Abstract
We reviewed 261 patients with chronicphase chronic myelogenous leukemia (CML) after interferon-α (IFN-α) failure treated with imatinib mesylate 400 mg daily. With a median follow-up time of 45 months, the major cytogenetic response rate was 73% and the complete cytogenetic response rate 63%. The estimated 4-year survival rate was 86%. Multivariate analysis for survival identified hematologic resistance to IFN-α (P = .01), splenomegaly (P = .03), and lack of any cytogenetic response after 3 months of therapy (P = .01) to have independent poor prognostic significance. Patients could be divided into good(no adverse factors), intermediate(1 adverse factor), and poor-risk groups (2 or 3 adverse factors; 12% of patients) with estimated 4-year survival rates of 96%, 86%, and 49%, respectively (P < .000 01). The 4-year cumulative major molecular response (quantitative reverse transcriptase–polymerase chain reaction [Q-PCR] = BCR-ABL/ABL less than 0.05%) rate was 43% and complete molecular response rate (BCR-ABL undetectable) 26%. Compared with a historical group of 251 similar patients treated with nonimatinib therapies, imatinib mesylate was associated with a better 4-year survival rate (86% versus 43%; P < .0001); the survival advantage was confirmed by multivariate analysis (hazard ratio, 0.19; P < .0001).
Introduction
The Philadelphia chromosome (Ph) molecular abnormalities have been implicated in the pathophysiology and development of chronic myelogenous leukemia (CML).1,2 Recent therapies in CML have attempted to suppress the Ph-positive cells or the Ph-related Bcr-Abl kinase activity to alter the natural course of the disease. Allogeneic stem cell transplantation (SCT) produced long-term event-free survival rates of 30% to 80% and 1-year mortality rates of 5% to 50% (depending on patient age, donor status, degree of matching, and other factors).3-6 Interferon-alpha (IFN-α) induced cytogenetic response rates of 30% to 70%, which were complete (0% Ph-positive cells) in 5% to 30%.7-13 Median survival durations with IFN-α were 5 to 7 years (9 years in patients with “good-risk” disease). Achievement of a complete cytogenetic response was associated with 10-year survival rates of 70% to 85%.14-16
Imatinib mesylate (STI571; Gleevec) is a selective inhibitor of the Bcr-Abl tyrosine kinase activity.17,18 Imatinib inhibits the binding site for adenosine triphosphate (ATP) to the Abl kinase, thus blocking the phosphorylation of tyrosine on substrate protein. Imatinib has shown encouraging results in all 3 CML phases—chronic, accelerated, and blastic.19-28 In chronic-phase CML after IFN-α failure, imatinib mesylate induced major cytogenetic response rates of 60%, complete cytogenetic response rates of 40%, and 18-month estimated freedom-from-progression and survival rates of 89% and 95%, respectively.21
Several issues remain unanswered. These include (1) whether the complete cytogenetic responses will be sustained or even improve with long-term follow-up; (2) whether the low annual mortality rates will persist in later years; (3) whether a prognostic model, based on pretreatment characteristics and early response to therapy, could accurately predict patients who ultimately achieve complete cytogenetic response; (4) the incidences of major molecular (BCR-ABL/ABL ratios less than 0.05%) and complete molecular response (undetectable BCR-ABL), which predict for excellent long-term prognosis; and (5) the significance of chromosomal abnormalities other than Ph. These questions are the subject of this analysis.
Patients and methods
Study group
Patients were treated on 2 Novartis-sponsored studies: the phase 2 pivotal study of chronic-phase CML after IFN-α failure (Study 110, 149 patients)21 and the expanded-access study (Study 113, 112 patients). Entry criteria were similar for both trials.21,24 Eligible patients were 18 years or older and had adequate performance status (a score of 0 to 2 on an Eastern Cooperative Oncology Group scale), renal function (creatinine level of less than twice the upper limit of normal), hepatic function (bilirubin, aspartate aminotransferase, and alanine aminotransferase levels of less than twice the upper limit of normal), and cardiac function (those with New York Heart Association grades 3 to 4 cardiac disease were excluded). Hydroxyurea within the previous 7 days, IFN-α or cytarabine within the previous 14 days, or investigational agents within 28 days of starting imatinib mesylate were not allowed. Women of childbearing age were required to have a negative pregnancy test, and all patients were required to use barrier contraception during therapy. Patients provided written informed consent. Studies were approved by the Internal Review Board of the institution and conducted in accordance with the Declaration of Helsinki.
Patients were required to have Ph-positive chronic-phase CML after IFN-α failure because of hematologic or cytogenetic resistance or relapse or because of IFN-α toxicities.
Chronic-phase CML was defined as the presence, in the peripheral blood, of blasts less than 15%, basophils less than 20%, blasts together with promyelocytes less than 30%, and platelets more than 100 × 109/L. Failure of hematologic response of IFN-α was defined as hematologic resistance (failure to achieve complete hematologic response [CHR] after at least 6 months of IFN-α) or relapse (disease recurrence after CHR). Cytogenetic failure of IFN-α was defined as resistance (Ph-positive metaphases at least 65% after more than 12 months of IFN-α) or relapse (> 30% increase in Ph-positive metaphases on 2 occasions, or ≥ 65% increase in Ph-positive metaphases on 1 occasion). Intolerance of IFN-α therapy was defined as grade 3 or 4 nonhematologic toxicity (according to the National Cancer Institute Common Toxicity Criteria). Eligibility criteria and definitions had been previously detailed.21,24
Treatment and dose modifications
Imatinib mesylate 400 mg was given orally daily. Criteria for dose modifications were previously detailed.21,24 Escalation to 400 mg twice daily was considered for patients who did not obtain a CHR after at least 3 months of therapy, those who relapsed after achieving CHR, those who did not achieve a major cytogenetic response (Ph-positive metaphases less than 35%) after 12 months of therapy, or those with cytogenetic relapse (increase of Ph-positive metaphases by at least 30%).
Dose reductions of imatinib mesylate for nonhematologic or hematologic toxic effects were as follows. For grade 2 persistent nonhematologic toxic effects, imatinib mesylate was interrupted until recovery to grade 1 or lower and resumed at the original dose level. If grade 2 toxicity recurred, treatment was interrupted again until recovery and resumed at a daily dose of 300 mg. For grade 3 or 4 nonhematologic toxicity, therapy was interrupted until recovery to grade 1 or lower and resumed at a daily dose of 300 mg. For a grade 3 or 4 hematologic effect (granulocyte count of less than 109/L or a platelet count of less than 50 × 109/L), therapy was interrupted until the effect resolved to grade 1 or lower. If the toxic effect resolved within 2 weeks, treatment was resumed at the original dose of 400 mg daily. If grade 3 or 4 toxicities reappeared or persisted for more than 2 weeks, therapy was interrupted until the effect resolved to grade 1 or lower and resumed at a reduced daily dose of 300 mg. Patients developing anemia received transfusions of blood or blood products at the discretion of the investigator or 40 000 units of erythropoietin subcutaneously once a week until the hemoglobin level increased to at least 120 g/L (12 g/dL).
Complete blood counts and serum chemistry evaluations were performed weekly during the first 12 weeks, every other week for the next 3 months, and every 6 weeks thereafter. Marrow studies, including morphologic and cytogenetic or interphase fluorescent in situ hybridization (iFISH) analysis, were performed every 3 months in the first year and then every 6 months.
Response criteria and statistical considerations
Response criteria have been described previously.7,8 A CHR was defined as a white blood cell count of less than 10 × 109/L, a platelet count of less than 450 × 109/L, no immature cells (blasts, promyelocytes, myelocytes) in the peripheral blood, and disappearance of all signs and symptoms related to leukemia (including palpable splenomegaly) lasting for at least 4 weeks. A CHR was further categorized by the best cytogenetic response: complete, Ph-positive 0%; partial, Ph-positive 1% to 34%; minor, Ph-positive 35% to 90%. Major cytogenetic response included complete plus partial cytogenetic responses (ie, Ph-positive less than 35%). Cytogenetic response was judged by standard cytogenetic analysis, not by iFISH. Time to disease transformation was calculated from the time the treatment began until the first reported appearance of acceleratedor blastic-phase disease or death.29,30 Progression-free survival was similar to time to transformation (ie, time to acceleratedor blastic-phase or death) but also included patients who discontinued therapy because of an unsatisfactory response. Survival was calculated from the time the treatment began until death from any cause or last follow-up.
Univariate and multivariate analyses were performed to identify potential prognostic factors associated with complete cytogenetic response and survival. The χ2 test was used to identify prognostic factors, which were then included as variables in a multivariate regression model for response. Factors retaining significance in the multivariate model were interpreted as being independently predictive of complete cytogenetic response. Multivariate analysis of survival used the Cox proportional hazard model.31-33
Cytogenetic and polymerase chain reaction (PCR) analysis
Cytogenetic analysis was performed by the G-banding technique. Marrow specimens were examined on direct short-term (24-hour) cultures; at least 20 metaphases were analyzed. BCR-ABL transcripts were detected by real-time quantitative reverse transcriptase–polymerase chain reaction (Q-PCR) analysis on peripheral blood and/or marrow aspirate, and negative results (ie, undetectable transcript) were confirmed by nested PCR as previously reported.34 The lower limit of detection in the nested PCR assay is approximately 1 BCR-ABL–expressing cell per 100 000. Normalization ratios of BCR-ABL transcript level were done by comparison with the level of ABL transcript. For the purpose of this analysis, a complete molecular response was defined as negative (undetectable) BCR-ABL transcript levels. A major molecular response was defined as BCR-ABL/ABL ratios less than 0.05%. This cutoff was based on previous studies identifying BCR-ABL/ABL ratios of 0.045% to 0.1% to be prognostically important.35,36 A BCR-ABL/ABL ratio of 0.05% is roughly equivalent to a 3-log reduction in BCR-ABL transcript levels compared with that seen in samples before imatinib mesylate treatment.37
Historical control group
To evaluate the efficacy of imatinib mesylate versus “standard therapy” of chronic-phase CML after IFN-α failure, we compared the results of the study group with a historical control group of patients in early chronicphase CML treated on institutional protocols from 1982 until 1995 and whose disease subsequently progressed while on IFN-α therapy and in chronic phase. A total of 251 patients constituted the historical control group. Their median age was 45 years (range, 19 to 77 years). Their characteristics are shown in Table 1. Subsequent therapies were hydroxyurea and/or busulfan, 110 patients; homoharringtonine, 42 patients; chemotherapy combination regimens, 27 patients; allogeneic SCT, 38 patients; and other, 34 patients. The median time from diagnosis to therapy refers to the time the first therapy after IFN-α failure (specified in “Study group”) was implemented.
Results
Patients
A total of 261 patients with chronic-phase CML after IFN-α failure treated with imatinib mesylate were analyzed. Their characteristics are summarized in Table 1. Of the 256 patients evaluable for response (Table 2), 146 patients had active disease and 110 patients were in CHR. The median duration of chronic phase (CML diagnosis to start of imatinib mesylate therapy) was 33 months (range, 1 to 221 months). The median follow-up time was 45 months; for Study 110 it was 48 months (range, 27 to 50 months), and for Study 113 it was 35 months (range, 6 to 43 months). At the time of last follow-up, 230 of the 261 patients (88%) were alive, 196 (75%) were in chronic phase on imatinib mesylate therapy, and 31 patients had died.
Response
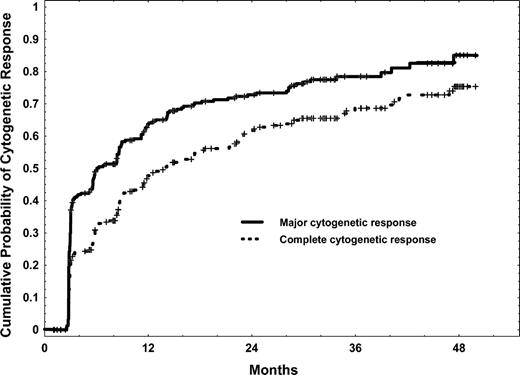
Responses to imatinib mesylate are shown in Table 2. The CHR rate was 96% in patients with active disease. The cytogenetic response rate was 79%, the major cytogenetic response rate was 73%, and the complete cytogenetic response was 63%. The cumulative rates of major and complete cytogenetic response are shown in Figure 1. There was a continued increase in the cumulative major and complete cytogenetic response rates with therapy, even after 2 years. Associations between pretreatment characteristics and later probability of achievement of complete cytogenetic response are shown in Table 3. The associations between response at 3, 6, 12, and 15 to 18 months and later complete cytogenetic response are shown in Table 4. Of note, among patients who had achieved only a minor cytogenetic response after 3 to 12 months of therapy, the subsequent complete cytogenetic response rates were 62% to 76%. That rate decreased to only 10% in patients who still had a minor cytogenetic response after 12 months of therapy. However, patients in partial cytogenetic response after 12 months of therapy still had a 73% incidence of later achieving a complete cytogenetic response. Also, as shown in Table 4, patients without a cytogenetic response at 3 to 6 months, and those with only a minor cytogenetic response (Ph-positive, 35% to 90%) at 12 to 18 months, had worse estimated 4-year survival rates (70% to 79%) compared with those with better cytogenetic response (estimated 4-year survival rates, 88% to 100%).
In the multivariate analysis, summarized in Table 5, the percentage of Ph-positive cells before therapy and platelet counts were independently associated with achievement of complete cytogenetic response. Patients could be assigned to 1 of 3 prognostic risk groups: good (no adverse factors), intermediate (1 adverse factor), or poor (2 adverse factors), with predicted complete cytogenetic response rates of 94%, 58%, and 39%, respectively. These risk groups were also predictive for differences in 4-year progression-free status (P < .0001) and survival (P = .007) (Table 6).
Seventy-four of the 261 patients had dose escalations of imatinib mesylate as per protocol guidelines for unsatisfactory response, after a median duration of 18.5 months of imatinib mesylate (range, 3 to 47 months). Dose escalation was to 800 mg daily (n = 60) or to 600 mg daily (n = 14). Among 23 patients dose-escalated for cytogenetic relapse, 16 (70%) improved their cytogenetic response to complete (n = 9), partial (n = 6), or minor (n = 1); these improved cytogenetic responses were sustained in 7 patients (30%). Among 28 patients treated for cytogenetic refractoriness, 16 (57%) improved their cytogenetic response to complete (n = 6), partial (n = 6), or minor (n = 4); these improved cytogenetic responses were sustained in 7 patients (25%). Among 23 patients treated for hematologic resistance, 9 (39%) achieved a complete hematologic response, 2 of them with cytogenetic response (1 partial, 1 minor), and 5 patients (22%) had a partial hematologic response. Five patients (22%) are in sustained complete hematologic response.
Time to transformation, progression-free survival, and survival
Of the 261 patients treated, 196 (75%) remain alive in chronic phase and on imatinib mesylate therapy (31 have died; 34 are alive in different CML phases on other therapies as discussed later). The 65 patients who stopped therapy did so because of the following: development of cytogenetic resistance (n = 5) or hematologic resistance (n = 21); transformation to accelerated or blastic phase (n = 16); toxic side effects (n = 7: 3 liver function abnormalities, 2 renal failure, 2 other); or other causes (n = 16: 5 intercurrent illness, 2 pregnancies, 1 sudden unrelated death, 1 death outside the institution, 7 noncompliance). Five patients (median age, 44 years; range, 33 to 69 years) received allogeneic SCT after imatinib mesylate failure. Three patients in chronic phase underwent matched unrelated donor (MUD) SCT (n = 2) and matched related SCT (n = 1); 2 are alive at 2 or more (related) and 25 or more months (MUD). One patient in accelerated phase is alive 38 or more months after MUD SCT; 1 patient who underwent transplantation in second chronic phase from a related donor died 7 months after SCT. Thirty-one patients have died, 27 after discontinuation of imatinib mesylate and 4 during imatinib mesylate therapy. Eighteen patients have died after disease progression to accelerated (n = 5) or blastic phase (n = 13); 13 patients died of other causes (2 from infections, 3 from cardiac events, 2 after allogeneic SCT, and 6 from other causes).
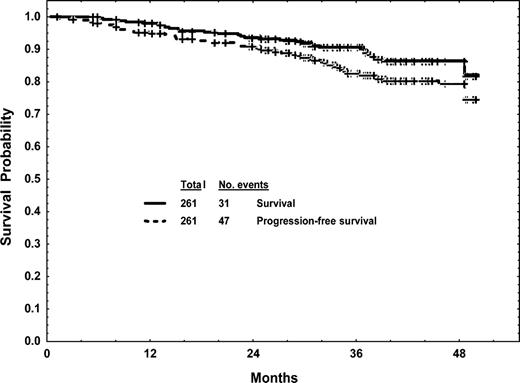
The estimated 4-year freedom-from-transformation rate was 80%; the estimated 4-year progression-free survival rate was 80%; and the estimated 4-year survival rate was 86% (Figure 2). Factors associated with survival in univariate analysis are shown in Table 3. A multivariate analysis for survival selected the following to be independent adverse factors (P < .05): marrow blasts at least 5%, splenomegaly, and prior hematologic resistance to IFN-α.
A landmark analysis was conducted on patients who were alive and still on imatinib mesylate at 3 months and at 6 months in relation to survival. The results are shown in Figures 3 and 4.
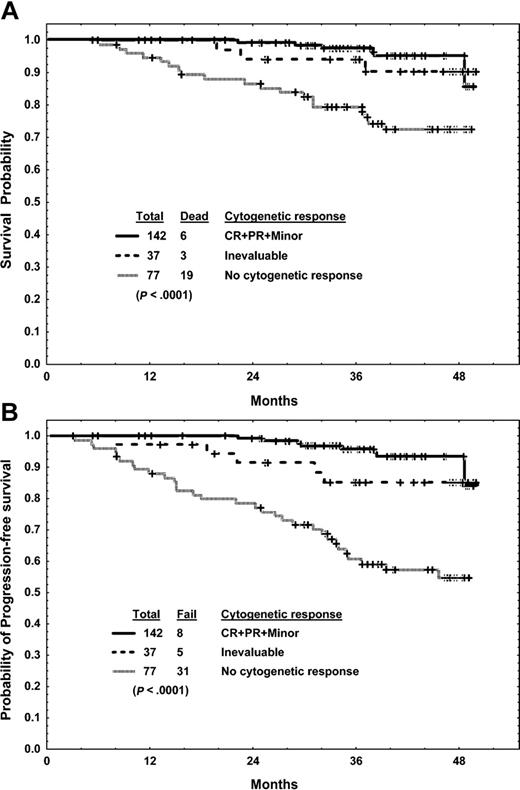
Three-month landmark analysis. Analysis of the association of cytogenetic response with survival (A) and progression-free survival (B).
Three-month landmark analysis. Analysis of the association of cytogenetic response with survival (A) and progression-free survival (B).
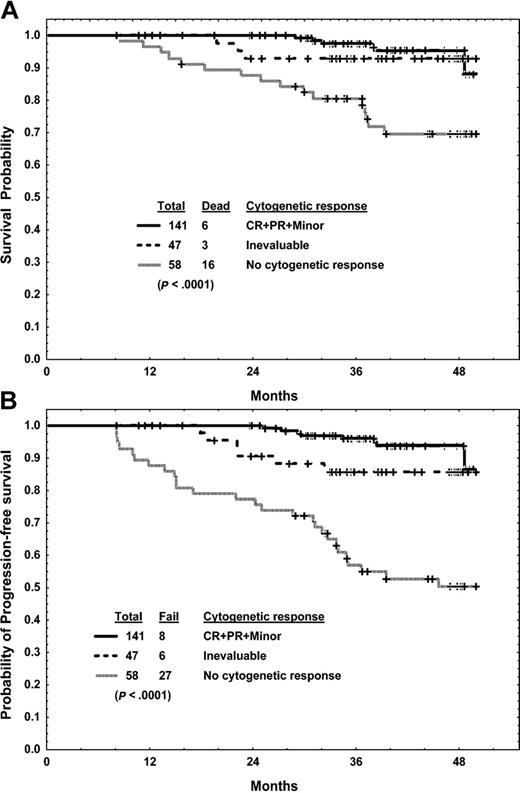
Six-month landmark analysis. Analysis of the association of cytogenetic response with survival (A) and progression-free survival (B).
Six-month landmark analysis. Analysis of the association of cytogenetic response with survival (A) and progression-free survival (B).
Among the 256 patients who were alive and taking imatinib mesylate at 3 months, 219 had evaluable cytogenetic studies and 142 (55% of total) had achieved a cytogenetic response (complete, partial, or minor). The estimated 4-year survival rates were 95% for those who had achieved a cytogenetic response and 72% for those who did not (P < .0001) (Figure 3A). Similarly, the 4-year progression-free survival rate was better for patients who had achieved a cytogenetic response than for those who had not (93% versus 55%; P < .0001) (Figure 3B).
Among the 246 patients who were alive and taking imatinib mesylate at 6 months, 199 had evaluable cytogenetic studies and 112 (46% of total) had achieved a major cytogenetic response. The estimated 4-year survival (95% versus 78%; P = .001) and the progression-free survival rate (93% versus 65%; P < .0001) were better in patients who had achieved a major cytogenetic response. Achieving any cytogenetic response at 6 months was associated with a 4-year survival rate of 96% versus 70% (P < .0001; Figure 4A) and a 4-year progression-free survival rate of 94% versus 51% (P < .0001; Figure 4B).
A multivariate analysis including cytogenetic response at 3 months (any versus other) and at 6 months (any versus other) identified early response at 3 months (P = .03) and at 6 months (P = .01) to remain as important prognostic factors for survival. Any cytogenetic response at 6 months was more predictive of prognosis than a major cytogenetic response (P = .06 by multivariate analysis). The multivariate analysis including cytogenetic response at 3 months identified the following to be independent poor prognostic factors for survival: hematologic resistance to IFN-α (P = .01), splenomegaly (P = .03), and lack of cytogenetic response at 3 months into therapy (P = .01). Patients could be divided into good- (no unfavorable factors), intermediate- (1 unfavorable factor), and poor- (2 or 3 unfavorable factors) risk groups with estimated 4-year progression-free survival rates of 94%, 72%, and 30% (P < .0001), respectively, and survival rates of 96%, 86%, and 49% (P < .0001), respectively (Table 7).
Durability of cytogenetic responses
Among the 162 patients who achieved a complete cytogenetic response, 4 did not have follow-up at our institution; 147 of the remaining 158 patients (93%) continue to have a durable complete (n = 122) or partial (n = 25) cytogenetic response at the time of last follow-up, while 11 (7%) showed loss of major cytogenetic response (defined as an increase of Ph-positive cells by 30% or more, documented on 2 occasions, or an increase to 65% or more, documented once).
Among 26 patients with partial cytogenetic response, 10 (38%) lost the response (as defined in the above paragraph). Among the 14 patients who achieved a minor cytogenetic response, 9 (64%) have so far shown loss of cytogenetic response (defined as an increase of Ph-positive cells to more than 90%).
Comparison of survival with imatinib mesylate to previous historical experience
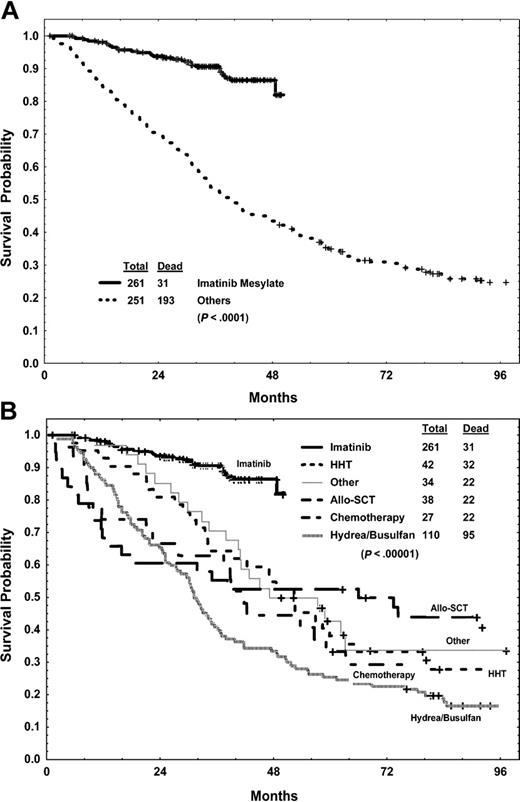
Whether imatinib mesylate improves survival in patients with chronic-phase CML after IFN-α failure is unlikely to be the subject of a randomized comparative study given the significant activity of imatinib mesylate. We thus compared this study group with 251 patients with early chronic CML treated with IFN-α–based regimens on our institutional protocols from 1982 until 1995, and who later had CML failure on IFN-α therapy while still in chronic phase, and who were given other therapies. Compared with the historical control group of 251 patients (Table 1), the 261 patients in the imatinib mesylate study group were older (P < .0001) and had longer CML duration (P = .002), but they had lower incidences of hematologic resistance or relapse (P < .0001), splenomegaly (P = .0003), anemia (P = .003), and leukocytosis (P < .0001) as well as a lower percent of Ph-positive cells before therapy (P < .0001). Survival was significantly better among the imatinib mesylate group than among the “other-treatments” group (Figure 5); the estimated 4-year survival rates were 86% versus 43% (P < .0001). A multivariate analysis conducted in the total group identified longer duration of chronic phase, peripheral blasts, prior IFN-α response, and thrombocytosis to be independent significant predictors for survival (all with P values less than .05). Type of therapy (imatinib mesylate versus other) remained the most significant independent prognostic factor for survival, favoring imatinib mesylate over other regimens (hazard ratio, 0.19; P < .0001).
Survival of 261 patients with CML after failed response to IFN-α treated with imatinib mesylate and 251 historical patients with similar failed response to IFN-α treated with other regimens. Overall (A) and by therapy (B).
Survival of 261 patients with CML after failed response to IFN-α treated with imatinib mesylate and 251 historical patients with similar failed response to IFN-α treated with other regimens. Overall (A) and by therapy (B).
An important question is whether survival with imatinib mesylate is similar or better than with other therapies within defined cytogenetic response categories. We thus analyzed patients within cytogenetic response categories achieved at 3 and 6 months for subsequent survival on imatinib mesylate versus other treatments. As shown in Table 8, survival was significantly better with imatinib mesylate within each response category.
Molecular responses
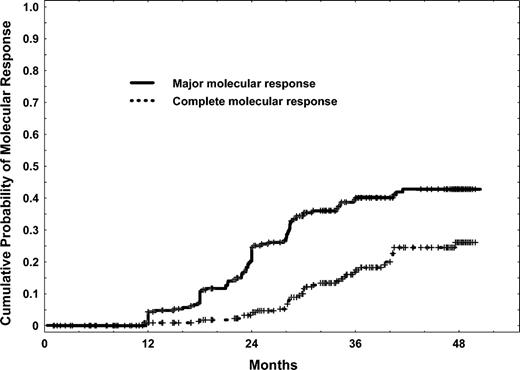
Among the 167 patients who started in, or later achieved a complete cytogenetic response, 152 had quantitative PCR analysis: 82 patients (49% of complete cytogenetic responses; 31% of the total study group) achieved a major molecular response (BCR-ABL/ABL less than 0.05%), and 40 (24% of complete cytogenetic responses; 15% of the study group) had a complete molecular response (undetectable BCR-ABL transcripts). Twenty-four of the 40 patients remain in complete molecular response and 9 in major molecular response; the other 5 patients eventually showed a Q-PCR value of at least 0.05%, but none had a cytogenetic relapse. The cumulative incidences of major and complete molecular responses for the total study group are shown in Figure 6. The estimated 4-year cumulative rates of major and complete molecular responses were 43% and 26%, respectively, in the total study group.
Cumulative rates of major (BCR-ABL/ABL less than 0.05%) and complete (undetectable BCR-ABL transcripts) molecular responses.
Cumulative rates of major (BCR-ABL/ABL less than 0.05%) and complete (undetectable BCR-ABL transcripts) molecular responses.
Side effects
No new significant side effects were noted with the longer-term follow-up. Side effects of imatinib mesylate were similar to those previously reported. The severe (grade 3-4) toxic effects are shown in Table 9. Only 7 patients had to discontinue imatinib mesylate therapy for unacceptable severe toxic effects; no patients died in chronic phase from imatinib mesylate-related complications.
Cytogenetic clonal evolution in Ph-positive cells and chromosomal abnormalities in Ph-negative cells
Among 24 patients with pretreatment cytogenetic clonal evolution, 10 (42%) achieved a complete cytogenetic response: 5 remain in complete cytogenetic response; 3 remain in partial cytogenetic response; 2 have died after discontinuation of imatinib mesylate. The other 14 patients are in chronic phase on therapy with minor (n = 1) or no cytogenetic response on imatinib mesylate (n = 4), in chronic phase on other therapy (n = 3), or have transformed (n = 1) or died (n = 5). The 4-year survival rate of the 24 patients with pretreatment clonal evolution was 69% versus 88% for others (P = .007). However, this variable was not selected by multivariate analysis, probably because of the small number of patients.
On therapy, 42 patients (16%) developed clonal evolution in Ph-positive cells, which was transient (n = 26) or persistent (n = 8); the other 8 patients had clonal evolution in the last analysis and were not yet evaluated on follow-up. Twenty patients continue to be in major (n = 15) or minor cytogenetic response (n = 5). Fourteen patients have developed resistant or transformed CML (n = 2) or have died (n = 12).
Forty-one patients have developed chromosomal abnormalities in Ph-negative cells (trisomy 8, 8; chromosome 5 or 7 abnormalities, 11; 20q, 4; others, 18). These were transient in 29 (71%). Thirty-six of the 41 patients remain in cytogenetic response on imatinib mesylate (31 major, 5 minor); only 1 patient has developed accelerated-phase CML. Twenty of the 41 patients (49%) have had grade 3-4 myelosuppression; no patient has developed myelodysplastic syndrome or acute myeloid leukemia.
Discussion
The longer-term follow-up results of patients with chronic-phase CML after IFN-α failure treated with imatinib mesylate remain very encouraging. With a median overall follow-up of 45 months, 73% of patients achieved a major cytogenetic response, which was complete in 63%. The estimated 4-year progression-free survival and survival rates were 80% and 86%, respectively. The major and complete molecular response rates were 31% and 15%, respectively. Thus, there is continued increase in the rates of major and complete cytogenetic responses and of major and complete molecular responses. The annual mortality rates were 2%, 6% and 9% in the first 3 years.
Lower complete cytogenetic response rates to imatinib mesylate were observed in patients with hematologic resistance to IFN-α, with more aggressive forms of disease (high platelet counts, high blast and basophil levels, cytogenetic clonal evolution), with high tumor burden (splenomegaly, active disease, no Ph suppression), and in those treated after 12 months from diagnosis. These results suggest that frontline therapy with imatinib mesylate, either alone or in combinations, may further improve the complete cytogenetic and molecular response rates. This was indeed the case in the International Randomized study of Interferon-α versus STI571 (IRIS) study and in other frontline studies with standard (400 mg daily) or high-dose imatinib mesylate (800 mg daily). With standard-dose imatinib mesylate, the complete cytogenetic response rates range from 65% to 75% and the major and complete molecular response rates are 35% to 40% and 6% to 10%, respectively.27,28,39 With high-dose imatinib mesylate in newly diagnosed CML, the estimated 3-year rate of complete cytogenetic response was 90% and of major and complete molecular responses was 70% and above 40%, respectively.39 These surrogate end points have been previously associated with high long-term survival rates.37 Complete cytogenetic responses after IFN-α therapy have been associated with 10-year survival rates as high as 80%.14-16
In a multivariate analysis, we identified the following to be independent adverse prognostic factors for achieving a complete cytogenetic response: high platelet counts and percentage Ph positivity more than 90% prior to starting imatinib mesylate. Patients could be thus divided into good-, intermediate-, and poor-risk groups with estimated complete cytogenetic response rates of 94%, 58%, and 39%, respectively (P < .0001). Several studies are now incorporating additional therapies to imatinib mesylate (eg, vaccines, homoharringtonine, others) for “unsatisfactory” response to imatinib mesylate.40-43 In our study, patients who had a minor cytogenetic response at 3 to 12 months still had a probability of later complete cytogenetic response of 62% to 76% with continuation of single-agent imatinib mesylate. Those with major cytogenetic response at 12 to 18 months had a later rate of complete cytogenetic response of about 70% (Table 4). These findings should be considered in the efficacy analysis of therapies added to imatinib mesylate.
In other cancers, the introduction of a highly effective therapy (eg, cisplatin regimens in testicular cancer, cladribine in hairy cell leukemia) has reduced or eliminated the significance of previously established prognostic patientand tumor-associated characteristics and emphasized the tumor response to the new therapy as a major prognostic determinant. This is also evident with imatinib mesylate therapy in CML, where several established poor prognostic factors (eg, older age or cytogenetic clonal evolution)44-46 as well as established prognostic models (eg, Sokal and Hasford models)27 are becoming less relevant. In contrast, response to imatinib mesylate therapy (hematologic, cytogenetic, molecular) at 3, 6, or 12 months is emerging as a major determinant of prognosis and a guide to treatment changes (eg, consideration of allogeneic SCT). O'Dwyer et al reported major differences in treatment outcomes on imatinib therapy by the degree of cytogenetic response at 3 or 6 months.46 Similarly, the IRIS trial demonstrated significant differences in progression-free survival by the 12-month response to imatinib mesylate: patients not achieving a complete cytogenetic response by then had an 80% progression-free survival rate; those achieving a 3-log or greater reduction of molecular burden had a 100% progression-free survival rate.27 In our analysis, similar findings were noted (Figures 3 and 4; Table 4). Patients who had not achieved any cytogenetic response at 3 to 6 months, or major cytogenetic response at 12 to 18 months, had worse estimated 4-year survival rates (70% to 79% versus 88% to 100%; Table 4; Figures 3A and 4A) and progression-free survival rates (Figures 3B and 4B) than those with better cytogenetic responses at these time points.
A multivariate analysis for survival, including early response to imatinib mesylate, selected the following to be independent poor prognostic factors for survival: prior hematologic resistance to IFN-α (P = .01), splenomegaly (P = .03), and lack of cytogenetic response after 3 months of imatinib mesylate therapy (P = .01). The estimated 4-year survival rates for good-, intermediate-, and poor-risk patients were 96%, 86%, and 49%, respectively (P < .0001). Thus, patients with 2 or 3 such factors (12% of the study group; eg, splenomegaly and IFN-α resistance before imatinib mesylate, or either factor before treatment, and Ph positivity more than 90% after 3 months of imatinib mesylate therapy) could be offered allogeneic SCT or alternative therapies, including imatinib mesylate combinations. A previous study by Marin et al suggested that patients with CML on imatinib mesylate could be divided into different risk groups for transformation or survival based on their 3-month cytogenetic response (Ph more than 65% versus less than 65%) and neutropenia less than 109/L on days 45 to 90 of therapy.38 In our study group, this risk model did not have a good fit (Table 7), perhaps because of different study group characteristics.
In previous studies, the estimated annual mortality rates were 10% to 15% for patients in chronic-phase CML after IFN-α failure. With the positive experience with imatinib mesylate, randomized comparative trials of imatinib mesylate versus “standard of care” for CML after IFN-α failure are not feasible for ethical and practical reasons. We thus compared the results of imatinib mesylate after IFN-α failure with our historical experience in CML after IFN-α treated with other approaches. Keeping in mind several significant differences in the characteristics of the study and historical control groups (which is difficult to avoid in such a retrospective analysis, and which we hope is partly accounted for in the multivariate analysis), our findings suggest the superiority of imatinib mesylate over other regimens, with estimated 4-year survival rates of 86% versus 43% (Figure 5; P < .0001). This was confirmed by multivariate analysis (hazard ratio, 0.19; P < .0001). Another important question is whether survival of patients who achieve a particular response is similar or better with imatinib mesylate versus other therapies. As shown in Table 8, survival with imatinib mesylate was better regardless of the cytogenetic response achieved with imatinib mesylate versus with other modalities. This is in contrast to a British study that suggested a survival advantage for imatinib mesylate only in imatinib mesylate cytogenetic responders, while nonresponders appeared to have a worse outcome than historical controls.47 This impacts on future salvage trials as to whether they should always include imatinib mesylate combinations (even after imatinib mesylate failure) or whether new single agents should be evaluated in short “therapeutic windows” trials (eg, 6 months) before reinstituting imatinib mesylate-based therapies.
Dose escalation of imatinib mesylate has been associated with better results in both preclinical and clinical studies,17,18,22,39,48,49 perhaps because higher doses of imatinib mesylate may overcome some of the known mechanisms of imatinib mesylate resistance.50-52 We had previously reported that some patients with chronic-phase CML after IFN-α failure who became resistant to imatinib mesylate 400 mg daily regained their response after the dose of imatinib mesylate was increased.49 In this update, we confirm our previous results in 74 patients who had imatinib mesylate dose escalation. In patients with cytogenetic refractoriness or relapse, the improved cytogenetic response rates were 57% to 70% and were sustained in 25% to 30%. With hematologic resistance, the CHR rate was 39% and was sustained in 22%.
Complete and major (Q-PCR reduction to less than 0.05% or by 3 logs or more) molecular responses have been associated with excellent prognosis with both IFN-α and imatinib mesylate therapy.14-16,26,27,35-37,39 While the incidence of major molecular response is reasonable (about 30% to 70%),37,39 complete molecular responses have been reported at a lower frequency of 7% to 10% with standard-dose imatinib mesylate in newly diagnosed CML with short-term follow-ups.37 Several updates are now showing a higher frequency of complete molecular responses. Rosti et al reported 22 complete molecular responses among 191 patients (12%) treated in late chronic-phase CML after IFN-α failure (median follow-up time, 26 months);53 the incidence in our study was 26% (median follow-up time, 45 months).
In summary, the results of imatinib mesylate therapy in chronic-phase CML after IFN-α failure continue to improve in terms of the rates and durability of complete cytogenetic response, major and complete molecular response, and in terms of improved survival compared with previous accepted treatments for CML.
Prepublished online as Blood First Edition Paper, June 15, 2004; DOI 10.1182/blood-2004-02-0711.
Supported by The Betty Foster Leukemia Research Fund and a grant from Novartis Pharmaceuticals, East Hanover, NJ.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.