecular consequence of this translocation is the production of a constitutively activated Bcr-Abl tyrosine kinase. This oncogene induces leukemia in mice, implicating Bcr-Abl as the cause of CML.3,4 Importantly, the kinase activity of Bcr-Abl is essential for transformation.3 Therefore, Bcr-Abl is an ideal target for therapy and this has been confirmed in clinical trials using imatinib mesylate (Gleevec; Novartis, East Hanover, NJ), a selective inhibitor of the Bcr-Abl tyrosine kinase.5 Impressive responses have been reported in patients with chronic-phase disease after failure of interferon (IFN) treatment, with 95% achieving a complete hematologic response and 60% achieving a major cytogenetic response within 18 months.6 However, follow-up is still short and the exact place of imatinib mesylate in chronic-phase CML treatment algorithms remains to be determined. Several important questions remain unanswered, including the durability of responses, factors associated with relapse, and whether cytogenetic responses correlate with improved survival. As a corollary, if cytogenetic responses do result in improved survival, what constitutes an adequate trial of therapy after which achieving a cytogenetic response becomes unlikely? In this report we analyzed the pretreatment characteristics that correlate with probability of hematologic relapse in patients with chronic-phase CML treated with imatinib mesylate. We also determined whether achievement of a major cytogenetic response at any time on treatment influenced the risk of hematologic relapse. Finally, we assessed the probability of achieving a cytogenetic response over time.
Patients, materials, and methods
Patients and laboratory data
As part of multi-institutional phase 1 and 2 clinical trials in patients with chronic-phase CML in whom IFN treatment failed (Novartis studies 001, 110, and 113), we have followed 141 patients treated with doses of imatinib mesylate greater than 300 mg. As part of routine follow-up, patients underwent bone marrow evaluations, including metaphase cytogenetics every 3 to 6 months. Cytogenetic responses, based on metaphase analysis of at least 20 cells, were defined as complete (Ph–), partial (1%-35% of cells Ph+), minor (36%-65% of cells Ph+), and absent or no response (> 65% Ph+). Major cytogenetic response includes complete and partial (0%-35% Ph+).
Statistics
Descriptive statistics (n, proportion, median, range) were used to summarize the pretreatment and baseline characteristics of the patients (Table 1). The Kaplan-Meier method was used to estimate the time to complete cytogenetic response, major cytogenetic response, complete hematologic response, hematologic relapse, and duration of hematologic response. Time to hematologic relapse is defined as the time from the start of the imatinib mesylate treatment to day of loss of hematologic response or day of last contact, whichever occurred first. Duration of hematologic response was defined as the time interval between day of complete hematologic response and day of loss of hematologic response or day of last contact, whichever occurred first. Those without hematologic relapse were censored at day of last contact. Complete hematologic response was attained in 98.6% of the patients within 18 months from the start of the imatinib mesylate treatment. Complete hematologic response was defined as normalization of white blood cell (WBC) counts to less than 10 × 109/L with normal differential, normalization of platelet counts to less than 450 × 109/L, and disappearance of all signs and symptoms of the disease for 4 weeks or longer. Using Cox regression analysis, the following pretreatment variables were evaluated to identify pretreatment risk factors for hematologic relapse: response to IFN therapy (hematologic failure, cytogenetic failure, IFN intolerance), age (< 60 years old, ≥ 60 years old), sex, weight (< 70 kg, ≥ 70 kg), splenomegaly, time since diagnosis of CML (< 3 years, ≥ 3 years), hemoglobin level (< 120 g/L [< 12 g/dL], ≥ 120 g/L [≥ 12 g/dL]), WBC count (< 10 000/mm3 [< 10 × 109/L], ≥ 10 000/mm3 [≥ 10 × 109/L]), platelet count (< 450 000/mm3 [< 450 × 109/L], ≥ 450 000/mm3 [≥ 450 × 199/L]), basophils (< 7%, ≥ 7%), bands (< 6%, ≥ 6%), blasts in peripheral blood (0%, > 0%), blasts in bone marrow (< 5%, ≥ 5%), Ph+ cells in metaphase (< 99%, ≥ 99%), clonal evolution, and myelosuppression. Major cytogenetic response at any time was evaluated as a time-dependent covariate. The stepwise procedure was used to identify independent prognostic factors for hematologic relapse among all pretreatment and time-dependent (major cytogenetic response) variables. The Kaplan-Meier method was used to estimate the probability of major (Ph+ cells ≤ 35%) or complete (Ph+ 0%) cytogenetic response among patients who had no cytogenetic response (defined as either Ph+ > 65% in one case or Ph+ cells > 95% in the other) in earlier months. Because there were no competing events, the probability can be interpreted as the conditional probability of a subsequent major or complete cytogenetic response given no response in earlier months.
Results
Of the 141 patients analyzed in this study, 134 (95.0%) achieved a complete hematologic response within 6 months and 139 (98.6%) within 18 months. The median time to achievement of a complete hematologic response was 0.92 months (range, 0-18.14 months). Sixty-six patients (47%) achieved a major cytogenetic response and 32.4% a complete cytogenetic response within 12 months (Table 2). These results are similar to those reported in the larger phase 15 and 2 studies of chronic-phase patients in whom IFN therapy failed, although there is a trend toward slightly inferior results than those published by Kantarjian et al.6 With a median follow-up of 15.9 months (range, 1.8-47.2 months), a total of 22 patients (15.6%) have lost a complete hematologic response, defined as a persistently elevated WBC or platelet count (n = 9) or progression to accelerated (n = 10) or blast phases (n = 3). The median time to loss of hematologic response for all patients has not yet been attained (range, 0-47.18 months). Similarly, the median duration of hematologic response has not yet been attained (range, 0-34.43 months). Considering only those patients who did have a relapse, the median time to loss of hematologic response was 9.79 months (range, 2.5-24.9 months) and median duration of hematologic response 8.59 months (range, 1.81-24.18 months).
In a univariate analysis the only pretreatment characteristics that increased the risk of hematologic relapse were a low hemoglobin level, less than 120 g/L (12g/dL) (P = .02), increased bands in the peripheral blood (P = .01), and evidence of cytogenetic clonal evolution (P < .0001). In a multivariate analysis, an elevated platelet count (P = .03) and clonal evolution (P < .0001) were the only significant pretreatment factors for relapse. Overall, patients with elevated platelet counts (≥ 450 000 mm3 [≥ 450 × 109/L]) had 2.74 (95% CI, 1.13-6.64) times greater risk of hematologic relapse than those without an elevated platelet count, and those with clonal evolution (including duplication of the Ph chromosome) had 6.84 (95% CI, 2.83-16.57) times greater risk of hematologic relapse than those without clonal evolution (Table 3).
Relapse was more frequent in patients who failed to achieve a major cytogenetic response at any time on therapy (Figure 1). On the average (ie, average over time), the attainment of a major cytogenetic response at any time significantly reduced the likelihood of hematologic relapse (hazard ratio = 0.12; 95% CI = 0.03, 0.52; P = .0047). When we performed the stepwise Cox regression using all the pretreatment and time-dependent variables that were significant in the univariate analysis (ie, hemoglobin level ≥ 120 g/L [≥ 12 g/dL], bands ≥ 6%, clonal evolution, and major cytogenetic response at any time [time-dependent covariate]), the model selected clonal evolution and major cytogenetic response as independent predictors of hematologic relapse (P = .0033 by log-rank test). Three of the 4 patients with major cytogenetic responses who had a relapse had discontinued therapy, one due to recurrent grade 3 elevations in liver function tests (LFTs), the other 2 voluntarily. Two of these patients regained major cytogenetic responses after restarting imatinib mesylate therapy.
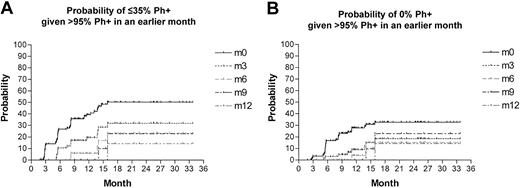
Having established that achieving a major cytogenetic response appears to be important in reducing risk of hematologic relapse, we were anxious to determine how long treatment should be continued to achieve such a response. Table 4 and Figure 2 show the probability of achieving a major cytogenetic response (≤ 35% Ph+) by 15 months if no cytogenetic response (> 65% Ph+) had been previously obtained by a specified month since the start of the treatment. For example, among patients who were more than 65% Ph+ in the first 3 months, their probability of subsequently achieving a major cytogenetic response by 15 months is 35% (95% CI, 23%-47%). The probability of a subsequent major cytogenetic response by 15 months decreased as the duration of Ph+ more than 65% increased. For patients more than 65% Ph+ at 6 and 9 months the probability of a subsequent major response by 15 months was less than 25%. The probability of achieving a major cytogenetic response for patients more than 65% Ph+ after at least 3 months of treatment was highest at 3 to 6 months, 38% (95% CI, 25%-50%), and appeared to decline after 9 months. In contrast, only 10% of patients achieved a major cytogenetic response by 15 months if they were more than 65% Ph+ at 12 months (95% CI, 0-48%). For patients more than 95% Ph+ at 3, 6, and 12 months the probability of a subsequent major response by 15 months was 28% (95% CI, 15%-41%), 17% (95% CI, 1%-33%), and 0% (95% CI, 0%-0%), respectively. Thus, patients who are 95% Ph+ at 6 months still have a 17% chance of having a subsequent major cytogenetic response. This combined with a large confidence interval means that we cannot make a firm statement about when one should consider that a cytogenetic response would be unlikely. Interestingly, the probability of achieving a complete cytogenetic response within 12 months falls dramatically if there is no evidence of any cytogenetic response (residual Ph > 65%) within 3 months, though again the limitations of the small patient numbers is recognized (Figure 2).
Discussion
In this study we sought to identify pretreatment factors associated with hematologic relapse in patients with chronic-phase CML treated with imatinib mesylate following failure of IFN treatment. We also wished to determine whether cytogenetic response reduced the risk of hematologic relapse and, finally, the probability of such a response over time. In terms of response, our results are similar to those reported in the larger phase 15 and 2 studies of patients with chronic-phase disease in whom IFN therapy failed, although there is perhaps a trend toward slightly inferior results than those published by Kantarjian et al.6 Of note, the pretreatment risk factors of the patients in this report are very similar to those reported by Kantarjian et al.
From this analysis, we conclude that the major prognostic factors for relapse are the presence of cytogenetic clonal evolution and failure to achieve a major cytogenetic response (Table 3). Additional clonal abnormalities observed in our patients were trisomy 8 (4 patients, 2 of whom relapsed), additional Ph (7 patients, 2 of whom relapsed), chromosome 17 abnormalities (5 patients, 3 of whom relapsed), and other random abnormalities (6 patients, 4 of whom relapsed). Although the numbers are small there is a suggestion of inferior outcome with chromosome 17 abnormalities in line with previous experience with IFN-based regimens.7
That clonal evolution emerged as a major prognostic factor for relapse is perhaps not surprising because it is considered in many CML classification systems to be a feature of accelerated-phase disease. We have previously reported that the presence of clonal evolution prior to therapy with imatinib mesylate results in inferior progression-free survival in patients with other accelerated-phase features.8 However, in a small subset of patients with clonal evolution as the sole criterion of disease acceleration, treatment with 600 mg resulted in superior outcome; with a median follow-up of 12 months, the major cytogenetic response rate was 80% (12 of 15), with a complete cytogenetic response rate of 67% (10 of 15).8 Only one of these patients has had a relapse. Thus, it would appear that patients with clonal evolution in the absence of other accelerated-phase features might have a prognosis intermediate between that of typical chronic-phase and accelerated-phase disease. Although this was a small study, the superior outcome of patients with clonal evolution compared with those in the present report suggests that more aggressive treatment (eg, higher doses of imatinib mesylate) is warranted in patients with clonal evolution in the absence of other accelerated-phase features.
Because few patients achieving a major cytogenetic response subsequently relapsed, we believe that achieving a major cytogenetic response should be an essential goal of therapy with imatinib mesylate. Moreover, because no patient achieving a major cytogenetic response within 6 months has had a relapse, we believe that achieving an early major cytogenetic response is highly desirable. A recently published report suggests that early cytogenetic response (within 3 months) takes over the significance of clonal evolution, at least for survival.9 However, because only one of our early cytogenetic responders had clonal evolution, we are unable to confirm this observation. Our data do strongly suggest, however, that clonal evolution is an independent risk factor for those without an early major cytogenetic response (Table 5). Although confidence intervals are high due to small patient numbers, it appears that cytogenetic responses are very unlikely in patients more than 65% Ph+ after 12 months of treatment. Thus, our recommendation for these patients would be to consider alternative treatment approaches, including escalation of the dose of imatinib mesylate to 800 mg,10 or allogeneic stem cell transplantation, where this is appropriate. Of note, approximately one third of patients in this study who underwent dose escalation at 12 months for lack of cytogenetic response subsequently achieved a major cytogenetic response. Because the likelihood of obtaining a major cytogenetic response declines over time, we view this as important information to guide decision-making in patients who might otherwise be candidates for transplantation. As more data become available, particularly in newly diagnosed patients, these recommendations are likely to evolve.11
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-02-0371.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.