Abstract
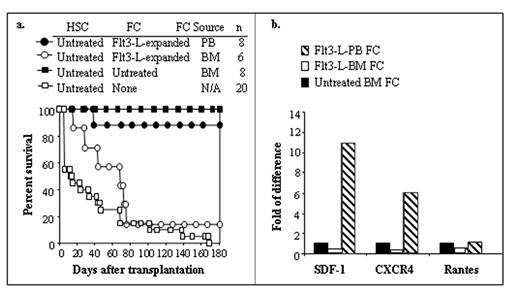
Facilitating cells (FC) are a CD8+/TCR− subpopulation of bone marrow (BM) cells that enhance engraftment of hematopoietic stem cells (HSC) in allogeneic recipients without causing graft-versus-host disease. They also significantly enhance engraftment of limiting numbers of HSC in syngeneic recipients. Treatment of mice with Flt3 ligand (Flt3-L) induces significant expansion of FC and HSC in the BM and their mobilization into peripheral blood (PB). In the present study, we evaluated the function of Flt3-L expanded FC and potential mechanism of action. Flt3-L treatment resulted in an 8.5-fold increase of FC in BM and a 100-fold increase in PB compared to untreated mice. When FC from the PB and BM of Flt3-L-treated donors were transplanted with HSC into allogeneic recipient mice, BM FC exhibited significantly impaired function while PB FC were potently functional (Fig. a). Strikingly, this correlated with an increase in mRNA for the chemokine SDF-1 and its receptor, CXCR4, which were significantly increased in PB FC (≥10 fold) and decreased in Flt3-L-expanded BM FC as compared to untreated control BM FC (Fig. b). The impaired function for BM FC was restored within 5 days after cessation of Flt3-L-treatment. Taken together, these data suggest that FC can be mobilized efficiently with Flt3-L and their engraftment facilitating function is associated with upregulation of transcripts for SDF-1 and CXCR4, suggesting that FC may chemoattract HSC by secreting SDF-1 and enhance their homing to the new microenvironment after transplantation. The fact that FC express CXCR4 suggests that they may co-migrate with HSC to the hematopoietic microenvironment after transplantation, being chemoattracted by BM-derived SDF-1. In summary, we provide evidence that FC are mobilized with HSC; the SDF-1-CXCR4 axis is involved in FC:HSC interaction; and FC co-migrate with HSC in response to an SDF-1 gradient.
Author notes
Corresponding author