Abstract
Background/Aim Despite great improvements in the treatment of childhood Acute Lymphoblastic Leukemia (ALL), resistant to treatment still remains a leading cause of cancer-related death in children. Although the underlying mechanism is not well understood, studies have shown that polymorphisms in those genes involved in drug metabolism may not only modify the susceptibility to the cancer but also influence the risk of relapse. Knowledge of patient’s pharmacogenetic make-up thus becomes important as we will move into a new era of individualizing therapy within the next decade. With the completion of the Human Genome Project, there is huge information on the SNPs which are involved in the drug metabolism. Hence, a highly efficient genotyping strategy is required to study multiple candidate genes on a single platform. This will provide timely reports for oncologists to tailor treatments for patients based on their pharmacogenetic constitution. Therefore, we aimed to develop an integrative genotyping platform, a DNA chip capable of detecting several mutations simultaneously. We plan to use this new tool to investigate the roles of 12 polymorphisms in 6 important drug-metabolizing genes (Table 1) in the risk of ALL as well as their impact on the treatment outcome in our Southeast Asian population, where only a few pharmacogenetic studies have been reported.
Methods Two allele-specific primers are designed to interrogate each polymorphism and all primers for wild-type or mutant are pooled to obtain 2 mixtures. Eleven fragments spanning 12 polymorphic alleles are amplified in 3 multiplex PCR and pooled, followed by multiplex allele-specific primer extensions using 2 primer mixtures respectively. Only fully matched primers will be extended with TAMRA-labelled ddNTP. The products are subsequently loaded on DNA chip and the extended primers are captured by corresponding complementary oligo tags. The fluorescence will then disclose the genotype in imaging system. Currently we have used 40 DNA control samples to validate this platform.
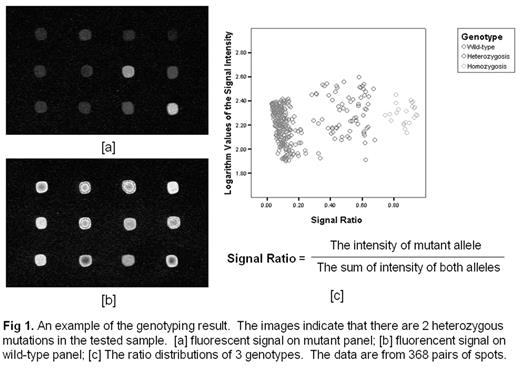
Results Homozygosity displayed fluorescence from only 1 allele while signals from both indicated heterozygosity (Fig 1). We have performed PCR-RFLP tests on NQO1, MTHFR and GSTP1, and used those results to verify the accuracy of the chip. 96.7% accuracy has been achieved.
Conclusion Our preliminary results have demonstrated the validity of this novel genotyping strategy which has great potential to become a simple and reliable method for pharmacogenetics study.
Future Plan Further verification and optimization are in progress. We will finally apply this genotyping strategy to screen childhood ALL patient samples to study the influence of these polymorphic genes on the disease.
The 6 polymorphic genes involved in drug metabolism
Figure
| Gene . | Polymorphism(s) . | Drugs affected . |
|---|---|---|
| TPMT | *3A (G460A, A719G), *6 (A539T) | 6-mercaptopurine, 6-thioguanine |
| NQO1 | *2 (C609T) | Alkylators |
| CYP1A1 | *2A (T6235C), *2B (A4889G, T6235C), *4 (C4887A) | others |
| CYP2D6 | *3 (A2637 deletion), *4 (G1934A) | others |
| GSTP1 | A105G | Glucocorticoids |
| MTHFR | C677T, A1298C | Methotrexate |
| Gene . | Polymorphism(s) . | Drugs affected . |
|---|---|---|
| TPMT | *3A (G460A, A719G), *6 (A539T) | 6-mercaptopurine, 6-thioguanine |
| NQO1 | *2 (C609T) | Alkylators |
| CYP1A1 | *2A (T6235C), *2B (A4889G, T6235C), *4 (C4887A) | others |
| CYP2D6 | *3 (A2637 deletion), *4 (G1934A) | others |
| GSTP1 | A105G | Glucocorticoids |
| MTHFR | C677T, A1298C | Methotrexate |
Author notes
Corresponding author