Abstract
The influence of graft composition on clinical outcomes after reduced-intensity conditioning is not well-characterized. In this report we prospectively enumerated CD34+, CD3+, CD4+, and CD8+ cell doses in granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cell (G-PBMC) allografts in 63 patients who received transplants following non-myeloablative conditioning with total body irradiation 200 cGy plus fludarabine as treatment for malignant diseases. Donors were HLA-identical siblings (n = 38) or HLA-matched unrelated individuals (n = 25). By univariate analyses G-PBMC CD8+ T-cell dose in at least the 50th percentile favorably correlated with full donor blood T-cell chimerism (P = .03), freedom from progression (P = .001), and overall survival (P = .01). No G-PBMC cell dose influenced grade II to IV acute or extensive chronic graftversus-host disease. In multivariate analysis only G-PBMC CD8+ T-cell dose (P = .003; RR = 0.2, 95% CI = 0.1-0.6) was associated with improved freedom from progression. Infusion of low G-PBMC CD8+ T-cell dose for reduced-intensity allografting may adversely affect T-cell engraftment and survival outcome.
Introduction
It is recognized that graft composition is an important determinant of a variety of clinical outcomes following myeloablative allogeneic hematopoietic cell transplantation (AHCT). For example, numerous studies have reported that following myeloablative HLA-identical sibling transplantation, higher doses of CD34+ cells are associated with a more rapid platelet and neutrophil recovery irrespective of whether bone marrow or granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cell (G-PBMC) products are used. Additionally, the number of donor CD3 T cells is critical in determining the risk of developing acute graft-versus-host disease (aGVHD) and graft failure following HLA-identical sibling transplantation. Recipients of T-cell–depleted products by CD34+ selection, typically less than 105 CD3+ cells/kg recipient weight, benefit from a negligible risk for aGVHD but are subject to a high rate of graft failure.1 Yet recipients of bone marrow compared with G-PBMC have virtually identical risks of aGVHD, probably reflecting that further increases beyond a critical threshold of CD3+ T cells does not result in an incrementally higher incidence of acute GVHD.2,3 Higher CD34+ cell doses have been shown to correlate with more frequent chronic GVHD (cGVHD) following myeloablative AHCT, although several recent studies dispute this prognostic connection.4-7
The influence of graft composition on clinical outcomes after reduced-intensity conditioning is less well-characterized. With emphasis shifting from myeloablative chemoradiation toward alloimmune effects of the graft, an understanding of the relationships, if any, between donor graft cell subpopulation contents and various outcomes may be particularly important. To gain insight in these regards, the present study evaluated the influence of donor G-PBMC CD34+, CD3+, CD4+, and CD8+ cell doses on engraftment kinetics, acute and chronic GVHD, and survival in 63 patients treated with reduced-intensity AHCT using low-dose total body irradiation (TBI) and fludarabine for malignant diseases.
Patients, materials, and methods
Patients and eligibility
Between July 21, 1998, and August 22, 2002, a total of 107 consecutive patients were treated with nonmyeloablative AHCT using G-PBMC allografts at the Stanford Hospital and Clinics. Excluded from this analysis were 29 patients with a diagnosis of multiple myeloma participating in a tandem transplant protocol in which myeloablative autologous HCT preceded nonmyeloablative G-PBMC allografting8 ; 11 patients conditioned with a regimen of total lymphoid irradiation and antithymocyte globulin; 1 patient conditioned with dose-intensified TBI plus fludarabine as salvage therapy for a prior failed allogeneic transplant; and 3 patients for whom G-PBMC T-cell dose information was not available. After these exclusions, evaluable were 63 patients with various malignant disorders uniformly conditioned with low-dose TBI 200 cGy with or without fludarabine followed by transplantation of unmanipulated G-PBMC allografts. According to the Declaration of Helsinki, all patients provided informed consent for treatment under protocols reviewed and approved by the Stanford University Administrative Panel on Human Subjects in Medical Research. As previously described, inclusion in these protocols required ineligibility for conventional myeloablative allografting due to advanced age or comorbid conditions.9,10
Patient characteristics for the 63 patients comprising the study group are summarized in Table 1. The median age was 53 years. The contraindications for myeloablative allografting among the 15 patients younger than 50 years of age were poorly controlled medical conditions (n = 8), unresolved fungal infection (n = 3), prior autologous transplantation (n = 2), and prior nephrectomy (n = 2). Almost all patients were treated for a hematologic malignancy. Exceptions were 4 patients with renal cell carcinoma and a single patient with paroxysmal nocturnal hemoglobinuria. Donors were unrelated in 25 patients (40%) and patient siblings in the remainder. Unrelated donors were significantly younger than sibling donors (P < .005) but did not differ with regard to gender nor donor-patient gender mismatch in either direction (data not shown).
For this analysis, a disease status category was assigned to each patient according to the underlying malignancy and remission status at transplantation. Patients considered to have advanced disease were those with acute leukemia beyond first remission, refractory anemia, or chronic myelomonocytic leukemia with excess blasts, chronic myeloid leukemia beyond first chronic phase, agnogenic myeloid metaplasia with excess blasts, or a lymphoproliferative disorder either beyond second remission or refractory. Twenty-four (38%) were categorized as having advanced disease.
HLA typing and donor matching
Confirmatory HLA typing and matching at HLA-A, HLA-B, and HLA-DRB1 was performed for all patients by the Stanford Medical School Histocompatibility Laboratory prior to transplantation. Patients with sibling donors were serologically matched for HLA-A and HLA-B and low resolution matched by DNA typing for HLA-DRB1.
Patients with unrelated donors were serologically matched for HLA-A and HLA-B (n = 13) until June 3, 2001, after which HLA-A and HLA-B alleles were matched at high type resolution (n = 12) by DNA sequencing. HLA-DRB1 was matched at the allele level for all patients. Low-resolution molecular HLA-C and HLA-DQB1 allele group typing also was performed and, although not used as criteria for unrelated donor matching throughout the study period, revealed 3 patients with a single HLA-C mismatch and an additional 2 patients with a single HLA-DQB1 mismatch.
Nonmyeloablative regimen and postgraft immunosuppression
On day 0, all patients received irradiation at a dose of 200 cGy delivered in a single fraction using 15 MV photons to the total body. As previously reported and due to evolving treatment protocols during the study period, the first 8 patients received TBI 200 cGy alone, whereas the remainder (n = 55) additionally received intravenous fludarabine 30 mg/m2/d given on days -4, -3, and -2 before G-PBMC transplantation.11 Immunosuppressive therapy with oral cyclosporine (6.25 mg/kg twice per day) was started on day -3, and mycophenolate mofetil (15 mg/kg twice per day) was started 12 hours after the infusion of donor G-PBMC. Subsequent tapering of immunosuppressive therapy was partially individualized depending on the particular treatment protocol, development of acute or chronic GVHD, and persistent or recurring malignancy, but was generally completed within 6 months of transplantation.12 Routine supportive care was implemented for all patients, including antibiotic prophylaxis for bacterial, Pneumocystis, and herpes zoster/simplex infections; prophylaxis and surveillance for cytomegalovirus (CMV) infection and reactivation; and blood component transfusions.
Collection of GCSF-mobilized peripheral blood
Sibling donors received a 5-day course of subcutaneous G-CSF 16 μg/kg/d. Starting on the fourth day of G-CSF, daily 12- to 16-liter blood volume leukapheresis was performed until a target CD34+ cell dose of at least 5 × 106 cells per kilogram of patient body weight was collected. Unrelated donors were given subcutaneous G-CSF 10 μg/kg/d for 5 days. After the last dose of G-CSF, two 12-liter blood volume leukapheresis collections were obtained on consecutive days as tolerated according to unrelated donor registry protocol. Collections were pooled without backup and given to patients as a single infusion on day 0 after low-dose TBI.
Flow cytometry evaluation of graft cell subset
CD34+, CD3+, CD4+, and CD8+ cell enumerations were prospectively performed on freshly harvested G-PBMC products on either a FACScan or FACSCalibur cytometry system (Becton Dickinson, San Jose, CA) by the Flow Cytometry Laboratory of the Stanford Hospital and Clinics. CD34+ cell count determination was performed according to standard International Society of Hematotherapy and Graft Engineering (ISHAGE) guidelines using a commercial anti-CD34+ monoclonal antibody (581, Beckman Coulter, Fullerton, CA).13
CD3+, CD4+, and CD8+ cells were enumerated using a protocol adapted from guidelines released by the Centers for Disease Control and Prevention.14 G-PBMC cells were labeled with a 4-color cocktail of monoclonal antibodies directed against the CD3ϵ (SK7, Becton Dickinson), CD4 (SK3, Becton Dickinson), CD8α (SK1, Becton Dickinson), and CD45 (2D1, Becton Dickinson) cell surface antigens. Absolute CD3+ T-cell numbers were calculated as the percentage of CD3+ cells, within the low side scatter and CD45+ lymphocyte gate, multiplied by an absolute total nucleated cell number adjusted for viability by 7-amino actinomycin D dye exclusion. CD4+ and CD8+ T-cell numbers were similarly calculated, respectively, based on the CD3+CD4+ and CD3+CD8+ double-staining cell population.
Donor chimerism determination
Donor hematopoietic chimerism was serially determined after transplantation by DNAgenotyping of simple sequence length polymorphic markers encoding short tandem repeats (STRs) by the Stanford Medical School Histocompatibility Laboratory, as previously described in detail.15 In brief, DNA purified from immunomagnetically separated patient peripheral blood CD3+ T cells (SPV-T3b, Dynal Biotech, Oslo, Norway) and CD15+ granulocytes (BRA-4F1, Dynal Biotech) were PCR amplified for informative DNAmarkers and size fractionated on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). STR donor chimerism was evaluated on day 28, day 56, day 100, day 180, and day 365, then annually thereafter.
Study definitions
The main study outcomes were donor engraftment, GVHD, and survival. Full donor engraftment in patient peripheral blood T cells and granulocytes was defined as the attainment of at least 95% donor chimerism any time after transplantation. Primary graft rejection was defined as the failure to exceed 5% donor chimerism or a decline to below that level in the instance of secondary graft rejection. GVHD was diagnosed on the basis of clinical evidence with histologic confirmation where possible and graded according to standard criteria.16 All patients were considered evaluable for acute GVHD. Patients surviving beyond day +100 after transplantation were additionally considered evaluable for chronic GVHD. Overall survival (OS) was defined as days from transplantation to death from any cause. Freedom from progression (FFP) was defined as days from transplantation to disease progression, censoring deaths from nonprogressive causes. Patients were additionally censored at date of last clinic follow-up but not at date of a second transplantation, which occurred for 5 patients because of disease relapse and 2 patients because of graft rejection.
Statistical methods
Statistical analysis, as well as prospective collection of transplant patient characteristic and outcome data, was performed by the Stanford University Bone Marrow Transplantation Biostatistics and Data Management Core. Cox proportional hazards univariate and multivariate regression models were used to identify predictors of engraftment, GVHD, and survival.17 The day 100 after transplantation was applied as a landmark point for analysis of chronic GVHD as a correlate of study endpoints. The Kaplan-Meier method was used to obtain actuarial estimates of patient events and differences between patient groups were assessed with the log-rank test.18 Differences in the medians and proportions of patient characteristics were determined, respectively, with the Wilcoxon rank sum test and the Fisher exact test.19 The correlation between G-PBMC graft cell doses was estimated with the Spearman rank correlation coefficient.
Results
G-PBMC graft composition
The absolute CD34+, CD3+, CD4+, CD8+, and total nucleated cell numbers from each G-PBMC product infused after patient conditioning are shown in Table 2 and Table S1, available on the Blood website (see the Supplemental Tables link at the top of the online article). The CD3+, CD4+, and total nucleated cell doses were significantly lower in G-PBMC allografts harvested from unrelated donors. In contrast, the absolute CD34+ and CD8+ cell numbers did not differ between the 2 donor groups. For all patients there was near absolute concordance between the CD3+ and CD4+ cell doses (P < .001) as well as between the CD3+ and CD8+ cell doses (P < .001). In contrast, there was considerable discordance between CD4+ and CD8+ T-cell doses, which enabled independent evaluations of these subsets on clinical outcomes. The median ratio between the CD4+ and CD8+ cell doses was 2.0, with a broad range extending from 0.7 to 6.1. The CD34+ cell dose exceeded 1.9 × 106/kg of patient weight in all cases, although 8 patients received G-PBMC products containing less than the targeted CD34+ cell dose of 5 × 106/kg.
Univariate analysis of G-PBMC cell dose for transplant outcome
Results from univariate analyses of CD34+, CD3+, CD4+, and CD8+ cell doses as correlates for donor engraftment, GVHD, and survival are summarized in Table 3. G-PBMC cell doses were analyzed as categorical variables comparing the lower versus greater than or equal to the upper 50th percentile of each cell dose range. Due to sample size considerations, patients with unrelated as opposed to sibling donors were not evaluated separately.
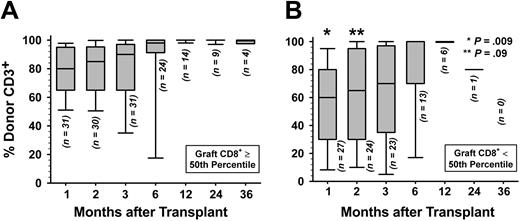
Donor engraftment. Complete STR chimerism data were not available for 4 patients who died because of nonrelapse causes at days 18, 22, 34, and 60 as shown in Table S2. A fifth patient, the first one to receive a transplant in this patient series, had only nonquantitative chimerism assessment performed prior to expiring at day 51. The remaining 58 patients were evaluated for attainment of full donor T-cell and granulocyte chimerism after transplantation. Only G-PBMC CD8+ T-cell doses at least at the 50th percentile predicted greater likelihood for attainment of full donor chimerism in patient peripheral blood T cells (P = .03). Figure 1 shows that percent donor T cells differed significantly at 1 month (P = .009) but not at 2 months (P = .09) and at subsequent posttransplant timepoints, when comparing patients grafted with G-PBMCs containing at least versus less than the median of the CD8+ T-cell dose. Among patients evaluable for engraftment, secondary graft rejection in the absence of disease relapse occurred with 3 patients who received a CD8+ T-cell dose in the lower 50th percentile and in 1 patient given a CD8+ T-cell dose greater than that.
Donor T-cell engraftment. Shown are percent donor CD3+ cells in peripheral blood by STR chimerism analysis for patients infused with G-PBMCs containing (A) at least the 50th percentile or (B) less than the 50th percentile of the CD8+ T-cell dose. For each time point and with the number of evaluable patients as indicated, horizontal bar within each box-and-whisker plot gives the median of percent donor T cells; top and bottom of box gives 25th and 75th percentiles, respectively; and top and bottom of the whisker gives the 5th and 95th percentiles, respectively. P values derived from Wilcoxon rank sum test comparing percent donor CD3+ cells of patients receiving at least versus less than 50th percentile CD8+ T-cell dose at each time point.
Donor T-cell engraftment. Shown are percent donor CD3+ cells in peripheral blood by STR chimerism analysis for patients infused with G-PBMCs containing (A) at least the 50th percentile or (B) less than the 50th percentile of the CD8+ T-cell dose. For each time point and with the number of evaluable patients as indicated, horizontal bar within each box-and-whisker plot gives the median of percent donor T cells; top and bottom of box gives 25th and 75th percentiles, respectively; and top and bottom of the whisker gives the 5th and 95th percentiles, respectively. P values derived from Wilcoxon rank sum test comparing percent donor CD3+ cells of patients receiving at least versus less than 50th percentile CD8+ T-cell dose at each time point.
Graft-versus-host disease. The actuarial incidence of grade II to IV aGVHD was 16.3% (95% CI: 6.9-25.8). Among the 55 patients evaluable for extensive cGVHD, the actuarial incidence was 42.9% (95% CI: 27.8-57.9). There was no significant correlation between any of the G-PBMC cell doses with the development of either grade II to IV acute GHVD or extensive chronic GVHD.
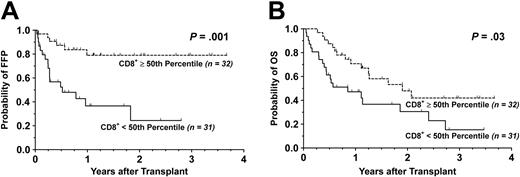
Survival. With a median follow-up time of 13 months (range, 5-45 months) for surviving patients, the FFP was 55.2% (95% CI: 39.7-70.7) and the OS was 29.0% (95% CI: 14.0-44.0) at 3 years. By univariate analysis, grafting with a G-PBMC product containing greater than the median of the CD8+ T-cell doses strongly correlated with an improved FFP (P = .001). Likewise, the presence of a donor CD8+ T-cell dose above the 50th percentile favorably influenced OS (P = .03). The Kaplan-Meier survival curves for FFP and OS stratified by the median of CD8+ T-cell doses are shown in Figure 2. Among patients receiving donor CD8+ T-cell doses below the median, the most common cause of death was relapse (n = 14), as shown in Table 4 and Table S3. Causes of death in patients given at least the 50th percentile of CD8+ T-cell doses were more evenly distributed among nonrelapse causes including acute and chronic GVHD (n = 7) and toxicity (n = 5). There was a trend toward significance for higher CD3+ T-cell dose and better OS (P = .06), in part due a tight correlation with the CD8+ T-cell dose. In contrast, a complete lack of significant association between OS and CD4+ T-cell dose was observed. Despite a strong correlation among CD3+ and CD4+ T-cell doses, considerable discordance between CD4+ and CD8+ T-cell doses allowed the influence, or lack thereof, by CD4+ T-cell doses on clinical outcomes to be independent of CD8+ T-cell doses.
Kaplan-Meier survival curves stratified by G-PBMC CD8 T-cell dose. Shown are cumulative proportion surviving with respect to (A) FFP and (B) OS. Dotted line indicates G-PBMCs containing at least the 50th percentile; solid line, those containing less than the 50th percentile.
Kaplan-Meier survival curves stratified by G-PBMC CD8 T-cell dose. Shown are cumulative proportion surviving with respect to (A) FFP and (B) OS. Dotted line indicates G-PBMCs containing at least the 50th percentile; solid line, those containing less than the 50th percentile.
Univariate analysis of patient characteristics for transplant outcome
Confounding patient characteristics were considered as prognostic indicators, with results summarized in Table 5. The presence versus absence of any donor patient gender mismatch (P = .81) and whether the donor was a sibling as compared to an unrelated individual (P = .48) had no influence on the attainment of full donor peripheral blood T-cell chimerism in patients.
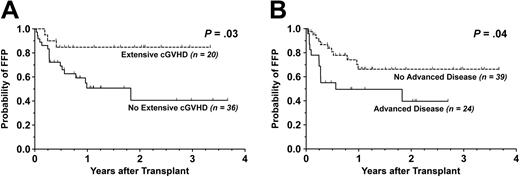
Pertaining to survival, the development of extensive cGVHD was found to be significantly beneficial for FFP (P = .03) but without any effect on OS (P = .30). Similarly, a diagnosis of advanced disease status correlated with worse FFP (P = .04) without significantly influencing OS (P = .06). The Kaplan-Meier curves for FFP stratified by extensive cGVHD and advanced disease are shown in Figure 3. Patients who developed grade II to IV aGVHD had similar FFP (P = .96) and OS (P = .38) compared to those who did not.
Kaplan-Meier survival curves stratified by extensive chronic GVHD. For patients evaluable for chronic graft-versus-host disease (n = 55), shown are cumulative proportion surviving with respect to (A) FFP and (B) OS. Dotted line indicates patients with extensive cGVHD; solid line, those without extensive cGVHD.
Kaplan-Meier survival curves stratified by extensive chronic GVHD. For patients evaluable for chronic graft-versus-host disease (n = 55), shown are cumulative proportion surviving with respect to (A) FFP and (B) OS. Dotted line indicates patients with extensive cGVHD; solid line, those without extensive cGVHD.
Multivariate analysis for progression-free survival
Donor CD8+ T-cell dose, advanced disease status, and extensive cGVHD were then evaluated as covariates in multivariate regression for FFP, with results shown in Table 6. Only CD8+ T-cell dose in at least the 50th percentile (P = .003) remained independently prognostic of better FFP. The risk ratio for disease progression was 0.2 (95% CI: 0.1-0.6) for patients grafted with a G-PBMC product containing at least the 50th percentile of the CD8+ T-cell dose.
Discussion
This study analyzed the influence of G-PBMC graft CD34+, CD3+, CD4+, and CD8+ cell doses on clinical outcomes following reduced-intensity AHCT. Patients were uniformly conditioned with low-dose TBI, with or without fludarabine, followed by postgraft immunosuppression with cyclosporine and mycophenolate mofetil. The infusion of higher donor CD8+ T-cell dose in G-PBMC products was found to favorably influence the attainment in patients of full donor T-cell chimerism, correlated with a lower incidence of disease progression, predicted improved OS, and did not adversely affect the occurrence of acute or chronic GVHD. CD34+ cell dose was not significantly associated with any outcome evaluated in this study. With regard to FFP, G-PBMC CD8+ T-cell dose was prognostic independent of extensive chronic GVHD and advanced disease status.
The association of higher G-PBMC CD8+ cells and establishment of full donor T-cell chimerism in the present study concurs with several lines of evidence suggesting that donor CD8+ cells exert beneficial effects on engraftment after AHCT. In a myeloablative murine model of transplantation, both CD4+ and CD8+ cells from the donor can cause GVHD, but donor CD8+ cells were more than 5-fold more effective than CD4+ cells for preventing graft rejection mediated by host immune cells remaining after myleoablation.20,21 Similarly, other experimental models have shown that donor-derived bone marrow–resident CD8+ cells, but not CD4+ cells, directly facilitate engraftment of purified hematopoietic stem cells.22,23 In humans, CD4+ cell removal from donor bone marrow does not hinder engraftment, whereas a correlation between graft failure and depletion of donor CD8+ cells has been reported following myeloablative AHCT.24,25 Conversely, the addition of fixed CD8+ cell numbers to T-cell–depleted bone marrow can effectively prevent graft loss.26
The impact of CD8+ cell dose on engraftment also has been observed following reduced-intensity transplantation. In a murine model, CD8+ cells were shown to potently enhance engraftment of T-cell–depleted bone marrow transplanted into sublethally irradiated recipients.27 In humans, 2 studies are of particular interest because, like the present study, patients from both groups were treated with the TBI 200 cGy plus fludarabine nonmyeloablative regimen. Baron et al28,29 directly evaluated CD8+-depleted G-PBMC and reported a 21% estimated incidence of graft rejection, compared with 10% in an unmanipulated G-PBMC comparison group but, due to small patient number and short follow-up time, were not able to firmly conclude whether CD8+ depletion adversely affected engraftment. Reporting on a larger series, Panse and colleagues30 evaluated 125 patients who received G-PBMC grafts from HLA-identical related donors. In contrast to the present study, a correlation between CD8+ cell dose and day 28 T-cell chimerism was not identified. One possibility for these incongruent findings may have been the inclusion of 29 myeloma patients treated with the planned “tandem auto/allo” HCT program. Similar patients were excluded from analysis in the present study to avoid skewing in transplant characteristics and outcome of the study population. As reported by Maloney et al,8 these transplant outcomes included uniformly and rapidly attained full donor T-cell chimerism, possibly due to the immediately preceding myeloablative therapy.31
In addition to the beneficial effect on T-cell engraftment, another finding in this report was significant associations between high G-PBMC CD8+ cell doses and improved survival endpoints. While correlations with both FFP and OS suggest a link between survival and protection from disease progression, the biologic basis for the improved clinical outcomes is not clear. It may be that infused donor CD8+ T cells directly contribute to specific antitumor responses. Alternatively, donor CD8+ T cells may promote tumor eradication indirectly through facilitation of donor engraftment. In experimental models, conversion to full donor T-cell chimerism facilitated by CD8+ cells most effectively induces tumor clearance.27,32 In keeping with these observations, mixed rather than full donor T-cell chimerism following reduced-intensity AHCT in patients has been associated with more disease progression.33,34
A number of prognostic factors previously identified as significant for survival outcomes after reduced-intensity AHCT have been described. Giralt et al35 reported that patients with good or intermediate disease risk categories had better disease-free survival with AHCT following a melphalan and purine analog–containing preparative regimen. Similarly, evaluation of outcome for 188 patients with lymphoma treated with reduced-intensity AHCT significantly characterized chemotherapy-resistant and aggressive disease as major predictors for disease progression, progression-free survival, and OS.36 Lee et al37 studied outcome among patients with multiple myeloma undergoing reduced-intensity AHCT and found chronic GVHD to be favorably predictive of an improved event-free survival. While both advanced disease status and extensive chronic GVHD correlated with improved FFP in the present study, neither were significantly associated with OS, and in multivariate analyses only G-PBMC CD8+ cell dose was prognostic for FFP.
Studies evaluating the impact of CD34+ cell dose on clinical outcomes following reduced-intensity AHCT have yielded conflicting results. One group reported more frequent cGVHD with higher CD34+ cell dose after reduced-intensity transplantation, while others found no such association.10,30,38-40 Additionally, high CD34+ cell dose was identified as an adverse predictive factor influencing survival in some instances but, again, not in others.30,41-43 We report no significant association between CD34+ cell dose with clinical outcome. In the current report, few patients actually received grafts containing low CD34+ cell numbers because of institutional practices targeting CD34+ cell dose of more than 5 × 106/kg recipient weight at G-PBMC collection. Possibly, our comparison groups were above thresholds whereby clinical differences could be detected.
The development of acute and chronic GVHD was not influenced by any of the G-PMBC cell doses evaluated in the present study, including CD3+ or CD8+ T-cell content. It has been suggested that increases beyond a critical threshold of T-cell dose necessary to induce GVHD, perhaps 1 × 105/kg to 1 × 106/kg of patient body weight, does not necessarily result in an incrementally higher incidence of GVHD.44,45 Support for this contention is exemplified in studies comparing incidences of acute and chronic GVHD following transplantation of bone marrow versus G-PBMC. Despite a 1 log10 lower T-cell content in bone marrow grafts, the use of G-PBMC does not increase the risk of acute GVHD, and associations with increased chronic GVHD have been inconsistent.6,46
Subtle differences in G-PBMC products harvested from sibling versus unrelated donors were reported in the present study. The higher total nucleated and CD3+ cell doses collected from sibling donors may be due to a greater cumulative leukapheresis volume and granulocyte colony-stimulating factor dosing.47 Older patient age in sibling donors additionally contributed to a higher CD4+ cell dose in G-PBMC products donated by these individuals.48 Importantly, the CD34+ and CD8+ cell doses collected from sibling and unrelated donors did not differ and hence did not confound interpretation of findings in this study.
In the present study a higher G-PBMC CD8+ cell dose was found to favorably influence T-cell engraftment, FFP, and OS following reduced-intensity AHCT. The 1.0 × 108/kg median value was used to characterize high versus low infused CD8+ T-cell doses. Due to limited sample size, however, it is difficult to suggest a threshold of CD8+ T-cell dose above which beneficial effects might be anticipated. Given the heterogeneity in patient diagnoses, it is not possible to identify whether the CD8+ T-cell dose effect is specific for any disease in particular. Patients were selected for analysis based on uniform treatment with low-dose TBI-based regimens with or without fludarabine. Hence, there is additional uncertainty as to whether this effect extends to other reducedintensity AHCT regimens. Last, the present study evaluated T-cell subsets only with CD4+ and CD8+ markers. It is possible that other G-PBMC immune cell populations may prove to be prognostically informative if studied. G-PBMC CD8+ T-cell doses cannot be altered without graft manipulation. Still, it is not difficult to speculate, for instance, about adding minimal leukapheresis CD8+ target doses to ones already existing for CD34+ cell dose. Additional insight with respect to the above considerations may be important for optimizing outcome with reduced-intensity AHCT and should be addressed in future studies.
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-04-1473.
Supported in part by grant 2PO1CA049605 from the National Institutes of Health.
The online version of the article includes a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are indebted to the nursing staff, case managers, patient counselors, house staff, and other Stanford Hospital and Clinics personnel for their outstanding care of these patients.