Meis1 is a homeodomain transcription factor coexpressed with Hoxa9 in most human acute myeloid leukemias (AMLs). In mouse models of leukemia produced by Hoxa9, Meis1 accelerates leukemogenesis. Because Hoxa9 immortalizes myeloid progenitors in the absence of Meis1 expression, the contribution of Meis1 toward leukemia remains unclear. Here, we describe a cultured progenitor model in which Meis1 programs leukemogenicity. Progenitors immortalized by Hoxa9 in culture are myeloid-lineage restricted and only infrequently caused leukemia after more than 250 days. Coexpressed Meis1 programmed rapid AML-initiating character, maintained multipotent progenitor potential, and induced expression of genes associated with short-term hematopoietic stem cells (HSCs), such as FLT3 and CD34, whose expression also characterizes the leukemia-initiating stem cells of human AML. Meis1 leukemogenesis functions required binding to Pbx, binding to DNA, and a conserved function of its C-terminal tail. We hypothesize that Meis1 is required for the homing and survival of leukemic progenitors within their hematopoietic niches, functions mediated by HSC-specific genes such as CD34 and Fms-like tyrosine kinase 3 (FLT3), respectively. This is the first example of a transcription factor oncoprotein (Meis1) that establishes expression of a tyrosine kinase oncoprotein (FLT3), and explains their coexpression in human leukemia. This cultured progenitor model will be useful to define the genetic basis of leukemogenesis involving Hoxa9 and Meis1.
Introduction
Hoxa9 and Meis1 are homeodomain-containing transcription factors that control progenitor abundance in hematopoiesis and in leukemogenesis. In hematopoiesis, expression of Hoxa9 and Meis1 is high in CD34+, Sca-1+, lineage-negative (Lin-) bone marrow populations that are enriched in hematopoietic stem cells (HSCs) and in lineage-committed progenitors (LCPs), and is down-regulated coincident with transition to the CD34- stage of early progenitor differentiation.1,2 Progenitor-specific expression of Hoxa9 and Meis1 is linked to positive maintenance of progenitor numbers. Mice harboring null mutation in Hoxa9 have significant reductions in myeloid and pre–B cell progenitors, and they contain 5- to 10-fold reductions in their numbers of marrow HSCs.2 Elimination of Meis1 also results in strong reductions in numbers of myeloid, lymphoid, and multipotent progenitors.3 By contrast, retroviral expression of Hoxa9 produces a 10-fold increase in the number of long-term repopulating HSCs (LT-HSCs), which is followed, inevitably, by development of myeloid leukemia.4
In murine and human leukemias, Hoxa9 and Meis1 function as dominant cooperating oncoproteins, yet the mechanism of cooperativity is unclear. In spontaneous acute myeloid leukemia (AML) arising in BXH2 mice, intracisternal particles activate transcription of Meis1 in combination with either Hoxa7 or Hoxa9.5,6 The cooperating nature of Meis1 and Hoxa9 in leukemia was verified by using retrovirus to express these genes in primary bone marrow and then using the infected marrow cells to reconstitute the hematopoietic compartment of lethally irradiated mice. In this model, expression of Meis1 alone does not cause leukemia, expression of Hoxa9 eventually causes AML only after a long latency (20 weeks), and coexpression of Hoxa9 plus Meis1 yields rapid AML that emerges within 8 weeks.7 While Hoxa9 and Meis1 can interact physically,8 it is clear that each impacts at least partly distinct genetic pathways that yield different phenotypes. Retroviral expression of Hoxa9 alone blocks differentiation of granulocyte-macrophage colony-stimulating factor (GM-CSF)–dependent myeloid progenitors in the absence of coexpressed Meis1, Meis2, or Meis3; however, such progenitors are incapable of initiating leukemia in sublethally irradiated mice, suggesting that a second, Meis-dependent genetic response is required to program leukemia-initiating properties.9 Expression of Meis1 in GM-CSF–dependent Hoxa9-immortalized myeloid progenitors induces responsiveness to stem cell factor (SCF) and suppresses neutrophil differentiation by G-CSF, yet these progenitor as well cannot initiate leukemia.10 Thus, while Hoxa9 and Meis1 can deregulate facets of progenitor proliferation and differentiation, there is no cultured cell line model that correlates these properties with accelerated leukemogenesis, and the genetic mechanism through which Meis1 contributes to leukemia remains unclear and difficult to approach.
Persistent expression of Hoxa9 and Meis1 is also proposed to mediate human leukemogenesis. Hoxa9 and Meis1 are expressed in more than 80% of human AML,11,12 as well as in human acute lymphoid leukemias (ALLs) containing MLL chromosomal translocations. Evidence that Meis1 cooperates with Hoxa9 in establishing genetic changes underlying human leukemia was suggested by studies of the translocation protein mixed-lineage leukemia–eleven-nineteen leukemia (MLL-ENL). MLL normally controls Hox gene expression.13 Chromosomal translocations produce MLL fusion proteins that have acquired persistent activation function, such as MLL-ENL. Both the human leukemias that express MLL-ENL and the cultured myeloid progenitors that are immortalized by retroviral MLL-ENL14 exhibit robust expression of Hoxa9 and Meis1, suggesting that MLL-ENL might immortalize progenitors through the functions of subordinate Hoxa9 genes.13 Consistent with this hypothesis, myeloid progenitors from Hoxa9-/- knockout mice cannot be immortalized by MLL-ENL, demonstrating that the endogenous Hoxa9 gene mediates an important aspect of progenitor immortalization by MLL-ENL.15 The human AML oncoprotein Nup98-Hoxa9 encodes a transcriptionally activated version of Hoxa9 and also sustains expression of endogenous Hoxa9 and Meis1 genes in cultured progenitors16 that evoke rapid AML in mice.17 Thus, human leukemias contain oncogenes that maintain the expression, and hence the transforming functions, of Hoxa9 and Meis1.
FLT3 ligand (FL) is a transmembrane protein produced by stromal cells of the hemopoietic compartment,18 and plays an important role in the expansion of both normal and leukemic progenitors by activating the tyrosine protein kinase receptor FLT3. FLT3 expression is low in murine LT-HSCs19 but present in human LT-HSCs,20 and is up-regulated in short-term HSCs (ST-HSCs) and LCPs and down-regulated in the early stages of LCP differentiation.19 Knockout mice reveal the importance of the FL-FLT3 interaction in vivo: Flt3-/- stem cells are deficient in lymphoid and myeloid reconstituting potential21 and FL-/- mice display 10-fold reductions in common lymphoid progenitors22 and reduced numbers of myeloid progenitors.23 Virtually all human AMLs also express FLT3,24 suggesting that leukemia cells use stromal cytokines to maintain viability and proliferation in the marrow. In AML, FLT3 signaling is activated by receptor mutations, such as FLT3–internal tandem duplication (ITD), in approximately one-third of AML, or by autocrine FL production.25,26 The mechanism, by which FLT3 transcription is up-regulated transiently in normal hematopoiesis while persistently in leukemia, is undefined.
Here we develop a novel cultured cell model that demonstrates how Meis1 cooperates with Hoxa9 in programming cell phenotype and acute leukemic characteristics. Hoxa9-immortalized progenitors that were myeloid restricted exhibited a low degree of leukemic potential—only 1 of 9 mice acquired AML within 280 days of injection of progenitors immortalized by Hoxa9 alone. Meis1 cooperated with Hoxa9 to immortalize an earlier progenitor resembling the ST-HSCs that induced rapid AML in mice within 3 months, and these AML progenitors expressed FLT3, CD34, and Itgb7. Transcription of these same genes was induced by Meis1 in Hoxa9-immortalized progenitors. We propose that Meis1 endows progenitors with leukemia stem-cell character by activating transcription of genes that permit homing to, and proliferate within, hemopoietic microenvironments that produce FL.
Finally, we develop an assay in which cultivation in FL selects for leukemia-initiating FLT3+ progenitors coexpressing exogenous Hoxa9 and Meis1, and use this assay to define the essential biochemical domains of Meis1. In order to cooperate with Hoxa9 to maintain FLT3 expression and initiate AML, Meis1 required binding to Pbx and binding to DNA, as well as a unique function of sequences C-terminal to its homeodomain (HD). Together, these results suggest that FLT3 transcription, expansion of normal ST-HSCs, and expansion of leukemia-initiating progenitors in vivo is regulated by Meis1-Pbx complexes.
Materials and methods
Infection and culture of primary hematopoietic cells
Helper-free retrovirus was prepared by cotransfection of retroviral expression vectors, together with an ecotropic murine leukemia virus (MuLV) packaging vector, into 293T cells, using calcium phosphate coprecipitation. Retroviral titers were calculated by enumerating G418-resistant colonies. Sca+Lin- progenitors were enriched by negative selection against lineage markers (StemCell Technologies, Vancouver, BC, Canada) and cultured in OptiMEM base medium (contains 15% fetal bovine serum, penicillin, streptomycin, and glutamine) that was supplemented with 10 ng/mL of stem cell factor (SCF; from Chinese hamster ovary [CHO] producer cells), 5 ng/mL interleukin-3 (IL-3; Peprotech, Rocky Hill, NJ), and 5 ng/mL interleukin-6 (Peprotech) for 2 days. To examine the impact of Hoxa9 and Meis1 on the proliferation and differentiation of expanded progenitors, 1 × 105 progenitors were subjected to 1 round of spinoculation with 1 mL retrovirus supernatant (provirus titration: 105-106/mL) in a total volume of 1.25 mL, and plated in OptiMEM base medium (Gibco BRL, Carlsbad, CA) containing 10 ng/mL SCF (SCF media) plus 1 mg/mL G418 (for murine stem-cell virus (MSCV) Neo constructs) or 1 μg/mL puromycin (for MSCVPuro). After drug selection, expression of Hoxa9 and Meis1 was confirmed by Western blotting. Equivalent numbers of G418-resistant progenitors were plated in fresh SCF media for proliferation assays. In some cases, IL-7 (10 ng/mL from J558 cell supernatant) was also added to primary infections. To assess proliferation in FL, 2 × 105 progenitors were spin-infected and plated in OptiMEM base medium containing 5 ng/mL FL (Sigma, St Louis, MO) and 50μM of β-mercaptoethanol. One-half the media was changed every 3 days. Murine recombinant SCF and G-CSF were purchased from Peprotech. Flow cytometry was performed as described.27 Cultures are initially polyclonal because infection of as few as 2 × 102 progenitors yielded outgrowths. Within 8 weeks of infection, all cultures evaluated (3 of 3) were monoclonal, likely due to the expansion of clones expressing optimal levels of Hoxa9 and Meis1, or of other cellular cofactors.
Leukemogenesis assay
A total of 1 to approximately 2 × 106 retrovirally transduced progenitors were introduced by tail-vein injection into sublethally irradiated (450 rads) 8- to 12-week-old female Balb/c mice as previously described.28
Meis1-induced FL responsiveness assay
SCF-dependent Hoxa9-immortalized progenitors were infected with provirus made from Mscv-puromycin vector encoding Flag-Meis1 or from empty vector as a negative control. After 2 days of selection with 2 ng/mL puromycin, 2 × 105 puromycin-resistant progenitors were transferred to media with 5 ng/mL FL as sole cytokine for 48 hours, and the number of live and dead cells were enumerated. Frequency of FL responsiveness is defined as the percentage of live cells in this 48 hour population. Acquisition of Wright-Giemsa stain image was achieved by using Olympus microscope digital camera system (model BX341, Olympus, Melville, NY) at total magnification 500 × (objective lens, 50 ×). The images were modified and assembled with Adobe Photoshop (San Jose, CA).
Electrophoretic mobility shift assay
Radiolabeled oligonucleotides containing a Pbx1-Meis consensus element (PCE: tcacggTGATTGACAGgcgactgctcgg; binding site capitalized) were subject to electrophoretic mobility shift assay (EMSA) as described,9 using 5 μL of Pbx1a and Meis1a produced by coupled transcription/translation.
Microarray analysis
Hybridization and quantitation of array signals were performed by the UCSD gene chip core laboratory (UCSD), using the GeneChip Scanner 3000, enabled for high-resolution scanning, coupled with GeneChip operating software (GCOS). Array data were normalized to internal controls, and the overall chip signal intensity was normalized to the mean signal intensity across the group of 6 chips (3 probed with RNA from Hoxa9-immortalized progenitors and 3 with RNA from Hoxa9/Meis1 progenitors). Data were analyzed using “perfect match minus mismatch” algorithms, and signature genes identified based on a minimum 4-fold difference in signal intensity between the averages of each group of 3 samples. Normalization and processing of GCOS data were performed using dCHIP software.
Semiquantitative polymerase chain reaction
cDNA from each sample of total RNA was synthesized using the Superscript reverse transcription kit (Invitrogen, Carlsbad, CA). Polymerase chain reaction (PCR) amplification of genes of interests was performed using primers for products spanning over at least one exon/intron boundary, using appropriate PCR conditions and serial 1:10 dilutions of input cDNA template. cDNA was normalized by PCR amplification of housekeeping gene G3PDH.
Description of plasmid construction and protocols followed for Northern and Southern blotting, immunoblotting, and immunoprecipitation are outlined in Supplemental Document S1, available on the Blood website; see the supplemental Document link at the top of the online article.
Results
Meis1 cooperates with Hoxa9 to immortalize a distinct target cell that exhibits rapid cell division and an early progenitor phenotype
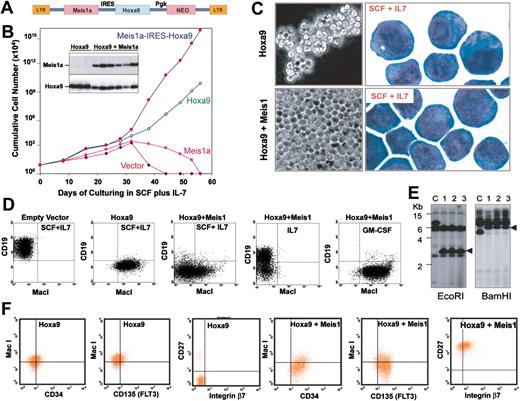
To derive immortalized progenitors expressing Hoxa9 only or coexpressing Hoxa9 plus Meis1, Sca1+/Lin--enriched marrow cells were infected with Hoxa9 or Meis1–internal ribosome entry site (IRES)–Hoxa9 retrovirus (Figure 1A) and cultured in medium containing SCF plus IL-7. The efficiency of retroviral transduction was approximately 25%, based on the efficiency of drug-resistant proliferation. Progenitors infected with the “empty” retroviral vector or with virus encoding Meis1 alone failed to become immortalized. The characteristics of SCF-dependent progenitors immortalized by Hoxa9 alone differed from those immortalized by Hoxa9 plus Meis1. Progenitors coexpressing Hoxa9 and Meis1 grew more rapidly (Figure 1B) and formed single layers across the bottom of culture flasks, while those immortalized by Hoxa9 grew as suspended cell clumps and exhibited fewer azurophilic granules (Figure 1C), suggesting that progenitors coexpressing Hoxa9 and Meis1 represented an earlier stage in differentiation. Fluorescence-activated cell-sorting (FACS) analysis confirmed that the 2 progenitor populations were different: progenitors immortalized by Hoxa9 alone were B220-, CD19-, with varying degrees MacI+ (high in Figure 1D, low in Figure 1F), whereas the vast majority of those immortalized by Hoxa9 plus Meis1 were Lin- and coexisted with a small population of MacIvariable progenitors (Figure 1D). Retroviral integration analysis demonstrated that by 8 weeks after infection, these cultures became clonal (Figure 1E), suggesting that a dominant clone emerged rapidly. As consistent high levels of Hoxa9 and Meis1 are always observed in these immortalized cultures, it is likely that different retroviral integrations in clones express variable levels of Hoxa9 and Meis1, and that clones with the highest levels emerge. Identical populations were immortalized using medium containing SCF alone. Collectively, these results demonstrated that Hoxa9-immortalized progenitors are committed to myeloid differentiation, but that progenitors immortalized by coexpressed Meis1 plus Hoxa9 are not yet lineage-committed.
Meis1 coexpression with Hoxa9 immortalizes a distinct hematopoietic progenitor that exhibits multilineage differentiation potential and rapid monolayer proliferation. (A) MSCV retroviral vector used to achieve expression of Hoxa9 and Meis1a. LTR indicates long-term repeat, and NEO, neomycin-resistant gene. (B) Proliferation rates of Lin- marrow progenitors cultured in SCF and infected with empty MSCV, or MSCV encoding Hoxa9, Meis1, or Meis1 plus Hoxa9. The inserted blot shows expression of Meis1 (top) and Hoxa9 (bottom) by Western blot. (C) Morphology under phase-contrast microscopy (left) or following staining with Wright-Giemsa (right) of progenitors immortalized by Hoxa9 or by Hoxa9 plus Meis1. (D) Flow cytometric analysis of Lin- progenitors cultured in SCF plus IL-7 for 3 weeks following infection by empty vector (plot 1), Hoxa9 (plot 2), or Hoxa9 plus Meis1 (plots 3-5). Cells depicted in plots 4 and 5 were shifted from SCF into IL-7 or GM-CSF for 5 days prior to FACS analysis. (E) Monoclonality of progenitors immortalized by Hoxa9 and Meis1 depicted in panel D, demonstrated by analysis of retroviral integration using a Hoxa9 cDNA probe. Resolved on the gel are DNA from a different clone as a control (lane C), and from the same Hoxa9 plus Meis1-coexpressing cells grown in SCF, IL-7, or GM-CSF that were analyzed in Panel D (lanes 1-3, respectively). (F) FACS analysis demonstrating up-regulation of CD34, FLT3 (CD135), CD27, and integrin β7 on progenitors expressing Meis1.
Meis1 coexpression with Hoxa9 immortalizes a distinct hematopoietic progenitor that exhibits multilineage differentiation potential and rapid monolayer proliferation. (A) MSCV retroviral vector used to achieve expression of Hoxa9 and Meis1a. LTR indicates long-term repeat, and NEO, neomycin-resistant gene. (B) Proliferation rates of Lin- marrow progenitors cultured in SCF and infected with empty MSCV, or MSCV encoding Hoxa9, Meis1, or Meis1 plus Hoxa9. The inserted blot shows expression of Meis1 (top) and Hoxa9 (bottom) by Western blot. (C) Morphology under phase-contrast microscopy (left) or following staining with Wright-Giemsa (right) of progenitors immortalized by Hoxa9 or by Hoxa9 plus Meis1. (D) Flow cytometric analysis of Lin- progenitors cultured in SCF plus IL-7 for 3 weeks following infection by empty vector (plot 1), Hoxa9 (plot 2), or Hoxa9 plus Meis1 (plots 3-5). Cells depicted in plots 4 and 5 were shifted from SCF into IL-7 or GM-CSF for 5 days prior to FACS analysis. (E) Monoclonality of progenitors immortalized by Hoxa9 and Meis1 depicted in panel D, demonstrated by analysis of retroviral integration using a Hoxa9 cDNA probe. Resolved on the gel are DNA from a different clone as a control (lane C), and from the same Hoxa9 plus Meis1-coexpressing cells grown in SCF, IL-7, or GM-CSF that were analyzed in Panel D (lanes 1-3, respectively). (F) FACS analysis demonstrating up-regulation of CD34, FLT3 (CD135), CD27, and integrin β7 on progenitors expressing Meis1.
Progenitors immortalized by coexpression of Meis1 plus Hoxa9 induced acute myeloid leukemia, while those immortalized by Hoxa9 alone do not
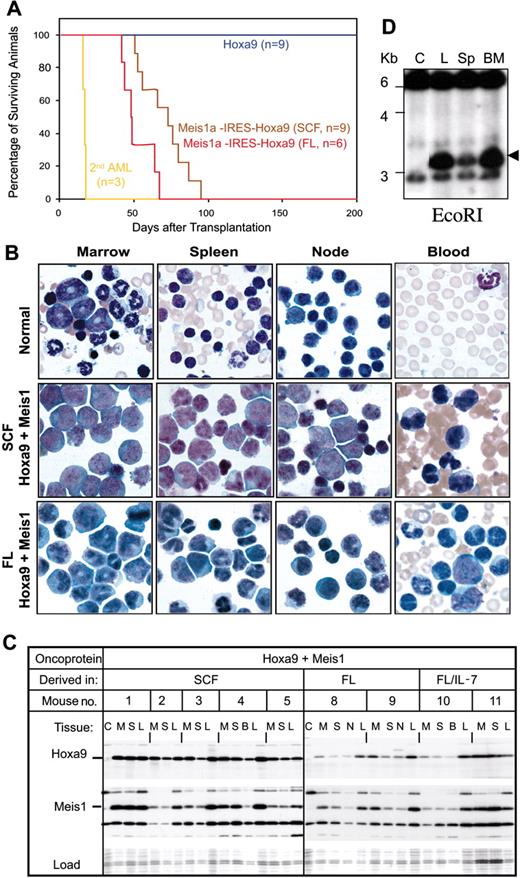
Progenitors immortalized by Hoxa9 or by Hoxa9 plus Meis1 were introduced into sublethally irradiated syngenic recipients to determine their leukemic potential. Those immortalized by Hoxa9 plus Meis1a caused acute leukemia after 72 plus or minus 15 days (Figure 2A), with a characteristic presentation. Bone marrow averaged more than 85% MacI+/B220- blasts, spleens were enlarged 4-fold and contained more than 65% myeloid blasts, and an 18- to 43-fold increase in the levels of circulating myeloid cells was observed (myeloblasts, metamyelocytes, banded and segmented stages; Figure 2B, Table 1). Progenitor cell lines immortalized by Hoxa9 exhibited only weak leukemogenicity, with only 1 of 9 mice acquiring AML within 280 days of injection. The length of time that progenitors spent in culture did not alter the outcome of their disease kinetics, with those cultured for 2, 6, or 12 weeks following infection with Meis1-IRES-Hoxa9 retrovirus yielding AML with kinetics that could be superimposed (data not shown). Leukemic cells from all tissue expressed Hoxa9 and Meis1 at an abundance comparable with the parental immortalized cell lines (Figure 2C), indicating that leukemic properties in vivo required the same level of Meis1 and Hoxa9 expression as was required for progenitor immortalization in culture. Injected progenitors contained the same retroviral integration as derivative leukemias (Figure 2D), verifying that leukemias arose from injected cell lines. Transfer of leukemic progenitors to secondary recipients produced AML with much shorter latency (average, 17 days; Figure 2A), suggesting that additional genetic events contribute to leukemias initiated by Hoxa9 plus Meis1. Leukemic blasts from primary or secondary AML were factor dependent. Collectively, these data demonstrate that there is a profound difference in the leukemogenic potential of cultured progenitors immortalized by Hoxa9 versus those immortalized by Hoxa9 plus Meis1.
Progenitors immortalized by Hoxa9 plus Meis1 induce overt AML, while those immortalized by Hoxa9 do not. (A) Survival curve for cohorts of sublethally irradiated mice injected with 2 million progenitors immortalized by Hoxa9 or Hoxa9 plus Meis1 and derived in either SCF or FL. Three different Hoxa9-immortalized cell lines (3 mice each) compose the control cohort. Three different Hoxa9/Meis1 cell lines (3 mice each) compose the SCF cohort, and 2 different Hoxa9/Meis1 cell lines (3 mice each) compose the FL cohort. The secondary serial injection was performed using leukemic cells extracted from bone marrow of mice with AML induced by Hoxa9/Meis1 progenitors immortalized in SCF. (B) Comparison of the morphologies of hemopoietic cells in bone marrow, spleen, lymph node, or peripheral blood from normal mice and those bearing AML characterized in panel A. (C) Consistent expression of Hoxa9 and Meis1 in mice with AML induced by Hoxa9 plus Meis1-expressing cell lines, detected by immunoblotting with anti-Hoxa9 or anti-FLAG (for Meis1) antibodies. Cell lysates were derived from marrow (M), spleen (S), blood (B), and leukemic cell lines derived from AML spleen tissue (L). Hoxb8-immortalized progenitors (lane C) served as negative control. (D) Retroviral integration analysis by Southern blot. Genomic DNA was isolated from control cells (lane C), from injected progenitors (L), and from leukemic myeloblasts from the spleen (Sp) or bone marrow (BM) of mice injected with the immortalized progenitors. DNA was digested by EcoRI and probed with Hoxa9 cDNA.
Progenitors immortalized by Hoxa9 plus Meis1 induce overt AML, while those immortalized by Hoxa9 do not. (A) Survival curve for cohorts of sublethally irradiated mice injected with 2 million progenitors immortalized by Hoxa9 or Hoxa9 plus Meis1 and derived in either SCF or FL. Three different Hoxa9-immortalized cell lines (3 mice each) compose the control cohort. Three different Hoxa9/Meis1 cell lines (3 mice each) compose the SCF cohort, and 2 different Hoxa9/Meis1 cell lines (3 mice each) compose the FL cohort. The secondary serial injection was performed using leukemic cells extracted from bone marrow of mice with AML induced by Hoxa9/Meis1 progenitors immortalized in SCF. (B) Comparison of the morphologies of hemopoietic cells in bone marrow, spleen, lymph node, or peripheral blood from normal mice and those bearing AML characterized in panel A. (C) Consistent expression of Hoxa9 and Meis1 in mice with AML induced by Hoxa9 plus Meis1-expressing cell lines, detected by immunoblotting with anti-Hoxa9 or anti-FLAG (for Meis1) antibodies. Cell lysates were derived from marrow (M), spleen (S), blood (B), and leukemic cell lines derived from AML spleen tissue (L). Hoxb8-immortalized progenitors (lane C) served as negative control. (D) Retroviral integration analysis by Southern blot. Genomic DNA was isolated from control cells (lane C), from injected progenitors (L), and from leukemic myeloblasts from the spleen (Sp) or bone marrow (BM) of mice injected with the immortalized progenitors. DNA was digested by EcoRI and probed with Hoxa9 cDNA.
Clonal progenitors immortalized by Hoxa9 plus Meis1 demonstrate multilineage differentiation potential and express FLT3 and IL-7 receptor
The fact that cultured progenitors immortalized by Hoxa9 plus Meis1 initiated AML, while those immortalized by Hoxa9 did not, led us to question whether each population exhibited differences in proliferation or differentiation in response to cytokines in the marrow microenvironment that might explain their different behavior in vivo. Both groups of progenitors proliferated well in GM-CSF (Figure 3A-B). While Hoxa9 progenitors differentiated to neutrophils in SCF plus G-CSF or in G-CSF alone, those coexpressing Meis1 proliferated as myeloblasts in SCF plus G-CSF and failed to differentiate in G-CSF alone (Figure 3A-B). Thus, Meis1 suppresses differentiation initiated by G-CSF signaling. Strikingly, progenitors immortalized by Hoxa9 plus Meis1 proliferated in response to FL, as well as to IL-7, while those immortalized by Hoxa9 alone died within 24 hours when cultured in FL or in IL-7, kinetics identical to those obtained when these progenitors were cultured in no cytokine. Proliferation of clonal progenitors coexpressing Hoxa9 plus Meis1 in GM-CSF resulted in outgrowth of MacI+ progenitors, while proliferation in IL-7 produced outgrowths of CD19+ progenitors (Figure 1D). Both the myeloid-shifted and lymphoid-shifted populations contained the same single retroviral integration site as their monoclonal parents (Figure 1E; lane 1 vs lanes 2 and 3). Thus, in SCF, coexpression of Meis1a plus Hoxa9 established an early differentiation block that permitted progenitors to retain both myeloid and lymphoid differentiation potential upon stimulation by appropriate cytokines. This phenotype is similar to the murine ST-HSC, which expresses FLT3 and retains both lymphoid and myeloid reconstitution potential when cultured in SCF.29
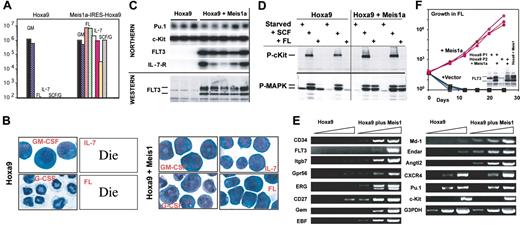
Meis1 induces FLT3 transcription and permits responsiveness to FL. (A-B) Proliferation of 2 progenitor lines immortalized by Hoxa9 and 2 progenitor lines immortalized by coexpressed Hoxa9 and Meis1, cultured in the presence of various cytokines (GM-CSF, FL, IL-7, or SCF plus GCSF). Cells were enumerated 7 days after plating in the indicated cytokines (A), and stained for morphology by Wright-Giemsa (B). (C) Northern blotting demonstrates expression of FLT3 and IL-7-R specifically in progenitors immortalized by coexpressed Hoxa9 plus Meis1, and immunoblotting demonstrates specific production of FLT3 in Hoxa9-immortalized progenitors that coexpress Meis1. (D) Immunoblot demonstrating that FL-induced MAP kinase (MAPK) phosphorylation is restricted to progenitors coexpressing Hoxa9 plus Meis1, while SCF induces receptor phosphorylation and MAP kinase phosphorylation in progenitors immortalized by Hoxa9 alone, as well as those immortalized by Hoxa9 plus Meis1. (E) Confirmation of microarray analysis by semiquantitative PCR, using 1:10 serial dilutions of cDNA as template. (F) Expression of Meis1 in 3 different Hoxa9-immortalized progenitor lines induces expression of FLT3 (inset) and robust proliferation in response to recombinant FL as the sole cytokine.
Meis1 induces FLT3 transcription and permits responsiveness to FL. (A-B) Proliferation of 2 progenitor lines immortalized by Hoxa9 and 2 progenitor lines immortalized by coexpressed Hoxa9 and Meis1, cultured in the presence of various cytokines (GM-CSF, FL, IL-7, or SCF plus GCSF). Cells were enumerated 7 days after plating in the indicated cytokines (A), and stained for morphology by Wright-Giemsa (B). (C) Northern blotting demonstrates expression of FLT3 and IL-7-R specifically in progenitors immortalized by coexpressed Hoxa9 plus Meis1, and immunoblotting demonstrates specific production of FLT3 in Hoxa9-immortalized progenitors that coexpress Meis1. (D) Immunoblot demonstrating that FL-induced MAP kinase (MAPK) phosphorylation is restricted to progenitors coexpressing Hoxa9 plus Meis1, while SCF induces receptor phosphorylation and MAP kinase phosphorylation in progenitors immortalized by Hoxa9 alone, as well as those immortalized by Hoxa9 plus Meis1. (E) Confirmation of microarray analysis by semiquantitative PCR, using 1:10 serial dilutions of cDNA as template. (F) Expression of Meis1 in 3 different Hoxa9-immortalized progenitor lines induces expression of FLT3 (inset) and robust proliferation in response to recombinant FL as the sole cytokine.
Distinct responses to FL and IL-7 were attributable to unique expression of the FLT3 and IL-7 receptor in these progenitors (Figure 3C; Northern). Both the cell-surface, glycosylated form of FLT3 (upper band) and the internal, unglycosylated form of FLT3 were evident exclusively in progenitors coexpressing Hoxa9 plus Meis1 (Figure 3C; Western). Signaling in response to FL was also found exclusively in progenitors coexpressing Hoxa9 plus Meis1, as reflected by the restricted activation of mitogen-activated protein (MAP) kinase by FL only in these cells (Figure 3D). Consistent with the SCF dependence of all cell lines, the SCF receptor c-Kit was expressed in all progenitors at levels unrelated to coexpressed Meis1 (Figure 3C).
Meis1 induces expression of the FLT3 gene in Hoxa9-immortalized, SCF-dependent progenitors
There are 2 possible mechanisms by which Meis1 could maintain FLT3 expression in a marrow-derived progenitor. First, Meis1 could activate FLT3 transcription directly, through binding the FLT3 promoter, or indirectly, by maintaining expression of other transcriptional activators within an activation cascade. Second, Meis1 could establish an earlier block to progenitor differentiation that prevents a cascade of transcriptional events resulting in down-regulation of the FLT3 promoter. The second case postulates that Meis1 plays no role in activating FLT3 transcription. If the first hypothesis were correct, Meis1 might be able to induce FLT3 expression in Hoxa9-immortalized progenitors. But if the second hypothesis were correct, we would not expect Meis1 to reactivate FLT3 transcription. Indeed, we found that retroviral expression of Meis1 in Hoxa9-immortalized, SCF-dependent progenitor cultures (5 of 5) induced high levels of FLT3 protein and enabled proliferation in FL as the sole cytokine (Figure 3F). Quantitative PCR analysis confirmed strong activation of FLT3 gene transcription in the Meis1-expressing derivatives. These Meis1-expressing progenitors also initiated rapid AML (70 days after injection in 4 of 4 mice; data not shown). Thus, Meis1 lies within a cascade that activates FLT3 transcription, and this function parallels the ability of Meis1 to produce AML-initiating progenitors. In leukemia, enforced transcription of Meis1 and consequential activation of FLT3 would promote survival of leukemic progenitors in stromal niches that express FL and would maintain responsiveness of myeloid progenitors to mutations that activate autocrine FL production or that activate FLT3 receptor function (eg, FLT3-ITD).
SCF-dependent progenitors immortalized by Hoxa9 plus Meis1 have a stem cell-like genomic signature
Analysis of Affymetrix mouse genome arrays (430 2.0 Array; Santa Clara, CA; display more than 39 000 transcripts, including more than 34 000 for characterized mouse genes) revealed 50 genes exhibiting more than 5-fold difference in expression level among 3 cell lines immortalized by Hoxa9 plus Meis1 versus 3 others immortalized by Hoxa9 alone. This analysis considered genes expressed as low as 1/30 that of c-Myb and one-third that of Bmi-1 (Table 2). Among genes whose expression did not change significantly were those encoding cyclin A2 and cyclin B, which regulate G1/S and G2/M progression; those encoding transcription factors Bmi-1 and MLL, which control progenitor self-renewal; and those encoding receptors CD44 and CXC chemokine receptor 4 (CXCR4), which control homing of progenitors to the marrow through their interaction with hyaluronic acid and stromal cell-derived factor 1 (SDF-1).30 Progenitors immortalized by Hoxa9 alone exhibited significant myeloid character (MPOhigh, Prtn3high, NB-1high, NEhigh, Csf2rahigh, and TFEChigh) and no evidence of nonmyeloid lineage-specific markers. Therefore, these immortalized progenitors are different—both in maturity and leukemic potential—from marrow progenitors freshly transduced with Hoxa9 retrovirus, which contribute to multilineage hematopoiesis and ultimately lead to leukemia. Progenitors immortalized by coexpressed Meis1 expressed fewer myeloid antigens (MPOhigh, Prtn3high, Csf2ralow, TFEClow, NB-1-, NE-) (genes listed in Table 2) and evidenced lymphoid antigens (MD-1high, EBF+, IL-7Rαhigh). Strikingly, progenitors immortalized by Hoxa9 plus Meis1 expressed genes in addition to FLT3 that are indicative of ST-HSC (CD34, CD27, ERG1, Gpr56, CRLR, and C1qR1/AA4.1). Therefore, Hoxa9 progenitors represent a more mature myeloid cell, while Hoxa9 plus Meis1 progenitors express myeloid, lymphoid, and early stem-cell antigens. FACS analysis confirmed specific expression of FLT3, CD34, CD27, and integrin β7 (Figure 1F).
Distinct differences in transcription factor gene expression accompanied each overall genomic signature
Transcription factor genes Tilz1b, ERG1, PLZF, Satb1, and RIP140 were expressed uniquely in progenitors immortalized by Hoxa9 plus Meis1, while Tfec was expressed uniquely in progenitors immortalized by Hoxa9 alone. Specific gene up-regulation, as detected by microarrays, was measured as more than 50-fold using semiquantitative PCR (Figure 3E).
FL-dependent immortalization represents a novel assay that detects Hoxa9 and Meis1 functions that produce leukemia-initiating progenitors
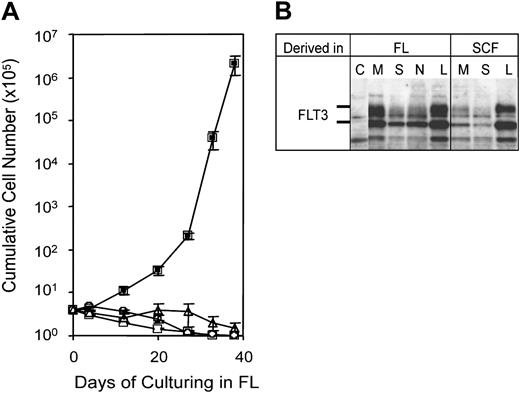
The exclusive ability of leukemia-initiating Hoxa9/Meis1 progenitors to proliferate in FL suggested they could be selected initially by cultivation of retrovirally infected progenitors in FL. This would represent a powerful assay for mapping physical domains and identifying associated biochemical functions that are required for Meis1 to cooperate with Hoxa9 in leukemogenesis. To test this possibility, Sca+/Lin- progenitors were infected by retrovirus encoding Neo, Hoxa9, Meis1a, or Hoxa9 plus Meis1a, and plated in culture medium supplemented with FL as the sole cytokine (Figure 4A). Cultures infected with retrovirus expressing Neo, Meis1, or Hoxa9 exhibited no proliferation, and evidenced a progressive differentiation and decline in viable cells. By contrast, progenitors infected by retrovirus coexpressing Hoxa9 plus Meis1 expanded as immortalized progenitors that expressed FLT3. Similar to progenitors derived in SCF plus IL-7, those derived in FL were mainly Lin- but did coexpress Mac1 or B220 on subpopulations. Introduction of these FL-dependent immortalized progenitors into mice resulted in myeloid leukemia (Table 1) with kinetics similar to previous experiments using SCF-dependent progenitors (50 days; Figure 2A). Myeloid blasts from AML initiated by FL-dependent progenitors evidenced the same morphology, tissue tropism in vivo, and property of producing elevated levels of circulating progenitors as did the AMLs induced by cognate progenitors immortalized in SCF (Figure 2B; Table 1). Leukemic tissues expressed Hoxa9 and Meis1 (Figure 2C), and leukemias produced by progenitors immortalized in either SCF or in FL expressed FLT3 (Figure 4B). We conclude that the leukemia-initiating target cell immortalized by Hoxa9 plus Meis1 is selected effectively by growth in FL.
Meis1a requires binding to Pbx and DNA, as well as a novel function of its carboxyl terminus to immortalize FL-dependent progenitors and to produce leukemia-initiating progenitors
Next, we used both the FL-dependent and SCF-dependent immortalization assays to identify biochemical functions of Meis1 required for expansion of FLT3-expressing, leukemia-initiating progenitors (Diagram of mutations in Figure 5A,C). Each mutant form of Meis1 was tested for its ability to bind Pbx1a in the absence of DNA (Figure 5B), to heterodimerize with Pbx1a on DNA (Figure 5D), to immortalize FL-dependent progenitors (Figure 5F-G), and to cause leukemia in vivo (Figure 5H). Expression of Meis1 mutants and coexpressed Hoxa9 was verified by Western blot using the SCF-dependent progenitors after G418 selection following retroviral infection (Figure 5E). Two regions of Meis1 preceding the homeodomain were dispensable for proliferation in FL: the first 64 residues (Meis1Δ1-64), and residues 202-260, which reside between the M2 motif and the homeodomain (Meis1Δ202-260). Meis1Δ1-64 also retained its ability to cause rapid AML (Figure 5H; Meis1Δ202-260 not tested). Both these deletion mutants preserved the ability of Meis1 to bind DNA and heterodimerize with Pbx (Figure 5B,D).
A simple FL-dependent proliferation and immortalization cell system can evaluate cooperation between Meis1 and Hoxa9. (A) Growth curve of progenitors cultured in FL following infection with empty vector (▵), Hoxa9 (□), Meis1 (○), or Meis1 plus Hoxa9 (▪). Error bar indicates standard deviation of data from 3 repeated experiments. (B) Anti-FLT3 immunoblot demonstrating strong FLT3 expression in representative leukemias arising from Hoxa9/Meis1-immortalized progenitors derived in either SCF or FL. Immunoblots were performed on cell extracts from a control cell line (C), from the bone marrow (M), spleen (S), and lymph nodes (N) of leukemic mice, and from cultured leukemic blasts from splenic tissue (L).
A simple FL-dependent proliferation and immortalization cell system can evaluate cooperation between Meis1 and Hoxa9. (A) Growth curve of progenitors cultured in FL following infection with empty vector (▵), Hoxa9 (□), Meis1 (○), or Meis1 plus Hoxa9 (▪). Error bar indicates standard deviation of data from 3 repeated experiments. (B) Anti-FLT3 immunoblot demonstrating strong FLT3 expression in representative leukemias arising from Hoxa9/Meis1-immortalized progenitors derived in either SCF or FL. Immunoblots were performed on cell extracts from a control cell line (C), from the bone marrow (M), spleen (S), and lymph nodes (N) of leukemic mice, and from cultured leukemic blasts from splenic tissue (L).
Meis1 required binding to Pbx and to DNA to cause FL-dependent proliferation and AML. Meis1 required active DNA-binding. Two homeodomain mutations, Meis1-HDN51S or a more dramatic HD mutant, Meis1-HDN51S/RRR53-55AEE, did not alter interaction with Pbx in the absence of DNA (Figure 5B, lane 10 vs lane 4) but strongly suppressed DNA binding by Meis1-Pbx heterodimers (Figure 5D, lanes 8-9 vs lane 4) and precluded FL-dependent immortalization. The SCF-dependent progenitors that arose through the function of coexpressed Hoxa9 expressed Meis1N51S efficiently (Figure 5E, lane 11) yet did not produce AML (Figure 5H). Interaction with Pbx was also essential. Deletion of the M1 and M2 motifs, which were previously shown to bind Pbx (Meis1Δ64-202; Figure 5B,D, lane 6), destroyed FL-dependent proliferation and leukemic function despite the fact that this deletion protein was expressed at high levels (Figure 5E, lane 6). The specific requirement for binding Pbx was further investigated by site-direct mutagenesis of the M1 and M2 leucine-zipper–like regions. Changing 3 or 4 residues in either the M1 or M2 helices was generally insufficient to disrupt interaction with Pbx or FL-dependent proliferation (M1ΔLFPLL or M2ΔLLEL; Figure 5D, lane 13; Figure 5G), while combining both M1 and M2 mutations within a single Meis1 protein (M1ΔLFPLL/M2ΔLLEL) or extending the disrupted helical interactions provided by a single M motif (M2ΔLRF/ΔLLEL or M2ΔIQVL/ΔLLEL) completely destroyed interaction with Pbx (Figure 5B,D), destroyed FL-dependent proliferation, and prevented leukemogenesis (Figure 5H) without altering Meis1 expression (Figure 5E, lane 15 vs lane 4).
The Meis1a C-terminal domain, composed of 49 residues downstream of the homeodomain, possessed an essential Meis1a leukemogenesis function independent of DNA binding or interaction with Pbx. This region contains 3 reiterated motifs that are highly conserved in Meis1, Meis2, and Meis3, but are not found in Prep1, a less-related family member (Figure 5C). Premature termination mutations were engineered to eliminate 1 (Meis1a370T), 2 (Meis1a357T), or all 3 (Meis1a341T) of these conserved motifs. Meis1a370T retained its ability to immortalize FL-dependent progenitors that caused rapid AML (Figure 5H). By contrast, Meis1a357T and Meis1a341T, despite exhibiting a wild-type ability to heterodimerize with Pbx in the presence of DNA (Figure 5D, lanes 11-12) and in the absence of DNA (Figure 5B, lanes 13-14), were incapable of immortalizing FL-dependent progenitors. Consistent with this observation, SCF-dependent progenitors that were immortalized by coexpressed Hoxa9 expressed Meis1341T efficiently (Figure 5E), yet failed to cause AML (Figure 5H).
Collectively, this mutational analysis demonstrates that Meis1 must bind DNA and interact with Pbx, and that Meis1 uses a yet-undefined function of its conserved C-terminus to maintain FLT3 expression and to establish the leukemic phenotype. This analysis also demonstrated an invariant link between the ability to immortalize FL-dependent progenitors and the ability to establish the leukemia-initiating phenotype.
Discussion
Here we present evidence that Meis1 activates expression of the myeloid proto-oncogene FLT3, that Meis1 has the general property of up-regulating genes characteristic of ST-HSCs, and that Meis1 confers an AML-initiating phenotype upon nonleukemic Hoxa9-immortalized progenitors. How might Meis1 establish the characteristics of a leukemia-initiating cell? Subpopulations of cancer cells in human leukemia and solid tumors that initiate cancer in xenografted nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice are denoted cancer stem cells. In culture, they are similar to their nontumorigenic counterparts in terms of their proliferation and apoptotic indices; however, they express distinctive surface antigens, such as CD44 in breast cancer31 or CD34 in both multiple myeloma and in AML.32 In the case of human AML, only CD34+ normal human progenitors migrate rapidly to bone marrow,33 and all human progenitors that initiate AML in NOD-SCID mice are restricted to CD34+ blasts.32 CD34 promotes homing34 and inhibits differentiation.35 The behavior of Meis1 is similar to that defined for a cancer stem cell gene because on a cellular level it directs development of AML in vivo, yet it is unnecessary for myeloblast proliferation in culture, and on a genetic level it induces expression of CD34 and FLT3, which are present on human AML-initiating progenitors. Three Meis1 effects we observe in cultured progenitors could establish leukemic stem cell characteristics in vivo—suppressing the differentiation in response to maturation cytokines such as G-CSF, establishing responsiveness to cytokines (FL, IL-7, and CD27 ligand, aka tumor necrosis factor [TNF] superfamily member 7), and promoting interactions within hematopoietic microenvironments through homing and adhesion proteins (CD34, integrin β7, and C1qR1 [AA4.1]). Responsiveness to FL would further augment responsiveness to SCF,21 although recent studies suggest that human AML blasts capable of providing long-term engraftment in NOD-SCID mice do not express cell-surface c-Kit, reiterating the central importance of FLT3.36 We have functional data for each of these proposed functions for Meis1 with the exception of engraftment potential.
The ability of Meis1 to promote FL-dependent proliferation correlates precisely with its ability to induce leukemogenesis. (A) Location of Meis1 mutations. Point mutations are shown in panel C. (B) Interaction between Meis1a and Pbx1a in the absence of DNA, demonstrated by coimmunoprecipitation. (C) Top panel shows designation of point mutant of Meis1 within the M1 and M2 alpha helices. An asterisk indicates the position of leucine or isoleucine. Bottom panel shows C-terminal sequences of Meis1, Meis2, Meis3, and Prep1 that lie downstream of homeodomain. The 3 repeated motifs are underlined. (D) Interaction between Meis1a and Pbx1a on DNA, demonstrated by EMSA. (E) Expression of Meis1 and Hoxa9 in primary Lin- marrow cells following retroviral infection and selection for G418 resistance. (F) FL-dependent proliferation demonstrating the requirement for C-terminal residues upstream of amino acid position 370. (G) FL-dependent proliferation demonstrating the importance of interaction with Pbx. Error bars in panels F and G indicate standard deviation of data from repeated experiments. (H) The ability of Meis1 to cooperate with Hoxa9 to cause AML correlates precisely with its ability to evoke FL-dependent proliferation.
The ability of Meis1 to promote FL-dependent proliferation correlates precisely with its ability to induce leukemogenesis. (A) Location of Meis1 mutations. Point mutations are shown in panel C. (B) Interaction between Meis1a and Pbx1a in the absence of DNA, demonstrated by coimmunoprecipitation. (C) Top panel shows designation of point mutant of Meis1 within the M1 and M2 alpha helices. An asterisk indicates the position of leucine or isoleucine. Bottom panel shows C-terminal sequences of Meis1, Meis2, Meis3, and Prep1 that lie downstream of homeodomain. The 3 repeated motifs are underlined. (D) Interaction between Meis1a and Pbx1a on DNA, demonstrated by EMSA. (E) Expression of Meis1 and Hoxa9 in primary Lin- marrow cells following retroviral infection and selection for G418 resistance. (F) FL-dependent proliferation demonstrating the requirement for C-terminal residues upstream of amino acid position 370. (G) FL-dependent proliferation demonstrating the importance of interaction with Pbx. Error bars in panels F and G indicate standard deviation of data from repeated experiments. (H) The ability of Meis1 to cooperate with Hoxa9 to cause AML correlates precisely with its ability to evoke FL-dependent proliferation.
We propose that while the SCF-dependent cell lines generated by Hoxa9 and Meis1 in culture may not perfectly reflect any particular normal hematopoietic progenitor within freshly isolated marrow, they nevertheless model normal functions of Hoxa9 and Meis1 within native HSCs and LCPs (Table 3). In the mouse, both FLT319,37 and CD3420 are expressed at low levels in quiescent stem cells, up-regulated in activated ST-HSCs and early LCPs, and down-regulated during differentiation, a transcriptional pattern similar to that of Meis1.1 This temporal coregulation is also consistent with the proposal that transcription of FLT3 and CD34 during normal hematopoiesis is also regulated by Meis1 and suggests that Meis1-/- mice contain lower numbers of ST-HSCs and LCPs because these cells express lower levels of FLT3 or may interact with their hematopoietic niches less efficiently due to altered expression of adhesion molecules.
An ability of Meis1 to activate transcription of FLT3 in AML would explain the association of FLT3 expression with expression of Hoxa9 and Meis1 in myeloid and lymphoid leukemias containing MLL translocation38,39 and would exemplify a novel form of oncoprotein cooperation in which a transcription factor oncoprotein (Meis1) enforces expression of a tyrosine kinase oncoprotein (FLT3-ITD). It would also imply that in human AML, mutations up-regulating MEIS1 and HOXA9 transcription precede mutations that activate signaling of the FLT3 receptor, such as FLT3-ITD25 or autocrine expression of FL.26,39 From a therapeutic standpoint, the observation that Meis1 activates FLT3 transcription makes FLT3 an exceptional drug target in AML because it should be expressed on the surface of all Meis1-expressing leukemia-initiating cells.
We hypothesize that the ability of Meis1 to program ST-HSC properties requires additional cooperating factors in immature hematopoietic progenitors, and that maturation cytokines can down-regulate these factors, promoting differentiation of the ST-HSCs within lineage-specific pathways. This is suggested by a number of observations. First, when Meis1 was expressed in Hoxa9 progenitors, only 5% to 30% of each population exhibited FL-dependent proliferation, despite the fact that all progenitor populations expressed high levels of Meis1 on immunoblots (data not shown). This suggests that only a subset of SCF-dependent Hoxa9 progenitors retained transcription of essential cofactor genes. Second, we found that Meis1 has no ability to induce either FLT3 expression or a leukemic phenotype in Hoxa9-immortalized progenitors cultured in GM-CSF, suggesting that maturation cytokines down-regulate transcription of factors that cooperate with Meis1 (K. R. Calvo, M.P.K., unpublished observations, May 2002). Based on our genomic analysis, cooperating transcription factors could include promyelocytic leukemia zinc finger (PLZF), receptor-interacting protein 140 (Rip140), special AT-rich sequence-binding protein 1 (Satb1), TSC22-related inducible leucine zipper 1b (Tilz1b), and CXXC finger 5 (CXXC5), which are invariantly coexpressed with Meis1. The argument that only a subset of cytokine signaling is compatible with Meis1-mediated leukemogenic function is also supported by the subsets of activated receptors that complement Meis1 function in human AML—FLT3 activation is frequent,25,26 G-CSFR activation is exceptionally rare,40 and GM-CSFR activation has never been observed.
The FL- and SCF-dependent progenitor immortalization systems described herein should provide a simple, useful tool for establishing the biochemical and genetic basis of leukemogenesis by Hoxa9 and Meis1, for identifying the important genetic targets required for leukemic stem cell behavior, and for determining the nature of the permissive transcriptional context provided by FL and SCF signaling. It is possible that analysis of oncoprotein domains required for leukemic function within this cell culture system could yield results somewhat different from those based on freshly transduced marrow progenitors in reconstituted irradiated mice, because in mice expression of all mutants of Meis1 or Hoxa9 are retained in LT-HSCs that engraft. Partially active Meis1 or Hoxa9 mutants could produce less aggressive leukemias with slower kinetics or could expand a progenitor pool that becomes susceptible to mutations that cause AML by mechanisms unrelated to the mutant Hoxa9 or Meis1 protein. In our assay, the wild-type functions of Hoxa9 and Meis1 provide the only selection determinants, and mutants with partial transforming function are likely to go undetected. Therefore, the cell culture assay we describe is likely to have a more stringent requirement for retention of transformation-related properties of Hoxa9 and Meis1.
Prepublished online as Blood First Edition Paper, March 8, 2005; DOI 10.1182/blood-2004-12-4664.
Supported by Public Health Service grant NIH CA56876 to M.P.K.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.