Abstract
Leptin is secreted by bone marrow (BM) adipocytes and stromal cells and was shown to stimulate myeloid proliferation. We here report that primary acute promyelocytic leukemia (APL) cells express high levels of the leptin-receptor (OB-R) long isoform. In cells with regulated promyelocytic leukemia–retinoic acid receptor (PML-RARα) expression, inducing PML-RARα was found to increase OB-R levels. We then investigated the effects of leptin produced by BM adipocytes on APL cells using a coculture system with mesenchymal stem cell (MSC)–derived adipocytes. In PML-RARα–expressing cells, all-trans retinoic acid (ATRA)– and doxorubicin-induced apoptosis were significantly reduced by coculture with adipocyte-differentiated MSCs. This antiapoptotic effect required direct cell-to-cell interactions, was associated with phosphorylation of signal transducer and activator of transcription-3 (STAT3) and mitogen-activated protein kinase (MAPK), and was reduced by blocking OB-R. This report provides a mechanistic basis for the BM adipocyte–leukemia cell interaction and suggests that OB-R receptor blockade may have therapeutic use in APL.
Introduction
Leptin is a 16-kDa protein produced by adipocytes and was originally identified as a cytokine that regulates fat metabolism.1 Adipocytes are abundant in the bone marrow (BM) microenvironment and might be responsible for the high concentration of leptin in BM.2 The leptin receptor (OB-R) has recently been detected on human hematopoietic progenitor cells expressing the CD34 antigen, and leptin was reported to induce proliferation and differentiation in these cells.3,4 Multiple isoforms of OB-R have been identified, including a long isoform and several isoforms with short cytoplasmic domains.5,6 Although all isoforms share an identical extracellular ligand-binding domain with homology to the class I cytokine receptor family, they differ at the C-terminus.6 Only the long OB-R isoform, which has the longest (303 amino acids) cytoplasmic domain and encodes all protein motifs, has the signaling capabilities of interleukin-6 (IL-6)–type cytokine receptors and can activate the Janus kinase–signal transducer and activator of transcription (JAK-STAT) signal transduction pathway.7-9 We have reported that leptin induces the proliferation and enhances the survival of primary leukemic cells from patients with acute myeloid leukemia (AML).10 We have further demonstrated the presence of leptin receptors (OB-Rs) on primary AML cells, with the highest expression of OB-R long isoform on acute promyelocytic leukemia (APL) cells, which was absent on normal promyelocytes.10 Given that APL is associated with increased body mass index (BMI)11 and leptin serum levels correlate with body fat percentage,12,13 leptin may play a role in the pathophysiology of APL. Alternatively, leptin secretion by BM adipocytes in the vicinity of leukemic cells could play a major role in the proliferation and survival of APL cells through paracrine interactions in the marrow microenvironment. The aims of this study were, therefore, to determine (1) whether inducing the promyelocytic leukemia–retinoic acid receptor (PML-RARα) gene in leukemic cells is associated with the up-regulation of OB-R; (2) whether leptin produced by BM stromal cell–derived adipocytes protects APL cells from apoptosis; (3) whether the soluble- or membrane-bound form of adipocyte-produced leptin mediates antiapoptotic effects in APL; and (4) which signal transduction pathways are activated by leptin in APL cells. Our results indicate that inducing PML-RARα is associated with the up-regulation of OB-R expression in APL cells. Leptin-producing mesenchymal stem cell (MSC)–derived adipocytes prevent the apoptosis of APL cells, perhaps through STAT3 and mitogen-activated protein kinase (MAPK) phosphorylation, and this effect requires direct cell-to-cell contact, suggesting that membrane-bound, not secreted, leptin is biologically active. Blocking the activation of OB-R by leptin may be useful for APL therapy.
Materials and methods
Cell cultures
BM and peripheral blood samples were obtained from patients with newly diagnosed APL with high (more than 70%) blast and promyelocyte counts. Informed consent was obtained according to institutional guidelines. Mononuclear cells were separated by Ficoll-Hypaque (Sigma Chemical, St Louis, MO) density-gradient centrifugation. Cell viability after Ficoll-Hypaque separation was greater than 95% in all samples tested. The engineered U937/PR9 cells, which express PML-RARα under the control of the zinc-inducible promoter, were kindly provided by Dr Pelicci (European Institute of Oncology, Milan, Italy).14 NB4 cells were a gift from Dr Lanotte (Hôpital Saint-Louis, Paris, France).15 APL patients' cells were maintained in AIM-V, and cell lines PR9 and NB4 were maintained in RPMI 1640 medium, each containing 10% fetal calf serum (FCS), 1% l-glutamine, and penicillin-streptomycin. PR9 cells were exposed to 200 μM ZnSO4 for 24 hours to induce PML/RARα. To analyze the effect of all-trans retinoic acid (ATRA, 1 μM; Sigma Chemical) and human recombinant leptin (100 ng/mL; R&D Systems, Minneapolis, MN), cells were cultured at 37°C in 5% CO2 at a starting concentration of 2 × 105 cells/mL for the indicated time. Leukemic cell proliferation was evaluated by cell counts using the trypan blue exclusion method.
Human MSCs were isolated from the BM of healthy donors undergoing BM harvest for allogeneic BM transplantation, as described.16,17 Mononuclear cells were separated by centrifugation over a Ficoll-Hypaque gradient and were suspended in α-modified essential medium (α-MEM) containing 20% fetal bovine serum, l-glutamine, and penicillin-streptomycin mixture, followed by plating at an initial seeding density of 1 × 106 cells/cm2. After 3 days, the nonadherent cells were removed by washing with phosphate-buffered saline (PBS), and monolayers of adherent cells were cultured until they reached confluence. Cells were then trypsinized, subcultured at densities of 5000 to 6000 cells/cm2, and used for experiments during passages 3 to 4. The isolated, cultured MSCs at passage 3 comprised a single phenotypic population, as determined by flow cytometric analysis of expressed surface antigens. They were uniformly positive for SH2 and SH3 and were negative for markers of hematopoietic lineage (including macrophages) CD14, CD34, and CD45. The same phenotype was described by Pittenger et al.16 For the induction of adipocytic differentiation, MSCs were cultured in 12-well, flat-bottom plates (Becton Dickinson Labware, Franklin Lakes, NJ) at a density of 0.3 × 105 cells/cm2 in adipocyte medium (α-MEM, 20% FCS, 1% L-glutamine and penicillin-streptomycin, and 0.01 mg/mL insulin). Under the adipogenic conditions, cells were allowed to differentiate for 3 to 4 days in medium consisting of 20% FCS, 1% L-glutamine and penicillin-streptomycin, 1 μM dexamethasone, 0.2 mM indomethacin, 0.01 mg/mL insulin, and 0.5 mM 3-isobutyl-methylxanthine, after which they were maintained in adipocyte medium for 3 to 4 days; the medium was changed every 3 to 4 days. The time of appearance of morphologically identifiable adipocytes with lipid droplets that stained with oil red O and the percentage of adipocytes among MSCs were determined during culture. At 2 and 3 weeks of adipocyte differentiation culture, approximately 20% and 70%, respectively, of MSCs had differentiated into mature adipocytes. Adipocyte-differentiated MSCs were cocultured with primary APL cells, PR9 cells, and NB4 cells (0.6 × 105 cells/mL) for the indicated time periods. In some experiments, cocultures were performed in the presence of ATRA (1 μM), doxorubicin (10 ng/mL or 50 ng/mL; American Pharmaceutical Partners, Los Angeles, CA), chimeric blocking leptin receptor/immunoglobulin G1 (IgG1) Fc (0.12 μg/mL, R&D Systems; 50% effective dose [ED50] 0.02-0.12 μg/mL), antihuman leptin antibody (3.0 μg/mL, R&D Systems; 50% neutralization dose [ND50] 0.5-3.0 μg/mL), MAPK inhibitor PD98059 (2′-amino-3-methoxyflavone; 20 mM; Calbiochem-Novabiochem, La Jolla, CA). In experiments comparing direct contact-to-noncontact conditions, APL cells were plated in 12-well plates containing adipocyte-differentiated MSCs, either in direct contact or separated by a 0.4-μM porous transwell insert (Corning, Corning, NY) that allows passage of soluble growth factors.
Flow cytometric analysis
At indicated time points, cell-cycle distribution was analyzed in primary APL, PR9, and NB4 cells (3 × 105), cultured under various conditions using flow cytometric analysis of propidium iodide (PI)–stained nuclei. Briefly, cells were washed twice with phosphate-buffered saline (PBS) buffer, fixed in ice-cold ethanol (70% vol/vol in water), and stained with the PI solution (25 μg/mL PI, 180 U/mL RNase, 0.15% Triton X-100, and 30 mg/mL polyethylene glycol in 4 mM citrate buffer, pH 7.8; all from Sigma Chemical). DNA content was determined using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). Cells with a hypodiploid DNA content (less than 2n) were counted as apoptotic. For the phosphatidylserine/annexin V–binding studies, fresh cells were washed twice with binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140 mM NaCl, and 5 mM CaCl2, pH 7.4; all from Sigma Chemical) and were stained with fluorescein isothiocyanate (FITC)–conjugated annexin V (Roche Diagnostic, Indianapolis, IN) for 15 minutes at room temperature. Stained cells were analyzed with a Becton Dickinson FACScan flow cytometer, and membrane integrity was simultaneously assessed by PI exclusion. The expressed surface antigens were determined on trypsinized cells. Cells were washed twice with PBS and incubated with phycoerythrin (PE)–conjugated anti-CD45, -CD34, and -CD14 (Becton Dickinson). Background staining was determined using PE-conjugated isotype control IgG1 antibody. Flow cytometric data obtained by FACScan flow cytometry were analyzed using Cell Quest software (Becton Dickinson), and results were expressed as the percentage of positive cells relative to background control levels.
Quantitative real-time RT-PCR
RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA). One microgram total RNA template was used per 10 μL reverse-transcriptase reaction by AMV reverse transcriptase (Roche Diagnostic) at 42°C for 1 hour. A reverse transcription (RT) kit was used to synthesize cDNA according to the manufacturer's instructions (Boehringer-Mannheim, Indianapolis, IN) using hexanucleotide random primers. Duplicate samples of 1 μL each cDNA were amplified by polymerase chain reaction (PCR) in the ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA). The Primer Express program (PE Applied Biosystems) was used to design primers and probes. Probes were labeled at the 5′ end with FAM and at the 3′ end with TAMRA, which served as quenchers. Amplification conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds and at 60°C for 60 seconds. The relative amount of gene expression was calculated using the expression of β2-microglobin as an internal standard. Primers for the long and short isoforms of the OB-R were designed to anneal to the unique sequence in the cytoplasmic portion of the receptor. Primers (forward [FP]; reverse [RP]) and probe sequences were as follows: OB-R long isoform (GenBank accession number U43168) FP, 5′-TGTTAAATACGCCACGCTGA-3′ (nucleotides 3142-3161); RP, 5′-GGCCTCTATCTCCCATGAGC-3′ (nucleotides 3273-3292); probe, 5′-CCAAGTGAAACTGGTGAAGAACAAGGGCTTAT-3′ (nucleotides 3176-3207); OB-R short-isoform (GenBank accession number U66495) FP, 5′-AACCCCAAGAATTGTTCCTG-3′ (nucleotides 2822-2841); RP, 5′-GGCACATTGGGTTCATCTGT-3′ (nucleotides 2903-2922); probe, 5′-CACAAGGACTTAATTTTCAGAAGAGAACGGACATTC-3′ (nucleotides 2844-2879); PML/RARα (GenBank accession number M73779) FP, 5′-TGGCTTCGACGAGTTCAAGG-3′ (nucleotides 1188-1205); RP, 5′-AAAGCAAGGCTTGTAGATGCG-3′ (nucleotides 1318-1338); probe, 5′-AGCCATTGAGACCCAGAGCAGCAGTTC-3′ (nucleotides 1248-1274); PPARγ (GenBank accession number NM_015869) FP, 5′-GGCTTCATGACAAGGGAGTTTC-3′ (nucleotides 1253-1274); RP, 5′-AACTCAAACTTGGGCTCCATAAAG-3′ (nucleotides 1303-1326); probe, 5′-AAAGAGCCTGCGAAAGCCTTTTGGTG-3′ (nucleotides 1276-1301); β2-microglobulin (GenBank accession number NM_004048) FP, 5′-AGCTGTGCTCGCGCTACTCT-3′ (nucleotides 34-53); RP, 5′-TTGACTTTCCATTCTCTGCTGG-3′ (nucleotides 113-134); probe, 5′-TCTTTCTGGCCTGGAGGGCATCC-3′ (nucleotides 55-71). The PCR cycle number that generated the first fluorescence signal above a threshold value (threshold cycle [CT]) was determined. Threshold was calculated as a value 10 SDs above the mean fluorescence generated during the baseline cycles. A comparative CT method (2-ΔΔCT method) was used to detect relative gene expression.18,19 The following formula was used to calculate the relative amount of the transcript of interest in the treated sample (X) and the control (calibrator) sample (Y), both normalized to an endogenous reference (β2-microglobulin): 2-ΔΔCT, where ΔCT is the difference in CT between the gene of interest and β2-microglobin, and ΔΔCT is the difference for sample X = ΔCT, X-ΔCT, Y. Untreated PR9 cells were used as calibrators for all PCR experiments. The CT data of duplicate PCRs using the same cDNA were averaged before calculating 2-ΔΔCT.
Western blot analysis
Leukemic cells were washed twice with PBS and lysed in cell lysis buffer (10 mM NaF, 1 mM Na3VO4, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 0.1% NaN3, 10 mM iodoacetamide, 3 mM phenylmethylsulfonyl fluoride [PMSF], and 1% Triton X-100) supplemented with a protease inhibitor cocktail (Roche Diagnostic). Equal amounts of lysate (equivalent to 5 × 105 cells) were separated on 7.5% polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). Proteins were transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England), immunoblotted with either RARα polyclonal antibody (Santa Cruz Biotechnology, CA), phospho-STAT3 (Tyr705) antibody (Upstate Biotechnology, Lake Placid, NY), STAT3 antibody (Upstate Biotechnology), phospho-p44/42MAPK (Thr202/Tyr204) antibody (Cell Signaling Technology, Beverly, MA), or polyclonal p42MAPK antibody (Santa Cruz) and were reacted with enhanced chemiluminescence (ECL) reagent (Amersham Pharmacia). An anti–β-actin or anti–α-tubulin blot was used in parallel as a loading control. Signals were detected by PhosphorImager (Storm 860, version 4.0; Molecular Dynamics, Sunnyvale, CA) and quantified by Scion Image software (Scion, Frederick, MD). For the coculture experiments, NB4 cells, ZnSO4-pretreated cells, or untreated PR9 cells were cultured alone (control) or cocultured with undifferentiated MSCs or adipocyte-differentiated MSCs at the indicated conditions. Results were expressed as P-STAT3/STAT3 or P-MAPK/ERK2 ratios.
Immunocytochemical staining
Cytospin slides of trypsinized MSCs before and after adipocyte differentiation were used for immunocytochemical analysis of leptin. Cytospin slides prepared from NB4 and PR9 cells, with or without PML/RARα induction, were subjected to OB-R immunocytochemical analysis. After formalin fixation, specimens were dehydrated and endogenous peroxidase was blocked with methanol containing 0.3% H2O2 for 30 minutes, after which the specimens were rehydrated in a graded series of ethanol. Following the manufacturer's protocol (Vectastain ABC Kit; Vector Laboratories, Burlingame, CA), specimens were preincubated with 2% normal serum for 15 minutes and were exposed to the primary antibody overnight at 4°C. Antibodies consisted of the goat antileptin antibody (0.1 mg/mL; R&D Systems) and the goat anti–OB-R antibody (0.1 mg/mL; R&D Systems). As a negative control, PBS was replaced with goat or mouse IgG. After exposure to secondary antibody for 1 hour at room temperature, specimens were incubated with ABC complex (Vectastain ABC Kit; Vector Laboratories) for 30 minutes, rinsed with PBS, and incubated with DAB reagent (3,3′-diaminobenzidine) for 10 minutes (Vector Laboratories). After the cell nuclei were counterstained with hematoxylin for 30 minutes, specimens were dehydrated through exposure to several concentrations of ethanol and then mounted with glycerin gelatin.
Leptin enzyme-linked immunosorbent assay
Leptin concentration in culture medium of adipogenic-differentiating MSCs was measured using enzyme-linked immunosorbent assay (ELISA), which was performed according to the manufacturer's instructions (Diagnostic Systems Laboratories, Webster, TX). MSCs were fed under the adipogenic conditions described in “Cell cultures,” and the culture supernatant was sampled 3 days after the medium was changed. This assay is sensitive to a concentration of 0.05 ng/mL leptin. The intra-assay coefficient of variation was 4.2%. Media were frozen at –80°C at the designated time points until the ELISAs were performed.
Statistical analysis
We analyzed data from 182 patients with newly diagnosed APL.20,21 The 77 patients who were not treated with ATRA were diagnosed between March 28, 1980, and February 24, 1992. The 105 patients who were treated with ATRA or cis-trans retinoic acid (cis-TRA) were diagnosed between September 19, 1991, and March 23, 2001. We used Cox proportional hazards regression22 to model overall survival and progression-free survival in 2 treatment groups (ATRA or cis-TRA vs no ATRA or cis-TRA) and BMI in a univariate fashion. BMI was defined as the weight in kilograms divided by the square of the height in meters; we used a BMI of 25 or more as the cutoff indicating obesity, following the classification of the World Health Organization Consultation on Obesity and the Dietary Guidelines for Americans.23 We estimated the overall survival and progression-free survival distributions with the Kaplan-Meier estimator.24 A 2-sample t test was used to compare the 2 treatment groups with regard to BMI.
Loge transformation of the data was used for correlation analysis between PML-RARα and OB-R mRNA expression levels of APL cells because there appeared to be some outliers among the PML-RARα data. The Mann-Whitney U test was used for statistical comparison of OB-R mRNA expression levels between NB4 cells and primary APL cells, and the Student paired t-test was used for other between-group statistical comparisons, with P values of less than .05 considered significant.
Results
PML/RARα induction is associated with up-regulation of OB-R in U937/PR9 cells
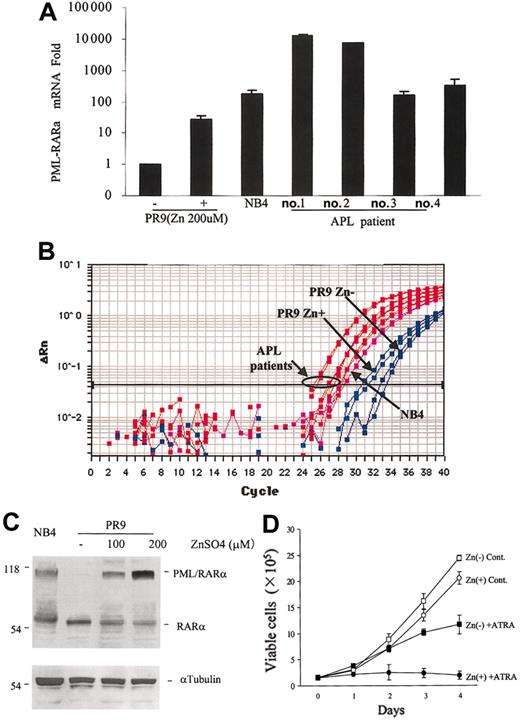
As a model of APL cells, we used U937/PR9 cells expressing PML-RARα in response to ZnSO4.14 Using this cell line, we investigated changes in OB-R expression following PML-RARα induction. In PR9 cells, 24 hours of 200 μM ZnSO4 exposure induced PML-RARα mRNA 26.6 ± 9.8-fold compared with baseline levels (n = 4), as determined by TaqMan PCR (Figure 1A-B).
PML-RARα mRNA induction in U937/PR9 cells and expression levels in primary APL cells. (A) TaqMan RT-PCR analysis showing the induction of PML-RARα mRNA expression in U937/PR9 cells treated with 200 μM ZnSO4 for 24 hours and the levels of PML-RARα mRNA in primary APL cells. PML-RARα mRNA expression of each sample was normalized to β2-microglobulin. PR9 cells without ZnSO4 were used as calibrators (PML-RARα mRNA level in PR9 cells without ZnSO4 = 1). APL cell line NB4 was used as a reference. (B) Amplification plot of PML-RARα cDNA expression in PR9 cells following ZnSO4 treatment in NB4 and primary APL cells. Blue lines indicate PR9; magenta lines, NB4; and red lines, primary APL cells. (C) Western blot showing the induction of PML-RARα protein expression in U937/PR9 cells treated with 100 μM or 200 μM ZnSO4 and in NB4 cells. (D) PR9 cells exposed to ZnSO4 (200 μM) were cultured in medium with or without 1 μM ATRA, and growth curves were constructed. Live cells were counted daily using the trypan blue exclusion method. Graphs represent the mean ± SD of the results obtained from 3 independent experiments. In the absence of ZnSO4, PR9 cells were cultured without ATRA (□) or with ATRA (▪). In the presence of ZnSO4, PR9 cells were cultured without ATRA (○) or with ATRA (•).
PML-RARα mRNA induction in U937/PR9 cells and expression levels in primary APL cells. (A) TaqMan RT-PCR analysis showing the induction of PML-RARα mRNA expression in U937/PR9 cells treated with 200 μM ZnSO4 for 24 hours and the levels of PML-RARα mRNA in primary APL cells. PML-RARα mRNA expression of each sample was normalized to β2-microglobulin. PR9 cells without ZnSO4 were used as calibrators (PML-RARα mRNA level in PR9 cells without ZnSO4 = 1). APL cell line NB4 was used as a reference. (B) Amplification plot of PML-RARα cDNA expression in PR9 cells following ZnSO4 treatment in NB4 and primary APL cells. Blue lines indicate PR9; magenta lines, NB4; and red lines, primary APL cells. (C) Western blot showing the induction of PML-RARα protein expression in U937/PR9 cells treated with 100 μM or 200 μM ZnSO4 and in NB4 cells. (D) PR9 cells exposed to ZnSO4 (200 μM) were cultured in medium with or without 1 μM ATRA, and growth curves were constructed. Live cells were counted daily using the trypan blue exclusion method. Graphs represent the mean ± SD of the results obtained from 3 independent experiments. In the absence of ZnSO4, PR9 cells were cultured without ATRA (□) or with ATRA (▪). In the presence of ZnSO4, PR9 cells were cultured without ATRA (○) or with ATRA (•).
Anti-RARα Western blot analysis showed a consistent, dose-dependent increase in the levels of PML-RARα protein after 24-hour ZnSO4 exposure in PR9 cells (Figure 1C) and remained at this level for 96 hours (data not shown). For the following experiments, we used 200 μM ZnSO4, which induced high level PML-RARα expression. We also confirmed that PML-RARα–expressing PR9 cells showed enhanced sensitivity to ATRA-induced cell growth inhibition and differentiation, consistent with published data.14 ZnSO4 treatment itself, without exposure to ATRA, had negligible effects on cell growth (Figure 1D) and apoptosis of PR9 cells (ZnSO4 untreated vs treated PR9 cells: 5.0% ± 1.6% vs 6.4% ± 2.8% sub-G1 cells; P = .46; n = 3; 96 hours).
We then analyzed the expression of OB-R in PML-RARα–expressing PR9 cells. Baseline short-isoform mRNA expression in untreated PR9 cells was 35.0 ± 10.2-fold higher than it was in long-isoform mRNA. PML-RARα induction was associated with the up-regulation of both OB-R isoforms detected by TaqMan PCR. Specifically, OB-R long-isoform mRNA levels were 20.0 ± 7.6-fold (n = 3) higher after 24 hours of ZnSO4 treatment compared with levels in PR9 cells not exposed to ZnSO4, whereas the induction of OB-R short-isoform mRNA was relatively weak (eg, 7.5 ± 0.5-fold higher after 24 hours [n = 2] of ZnSO4) (Figure 2A-B).
OB-R gene and protein expression in PR9 cells, NB4 cells, and primary APL cells. (A) OB-R long- and short-isoform mRNA levels before and after the induction of PML-RARα (200 μM ZnSO4 treatment) in U937/PR9 cells, in NB4 cells, and in primary APL cells (TaqMan RT-PCR). OB-R mRNA expression of each sample was normalized to β2-microglobulin. PR9 cells without ZnSO4 were used as calibrators (OB-R mRNA level in PR9 cells without ZnSO4 = 1). Percentages of blasts and promyelocytes in APL samples: no. 1, PB, 82%; no. 2, BM, 95%, no. 3, PB, 75%; no. 4, PB, 91% (PB indicates peripheral blood). Graphs represent the mean ± SD of results from 3 different experiments. (B) Amplification plot of OB-R long-isoform cDNA expression in PR9 cells after ZnSO4 treatment in NB4 and primary APL cells. Blue lines indicate PR9; magenta lines, NB4; and red lines, primary APL cells. (C) Representative immunocytochemistry results for OB-R expression in the cytosol of PR9 cells: (i) before ZnSO4 treatment and (ii) after 24 hours of ZnSO4 (200 μM) treatment. (iii) Immunoreaction of OB-R in cytosol of NB4 cells (original magnification, × 200).
OB-R gene and protein expression in PR9 cells, NB4 cells, and primary APL cells. (A) OB-R long- and short-isoform mRNA levels before and after the induction of PML-RARα (200 μM ZnSO4 treatment) in U937/PR9 cells, in NB4 cells, and in primary APL cells (TaqMan RT-PCR). OB-R mRNA expression of each sample was normalized to β2-microglobulin. PR9 cells without ZnSO4 were used as calibrators (OB-R mRNA level in PR9 cells without ZnSO4 = 1). Percentages of blasts and promyelocytes in APL samples: no. 1, PB, 82%; no. 2, BM, 95%, no. 3, PB, 75%; no. 4, PB, 91% (PB indicates peripheral blood). Graphs represent the mean ± SD of results from 3 different experiments. (B) Amplification plot of OB-R long-isoform cDNA expression in PR9 cells after ZnSO4 treatment in NB4 and primary APL cells. Blue lines indicate PR9; magenta lines, NB4; and red lines, primary APL cells. (C) Representative immunocytochemistry results for OB-R expression in the cytosol of PR9 cells: (i) before ZnSO4 treatment and (ii) after 24 hours of ZnSO4 (200 μM) treatment. (iii) Immunoreaction of OB-R in cytosol of NB4 cells (original magnification, × 200).
In the parental U937 cell line, OB-R mRNA expression was not changed by ZnSO4 treatment. The OB-R long isoform (1.6 ± 0.2-fold [n = 2]), and the OB-R short isoform was 0.31 ± 0.06-fold [n = 2]) compared with levels in U937 cells not exposed to ZnSO4. Cytoplasmic immunohistochemistry staining for OB-R in PR9 cells before ZnSO4 treatment showed only weak positivity, but protein expression increased after 24 hours of ZnSO4, reaching levels of expression found in NB4 cells (Figure 2C). Taken together, these results indicate that PML-RARα is associated with the up-regulation of OB-R.
Primary APL cells express high levels of OB-R long isoform
Using TaqMan PCR, we examined the PML-RARα and OB-R mRNA expression levels of primary APL cells in 4 patients with newly diagnosed disease (Figures 1A-B and 2A-B). OB-R long-isoform mRNA levels in primary APL cells were 193.6 ± 101.6-fold (n = 4) higher than in the APL cell line NB4 cells (P < .05; Mann-Whitney U test). Expression of OB-R short-isoform mRNA was similar to or lower in primary APLs compared with NB4 cells (0.36 ± 0.15-fold). In PR9, NB4, and primary APL cells, PML-RARα and OB-R long-isoform mRNA expression levels were highly correlated (r2 = 0.952; P < .001). There was no significant correlation between PML-RARα and OB-R short isoform.
Adipocyte-differentiated MSCs produce leptin
To mimic the effects of locally produced leptin on APL cells in the BM, we used an in vitro system consisting of leptin-producing preadipocytes and adipocytes derived from MSCs. After 7 days of culture under adipocyte-differentiation conditions, approximately 1% of the MSCs exhibited an adipocyte-like morphology consisting of cytoplasmic lipid-rich vacuoles that stain with oil red O (data not shown). After 14 days, approximately 20% of the MSCs showed an accumulation of multiple lipid droplets, and after 21 days, 70% of the MSCs were differentiated into adipocytes. We confirmed the adipogenic differentiation of MSCs by analyzing PPARγ, a well-established adipocyte-specific marker.25,26 During the adipocyte-differentiation culture period, MSCs showed increased levels in PPARγ mRNA as determined by TaqMan RT-PCR: 8.0-fold after 7 days, 17.1-fold after 14 days, and 26-fold after 21 days compared with undifferentiated MSCs. Macrophages also express PPARγ; however, flow cytometric analysis of MSC cultures demonstrated a phenotypically uniform population without contaminating CD14+ cells. Therefore, we concluded that detected PPARγ mRNA is produced solely by adipocytes. We next examined changes in leptin production in MSCs during adipogenic differentiation. The intensity of the immunoreactivity with antileptin antibody increased during adipocyte differentiation. Undifferentiated MSCs only weakly stained for leptin (Figure 3A,C). After differentiation, adipocytes with lipid droplets exhibited intense staining in the cytoplasm and cytoplasmic rim but not in the lipid droplets (Figure 3B,D). The concentration of leptin in the culture supernatant of MSCs increased significantly during adipogenic culture. Levels secreted by undifferentiated MSCs (0.5 ± 0.06 ng/mL) increased to 0.67 ± 0.12 ng/mL after 7 days, 2.52 ± 0.18 ng/mL after 11 days, and 4.2 ± 0.53 ng/mL after 16 days, and all concentrations reached plateaus afterward.
Immunocytochemical staining for leptin before and after induction of adipocyte differentiation of MSCs. Representative immunocytochemistry staining results for leptin expression in MSCs. (A) MSC monolayers before adipocyte differentiation culture and (B) after 14 days of adipocyte differentiation culture (original magnification, × 200). (C) Trypsinized MSC before adipocyte differentiation culture and (D) after 14 days of adipocyte differentiation culture (original magnification, × 400). After adipocyte differentiation, staining for leptin was more intense in cultured MSCs.
Immunocytochemical staining for leptin before and after induction of adipocyte differentiation of MSCs. Representative immunocytochemistry staining results for leptin expression in MSCs. (A) MSC monolayers before adipocyte differentiation culture and (B) after 14 days of adipocyte differentiation culture (original magnification, × 200). (C) Trypsinized MSC before adipocyte differentiation culture and (D) after 14 days of adipocyte differentiation culture (original magnification, × 400). After adipocyte differentiation, staining for leptin was more intense in cultured MSCs.
Decreased leptin mRNA levels in human BM stromal cells during the late phase of adipocyte differentiation has previously been reported.27 The decrease was likely caused by a local negative feedback mechanism regulated by leptin and OB-R in the cytoplasm and membrane of adipocytes.28 Therefore, we considered 14 days after plating, with 20% of adipocytes, the best time for adipogenic cultured MSCs to synthesize and replenish leptin.
Protection of APL cells from apoptosis by adipocyte-differentiated MSCs
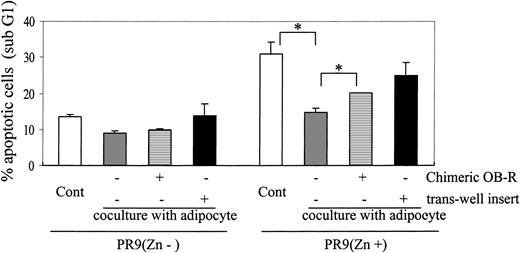
Because leptin stimulates the proliferation of normal hematopoietic and AML cells3,10 and a high level of OB-R expression was demonstrated in leukemic promyelocytes, we hypothesized that leptin produced by adipocyte-differentiated MSCs induces proliferation and protects APL cells from cell death. To assess the effects of adipocyte-produced leptin on APL cells, we conducted coculture experiments using 14-day adipogenic cultured MSCs with PR9 cells, with or without the induction of PML-RARα expression, NB4 cells, or primary APL cells. Cells were cultured in the presence of ATRA or doxorubicin or in serum-reduced conditions. Apoptosis was determined by PI/annexin V positivity or as “sub-G1” cell population by DNA flow cytometry after 72 hours, 96 hours, or both, of coculture. As shown in Figure 4, coculture with adipocyte-differentiated MSCs significantly prevented apoptosis in PML-RARα–expressing PR9 cells in the presence of 1 μM ATRA; the mean decrease in apoptotic cells was 16.3% ± 3.1% (P = .014) compared with controls. Consistently, ATRA-treated PML-RARα–expressing PR9 cells cocultured with adipocyte-differentiated MSCs showed significantly higher S-phase fractions than cells exposed to ATRA alone (16.3% ± 1.7% vs 4.2% ± 1.1%; P = .01). To determine whether the antiapoptotic effects of adipocyte-differentiated MSCs were mediated by the interaction of leptin and OB-R, cells were cocultured in the presence of chimeric OB-R protein, the competitive inhibitor of OB-R. The antiapoptotic effect of adipocyte-differentiated MSCs was partially but significantly blocked by chimeric OB-R (Figure 4; P < .05). To determine whether the antiapoptotic effect exerted by adipocytes requires direct cell-to-cell interactions or is mediated by secretion of soluble factors, we cultured PML-RARα–expressing PR9 cells separated from adipocyte-differentiated MSCs by transwell inserts. In noncontact conditions, PML-RARα–expressing PR9 cells were not significantly protected from apoptosis by adipocyte-differentiated MSC monolayers, suggesting that direct cell-to-cell contact is required for the observed antiapoptotic effects. In PR9 cells without PML-RARα expression, ATRA did not induce apoptosis. No significant changes in proliferation were found in cocultures of uninduced PR9 cells with adipocyte-differentiated MSCs, with or without chimeric OB-R or transwell insert, compared with control cells (Figure 4).
Adipocyte-differentiated MSCs protect PML-RARα–expressing PR9 cells from apoptosis. PR9 cells in the absence or presence of ZnSO4 pretreatment were cultured on adipocyte-differentiated MSC monolayers in the presence of 1 μM ATRA. After 96 hours of coculture, PR9 cells growing at the periphery or on top of the MSCs were harvested by extensive washing, and apoptosis was assessed by DNA flow cytometry (PI staining, sub-G1 region). ATRA-treated PR9 cells with or without ZnSO4 (200 μM) were cultured alone (□), cocultured in direct contact with adipocyte-differentiated MSCs (▦), cocultured in the presence of the chimeric OB-R protein (▤; OB-R competitive inhibitor), or separated from MSCs by a porous transwell inserts (▪). Graph represents mean ± SD of the results obtained in coculture experiments of PR9 cells without ZnSO4 (n = 2) and with ZnSO4 (n = 4). Statistically significant difference (*P < .05) was determined by paired Student t test; for all others, P ≥ .05.
Adipocyte-differentiated MSCs protect PML-RARα–expressing PR9 cells from apoptosis. PR9 cells in the absence or presence of ZnSO4 pretreatment were cultured on adipocyte-differentiated MSC monolayers in the presence of 1 μM ATRA. After 96 hours of coculture, PR9 cells growing at the periphery or on top of the MSCs were harvested by extensive washing, and apoptosis was assessed by DNA flow cytometry (PI staining, sub-G1 region). ATRA-treated PR9 cells with or without ZnSO4 (200 μM) were cultured alone (□), cocultured in direct contact with adipocyte-differentiated MSCs (▦), cocultured in the presence of the chimeric OB-R protein (▤; OB-R competitive inhibitor), or separated from MSCs by a porous transwell inserts (▪). Graph represents mean ± SD of the results obtained in coculture experiments of PR9 cells without ZnSO4 (n = 2) and with ZnSO4 (n = 4). Statistically significant difference (*P < .05) was determined by paired Student t test; for all others, P ≥ .05.
We then conducted experiments using the APL-derived cell line NB4. Adipocyte-differentiated MSCs maintained cell growth and reduced apoptosis induced by serum deprivation or treatment with low-dose doxorubicin (10 ng/mL). Chimeric OB-R and antileptin antibody blocked these antiapoptotic effects (Table 1). ATRA inhibited cell growth preferentially by cell-cycle arrest but did not induce apoptosis in NB4 cells, and adipocyte-differentiated MSCs did not alter the growth pattern.
In primary APL cells, adipocyte-differentiated MSC monolayers significantly prevented doxorubicin (50 ng/mL)–induced apoptosis (control, 27.8% ± 4.9% annexin V–positive cells; coculture, 11.4% ± 2.3%; P = .03; 72 hours; n = 3). Adipocyte-differentiated MSCs supported the growth of primary APL cells, and this effect was partially blocked by chimeric OB-R (67.4% ± 1.7% viability compared with cocultured cells).
During the coculture experiments, PML-RARα–expressing PR9 and NB4 cells spontaneously migrated beneath the adipocyte layer Adherent APL cells were detached from the adipocyte layer by trypsin/EDTA (ethylenediaminetetraacetic acid) after the nonadherent APL cells were removed, and apoptosis was determined using PI/annexin V labeling. Flow cytometric gates using the differences in relative size and CD45 staining (positive in APL cells, negative in adipocytes) were used to exclude adipocytes from analysis. Adherent NB4 and PR9 cells showed less than 2% PS/annexin V positivity, regardless of the apoptotic ratio of the floating fraction. This observation shows that cell-to-cell contact is necessary for the antiapoptotic effects to occur.
We then examined whether recombinant leptin exerts antiapoptotic effects similar to those of the adipocyte monolayer. In NB4 cells, human recombinant leptin, at a concentration equivalent to serum levels observed in obese persons (100 ng/mL),12 reduced the rate of serum deprivation–induced apoptosis from 27.0% ± 7.8% to 17.6% ± 5.7% (P = .01; n = 3) but did not affect sensitivity to ATRA (data not shown). Recombinant leptin did not protect PR9 cells (± Zn) from ATRA-induced apoptosis.
Because of the known positive correlation between BMI and serum leptin levels,27,29 we investigated the correlation between increased BMI and survival of APL patients. An increased BMI (with a cutoff of 25 kg/m2) did not significantly affect the overall and progression-free survival of APL patients, regardless of whether they were treated with ATRA (progression-free survival in no ATRA group, P = .388; in ATRA treatment group, P = .677).
Coculture with adipocyte-differentiated MSCs activates STAT3 and MAPK signaling in APL cells
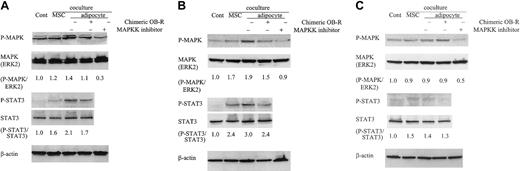
Because of the potential role of leptin in the activation of STAT-3 and MAPK pathways7-9,30 and because of our observation that adipocyte-differentiated MSCs prevented serum deprivation, doxorubicin- and ATRA-induced apoptosis in PML-RARα–expressing APL cells (Table 1; Figure 4), we investigated whether STAT3 and MAPK signaling pathways can be activated by adipocyte-produced leptin in APL cells. Coculture of NB4 cells with adipocyte-differentiated MSCs increased the phosphorylation of STAT3 and MAPK, and chimeric OB-R treatment reduced it. When NB4 cells were grown on undifferentiated MSCs, STAT3 and MAPK were phosphorylated to a lower degree than cocultivation with adipocytes (Figure 5A). Similarly, adipocyte-differentiated MSCs increased the phosphorylation of STAT3 and MAPK in PR9 cells with induced PML-RARα (Figure 5B), and STAT3 and MAPK phosphorylation were partially reduced by chimeric OB-R treatment. Coculture with adipocytes did not induce STAT3 or MAPK activation in ZnSO4-untreated PR9 cells (Figure 5C).
Coculture with adipocyte-differentiated MSCs stimulates the phosphorylation of STAT3 and MAPK. NB4 cells (A), ZnSO4 pretreated PR9 cells (200 μM, 24 hours) (B), and untreated PR9 cells (C) were cultured alone (control) or cocultured with undifferentiated MSCs or adipocyte-differentiated MSCs at the indicated conditions (with or without chimeric blocking OB-R or MAPK inhibitor) for 24 hours. Clarified lysates were probed with antibodies to phospho-MAPK, ERK2, STAT3, Tyr-705 phospho-Ab, STAT3, and β-actin by Western blotting. Results shown are representative of 3 experiments.
Coculture with adipocyte-differentiated MSCs stimulates the phosphorylation of STAT3 and MAPK. NB4 cells (A), ZnSO4 pretreated PR9 cells (200 μM, 24 hours) (B), and untreated PR9 cells (C) were cultured alone (control) or cocultured with undifferentiated MSCs or adipocyte-differentiated MSCs at the indicated conditions (with or without chimeric blocking OB-R or MAPK inhibitor) for 24 hours. Clarified lysates were probed with antibodies to phospho-MAPK, ERK2, STAT3, Tyr-705 phospho-Ab, STAT3, and β-actin by Western blotting. Results shown are representative of 3 experiments.
Discussion
This study demonstrates a strong correlation between long-isoform OB-R and PML-RARα mRNA expression levels in leukemic cells in both, an inducible cell line system in which the induction of PML-RARα resulted in increased OB-R transcript and protein concentrations, and in primary APL cells. Induced PML-RARα in U937/PR9 cells was associated with increased OB-R mRNA expression. We confirmed this finding by cytoplasmic immunostaining for OB-R in PR9 cells: leptin receptor protein levels increased after PML-RARα induction. Although mRNA of long- and short-isoform OB-R were induced in PML-RARα–expressing PR9 cells, the OB-R long isoform, which has signal transduction capabilities,7-9 increased more dramatically. We also observed that primary leukemic promyelocytes express significant high levels of the OB-R long-but not OB-R short-isoform mRNA.
We have previously reported the expression of the long and short isoforms of leptin receptors in normal progenitor cells (CD34+) and in most AMLs.10 In normal CD34–CD33+ and CD34–CD13+ cells, however, no long isoform and only weak expression of short-isoform OB-R were detected, which suggests the rapid down-regulation of OB-R expression during myeloid differentiation. Strikingly, long and short isoforms of OB-R were expressed at high levels in primary APL, even in CD34–33+ promyelocytes. Considering this differential expression of OB-R in malignant and normal promyelocytes, we propose that OB-R/leptin interactions may play a pathophysiologic role in APL. This is confirmed by our present finding of a strong association between PML-RARα and increased OB-R expression. OB-R expression in normal and AML CD34+ cells possibly reflects the undifferentiated status of these cells. Of interest, no expression of leptin receptor was found in a series of primary ALL samples by cDNA array,31 suggesting the restriction of functional effects of leptin to cells of the myeloid lineage.
We then examined the antiapoptotic effects of BM-produced leptin on APL cells. As the model system to produce leptin in vivo, we used adipocyte-differentiated MSCs.28 In primary cultures of human MSCs, leptin was expressed and secreted at high levels during adipocyte differentiation. MSC-derived adipocytes decreased apoptosis induced by serum deprivation or by doxorubicin in NB4 cells and protected primary APL cells from doxorubicin-induced apoptosis. This antiapoptotic effect was partially, but significantly reversed by blocking the OB-R/leptin interactions by either an OB-R/Fc chimeric fusion protein that binds leptin or by a leptin-neutralizing antibody. Similarly, adipocyte-differentiated MSCs protected PML-RARα–induced PR9 cells, with increased ATRA sensitivity, from ATRA-induced apoptosis and growth arrest. This effect was partially abrogated by the OB-R/Fc chimeric fusion protein. In PR9 cells without PML-RARα induction, adipocyte-differentiated MSCs did not prevent ATRA-induced apoptosis. To differentiate whether this antiapoptotic effect is mediated by secreted factors or depends on direct cell-to-cell interactions, we conducted coculture experiments in which Zn-induced PR9 cells were separated from adipocytes by transwell inserts, and we found that the antiapoptotic effect of adipocyte-differentiated MSCs is significantly diminished in noncontact conditions compared with direct contact culture. Strikingly, apoptosis was completely blocked in primary APL cells that migrated beneath the adipocytes. It has been reported that at least half of the tissue leptin may exist in membrane-bound form.32 Indeed, through immunostaining we found abundant leptin localized to plasma membrane–associated regions in adipocyte-differentiated MSCs. Furthermore, human recombinant leptin did not affect sensitivity to ATRA. In addition, no correlation was found between increased BMI and survival of APL patients, although we could not determine serum leptin levels in these patients. Therefore, we conclude that the production of membrane-bound leptin by BM adipocytes is involved in the BM cytokine network, which regulates APL cell proliferation, survival, and apoptosis through direct cell-to-cell contact.
We observed only partial abrogation of the protective effects of leptin by blocking OB-R/leptin interactions by OB-R/Fc chimeric fusion protein and anti–OB-R antibody. This suggests that other growth factors, in addition to leptin, are involved in APL cell survival and proliferation. The adipocyte-differentiated MSC monolayer contains fibroblast-shaped preadipocytes and undifferentiated MSCs that are known to produce cytokines other than leptin, including stromal-derived factor-1, macrophage colony-stimulating factor, granulocyte macrophage–colony-stimulating factor, stem cell factor, thrombopoietin, interleukin-6, leukemia inhibitory factor, and tumor growth factor β.33 Preadipocytes also strongly express fibronectin, an adhesive extracellular matrix protein that promotes tumor development.34
Adipocytes are the prevalent stromal cell type in adult BM, and they have been shown to play an important role in the hematopoietic environment. A positive correlation between adipocyte differentiation of stromal cells and the ability to support the growth of lymphoid cells has been documented.35 The molecular basis for the ability of adipocytes to support hematopoiesis is unknown. This is the first report demonstrating that leptin produced by MSC-derived adipocytes controls survival of APL cells.
We have further investigated molecular signaling mechanisms activated by leptin in APL cells that result in antiapoptotic effects. The activation of STAT-3 by leptin has been observed in the hypothalamus in vivo,9 and the phosphorylation of MAPK by leptin was shown in vitro and in vivo.36-38 In our study, an increase in phosphorylation of STAT3 and MAPK in PML-RARα–expressing APL cells was observed under the influence of leptin-producing, MSC-derived adipocytes in vitro. This activation was partially diminished by blocking OB-R to a level similar to that found after coculture with undifferentiated MSCs. PR9 cells without PML-RARα expression did not show the activation of STAT3 or MAPK by MSC-derived adipocytes. These data suggest that adipocyte-produced leptin phosphorylates STAT3 and activates the MAPK signaling pathway, specifically inAPL cells, through leptin/OB-R interactions. These actions are compatible with a role for MAPK signaling in regulating proliferation in AML.39,40 Of relevance, a recent study demonstrated that stroma contact promotes the proliferation of B-cell progenitors through the activation of ERK-2, which in turn modulates the cell-cycle regulators cdk2 and p27.41
Recently, Kawasaki et al42 reported that PML inhibits STAT3 activity by direct binding and that PML-RARα dissociated PML from STAT3. In their study,42 PML-RARα did not phosphorylate STAT3 by itself but potentiated gp130–mediated STAT3 activity.42 Furthermore, STAT3 activation by a newly identified STAT5b-RARα fusion protein in APL, which is responsible for resistance to ATRA, was reported by Dong and Tweardy.43 Considering these findings, our data suggest that STAT3 activation by BM adipocyte–produced leptin would critically affect the survival and proliferation of APL cells, which already have dysregulated STAT3 activity. Blocking STAT3 activation is reported to result in the inhibition of Bcl-XL,44 a well-established antiapoptotic protein.45 We previously observed that antiapoptotic effects of MS-5 stromal cells on NB4, HL60, and primary AML progenitor cells were mediated by the increased expression of Bcl-2 and Bcl-XL.46 Therefore, stimulating STAT3 signaling through leptin in APL may confer the upregulation of Bcl-XL that exhibits cytoprotective effects. This mechanism requires further investigation.
In conclusion, we here demonstrate that PML-RARα induction is associated with the up-regulation of the functional leptin receptor isoform, capable of transducing proliferative and antiapoptotic signals of BM adipocyte–produced leptin. Direct cell-to-cell contact between leptin-producing BM adipocytes and APL cells initiates leptin signaling events, including the activation of STAT3 and MAPK pathways. These mechanisms may be important components of the interactions between leukemic and stromal cells in vivo, with the potential of promoting chemoresistance in APL. Our results suggest that specific inhibitors of MAPK and STAT3 signaling pathways could be useful adjuncts to currently available therapeutics. In addition, pharmaceutical OB-R blockade may be a novel, testable hypothesis with potential therapeutic use in APL.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-03-0802.
Supported in part by National Institutes of Health grants CA55164, CA49639, and CA16654 and by the Stringer Professorship for Cancer Treatment and Research (M.A.).
Y. T. and M.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rosemarie Lauzon and Tena Horton for help in the preparation of the manuscript; and C. Ellen Jackson, R.N., B.S.N., and Kimberly Edwards, R.N., for the procurement of the clinical samples.