Abstract
Retinoids are potent inducers of cell cycle arrest and differentiation of numerous cell types, notably granulocytes. However the mechanisms by which retinoids mediate cell cycle arrest during differentiation remain unclear. We have used myeloid differentiation to characterize the molecular pathways that couple cell cycle withdrawal to terminal differentiation. Using primary cells from mice deficient for either the cyclin-dependent kinase inhibitor (CDKi) p27Kip1, the Myc antagonist Mad1, or both Mad1 and p27Kip1, we observed that signals mediated through retinoic acid receptor α (RARα), but not RARβ or γ, required both Mad1 and p27Kip1 to induce cell cycle arrest and to accelerate terminal differentiation of granulocytes. Although RARα did not directly regulate Mad1 or p27Kip1, the RARα target gene C/EBPϵ directly regulated transcription of Mad1. Induction of C/EBPϵ activity in granulocytic cells led to rapid induction of Mad1 protein and transcript, with direct binding of C/EBPϵ to the Mad1 promoter demonstrated through chromatin immunoprecipitation assay. These data demonstrate that cell cycle arrest in response to RARα specifically requires Mad1 and p27Kip1 and that Mad1 is transcriptionally activated by CCAAT/enhancer-binding protein ϵ (C/EBPϵ). Moreover, these data demonstrate selectivity among the RARs for cell cycle arrest pathways and provide a direct mechanism to link differentiation induction and regulation of the Myc antagonist Mad1.
Introduction
The roles of retinoids during hematopoiesis, particularly granulopoiesis, have been recognized for many years. Most strikingly, retinoids are powerful inducers of differentiation.1 This effect is central to the use of all-trans-retinoic acid (ATRA) in the treatment of acute promyelocytic leukemia (APL), a leukemia characterized by a chromosomal translocation always involving retinoic acid receptor α (RARα).2,3 Pharmacologic doses of ATRA are able to relieve the repression caused by the fusion proteins via directly binding and activating them and promoting cell cycle arrest and differentiation of the leukemic blasts.3 However, the mechanisms by which ATRA mediates cell cycle arrest as well as differentiation remain unclear. An understanding of the molecular basis of this effect will provide important insights into how cell cycle withdrawal is coupled to terminal differentiation and how retinoids interact with the cell cycle.
The effects of retinoid ligands are mediated through direct binding to the ligand-binding domain of RARs or retinoid X receptors (RXRs), members of the nuclear hormone receptor superfamily.4 RARs heterodimerize with RXRs, and this heterodimer binds DNA at retinoic acid response elements (RAREs) in the regulatory sequences of target genes. When unliganded, RAR/RXR heterodimers bind RARE and form repressive complexes through binding SMRT- and NCo-R–containing corepressor complexes.5 Ligand binding triggers the release of the corepressor complexes and coactivator complexes are recruited to the RAR/RXR dimer and activate gene transcription.6-8 Three isotypes of RARs and RXRs have each been identified: α, β, and γ, with each RAR isotype expressed as a number of isoforms.4 RARα and RARγ are believed to be most important in the regulation of granulopoiesis.4,9 This is most strikingly demonstrated in progenitor assays of bone marrow cells derived from RARα1–/–RARγ–/– mice. These cells displayed a block in granulopoiesis at the myelocyte stage of development, revealing an absolute requirement for RAR-mediated signaling in the terminal differentiation of granulocytes.9
Our understanding of the mechanisms through which RARα mediates cell cycle arrest and differentiation of myeloid cells is a question of particular relevance. First, RARα, but not RARβ or RARγ, is involved in the pathogenesis of myeloid leukemia.10 RARα is involved in leukemia both as a fusion partner, most commonly involving promyelocytic leukemia gene (PML) in APL, and point mutations in the RARα protein have also been reported to result in retinoid resistance.11 These data suggest a critical role for RARα-mediated signaling in regulating granulopoiesis. Second, related to the observations directly linking RARα to the pathogenesis of leukemia, is the development of high-specificity RARα agonists as therapeutics.12 The identification of the specific downstream effectors of RARα-mediated signaling and the ability to place these targets into molecular pathways allows insight into the mechanism of drug action of RARα-specific ligands and rational application of these compounds.
Cellular differentiation commonly involves a tight coupling of withdrawal from the cell cycle and the acquisition of specialized cell functions. Although the molecular mechanisms that regulate the cell cycle during mitotic cell division cycles have become increasingly clear, the mechanisms that regulate cell cycle withdrawal during differentiation remain poorly understood.13 Several families of proteins have been identified that may play a role in cell cycle withdrawal during differentiation, including the Mad family of transcriptional repressors14 and the Cip/Kip family of cyclin-dependent kinase inhibitors (CDKi's).13 How proteins such as these cooperate during cell cycle withdrawal remains a critical question to our understanding of differentiation.
The CDKi p21Cip1 contains a RARE in its promoter and is retinoid responsive, although the significance of this responsiveness during granulopoiesis remains unclear because p21Cip1 mutant mice have no reported granulocyte defects.15,16 We and others have recently demonstrated that retinoid treatment of granulocytic cell lines results in down-regulation of cyclin E/CDK2 activity and c-Myc and up-regulation of the Cdk2 inhibitor p27Kip1 and the Myc antagonist Mad1.17,18 These studies, as well as studies in primary cells from mutant mice, implicate roles for both Mad1 and p27Kip1 in regulating cell cycle exit during retinoid-induced granulocytic differentiation.17,19 In the current study we sought to determine the contributions of Mad1 and p27Kip1 to retinoid-induced cell cycle withdrawal pathways during granulocytic differentiation.
In this report we demonstrate that both Mad1 and p27Kip1 are absolutely required to mediate cell cycle arrest of granulocyte progenitors in response to RARα-specific stimulation. Requirement for both Mad1 and p27Kip1 is unique to RARα-mediated cell cycle withdrawal pathways because RARβ/γ selective ligand activity is sufficient to arrest Mad1–/–p27Kip1–/– progenitors with an efficiency equivalent to ATRA. Analysis of the promoter regions of Mad1 and p27Kip1 did not reveal strong candidate RAR/RXR-binding sites. We therefore focused on factors known to be important during terminal granulopoiesis that are also regulated by RARα. CCAAT/enhancer-binding protein ϵ (C/EBPϵ) is one such factor and analysis of the promoter of Mad1 revealed numerous putative C/EBP-binding sites. We demonstrate that C/EBPϵ can rapidly induce transcription of Mad1 RNA in the presence of cycloheximide and Mad1 protein, suggesting that C/EBPϵ can directly regulate Mad1 transcription. Furthermore, C/EBPϵ directly binds to the Mad1 promoter in vivo as determined by chromatin immunoprecipitation (ChIP) assays. Strikingly, granulocytic cell lines derived from Mad1–/–p27Kip1–/– animals are resistant to differentiation following induction of an inducible C/EBPϵ. These data demonstrate that cell cycle arrest in response to RARα specifically requires Mad1 and p27Kip1 and that Mad1 is a transcriptional target of C/EBPϵ. Moreover, these observations demonstrate selectivity among the RARs for cell cycle arrest pathways and provide a direct mechanism to link differentiation induction and regulation of the Myc antagonist Mad1.
Materials and methods
Retinoid ligands
ATRA was purchased from Sigma (St Louis, MO) and was dissolved in dimethyl sulfoxide (DMSO) and diluted with ethanol prior to use. The synthetic RARα-specific agonists AGN19407820 and AGN195183,20 the RARα-specific antagonist AGN194301,21 and the pan-RAR antagonist AGN19431022 were synthesized by Allergan (Irvine, CA).
Mice
p27Kip1 mutant mice were kindly provided by Dr J. Roberts (Fred Hutchinson Cancer Research Center, Seattle, WA).23 Mad1 mutant and Mad1/p27Kip1 double-mutant mice have been previously described.17,19 All wild-type mice were derived from within the breeding colony. RARA mutant mice (RARα and RARγ) have been previously described.24,25 Mice were used at 7 to 12 weeks of age.
Isolation and expression profiling of immature and mature granulocyte populations
Bone marrow from a wild-type mouse was collected and prepared as described previously.26 Samples were stained with phycoerythrin (PE) anti-CD11b and fluorescein isothiocyanate (FITC) anti–Gr-1 (PharMingen, San Diego, CA). Samples were sorted on a FACSStarplus flow cytometry system interfaced with Cellquest software (Becton Dickinson, San Jose, CA). Immunoblotting was performed on protein extracts lysed in sodium dodecyl sulfate (SDS) lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], pH 6.8; 1% SDS; 10% glycerol; 1 mM dithiothreitol [DTT]; protease inhibitors). Following SDS–polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Immobilon P; Millipore, Beverly, MA) and analyzed using the antisera listed and enhanced chemiluminescence (ECL) detection. The following antibodies were used: rabbit anti-p27KIP1 (sc-527, sc-528; Santa Cruz Biotechnology, Santa Cruz, CA), Mad1 (sc-222), ERα (sc-542) and mouse anti–α-tubulin (Sigma).
Quantitative real-time polymerase chain reaction (PCR) was performed following isolation of granulocyte fractions and on mouse promyelocytes (MPROs) differentiated with AGN195183. Cells were lysed in Trizol reagent (Invitrogen, Carlsbad, CA) and RNA prepared using standard procedures. cDNA was prepared following DNase treatment using random primers and Superscript II reverse transcriptase (Invitrogen) as described by the manufacturer. PCR was performed using quantitative real-time PCR, using the SYBR green dye detection method (Applied Biosystems, Warrington, United Kingdom). Primers were designed for Mad1, RARα, C/EBPϵ, and β2-microglobin cDNA using Primer Express software (Applied Biosystems).
Primary granulocyte cultures
Whole bone marrow (1 × 105/mL) was cultured in Dulbecco modified Eagle medium (DMEM) containing 20% fetal bovine serum (FBS; CSL, Melbourne, Australia), 15% baby hamster kidney cells–stem cell factor (BHK-SCF) cell-conditioned media (source of SCF, equivalent to 150 ng/mL, gift from S. Collins, Fred Hutchinson Cancer Research Center), 1000 U/mL recombinant human granulocyte colony-stimulating factor (rhG-CSF; Amgen, Thousand Oaks, CA) and either AGN195183 (10–6 M), ATRA (10–6 M), or control (ethanol). Cultures were analyzed by fluorescence-activated cell sorting (FACS) after 3 days using CD11b/Gr-1 staining and assessed morphologically.
Progenitor cell assays
Colony-forming cell (CFC) assays were performed using 3% methylcellulose-based media. Methylcellulose (3% in Iscove modified Dulbecco media; Gibco, Carlsbad, CA) supplemented with 20% FBS, 2 U/mL recombinant human erythropoietin (Janssen-Cilag, Sydney, Australia), 10% BHK-SCF cell-conditioned, 1% X63Ag8-653-IL3 cell-conditioned media27 (source of interleukin 3 [IL-3]; 1000 U/mL) and 1% IL-6 cell-conditioned media (source of IL-6, 1000 U/mL). Colonies were scored on day 7 of culture.
Where indicated, retinoids were directly added at a final concentration of: ATRA (10–6 M), AGN194078 (10–6 M), AGN195183 (10–6 M), AGN194301 (10–6 M), and AGN194310 (10–7 M). Ethanol (vehicle) was added as control.
Northern blot analysis
Northern blot analysis was performed on total RNA extracted using Trizol reagent (Invitrogen). Following electrophoresis and transfer to nylon membrane (Immobilon-Ny+; Millipore) membranes were probed using cDNA probes to Mad1, p27Kip1, C/EBPϵ, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Generation of MPRO cell lines and C/EBPϵ-ER MPRO cell lines
MPROs were generated from whole bone marrow of mice of the appropriate genotype and maintained in culture as previously described.19,28 Retroviral infection of MPRO cell lines with either pBabe or pBabeC/EBPϵ-ER was performed as described.17 Infected cells were selected with 2 μg/mL puromycin (Sigma) and maintained as bulk culture or cloned through 3% methylcellulose supplemented with 20% FCS and 1000 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF). For all C/EBPϵ-ER infections, at least 3 independent infections were performed and at least 6 clones from each infection screened for phenotypic response.
Confocal microscopy analysis of Mad1 expression
pBabeC/EBPϵ-ER and pBabe MPROs were treated with either AGN195183 or 4-OH-tamoxifen for 0, 14, and 24 hours. Following cytocentrifugation, cells were fixed (2% paraformaldehyde containing 0.1% Triton X-100 for 30 minutes at room temperature) and prepared for immunofluorescence analysis. Following incubation with the primary antibody against Mad1 (sc-222) cells were blocked in phosphate-buffered saline (PBS)/1% skim milk powder then Alexa Fluor 488-goat antirabbit antisera (Molecular Probes, Eugene, OR) was applied and DNA was stained with propidium iodide. Cells were examined by laser-assisted confocal microscopy (BioRad MRC1000, Hercules, CA).
ChIP analysis
C/EBPϵ-ER MPROs were treated with 4-OH-tamoxifen for 0, 2, and 4 hours. ChIP was carried out essentially as described with some modifications29 (Upstate Biotechnology, Lake Placid, NY). All assays were performed in triplicate using 3 × 106 cells per time point. Briefly, cells were pelleted following treatment with tamoxifen and then washed in PBS. Cells were collected and proteins cross-linked to DNA by adding formaldehyde to a final concentration of 0.8% for 10 minutes at 37°C. Cross-linking was quenched by the addition of 125 mM glycine/PBS for 10 minutes at room temperature. Cells were washed, resuspended in SDS lysis buffer, and sonicated to shear chromatin (300 to 1000 bp). The chromatin fraction was diluted with ChIP dilution buffer (Upstate Biotechnology) and precleared with 40 μL salmon sperm DNA-protein A agarose slurry. The soluble chromatin fraction was collected and 5 μg anti-C/EBPϵ antibody was added (sc-158). Samples were incubated overnight at 4°C with rotation. Immune complexes were retrieved, washed, and eluted with buffer. Protein-DNA cross-links were reversed by incubation at 65°C with NaCl. Samples were treated with proteinase K, DNA was recovered, and ethanol precipitated with the inclusion of glycogen (0.02 mg). DNA pellets were resuspended in Tris-ethylenediaminetetraacetic acid (TE) buffer. PCR was conducted using quantitative real-time PCR, using the SYBR green dye detection method (Applied Biosystems). Primers were designed to encompass putative C/EBP consensus-binding sites.
Statistical analyses
Statistical analyses were performed using an unpaired Student 2-tailed t test to assess differences between the means. The SEM was used to determine the deviation from the mean.
Results
Expression of Mad1 and p27Kip1 during terminal differentiation of primary granulocytes
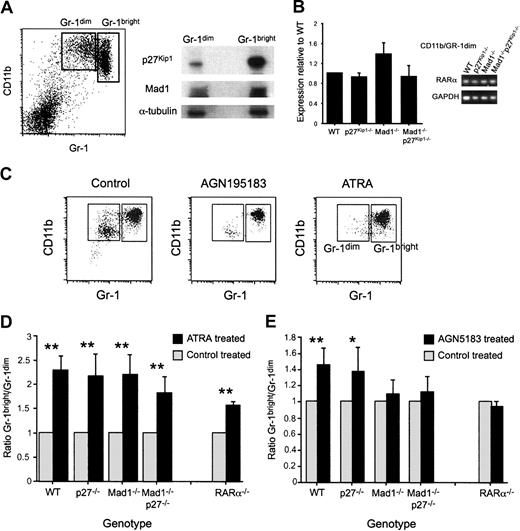
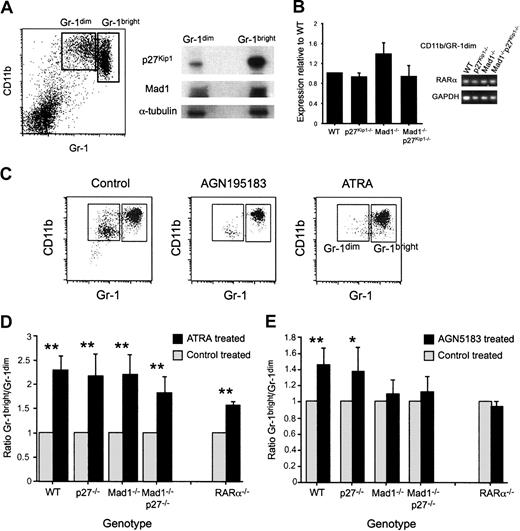
Using a well-characterized FACS-based isolation strategy,26 we isolated an immature granulocytic population (CD11b/Gr-1dim) and a mature granulocyte population (CD11b/Gr-1bright). We used these populations to analyze the expression of Mad1 and p27Kip1 during terminal differentiation of granulocytes by Western blotting. p27Kip1 was detected in both the immature and mature granulocyte populations, with a significantly increased amount detected in the terminally differentiated mature cell population (Figure 1A). Mad1, as for p27Kip1, was present at low levels in the immature population and at higher levels in the differentiated mature cells (Figure 1A). Within the immature population we were unable to detect c-Myc protein, consistent with the reciprocal regulation of c-Myc and Mad1. This expression pattern is consistent with a role for these proteins during terminal granulocytic differentiation.
Acceleration of granulocyte differentiation ex vivo by the RARα-specific agonist AGN195183 requires Mad1 and p27Kip1. (A) Immature (CD11b/Gr-1dim) and mature (CD11b/Gr-1bright) granulocytes were isolated as described in “Materials and methods.” Protein (36 μg) from each fraction was prepared in sample buffer and separated on a 10% SDS-PAGE gel. Proteins were then transferred to a PVDF membrane and probed with anti-Mad1, anti-p27Kip1, and anti–α-tubulin. (B) Expression of RARα in granulocyte subsets was analyzed using quantitative real-time PCR. Data shown are the expressions of RARα in the CD11b/Gr1dim population ± SEM, with a similar relative expression observed in the CD11b/Gr1bright and whole bone marrow populations (data not shown). (C) Whole bone marrow cells of the indicated genotype were cultured as described with either ethanol (control), the RARα-specific agonist AGN195183 (10–6 M), or ATRA (10–6 M). Following 3 days in culture, cells were isolated and phenotypically identified by CD11b/Gr-1 staining. Representative FACS dot plots following culture with ethanol (control) or retinoid agonist are shown. (D) ATRA can accelerate granulocyte differentiation independent of Mad1, p27Kip1, and RARα. (E) The RARα-specific agonist AGN195183 requires Mad1 and both Mad1 and p27Kip1 to accelerate granulocyte differentiation ex vivo. The specificity of AGN195183 is demonstrated by the lack of effect on RARα–/– cells; n > 6 for all treatments/genotypes. Data are graphed as ratio of Gr-1bright/Gr-1dim cells ± SEM (**P < .01, *P < .05).
Acceleration of granulocyte differentiation ex vivo by the RARα-specific agonist AGN195183 requires Mad1 and p27Kip1. (A) Immature (CD11b/Gr-1dim) and mature (CD11b/Gr-1bright) granulocytes were isolated as described in “Materials and methods.” Protein (36 μg) from each fraction was prepared in sample buffer and separated on a 10% SDS-PAGE gel. Proteins were then transferred to a PVDF membrane and probed with anti-Mad1, anti-p27Kip1, and anti–α-tubulin. (B) Expression of RARα in granulocyte subsets was analyzed using quantitative real-time PCR. Data shown are the expressions of RARα in the CD11b/Gr1dim population ± SEM, with a similar relative expression observed in the CD11b/Gr1bright and whole bone marrow populations (data not shown). (C) Whole bone marrow cells of the indicated genotype were cultured as described with either ethanol (control), the RARα-specific agonist AGN195183 (10–6 M), or ATRA (10–6 M). Following 3 days in culture, cells were isolated and phenotypically identified by CD11b/Gr-1 staining. Representative FACS dot plots following culture with ethanol (control) or retinoid agonist are shown. (D) ATRA can accelerate granulocyte differentiation independent of Mad1, p27Kip1, and RARα. (E) The RARα-specific agonist AGN195183 requires Mad1 and both Mad1 and p27Kip1 to accelerate granulocyte differentiation ex vivo. The specificity of AGN195183 is demonstrated by the lack of effect on RARα–/– cells; n > 6 for all treatments/genotypes. Data are graphed as ratio of Gr-1bright/Gr-1dim cells ± SEM (**P < .01, *P < .05).
RARα agonists, but not ATRA, require Mad1 and p27Kip1 to accelerate granulocytic differentiation ex vivo
To analyze the roles of Mad1 and p27Kip1 in retinoid-induced granulocyte differentiation we examined differentiation of primary granulocytes in short-term culture. Whole bone marrow was cultured in SCF and G-CSF with either ATRA or the RARα-specific agonist AGN195183.26 Compared with cultures of unstimulated cells, stimulation of the primary granulocytes with a retinoid agonist induced an increased number of mature granulocytes (Figure 1C) with no detectable difference in cell death (data not shown). These data demonstrate that stimulation of granulocyte precursors ex vivo with a retinoid agonist accelerates granulocyte differentiation and that under these conditions differentiation of the committed granulocyte progenitor is assessed. We hypothesized that if Mad1 and p27Kip1 are nonredundant mediators of cell differentiation induced by retinoids during granulocytic differentiation then primary granulocyte progenitors derived from Mad1–/–, p27Kip1–/–, or Mad1–/–p27Kip1–/– mice should be defective in their response to retinoids.
ATRA was a potent accelerator of granulopoiesis as assessed morphologically and by up-regulation of the granulocyte-restricted surface antigen Gr-1 in all genotypes (Figure 1C-D and data not shown). The RARα-specific agonist AGN195183 significantly accelerated granulocyte differentiation in wild-type and p27Kip1–/– cells (Figure 1E). Neither Mad1–/– nor Mad1–/–p27Kip1–/– cells, in contrast, showed a response to AGN195183 (Figure 1E). However, both Mad1–/– and Mad1–/–p27Kip1–/– cells maintained a normal response to ATRA, indicating that ATRA is capable of accelerating the differentiation of these cells independently of Mad1 and p27Kip1 status (Figure 1D). To further examine the specificity of the response to the RARα-specific agonist AGN195183, we determined the response of RARα–/– cells to this ligand. As anticipated, RARα–/– cells displayed no response to AGN195183, interestingly phenocopying the loss of Mad1 or Mad1 and p27Kip1, further confirming the specificity of the ligand and the interaction of RARα-dependent signaling pathways with Mad1. Taken together, these data implicate Mad1 as an important mediator of granulocytic differentiation in response to RARα-specific activation, but not of the effects of ATRA.
To determine if loss of p27Kip1, Mad1, or both Mad1 and p27Kip1 has an impact on the expression of RARα, the primary granulocyte fractions from deficient mice were analyzed for expression of RARα. As can be seen in Figure 1B, loss of these genes did not affect expression of RARα, as determined by quantitative real-time PCR.
Mad1 and p27Kip1 are required for inhibition of myeloid colony formation mediated by RARα
To more directly assess the contribution of Mad1 and p27Kip1 to retinoid-induced cell cycle arrest pathways, we undertook colonyforming cell assays. Unlike the differentiation assay, where cells may have already begun to exit the cell cycle before being analyzed in vitro, colony-forming unit (CFU) assays provide a more direct assay of the effects of retinoids on the cell cycle. Retinoids have been demonstrated to prevent colony formation by primary hematopoietic cells when directly added to semisolid cultures.30 This reduction in colony-forming efficiency is due to retinoid-induced cell cycle arrest of progenitors, preventing colony formation. To determine the contributions of Mad1 and p27Kip1 to inhibition of colony formation induced by retinoids, we used cultures of primary bone marrow cells in methylcellulose semisolid media. Cultures were incubated for 7 days and the numbers of CFUs determined.
When whole bone marrow cells from wild-type, p27Kip1–/–, Mad1–/–, and Mad1–/–p27Kip1–/– mice were stimulated with the pan-RAR agonistATRA, there was a significant decrease in the number and size of colonies formed (P ≤ .003, n > 4 for all genotypes; Figure 2A; Table 1). This was consistent with previous reports of the effects of ATRA when added directly into culture on CFU proliferation.30 ATRA also suppressed CFU proliferation from whole bone marrow from RARα or RARγ null mice, demonstrating that neither RARα nor RARγ alone mediates the effects of ATRA to reduce colony numbers (Figure 2A; Table 1). In contrast, the pan-RAR antagonist AGN194310 had no effect on colony proliferation in the genotypes tested except for a modest increase in CFUs in cultures of cells from Mad1–/– cells (Table 1 and Figure S1; see the Supplemental Figures link at the top of the online article on the Blood website).
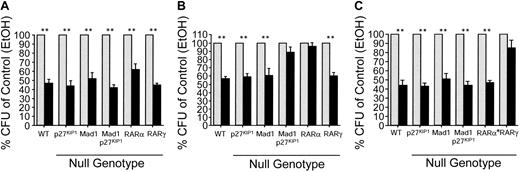
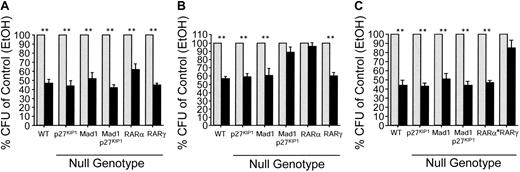
Mad1 and p27Kip1 are essential for RARα-mediated inhibition of CFU proliferation. Whole bone marrow of each genotype was plated at 5 × 104 cells/mL in methylcellulose supplemented with FCS, SCF, erythropoietin (Epo), IL-3, and IL-6. Ligands were added directly to the semisolid cultures. Cells were cultured for 7 days and then counted (colony defined as > 50 cells).  indicates control; and ▪, retinoid. (A) Colony formation in the presence of ATRA (10–6 M). Data are expressed as percent colonies of control ± SEM (ethanol-treated culture for each genotype). (B) Colony formation in the presence of an RARα-selective agonist (AGN195183, 10–6 M). (C) Colony formation in the presence of an RARβ/γ-selective agonist activity (ATRA and the RARα-specific antagonist AGN194301; 10–6 M). n ≥ 4 for all genotype/ligand combinations. **P ≤ .003.
indicates control; and ▪, retinoid. (A) Colony formation in the presence of ATRA (10–6 M). Data are expressed as percent colonies of control ± SEM (ethanol-treated culture for each genotype). (B) Colony formation in the presence of an RARα-selective agonist (AGN195183, 10–6 M). (C) Colony formation in the presence of an RARβ/γ-selective agonist activity (ATRA and the RARα-specific antagonist AGN194301; 10–6 M). n ≥ 4 for all genotype/ligand combinations. **P ≤ .003.
Mad1 and p27Kip1 are essential for RARα-mediated inhibition of CFU proliferation. Whole bone marrow of each genotype was plated at 5 × 104 cells/mL in methylcellulose supplemented with FCS, SCF, erythropoietin (Epo), IL-3, and IL-6. Ligands were added directly to the semisolid cultures. Cells were cultured for 7 days and then counted (colony defined as > 50 cells).  indicates control; and ▪, retinoid. (A) Colony formation in the presence of ATRA (10–6 M). Data are expressed as percent colonies of control ± SEM (ethanol-treated culture for each genotype). (B) Colony formation in the presence of an RARα-selective agonist (AGN195183, 10–6 M). (C) Colony formation in the presence of an RARβ/γ-selective agonist activity (ATRA and the RARα-specific antagonist AGN194301; 10–6 M). n ≥ 4 for all genotype/ligand combinations. **P ≤ .003.
indicates control; and ▪, retinoid. (A) Colony formation in the presence of ATRA (10–6 M). Data are expressed as percent colonies of control ± SEM (ethanol-treated culture for each genotype). (B) Colony formation in the presence of an RARα-selective agonist (AGN195183, 10–6 M). (C) Colony formation in the presence of an RARβ/γ-selective agonist activity (ATRA and the RARα-specific antagonist AGN194301; 10–6 M). n ≥ 4 for all genotype/ligand combinations. **P ≤ .003.
Similar to ATRA treatment of bone marrow cells, addition of either of the RARα-specific agonists AGN195183 (Figure 2B; Table 1) or AGN194078 (Figure S1 and Table 1) resulted in a significant decrease in the number and size of colonies formed from the wild-type, p27Kip1–/–, and Mad1–/– cells (P ≤ .003, n > 4 for all genotypes; Figure 2B; Figure S1; Table 1). Differential counts demonstrated a significant reduction in the numbers of GM-CFUs and G-CFUs with no apparent effect on other lineages (data not shown). Strikingly, colony formation by Mad1–/–p27Kip1–/– cells was not suppressed by either of the RARα-specific agonists (n = 5; Figure 2B and Figure S1). As anticipated, RARα–/– cells did not respond to AGN195183 but RARγ–/– cells remained sensitive to the effects of the RARα agonist, confirming both the specificity of the ligand and that loss of Mad1 and p27Kip1 results in a loss of only RARα-mediated cell cycle arrest (Figure 2B).
To determine if the defect in Mad1–/–p27Kip1–/– cells was restricted to RARα-mediated cell cycle arrest pathways, we combined the pan-RAR agonist ATRA and an RARα-specific antagonist (AGN194301). This combination of ligands results in activation of only RARβ and RARγ. The failure of RARγ–/– cells to respond to this combination of ligands demonstrates a requirement for RARγ but not RARβ in the inhibition of CFU formation and also the validity of this approach to specifically target RARγ and not RARα (Figure 2C; Table 1). As shown in Figure 2C, wild-type, p27Kip1–/–, Mad1–/–, and Mad1–/–p27Kip1–/– cells remained sensitive to RARβ/γ-mediated cell cycle arrest. These data show that loss of Mad1 and p27Kip1 results in a specific failure in RARα-mediated cell cycle arrest, but not of RARγ-mediated cell cycle arrest and demonstrate that the effects of RARα on myeloid cells absolutely requires Mad1 and p27Kip1.
RARα agonist induces accumulation of p27Kip1 protein
Having observed that both Mad1 and p27Kip1 accumulate during granulocyte differentiation and are required for the acceleration of granulopoiesis and inhibition of CFU proliferation by RARα-specific ligands, we sought to determine how RARα coordinates Mad1 and p27Kip1 during differentiation. For these studies we used a model murine granulocytic cell line (MPROs28 ). These cells have been well characterized and undergo a faithful reconstitution of normal murine granulocytic differentiation in response to retinoids.31,32
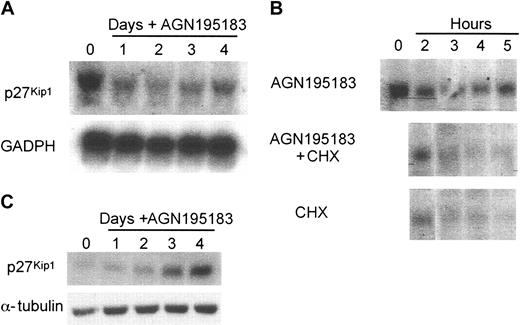
Analysis of the murine p27Kip1 promoter 15 kb upstream of the second exon did not reveal a strong candidate RARE, and differentiation induction with AGN195183 resulted in reduction of p27Kip1 mRNA levels during a 4-day differentiation time course (Figure 3A). Moreover, no induction of p27Kip1 transcript was observed in the presence of cycloheximide (Figure 3B), again suggesting that direct regulation of transcription is not the mechanism through which RARα regulates p27Kip1 during granulocytic differentiation. Previous studies in numerous cell types, including myeloid differentiation systems, have demonstrated that posttranscriptional processes are the major mechanism of regulation of p27Kip1 levels.18,33-35 Analysis of the levels of p27Kip1 protein in MPRO cells induced to differentiate with AGN195183 revealed a dramatic increase of p27Kip1 protein levels at 3 and 4 days after ligand addition (Figure 3C). This increase contrasts with the reduction observed in transcript levels (Figure 3A). Together these data demonstrate that RARα-activated differentiation results in an increase in cellular p27Kip1 protein, through posttranscriptional mechanisms.
p27Kip1 protein accumulation with no change in transcription in response to RARα-mediated granulocytic differentiation. The regulation of p27Kip1 during RARα-induced granulocytic differentiation was analyzed in the MPRO cells. (A) p27Kip1 transcript does not increase following RARα-induced granulocytic differentiation (AGN195183; 10–6 M). Total RNA (10 μg) was separated on a denaturing gel and transferred to a nylon membrane that was then probed for p27Kip1 and GAPDH. (B) p27Kip1 is not a direct transcriptional target of RARα. MPRO cells were treated with AGN195183, AGN195183, and cycloheximide (CHX; 10 μg/mL) or CHX alone for the indicated time points then RNA prepared and analyzed for p27Kip1 expression. (C) A significant accumulation of p27Kip1 protein occurs following differentiation through RARα. Cells were treated with AGN19183 and prepared as described, then probed for expression of p27Kip1 protein and α-tubulin as loading control.
p27Kip1 protein accumulation with no change in transcription in response to RARα-mediated granulocytic differentiation. The regulation of p27Kip1 during RARα-induced granulocytic differentiation was analyzed in the MPRO cells. (A) p27Kip1 transcript does not increase following RARα-induced granulocytic differentiation (AGN195183; 10–6 M). Total RNA (10 μg) was separated on a denaturing gel and transferred to a nylon membrane that was then probed for p27Kip1 and GAPDH. (B) p27Kip1 is not a direct transcriptional target of RARα. MPRO cells were treated with AGN195183, AGN195183, and cycloheximide (CHX; 10 μg/mL) or CHX alone for the indicated time points then RNA prepared and analyzed for p27Kip1 expression. (C) A significant accumulation of p27Kip1 protein occurs following differentiation through RARα. Cells were treated with AGN19183 and prepared as described, then probed for expression of p27Kip1 protein and α-tubulin as loading control.
Mad1 is directly induced by C/EBPϵ but not RARα
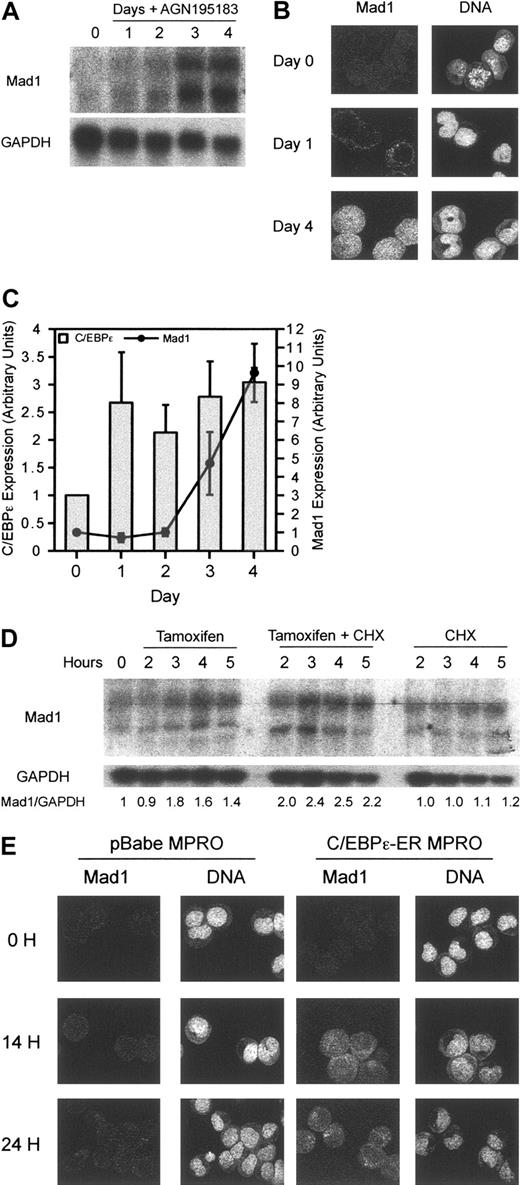
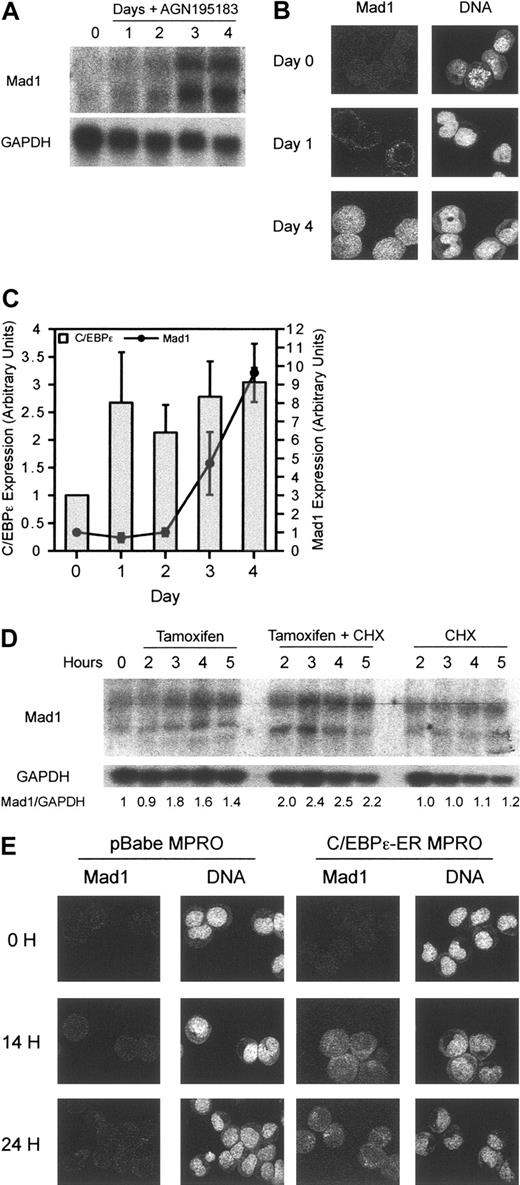
To determine the effects of RARα on Mad1, we examined transcript levels following differentiation of MPRO cells. Mad1 transcript can be seen to increase at 2 days and significantly by 3 days after differentiation induction by RARα (Figure 4A). Mad1 protein, correlating with transcript level, showed delayed kinetics of accumulation consistent with an indirect effect of RARα on Mad1 (Figure 4B). Consistent with the delayed inducing of Mad1 transcript, RARα agonists also failed to transcriptionally activate Mad1 in the presence of cycloheximide, suggesting that Mad1 is not a direct transcriptional target of RARα (data not shown). Examination of 8.5 kb upstream of the second exon of the murine Mad1 gene manually and by using the TRANSFAC computer program (Biobase GmbH, Wolfenbüttel, Germany) for RAR consensus sites4 failed to define a RARE, supportive of an indirect regulation of Mad1 by RARα. Further analysis of the murine Mad1 gene revealed possible binding sites for members of the CCAAT/enhancer-binding protein family, previously described retinoid target genes.36,37 Analysis of the induction of C/EBPϵ following treatment of MPRO cells with the RARα agonist AGN195183 demonstrated a rapid increase by 24 hours in the level of C/EBPϵ transcript that was sustained over the subsequent 72 hours (Figure 4C). The induction of C/EBPϵ temporally preceded that of Mad1, with Mad1 transcript increasing only after 72 hours of treatment with AGN195183 (Figure 4C).
Mad1 is a transcriptional target of the granulocyte-restricted, retinoid target gene C/EBPϵ. (A) Induction of Mad1 RNA is delayed following differentiation induction of wild-type MPRO cells with the RARα agonist AGN195183. Wild-type MPROs were treated for the indicated number of days with AGN195183 and Mad1 RNA levels were assessed by Northern blotting as described. (B) Expression of Mad1 protein is also delayed following treatment with AGN195183. Mad1 protein levels were analyzed using laser-assisted confocal microscopy at the indicated time points. (C) Induction of C/EBPϵ by the RARα agonist AGN195183 occurs rapidly and precedes the induction of Mad1. Quantitative real-time PCR was performed to determine the expression of C/EBPϵ and Mad1 following treatment of MPROs with AGN195183 for the indicated time periods. Results are normalized to the expression of β2-microglobulin. Error bars represent SEM from 3 PCR reactions. (D) Mad1 is a transcriptional target of C/EBPϵ. Wild-type C/EBPϵ-ER MPROs were treated with tamoxifen, tamoxifen and CHX, or CHX alone for the indicated time points; then RNA was prepared and analyzed for Mad1 expression. (E) C/EBPϵ-ER or pBabe MPROs were treated for 0, 14, and 24 hours with tamoxifen, fixed, and then expression of Mad1 protein analyzed using laser-assisted confocal microscopy.
Mad1 is a transcriptional target of the granulocyte-restricted, retinoid target gene C/EBPϵ. (A) Induction of Mad1 RNA is delayed following differentiation induction of wild-type MPRO cells with the RARα agonist AGN195183. Wild-type MPROs were treated for the indicated number of days with AGN195183 and Mad1 RNA levels were assessed by Northern blotting as described. (B) Expression of Mad1 protein is also delayed following treatment with AGN195183. Mad1 protein levels were analyzed using laser-assisted confocal microscopy at the indicated time points. (C) Induction of C/EBPϵ by the RARα agonist AGN195183 occurs rapidly and precedes the induction of Mad1. Quantitative real-time PCR was performed to determine the expression of C/EBPϵ and Mad1 following treatment of MPROs with AGN195183 for the indicated time periods. Results are normalized to the expression of β2-microglobulin. Error bars represent SEM from 3 PCR reactions. (D) Mad1 is a transcriptional target of C/EBPϵ. Wild-type C/EBPϵ-ER MPROs were treated with tamoxifen, tamoxifen and CHX, or CHX alone for the indicated time points; then RNA was prepared and analyzed for Mad1 expression. (E) C/EBPϵ-ER or pBabe MPROs were treated for 0, 14, and 24 hours with tamoxifen, fixed, and then expression of Mad1 protein analyzed using laser-assisted confocal microscopy.
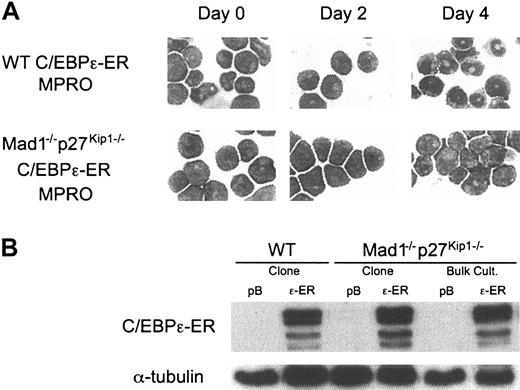
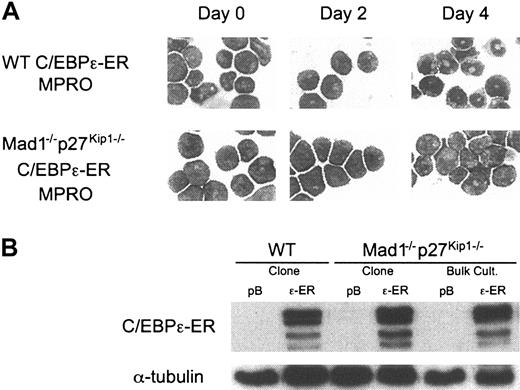
The CCAAT/enhancer-binding proteins (C/EBPs) are a family of transcription factors critically involved in the differentiation of numerous cell types.38 Of most importance during granulopoiesis are C/EBPα and C/EBPϵ. C/EBPα is required for myelopoiesis because loss of C/EBPα results in a failure to develop mature granulocytes and eosinophils as a result of differentiation arrest at the myeloid progenitor cell stage.39 C/EBPϵ is specifically expressed during the late stages of granulocyte differentiation. Loss of C/EBPϵ leads to a failure to produce terminally differentiated granulocytes, with functionally and morphologically abnormal cells being produced.40,41 Mice deficient for C/EBPϵ display no abnormalities in cells other than granulocytes, demonstrating a critical role for C/EBPϵ in regulating terminal granulopoiesis.40 C/EBPϵ is a target of RARα during granulocytic differentiation and its promoter contains a functional RARE.36 C/EBPϵ expression is repressed by PML-RARα, and enforced expression of C/EBPϵ in vivo in PML-RARα transgenic mice is able to overcome the block to differentiation and promote maturation of cells harboring the transgene.42-44 Using the MPRO cell line we generated cells containing a stably integrated 4-OH-tamoxifen–inducible C/EBPϵ construct (C/EBPϵ-ER). These cells differentiated into mature granulocytes on activation of C/EBPϵ, demonstrating that C/EBPϵ can functionally replace retinoid agonists and induce differentiation in the MPRO cell line (Figure 6A).
Mad1–/– p27Kip1–/– MPROs are refractory to C/EBPϵ-induced differentiation. (A) Wild-type and Mad1–/–p27Kip1–/– MPROs were infected with control (pBabe, pB) or C/EBPϵ-ER (ϵ-ER) retrovirus. Following selection in 2 μg/mL puromycin, cells were treated with 200 nM 4-OH-tamoxifen (Tam) and differentiation was assessed morphologically over 4 days. Original magnification, × 400. (B) Equivalent expression of C/EBPϵ-ER in cell lines was determined by Western blot analysis in both bulk culture and clonal cell lines using an anti-ERα antibody.
Mad1–/– p27Kip1–/– MPROs are refractory to C/EBPϵ-induced differentiation. (A) Wild-type and Mad1–/–p27Kip1–/– MPROs were infected with control (pBabe, pB) or C/EBPϵ-ER (ϵ-ER) retrovirus. Following selection in 2 μg/mL puromycin, cells were treated with 200 nM 4-OH-tamoxifen (Tam) and differentiation was assessed morphologically over 4 days. Original magnification, × 400. (B) Equivalent expression of C/EBPϵ-ER in cell lines was determined by Western blot analysis in both bulk culture and clonal cell lines using an anti-ERα antibody.
Using these cells, we sought to determine if C/EBPϵ was able to regulate expression of either p27KIP1 or Mad1. Analysis of mRNA after activation of C/EBPϵ in the presence and absence of cycloheximide revealed that, as with RARα, p27Kip1 was not transcriptionally regulated by C/EBPϵ during differentiation in these cells (data not shown). Mad1 transcript, however, was up-regulated rapidly by 3 hours on activation of C/EBPϵ and this occurred in the presence of cycloheximide, suggesting direct regulation of the Mad1 transcription (Figure 4D). This contrasts dramatically with the effects of RARα agonists on Mad1, where induction of Mad1 transcript begins at 48 hours (Figure 4A). Confocal microscopy clearly demonstrated that Mad1 protein was induced as early as 14 hours after the induction of C/EBPϵ and increased at 24 hours after activation (Figure 4E). This induction did not occur in vector control infected cells nor was Mad1 protein detectable at these time points during RARα agonist-induced differentiation of the MPRO cell line (Figure 4D-E), consistent with Mad1 being a direct transcriptional target of the granulocyte-restricted transcription factor C/EBPϵ, but not of RARα.
To determine if C/EBPϵ was capable of directly binding to the Mad1 promoter in vivo, we performed ChIP analysis on 4 putative C/EBP-binding sites in the 5′ region of the Mad1 gene. At 2 and 4 hours after activation of C/EBPϵ we performed ChIP analysis using a validated quantitative real-time PCR that accurately quantitates the level of DNA binding,45 revealing a specific accumulation of C/EBPϵ on the consensus site most proximal to the first exon of Mad1 (Figure 5A,C). This enrichment was specific for this site because other putative C/EBP consensus-binding sites within 3 kb of the first exon of Mad1 were not significantly bound in vivo by C/EBPϵ, nor was an unrelated promoter that does not contain C/EBPϵ consensus sites (Figure 5A,C; mUBF promoter; Figure S2). These data provide a mechanism linking differentiation to the direct induction of the MYC-antagonist Mad1.
C/EBPϵ can directly bind to the Mad1 promoter in vivo. C/EBPϵ-ER MPROs were treated with 200 nM 4-OH-tamoxifen (Tam). ChIP was performed using an anti-C/EBPϵ antibody and the binding of C/EBPϵ to the Mad1 promoter assessed by quantitative real-time PCR. (A) Schematic representation of the murine Mad1 gene, with consensus C/EBP-binding sites and PCR amplicons as indicated. (B) Representative quantitative PCR plot showing amplification of site D. NTC indicates no template control; input, total amount of input chromatin; no Ab, no antibody control immunoprecipitation. The arbitrary amplification threshold is depicted as the horizontal bar running across the graph. (C) Quantitation of C/EBPϵ binding to the Mad1 promoter 2 and 4 hours after the addition of Tam for 4 consensus C/EBP-binding sites. Data expressed as fold enrichment relative to 0 hour time point from a representative experiment (n = 3 experiments).
C/EBPϵ can directly bind to the Mad1 promoter in vivo. C/EBPϵ-ER MPROs were treated with 200 nM 4-OH-tamoxifen (Tam). ChIP was performed using an anti-C/EBPϵ antibody and the binding of C/EBPϵ to the Mad1 promoter assessed by quantitative real-time PCR. (A) Schematic representation of the murine Mad1 gene, with consensus C/EBP-binding sites and PCR amplicons as indicated. (B) Representative quantitative PCR plot showing amplification of site D. NTC indicates no template control; input, total amount of input chromatin; no Ab, no antibody control immunoprecipitation. The arbitrary amplification threshold is depicted as the horizontal bar running across the graph. (C) Quantitation of C/EBPϵ binding to the Mad1 promoter 2 and 4 hours after the addition of Tam for 4 consensus C/EBP-binding sites. Data expressed as fold enrichment relative to 0 hour time point from a representative experiment (n = 3 experiments).
The RARα target gene C/EBPϵ is unable to induce differentiation in Mad1–/–p27Kip1–/– MPRO cells
We had previously reported a failure in cell cycle arrest and differentiation of Mad1–/–p27Kip1–/– MPRO cell lines in response to retinoid.17 These studies demonstrated the requirement for loss of both Mad1 and p27Kip1 because single mutant cells respond to retinoid agonists by undergoing terminal granulocytic differentiation. To determine if C/EBPϵ, like retinoids, requires Mad1 and p27Kip1 to induce cell cycle arrest and terminal granulocytic differentiation, we generated wild-type and Mad1–/–p27Kip1–/– MPROs harboring the inducible C/EBPϵ-ER. Strikingly, C/EBPϵ was also unable to differentiate MPRO cells derived from Mad1–/– p27Kip1–/– mice, consistent with the hypothesis that C/EBPϵ is an important mediator of RARα cell cycle arrest pathways in granulocytes and is upstream of Mad1 and p27Kip1 (Figure 6).
Discussion
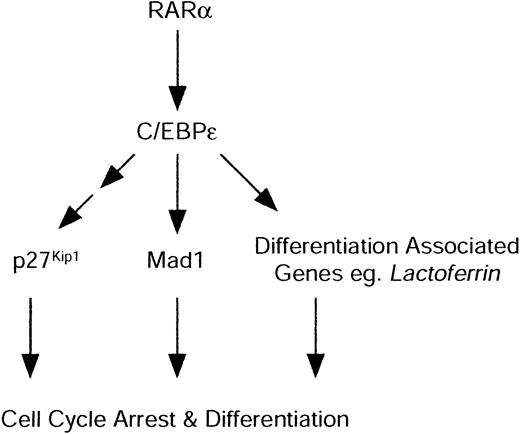
A common feature during the terminal differentiation of many cell types is withdrawal from the cell cycle. In general, failure to appropriately withdraw from the cell cycle results in a failure to achieve a terminally differentiated state, powerfully demonstrated by malignancy. The mechanisms through which differentiation and cell cycle arrest are so tightly coupled remain largely poorly understood. In this report we demonstrate that during differentiation of granulocyte progenitors induced by RARα, cell cycle withdrawal occurs through 2 distinct families of negative cell cycle regulators. We demonstrate that the CDK inhibitor p27Kip1 and Mad1, a member of the Max network of transcription factors, are both required to mediate cell cycle arrest by RARα during granulocytic differentiation. RARα agonist-induced differentiation results in an accumulation of p27Kip1 protein and induction of Mad1 protein via indirect means. Mad1 is an in vivo transcriptional target of the granulocyte-restricted transcription factor C/EBPϵ, a direct retinoid target. This is the first report to indicate specificity for the cell cycle machinery among the RARs and to provide a direct link between differentiation induction and the regulation of the Myc-antagonist Mad1 (Figure 7).
Proposed model of granulocytic cell cycle arrest and differentiation by RARα. RARα directly activates C/EBPϵ. C/EBPϵ in turn is able to directly activate differentiation-associated genes, such as lactoferrin, that are required for functionally competent mature granulocytes. C/EBPϵ also directly mediates induction of Mad1, coupling cell cycle arrest pathways and differentiation induction by RARα.
Proposed model of granulocytic cell cycle arrest and differentiation by RARα. RARα directly activates C/EBPϵ. C/EBPϵ in turn is able to directly activate differentiation-associated genes, such as lactoferrin, that are required for functionally competent mature granulocytes. C/EBPϵ also directly mediates induction of Mad1, coupling cell cycle arrest pathways and differentiation induction by RARα.
It has previously been reported that p27Kip1 is required to mediate the appropriate cell cycle withdrawal of oligodendrocyte precursor cells during differentiation and that this is a nonredundant role of p27Kip1.46,47 Consistent with these reports, we detected a significant accumulation of p27Kip1 protein during the differentiation of granulocytes, although the nature of this accumulation has not been fully elucidated in the current study. p27Kip1 appears in most circumstances to be regulated after transcription, either translationally33 or by ubiquitin-dependent proteolysis.35 Our data suggest that during granulopoiesis, translational control of p27Kip1 maybe the predominant regulation that occurs. When the stability of p27Kip1 protein was determined in cells treated with cycloheximide after 4 days of exposure to AGN195183, no significant increase in protein half-life could be detected compared to uninduced cells (C.R.W. and G.A.M., unpublished data, November 2002). Interestingly, we also observe a similar regulation of elevated p27Kip1 protein compared to transcript when MPRO cells are differentiated with a retinoid or induced to differentiate by activation of C/EBPϵ (data not shown). That p27Kip1 kinetics remains so similar under both conditions suggests that the regulation of p27Kip1 protein accumulation occurs downstream of RARα and C/EBPϵ (Figure 7).
The coupling of terminal cell cycle exit and differentiation is a feature common to many cell types. Since isolation, the C/EBP family of transcription factors has demonstrated functions in the regulation of differentiation of various tissues.48 C/EBP transcription factors directly bind DNA through a common motif37 and have a broad expression pattern that is regulated by both tissue- and stage-specific expression.38 Cumulative data place C/EBP family members as central components during the switch from a proliferative state to an arrested differentiated cell type.49-52 Recent data have demonstrated that C/EBPα can interact with the cell cycle through a number of distinct mechanisms. C/EBPα can directly regulate the cell cycle through protein-protein interactions with the critical cyclin-dependent kinases cdk2 and cdk4,53 and also through repression of E2F-dependent transcription.52 It has also been demonstrated that C/EBPα can regulate the proteasome-dependent degradation of cdk4 during growth arrest in hepatocytes, suggesting a more complex interaction with the cell cycle than a kinase inhibitory role.54 In this report we describe a direct transcriptional target of C/EBPϵ, Mad1, that directly links cell cycle withdrawal and differentiation.
The expression of Mad family members during terminal differentiation, and the reciprocal regulation of Myc proteins, has led to the suggestion that the Mad family is involved in regulating cell cycle withdrawal during differentiation. Consistent with this role for Mad proteins, Mad1–/– granulocyte precursors undergo ectopic cell divisions and have a delayed differentiation.19 In this report we describe a novel mechanism through which the granulocyte-restricted transcription factor C/EBPϵ can regulate cell cycle exit during granulocytic differentiation. Through the direct transcriptional activation of Mad1, a protein whose expression tightly correlates with the terminal exit from cell cycle and reciprocally with Myc, C/EBPϵ can regulate the transcriptional cascade required for the acquisition of specialized cell functions and mediate exit from the cell cycle. This provides an elegant model to couple terminal differentiation and cell cycle withdrawal, through the action of tissue-specific instructive transcription factors. These factors orchestrate a transcriptional cascade that directly leads to cell cycle arrest and the activation of the genes required for the terminal function of the given cell type. The regulation of Mad1 by C/EBPϵ may define a common pathway for cell cycle withdrawal in response to granulocyte differentiation stimuli, for example, G-CSF has been demonstrated to modulate C/EBPϵ during granulocyte differentiation.55 The requirement for p27Kip1 in the observed phenotypes reported here is consistent with a dual role for C/EBPϵ in regulating cell cycle exit, potentially through effects on cyclin-dependent kinases as have been ascribed for C/EBPα, in addition to the direct regulation of Mad1 reported here. By targeting these 2 families of distinct negative cell cycle regulators, C/EBPϵ may be able to impart a profound block to cell cycle that is required during terminal differentiation.
The mechanisms through which differentiating agents such as retinoids are able to induce cell cycle arrest are of significance to the clinical application of these compounds. One of the characteristics of malignancy is the loss of the capacity to withdraw from the cell cycle, often accompanied by loss or mutation of the regulatory proteins for the G1/S phase transition of the cell cycle.56,57 Our study reveals that loss of genes that are important in the withdrawal from the cell cycle can directly affect the efficacy of retinoids. RAR-specific retinoids are being developed and evaluated in the hope of improved specificity and a reduced side effect profile over conventional pan-RAR agonists.12,58 The identification of pathways specific for the distinct RARs, such as Mad1 and p27Kip1 for RARα-mediated cell cycle arrest pathways, may allow a more rational application of these compounds. Our study also reveals that RARγ-induced differentiation of granulocyte progenitors occurs independent of Mad1 and p27Kip1 status, highlighting potential differences in target genes among the RARs that may be able to be therapeutically exploited. This information can also be used to provide a rational basis for combination therapy with agents that target the cell cycle or to select patients who are more or less likely to respond to an RAR-specific ligand.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-07-2391.
Supported by a grant from the National Health and Medical Research Council (NHMRC) of Australia (G.A.M.). C.R.W. is a recipient of an Australian Postgraduate Award; L.E.P. and G.A.M. are Special Fellows of the Leukemia and Lymphoma Society.
Y.-D.Y. and R.A.S.C. have declared a financial interest in Allergan Inc, whose potential products were studied in the present work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge B. Pulverer for sharing unpublished data. We thank G. Poortinga, E. Baker, A. El-Osta, K. Herbert, D. Thomas, D. Germain, and M. O'Connell for discussion and comment; the PMCC Animal Facility Staff for care of experimental animals; and the FACS staff for assistance with FACS sorting.




 indicates control; and ▪, retinoid. (A) Colony formation in the presence of ATRA (10–6 M). Data are expressed as percent colonies of control ± SEM (ethanol-treated culture for each genotype). (B) Colony formation in the presence of an RARα-selective agonist (AGN195183, 10–6 M). (C) Colony formation in the presence of an RARβ/γ-selective agonist activity (ATRA and the RARα-specific antagonist AGN194301; 10–6 M). n ≥ 4 for all genotype/ligand combinations. **P ≤ .003.
indicates control; and ▪, retinoid. (A) Colony formation in the presence of ATRA (10–6 M). Data are expressed as percent colonies of control ± SEM (ethanol-treated culture for each genotype). (B) Colony formation in the presence of an RARα-selective agonist (AGN195183, 10–6 M). (C) Colony formation in the presence of an RARβ/γ-selective agonist activity (ATRA and the RARα-specific antagonist AGN194301; 10–6 M). n ≥ 4 for all genotype/ligand combinations. **P ≤ .003.