Although expression of vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells and its functional implications have been previously appreciated, VCAM-1 expression in other than endothelial cells, especially hematopoietic cells, has been recently recognized and has not been explored in detail. Using normal mice and mice with a conditional ablation of VCAM-1 through a Tie2-driven cre transgene, we have studied the biodistribution and the pattern of VCAM-1 expression in circulating versus tissue-residing cells before and after their enforced mobilization. In the normal mouse, both at basal hematopoiesis or following mobilization, VCAM-1 expression is confined to myeloid cells residing in hematopoietic tissues, whereas free cells in circulation or in body cavities are devoid of VCAM-1 messenger RNA (mRNA) and protein. However, following culture, proliferating myeloid cells, but not lymphoid cells, express VCAM-1. In the VCAM-1–ablated mouse, there is an increase in circulating progenitors as a consequence of their ongoing release from bone marrow, a process enhanced by splenectomy. We postulate that the main mechanism leading to their release is the ablation of VCAM-1 by fibroblastic and by endothelial cells. Ablation of VCAM-1 in fibroblasts by Tie2-driven cre is a novel finding and likely denotes their developmental ancestry by Tie2-expressing (mesenchymal?) progenitor cells during development.
Introduction
The vascular cell adhesion molecule 1 (VCAM-1), an immunoglobulin (Ig)–like transmembrane adhesion molecule, is highly conserved in evolution and participates in a variety of cellular functions in health and disease. Human VCAM-1 has 2 isoforms, the predominant 7 Ig-domain isoform and a minor, alternatively spliced isoform with 6 Ig domains, whereas in mice the second isoform consists of the first 3 domains attached to the cell membrane through a glycosylphosphatidylinositol (GPI) anchor.1,2 VCAM-1 is minimally expressed on most resting vascular endothelial cells and is inducible in many tissue vascular beds following injury or stress. Because of this activation, VCAM-1 has been implicated in the pathophysiology of certain autoimmune diseases, atherosclerosis, and allograft rejection.3-8 VCAM-1 is constitutively expressed in bone marrow (BM) stromal/endothelial cells and certain classes of hematopoietic cells (B cells, follicular dendritic cells, and macrophages9-15 ). Its major ligand is the integrin very late antigen 4 (VLA-4; α4β1) with binding sites located in the first and fourth Ig domains, whereas other ligands bind with less affinity and include α4β7, α9β1, and αDβ2.16-18 Deletion of the VCAM-1 gene produced a phenotype similar to deletion of the α4 gene, with embryonic lethality due to cardiac and placental developmental abnormalities, although few VCAM-1–/– mice survived.19,20 Several investigators then adopted approaches to overcome the embryonic lethality observed in VCAM-1–null mice. Cybulsky et al generated mice with a homozygous deletion of domain 4 (VCAM-1D4D),21 resulting in expression of the VCAM-1 isoform containing one binding site. Embryonic survival was significantly reduced and was strain dependent, but surviving mice showed a reduction of VCAM-1 protein to less than 10% of wild type, allowing informative studies in the adult animals.22-24 In other studies,25 Tie2-cre transgenic mice were bred with floxed VCAM-1 mice to allow VCAM-1 deletion in hematopoietic cells and endothelium through cre-loxP–mediated recombination. These viable mice exhibited mild leukocytosis with elevated immature B cells in the peripheral blood (PB), with reduced immature B cells in the BM, consistent with the role of VCAM-1 in the retention of immature lymphocytes in the marrow. In a similar approach, mice carrying the Mx-cre transgene bred with mice homozygous for the floxed VCAM-1 gene, subsequently deleted neonatally by interferon-mediated cre recombination, led to a reduction of immature B cells in the BM, and an increase of these cells in the circulation.26 Additional in vitro studies have shown that the VLA-4/VCAM-1 interaction is involved in the adhesion of human B cells to follicular dendritic cells of germinal centers in vitro and a link has been reported between VCAM-1 function and the rescue of germinal center B cells or thymocytes from apoptosis.13,14,27 VCAM-1 may also function in maturation and costimulation of T cells.28-30 In contrast to results from chimeric α4–/– mice showing postnatal dependency of B and T cells on α4 integrins,31 B and T lymphocytes can develop in the absence of VCAM-1.25,26 Later studies reported that neonatally induced inactivation of VCAM-1 impaired responses to Toxoplasma encephalitis.32
Herein, we explored the pattern of VCAM-1 expression among hematopoietic and nonhematopoietic cells from BM and from selective tissues, before and after the use of mobilizing agents. Several novel insights about VCAM-1 regulation in these cell populations are revealed by studying both control and VCAM-1–ablated animals and these are described in the present work.
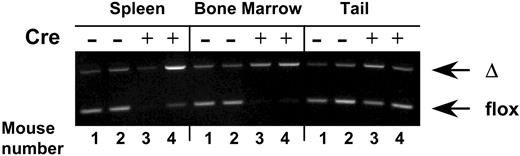
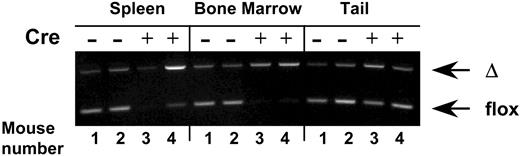
Virtually complete VCAM-1 gene ablation in hematopoietic cells from bone marrow and spleen in Tie2cre+ VCAM-1 mice (VCAM-1Δ/Δ). Genomic DNA was isolated from the spleen, bone marrow cells, and tails of VCAM-1f/f (nos. 1 and 2) or VCAM-1Δ/Δ (nos. 3 and 4) mice. PCR was performed using a set of VCAM-1– specific primers that allow distinction between floxed and deleted (Δ) alleles of the VCAM-1 gene. Note that in contrast to the findings with the tail, only cre+ hematopoietic cells are nearly completely ablated.
Virtually complete VCAM-1 gene ablation in hematopoietic cells from bone marrow and spleen in Tie2cre+ VCAM-1 mice (VCAM-1Δ/Δ). Genomic DNA was isolated from the spleen, bone marrow cells, and tails of VCAM-1f/f (nos. 1 and 2) or VCAM-1Δ/Δ (nos. 3 and 4) mice. PCR was performed using a set of VCAM-1– specific primers that allow distinction between floxed and deleted (Δ) alleles of the VCAM-1 gene. Note that in contrast to the findings with the tail, only cre+ hematopoietic cells are nearly completely ablated.
Materials and methods
Mice
VCAM-1 mice used in our experiments were created through interbreeding of VCAM-1 floxed (VCAM-1f/f) mice with Tie2cre transgenic mice.25 When VCAM-1f/f males are bred with Tie2cre+ females, all progeny, regardless of the presence of the cre transgene, have a constitutional deletion of 1 VCAM-1 allele (VCAM-1Δ). This scheme seems to afford a more complete deletion of VCAM-1 in hematopoietic tissues of Tie2cre+VCAM-1f/Δ versus Tie2cre+VCAM-1f/f animals following subsequent breeding.25 Consequently, mice used for our experiments had the Tie2cre+VCAM-1f/Δ genotype and controls were of cre–VCAM-1f/f genotype (Figure 1). Observations in these mice were carried out from a few weeks of age up to over a year. Mice bred and maintained under specific pathogen-free conditions in University of Washington facilities were provided with irradiated food and autoclaved water ad libitum.
Mobilization treatments
Granulocyte–colony-stimulating factor (G-CSF; Neupogen Filgrastim; Amgen, Thousand Oaks, CA) treatments were given via an osmotic pump (Alzet Model 2001; Durect, Cupertino, CA) for 5 days at 100μg/kg per day (3 experiments) or by intraperitoneal injection twice daily at 200 μg/kg for 4 days (1 experiment). All mice were tested on the fifth day. Flt-3 ligand alone (FL; 50 ng/kg per day; kind gift from S. Lyman, Immunex, Seattle, WA) or the combination of G-CSF (100 μg/kg per day) and FL was administered with osmotic pump for 7 days.
Antibodies
Anti–VCAM-1 (MK/2) antibodies, unconjugated for immunohistochemistry (IHC) and phycoerythrin (PE)–conjugated for FACS, were from Southern Biotech (Birmingham, AL). Fluorochrome-conjugated antibodies to B220, CD3, TER119, Mac-1, Gr-1, and CD117 (c-kit), and fluorescein isothiocyanate (FITC)–conjugated secondary antibodies and streptavidin allophycocyanin (APC) were from BD-Pharmingen (San Diego, CA). Antibodies used for endothelial cells were biotinylated CD105 (endoglin), MECA32 (BD-Pharmingen), biotinylated isolectin B4 (Vector Labs, Burlingame, CA), labeled acetylated low-density-lipoprotein (diI-Ac-LDL; Biomedical Technologies, Stoughton, MA), rabbit anti–human von Willebrand factor (VWF; Abcam, Cambridge, MA), and PE-conjugated anti–mouse CD202 (Tie2; eBioscience, San Diego, CA). Anti–rabbit Alexa 594, streptavidin 488 (Invitrogen/Molecular Probes, Carlsbad, CA), and streptavidin PE (Biomeda, Foster City, CA) were used as secondary reagents.
FACS analysis
Antibody-stained cells were analyzed on a FACSCalibur (BD Immunocytometry Systems, San Jose, CA) using the CELLQuest program.
Immunohistochemistry
Tissues for immunohistochemistry were fixed in 4% paraformaldehyde, sucrose treated, embedded in TissueTek (Sakura Finetechnical, Tokyo, Japan), and frozen in acetone/dry ice. Femurs/tibiae were similarly fixed, decalcified in 10% EDTA (ethylenediaminetetraacetic acid; 7 to 10 days at 4°C), sucrose treated, then embedded and frozen. Air-dried frozen sections were washed, blocked for endogenous peroxidase activity and nonspecific staining, stained with appropriately diluted antibodies, and processed following the manufacturer's instructions (ABComplex/HRP; Dako, Carpinteria, CA).
Culture–colony-forming unit assay
Culture–colony-forming unit (CFU-C) assays were performed using a methylcellulose mixture (Methocult GF; Stem Cell Technologies, Vancouver, BC, Canada), as described previously.33
Endothelial and fibroblast cell cultures
Endothelial cells from bone were derived by treating cleaned and flushed femurs with 0.125% Trypsin/0.2% EDTA for 15 minutes at 37°C; cells were discarded, bones minced, then incubated with 0.1% collagenase (1-3 hours at 37°C). After washing and removing bone fragments, cells were plated in collagen I–coated wells (BD BioCoat; BD Biosciences, Bedford, MA) using endothelial-cell medium as previously described,34 adding 250 ng/mL endothelial mitogen (Biomedical Technologies), 10 ng/mL recombinant murine vascular endothelial cell growth factor (rm-VEGF) and 7.5 ng/mL basic fibroblast growth factor (both from Peprotech, Rocky Hill, NJ). Nonadherent cells were removed after 24 hours and fresh medium was added. Adherent cells were dissociated with either 1:1 cell dissociation solution/phosphate-buffered saline (PBS) (Sigma Aldrich, St Louis, MO), or 1:10 TrypLE Express/PBS (Invitrogen). Bone fibroblasts were derived by plating bone fragments or cells from above in Dulbecco modified Eagle medium (DMEM) with 20% fetal bovine serum (FBS), cultured for 4 to 7 weeks, and dissociated with TrypLE Select. Endothelial cells from heart or lung were isolated by mincing, then washed extensively to remove blood, collagenase treated and plated in 2% gelatin-coated wells/dishes with endothelial cell medium. Endothelial cells or fibroblasts were used for evaluation by fluorescence-activated cell sorting (FACS) or for DNA and RNA studies.
Bone marrow, peripheral blood, and splenic lymphocyte cell cultures
Femurs from control and VCAM-1–ablated mice were flushed, and recovered cells were washed, incubated for 2 hours in Iscoves modified Dulbecco medium (IMDM) plus 10% FBS to remove adherent cells, counted and plated in IMDM with 10% FBS, 100 ng/mL murine stem-cell factor (Peprotech), 10 μL/mL of murine interleukin 3 (IL-3; kind gift from K. Kaushansky, San Diego, CA), 100 ng/mL G-CSF, 50 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; Peprotech), and 3 U/mL erythropoietin (Epoetin; Amgen, Thousand Oaks, CA).
Mononuclear cells from blood of mobilized control or ablated animals were isolated by density gradient separation (Histopaque 1083; Sigma Chemical), washed, and cultured in the same media as BM cells. Spleens from control and VCAM-1–ablated mice were teased, triturated, washed, and plated in 10% FBS, 100 ng/mL murine IL-2, 50 ng/mL murine IL-7 (both from Peprotech), and 10–4 M 2-mercaptoethanol (Sigma Chemical), or in the same cytokine cocktail as the PB cells. Samples were collected for analysis at day 0 and after 3 to 7 days in culture.
DNA/RNA studies
Genomic DNA and total RNA were isolated from mouse tails or cell cultures using DNA or RNA isolation kits (Gentra Systems, Minneapolis, MN) according to the manufacturer's instructions. Isolated RNA was reverse-transcribed using oligo-dT (Invitrogen) or random nonamer (Sigma Chemical) primer and SuperScript II enzyme (Invitrogen). The following primers were used in polymerase chain reactions (PCRs): for genomic DNA to distinguish between wild type, floxed, and Δ alleles of the VCAM-1 gene (5′-ATCGATCCCTGGATATGTCG-3′,5′-AGGCTATCAGTGAGACCCACA-3′,5′-TGGGCTGTCTATCTGGGTTC-3′); mVCAM-1 7 Ig domain-specific primers (sense, 5′-AAGTCTGTGGATGGCTCGT-3′; antisense, 5′-AGATGCGCAGTAGAGTGCAA-3′); VCAM-1/GPI-linked–specific primers (sense, 5′-GATGGGAGAATGAAGTCTCAGATTA; antisense, 5′-TTCAGCTTCCTCACAGAGCA); Tie2-specific primers (sense, 5′-TGTCAATCAGGCCTGGAAATAC-3′; antisense, 5′-GAGGAGGGAGAATGTCACTAAGC-3′)21 or (sense, 5′-CCTTCCTACCTGCTA-3′; antisense 5′-CCACTACACCTTTCTTTACA-3′)35 ;discoidindomainreceptor2(DDR2)–specific primers (sense, 5′-CTGTCGGATGAGCAGGTTAT-3′; antisense, 5′-CTCGGCTCCTTGCTGAAGAA-3′)36 ; and murine hypoxanthine phosphoribosyltransferase (HPRT)–specific primers (sense 5′-GCTGGTGAAAAGGACCTCT-3′; antisense 5′-CACAGGACTAGAACACCTGC-3′)22 or murine β-actin–specific primers (sense, 5′-GCTGTATTCCCCTCCATCGTG-3′, antisense, 5′-CACGGTTGGCCTTAGGGTTCAG-3′).
Enzyme-linked immunosorbent assay (ELISA)
Levels of stromal cell–derived factor 1 (SDF-1) and soluble VCAM-1 (sVCAM-1) were assayed in the cytokine analysis core facility of Fred Hutchinson Cancer Research Center, as previously described.37 SDF-1 and sVCAM-1 kits were purchased from R&D Systems (Minneapolis, MN).
Cell-cycle analysis
Bone marrow cells, permeabilized for 15 minutes in PBS with 10 mM EDTA and 0.1% Triton X-100, were mixed with propidium iodide at 2 μg/mL and analyzed by FACS using CELLQuest and ModFit LT (Verity, Topsham, ME) software.
Statistical tests
Tests of significance were performed using the Student t test (Microsoft Excel 2000 software; Microsoft, Redmond, WA).
Results
VCAM-1 expression in hematopoietic cells
VCAM-1 expression in hematopoietic cells was assessed by flow cytometry using well-characterized antibodies. In control mice of young or old age (α4f/f/cre–; Figure 1), nearly half of all BM cells were VCAM-1+ (Figure 2A). The proportion of VCAM-1+ cells was lower in the spleen. Cells from all hematopoietic lineages were represented in the BM VCAM-1+ population of VCAM-1f/f mice (Gr-1+, Mac-1+, CD3+, B220+, or TER119+ cells) and 59.6% of kit+ cells were VCAM-1+ (Figure 2A). We also examined the expression of VCAM-1 at the RNA level in BM sorted cells (Gr1+/VCAM-1+ and Gr1+/VCAM-1–) and by immunohistochemistry. In Figure 3 (top 2 panels), a broad VCAM-1 positivity in BM, consistent with scattered positive cells, is seen. In the spleen there is positivity throughout the red pulp, reflecting the presence of VCAM-1+ myeloid cells. Within the largely negative germinal centers, small groups of cells highly positive for VCAM-1 are seen, likely representing follicular dendritic cells.14 Importantly, BM and spleen from VCAM-1–deficient animals showed no staining with VCAM-1 (Figure 3, right panels) and proportions of VCAM-1+ cells by FACS were also very low (Figure 2).
In contrast to high VCAM-1 positivity in the hematopoietic tissues of normal mice, circulating cells were virtually devoid of VCAM-1 expression and as such, they were no different from VCAM-1–ablated mice (Figure 2B).
VCAM-1 expression in BM cells of several lineages and its absence from PB. (A) Dot blots of BM cells from control (f/f) and ablated (Δ/Δ) mice doubly labeled with anti-CD45, anti–c-kit, or lineage-affiliated markers (Gr1, Mac-1, B220, CD3, TER119) and with VCAM-1. Note the presence of VCAM-1 positivity in all lineages in normal BM cells and its virtual absence from VCAM-1–ablated animals. VCAM-1 expression is also seen at the RNA level (reverse transcriptase [RT]–PCR was performed on total RNA isolated from unsorted and sorted Gr1+/VCAM-1+ and Gr1+/VCAM-1– bone marrow cells; see designated boxes). (B) Expression of VCAM-1 in BM or PB kit+ cells before and 5 days after G-CSF mobilization in normal (VCAM-1f/f) mice. BM or PB cells were doubly labeled with anti–c-kit and anti–VCAM-1 and the expression levels of both are depicted in the dot blots. In BM, note the increase in kit+ cells, but the decreased proportion of VCAM-1+ cells after G-CSF–induced mobilization; in PB, note the absence of VCAM-1 positivity both before and after mobilization. Numbers in dot plots indicate the percentage of positive cells. (C) VCAM-1 RNA expression in mobilized and nonmobilized BM cells from a control animal. Total RNA was reverse transcribed and PCR for VCAM-1 was performed on serially diluted cDNA templates. Note the decrease in RNA expression for both 7-domain (VCAM-1 7D) and GPI-linked (VCAM-1/GPI) VCAM-1 isoforms in mobilized (+G/FL) versus nonmobilized (–G/FL) BM cells.
VCAM-1 expression in BM cells of several lineages and its absence from PB. (A) Dot blots of BM cells from control (f/f) and ablated (Δ/Δ) mice doubly labeled with anti-CD45, anti–c-kit, or lineage-affiliated markers (Gr1, Mac-1, B220, CD3, TER119) and with VCAM-1. Note the presence of VCAM-1 positivity in all lineages in normal BM cells and its virtual absence from VCAM-1–ablated animals. VCAM-1 expression is also seen at the RNA level (reverse transcriptase [RT]–PCR was performed on total RNA isolated from unsorted and sorted Gr1+/VCAM-1+ and Gr1+/VCAM-1– bone marrow cells; see designated boxes). (B) Expression of VCAM-1 in BM or PB kit+ cells before and 5 days after G-CSF mobilization in normal (VCAM-1f/f) mice. BM or PB cells were doubly labeled with anti–c-kit and anti–VCAM-1 and the expression levels of both are depicted in the dot blots. In BM, note the increase in kit+ cells, but the decreased proportion of VCAM-1+ cells after G-CSF–induced mobilization; in PB, note the absence of VCAM-1 positivity both before and after mobilization. Numbers in dot plots indicate the percentage of positive cells. (C) VCAM-1 RNA expression in mobilized and nonmobilized BM cells from a control animal. Total RNA was reverse transcribed and PCR for VCAM-1 was performed on serially diluted cDNA templates. Note the decrease in RNA expression for both 7-domain (VCAM-1 7D) and GPI-linked (VCAM-1/GPI) VCAM-1 isoforms in mobilized (+G/FL) versus nonmobilized (–G/FL) BM cells.
VCAM-1 expression in nonhematopoietic cells
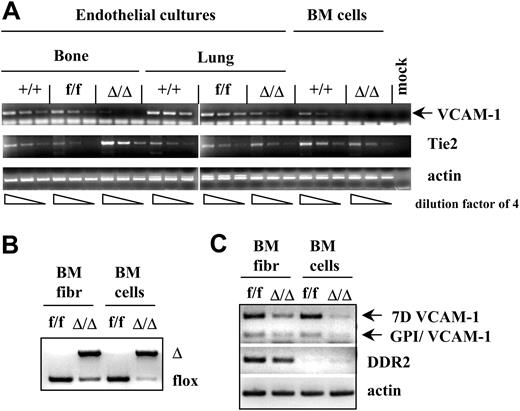
VCAM-1 expression in nonhematopoietic cells was initially assayed by immunohistochemical analyses of tissues. In control mice, endothelial cells in the BM, spleen, and lymphoid tissues (lymphatic and blood vessels) were clearly positive for VCAM-1, as were some stromal cells of the thymus (Figure 3). There were no VCAM-1+ endothelial cells in tissues from ablated mice, although rare, scattered positive cells could be seen. We next initiated endothelial cell cultures from bone, lung, or heart, and tested them after in vitro expansion. Expression of endothelial cell markers (Tie2, VWF, diI-Ac-LDL, and isolectin B4 uptake38 ) was seen in all cultures by immunofluorescence labeling, and the cells formed tubular networks in Matrigel (Becton Dickinson, Bedford, MA; see also Supplemental Figure S1B, available at the Blood website [see the Supplemental Materials link at the top of the online article]). VCAM-1 mRNA was detected in endothelial cell cultures from bone, lung, and heart (Figure 4 and data not shown) from control animals, and at much lower levels in endothelial cell cultures from ablated animals, in contrast to the virtual absence of VCAM-1 mRNA from hematopoietic cells residing in bone marrow from the same animals (Figure 4).
Immunohistochemistry of frozen sections from several tissues. Note the intense staining of myeloid cells in bone marrow (BM) and the red pulp of the spleen (SPL), and in addition, the labeling of blood and lymphatic vessels clearly seen in lymphoid tissues (Peyer patches [PP], thymus and lymph node [LN] stained with an anti–VCAM-1 antibody, as described in “Materials and methods”). Slides were permanently mounted, and photographs were taken using a Nikon COOLPIX 995 digital camera (Nikon USA, Melville, NY) with an adapter that fits into the eyepiece of an Olympus BH-2 microscope (Olympus America, Melville, NY). A 10×/0.25 objective was used for all except the sections from thymus, for which a 20×/0.40 objective was used. These were transferred directly to the computer, and Photoshop 7.0 (Adobe Systems, San Jose, CA) was used to adjust brightness, contrast, and color balance.
Immunohistochemistry of frozen sections from several tissues. Note the intense staining of myeloid cells in bone marrow (BM) and the red pulp of the spleen (SPL), and in addition, the labeling of blood and lymphatic vessels clearly seen in lymphoid tissues (Peyer patches [PP], thymus and lymph node [LN] stained with an anti–VCAM-1 antibody, as described in “Materials and methods”). Slides were permanently mounted, and photographs were taken using a Nikon COOLPIX 995 digital camera (Nikon USA, Melville, NY) with an adapter that fits into the eyepiece of an Olympus BH-2 microscope (Olympus America, Melville, NY). A 10×/0.25 objective was used for all except the sections from thymus, for which a 20×/0.40 objective was used. These were transferred directly to the computer, and Photoshop 7.0 (Adobe Systems, San Jose, CA) was used to adjust brightness, contrast, and color balance.
In addition to endothelial cell cultures, the level of VCAM-1 ablation was tested in stromal cell cultures (positive for DDR-2 and procollagen 1α1; Figure 4 and data not shown) from crushed bones. Unexpectedly, we found a significant level of VCAM-1 gene excision in fibroblast cultures from ablated animals (Figure 4), similar to that seen in some endothelial cell cultures. Parallel cultures of early passage fibroblasts from control animals showed VCAM-1 expression by virtually all CD45– cells (Supplemental Figure S2B). Also, Tie2 mRNA was detected in cultured fibroblasts (Supplemental Figure S2A), but at lower levels than in cultured endothelial cells.
Basal hematopoiesis in VCAM-1–ablated animals in the presence or absence of the spleen
Bone marrow cellularity and progenitor content were tested in VCAM-1–ablated young animals (9-14 weeks old) as well as in older animals (> 40 weeks old) to uncover age-related changes. As seen in Table 1, BM cellularity of young adult VCAM-1–ablated animals was lower than controls. This difference, likely attributable partially to their smaller (about 10%) weight, was maintained when older animals from both groups were compared. There was a moderate decrease in BM progenitor content in young ablated animals, but in older animals of either genotype, no significant differences were seen, although progenitors were significantly increased in both, consistent with previously published data.39 The proportion of lineage-affiliated cells, Gr-1+, Mac1+, and TER119+, in BM was not significantly different between the 2 groups (Supplemental Table S1). However, there were lower levels of T cells (P < .05) and B220+ cells.
A modest but significant increase was seen in spleen cellularity of ablated animals, which persisted with age, when the total cellularity increased in both groups (Table 1). There were also more splenic progenitors in ablated animals, both young and old, but no significant differences in the proportion of B cells, T cells, myeloid, or TER119+ erythroid cells in the spleen.
Significant and consistent differences in circulating cells were found between ablated and control groups. Circulating white blood cells (WBCs) were significantly higher in the ablated group (Table 1), with a higher proportion of B220+ cells and a relative decrease in Mac-1+ cells (Supplemental Table S1). More pronounced and sustained differences were seen in the number of circulating hematopoietic progenitors (CFU-Cs). It is notable that the progenitor differences in peripheral blood between VCAM-1–ablated animals and controls were maintained with age (Table 1).
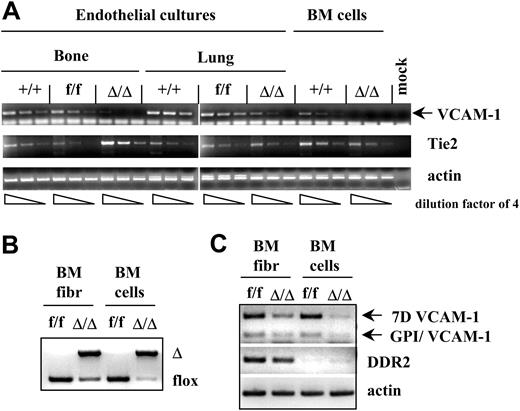
VCAM-1 ablation in endothelial and fibroblast cell cultures. (A) VCAM-1 RNA expression is significantly decreased in endothelial cultures from bone or lung of VCAM-1Δ/Δ mice compared with controls. By contrast, no significant differences are seen in Tie2 expression in all groups. Endothelial cultures were established from the bones and lungs of the wild type (+/+), VCAM-1f/f, and VCAM-1Δ/Δ mice. Total RNA was isolated from these cultures, as well as from uncultured bone marrow cells, and reverse transcribed using oligo-dT primer. PCR was performed on serially diluted RT templates (dilution factor of 4) with VCAM-1–, Tie2- or actin-specific primer sets. (B) Partial deletion of the VCAM-1 gene in the fibroblast cultures from the bone marrow of VCAM-1Δ/Δ mice. PCR analysis of genomic DNA isolated from fibroblast cultures and uncultured BM cells of VCAM-1f/f and VCAM-1Δ/Δ mice was done to show the floxed and Δ alleles of the VCAM-1 gene. (C) VCAM-1 RNA expression is decreased in fibroblast cultures of VCAM-1Δ/Δ mice compared with control-cultured fibroblasts. Total RNA was isolated from the bone marrow fibroblast cultures, as well as from uncultured BM cells of VCAM-1f/f and VCAM-1Δ/Δ mice, and reverse transcribed using oligo-dT primer. PCR was performed with VCAM-1–specific primers recognizing the long VCAM-1 isoform (7D VCAM-1) or GPI-linked VCAM-1 isoform (GPI/VCAM-1), as well as with primers for the fibroblast-specific receptor DDR2, and actin-specific primers as internal controls.
VCAM-1 ablation in endothelial and fibroblast cell cultures. (A) VCAM-1 RNA expression is significantly decreased in endothelial cultures from bone or lung of VCAM-1Δ/Δ mice compared with controls. By contrast, no significant differences are seen in Tie2 expression in all groups. Endothelial cultures were established from the bones and lungs of the wild type (+/+), VCAM-1f/f, and VCAM-1Δ/Δ mice. Total RNA was isolated from these cultures, as well as from uncultured bone marrow cells, and reverse transcribed using oligo-dT primer. PCR was performed on serially diluted RT templates (dilution factor of 4) with VCAM-1–, Tie2- or actin-specific primer sets. (B) Partial deletion of the VCAM-1 gene in the fibroblast cultures from the bone marrow of VCAM-1Δ/Δ mice. PCR analysis of genomic DNA isolated from fibroblast cultures and uncultured BM cells of VCAM-1f/f and VCAM-1Δ/Δ mice was done to show the floxed and Δ alleles of the VCAM-1 gene. (C) VCAM-1 RNA expression is decreased in fibroblast cultures of VCAM-1Δ/Δ mice compared with control-cultured fibroblasts. Total RNA was isolated from the bone marrow fibroblast cultures, as well as from uncultured BM cells of VCAM-1f/f and VCAM-1Δ/Δ mice, and reverse transcribed using oligo-dT primer. PCR was performed with VCAM-1–specific primers recognizing the long VCAM-1 isoform (7D VCAM-1) or GPI-linked VCAM-1 isoform (GPI/VCAM-1), as well as with primers for the fibroblast-specific receptor DDR2, and actin-specific primers as internal controls.
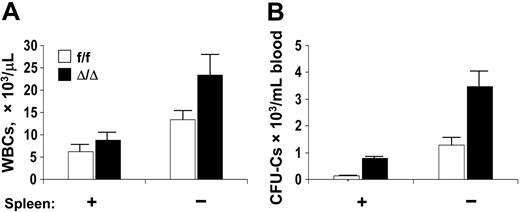
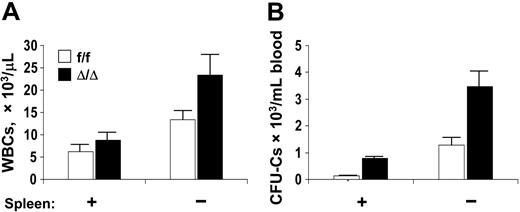
Since cellularity and progenitor content in the spleen were higher in VCAM-1–ablated mice, we suspected that the spleen continuously sequesters progenitor cells from circulation, so we studied splenectomized animals. Both WBCs and circulating progenitors increased (Figure 5) in splenectomized mice (5.3-fold for CFU-GMs, 22.4-fold for BFU-Es, and 6.2-fold for CFU-GEMMs). In the BM of splenectomized animals, there was an increased CFU-C content per femur (149 376 ± 15 131/femur in controls compared with 112 065 ± 17 447 in the ablated group), but no significant differences in the proportion of different lineages within this population. It is speculated that the increase in progenitor content in the marrow after splenectomy is attributable at least in part to some rehoming of circulating progenitor cells.40
Mobilization of hematopoietic progenitors in VCAM-1–deficient adult mice
To test the ability of VCAM-1Δ/Δ mice to mobilize hematopoietic progenitors beyond their increased levels at baseline, we treated them with several mobilizing agents. First, they were treated with anti–VLA-4 antibody. In contrast to the expected and observed responses in control animals, both in the WBCs (10 761 ± 1230 vs 5040 ± 480/μL at baseline) and in the CFU-Cs (736 ± 172/mL vs 47 ± 6.3 at baseline; n = 5; P < .05), there was no significant response from VCAM-1–ablated mice (WBCs, 9965 ± 1148/μL vs 9970 ± 1120; CFU-Cs, 972 ± 181 vs 683.6 ± 165/mL at baseline; n = 5; P = .28). These data provided assurance that the previously described anti–VLA-4–induced mobilization41 was due to the disruption of VLA-4 interaction with VCAM-1 rather than other α4 integrin ligands, and unlikely to be the result of extraneous antibody effects.
We then examined the response to G-CSF (G-CSF-5d; Figure 6). WBCs increased similarly in VCAM-1f/f and VCAM-1Δ/Δ mice (P > .5), but mobilized CFU-Cs in blood were significantly higher in VCAM-1–deficient animals (P < .05). At peak mobilization, BM cellularity was reduced both in VCAM-1f/f mice and VCAM-+Δ/Δ animals (P < .05), but progenitor content in BM was not significantly different between the two groups. Spleen cellularity increased in both groups, but was higher in controls than in VCAM-1–deficient spleens (P<.01). Spleen progenitor content was also significantly higher in controls (Figure 6) compared with ablated animals (P < .05). Consistent with the increased proliferative activity in spleen, the proportion of kit+ cells was higher in control spleens compared with VCAM-1–deficient spleens (29.3% ± 1.0% vs 16.76% ± 0.5%, respectively; P < .001). Furthermore, following G-CSF treatment, VCAM-1+ cells in the spleens of VCAM-1f/f animals were significantly increased compared with untreated animals (38.85% vs 22.26%). This increased expression was confirmed by immunohistochemistry after mobilization (data not shown). In contrast to spleen, control mice had a decrease in VCAM-1 protein expression in BM at the end of cytokine treatment (Figure 2B).
Since there was considerably less CFU-C accumulation in the spleens of VCAM-1Δ/Δ mice after G-CSF mobilization (Figure 6), the total progenitor level from all hematopoietic organs (total BM, total blood volume, and spleen) was significantly reduced between ablated animals and controls (1.82 ± 0.2 × 106 CFU-Cs in f/f vs 1.28 ± 0.1 × 106 in Δ/Δ; P = .04). To test whether the increased numbers in blood were simply due to decreased progenitor return to tissues, or decreased adhering to tissue vascular beds, rather than to increased progenitor release from the BM, we followed the kinetics of progenitor clearance from the blood after stopping G-CSF administration. As seen in Figure 6B, the rate of reduction in circulating progenitor cells after cessation of G-CSF treatment was similar in control and VCAM-1–ablated animals, suggesting that the higher CFU-C levels in blood are not the result of decreased migration to tissues and/or increased survival in circulation.
To further pursue the issue of decreased splenic response in ablated mice, we used more intense mobilization with combinations of G-CSF and FL.42-44 Following a 7-day mobilization with G-CSF plus FL, a strong response was elicited by both control and VCAM-1–ablated animals well above levels achieved with either cytokine alone (Figure 6). However, the total progenitor content in BM, spleen, and blood was again higher in control mice (7.64 ± 2.1 × 106 CFU-Cs in f/f vs 4.43 ± 1.35 × 106 in Δ/Δ; P = .21), largely as a result of differences in splenic progenitor content, and consistent with data after only G-CSF mobilization (Figure 6). Cell-cycle analyses in BM and spleen also suggested decreased cycling in spleens of ablated mice after mobilization (Supplemental Table S2). Moreover, in G-CSF plus FL–mobilized normal animals, there was a significant decrease in VCAM-1 protein (from 49.63% ± 2.87% to 14.8% ± 0.4% VCAM-1+ cells) and mRNA expression (Figure 2C) in BM compared with baseline levels.
Circulating WBCs and CFU-Cs in a cohort of control and VCAM-1–ablated mice, before and 8 weeks after splenectomy. (A) WBCs; (B) CFU-Cs. Mice were splenectomized at 9 to 14 weeks of age and tested 8 weeks after splenectomy (Spleen: –). Note significant increases in circulating CFU-Cs after splenectomy. Error bars indicate standard error of the mean (SEM).
Circulating WBCs and CFU-Cs in a cohort of control and VCAM-1–ablated mice, before and 8 weeks after splenectomy. (A) WBCs; (B) CFU-Cs. Mice were splenectomized at 9 to 14 weeks of age and tested 8 weeks after splenectomy (Spleen: –). Note significant increases in circulating CFU-Cs after splenectomy. Error bars indicate standard error of the mean (SEM).
Response of VCAM-1Δ/Δ animals and controls to treatments by single or combinations of mobilizing agents. (A) Controls or VCAM-1–ablated mice were treated with cyclophosphamide ([CY]; single dose and tested at day 8 after cyclophosphamide), G-CSF alone (G-5d), Flt-3 ligand alone (FL-7d), or G-CSF+FL (G+FL-7d) and evaluated at the end of treatments. BM values refer to calculated CFU-C content of total bone marrow on the basis of measured femur content and its contribution to total bone marrow.51 PB refers to total circulating CFU-Cs, assuming a blood volume of 2 mL, and spleen CFU-Cs are based on splenic cellularity. The large asterisk indicates P < .05 between f/f and Δ/Δ mice. Note that in the single treatments (G-CSF, FL, CY) circulating PB CFU-Cs were much higher in the ablated group. There was a trend for lower spleen values in ablated mice in all treatments, but statistical significance was seen only in G-CSF (5-day) treatments. A significant proliferative response in BM was seen in both sets of animals with FL treatments and less so in other treatments, but there were no significant differences between the 2 groups. (B) The kinetics of circulating CFU-C disappearance following cessation of G-CSF treatment were studied in one experiment and found to be similar between VCAM-1f/f and VCAM-1Δ/Δ mice. Error bars indicate SEM.
Response of VCAM-1Δ/Δ animals and controls to treatments by single or combinations of mobilizing agents. (A) Controls or VCAM-1–ablated mice were treated with cyclophosphamide ([CY]; single dose and tested at day 8 after cyclophosphamide), G-CSF alone (G-5d), Flt-3 ligand alone (FL-7d), or G-CSF+FL (G+FL-7d) and evaluated at the end of treatments. BM values refer to calculated CFU-C content of total bone marrow on the basis of measured femur content and its contribution to total bone marrow.51 PB refers to total circulating CFU-Cs, assuming a blood volume of 2 mL, and spleen CFU-Cs are based on splenic cellularity. The large asterisk indicates P < .05 between f/f and Δ/Δ mice. Note that in the single treatments (G-CSF, FL, CY) circulating PB CFU-Cs were much higher in the ablated group. There was a trend for lower spleen values in ablated mice in all treatments, but statistical significance was seen only in G-CSF (5-day) treatments. A significant proliferative response in BM was seen in both sets of animals with FL treatments and less so in other treatments, but there were no significant differences between the 2 groups. (B) The kinetics of circulating CFU-C disappearance following cessation of G-CSF treatment were studied in one experiment and found to be similar between VCAM-1f/f and VCAM-1Δ/Δ mice. Error bars indicate SEM.
There was very little VCAM-1 expression in circulating cells after mobilization in both control and deficient animals (Figure 2B). To explore whether VCAM-1 expression could be induced in mobilized cells, the latter were cultured in a cytokine cocktail. After 3 days in culture and at a time when there was no appreciable increase in cell number, there were significant increases in VCAM-1 mRNA (Figure 7) and protein expression (from < 1% to 10.1%). There was an additional increase (to 28.6%) in VCAM-1+ cells at day 6 following cell proliferation. To test whether all proliferating cells express or activate VCAM-1 expression, we also cultured splenocytes under IL-7 and IL-2 stimulation. Despite significant proliferation, the latter failed to show VCAM-1 expression (Figure 7). Further, since VCAM-1 can be up-regulated in nonproliferating endothelial cells by inflammatory stimuli, we also tested induction of VCAM-1 expression in mature hematopoietic cells from control mice following an inflammatory stimulus (thioglycolate-induced peritonitis). Cells accumulated in high numbers in the inflamed peritoneum, but no expression of VCAM-1 was seen at either 4 or 16 hours after thioglycolate injection (data not shown).
SDF-1 and sVCAM-1 levels after mobilization
Because of the previously emphasized role of SDF-1 in mobilization, we explored changes in SDF-1 levels in BM and PB after mobilization. Significant decreases in BM SDF-1 levels at the peak of mobilization were seen in wild-type, VCAM-1f/f, and VCAM-1Δ/Δ mice (Table 2). This is consistent with several previous studies45 and references therein. Moreover, sVCAM-1 was also decreased in BM of all G-CSF–treated animals (Table 2). Variable levels of SDF-1 were present in the PB, but increases in sVCAM-1 were seen in control animals (P < .001), as previously reported.36 VCAM-1–ablated animals showed lower levels of sVCAM-1 in BM and circulation, consistent with incomplete ablation of VCAM-1 in certain populations of VCAM-1–expressing cells.
Discussion
VCAM-1 expression in hematopoietic cells is tissue associated
Although VCAM-1+ cells of hematopoietic origin have been previously noted, no systematic studies of VCAM-1 expression patterns among hematopoietic cells at baseline and following stress have been reported.
VCAM-1 expression in normal BM is present among cells of all lineages, both immature and mature. The overall expression of VCAM-1 in almost half of BM cells is consistent with VCAM-1 expression in a subset of progenitor cells or kit+ cells, and likely a subset of primitive stem cells, although further studies are needed to document this notion. Thus, VCAM-1 expression in at least a common myeloid progenitor (CMP), may question a prior conclusion26 about the expression and functional influence of VCAM-1 only in late cells, beyond the stage of common progenitors.
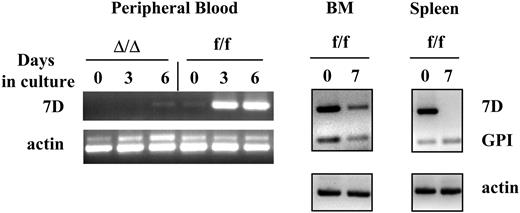
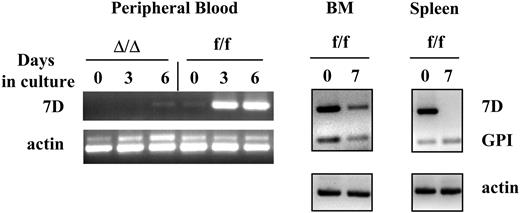
VCAM-1 is expressed in proliferating myeloid cells in vitro from BM or PB, but not in proliferating lymphoid cells. Mononuclear cells from the bone marrow or peripheral blood, or cells isolated from the spleen of VCAM-1f/f or VCAM-1Δ/Δ mice were cultured for up to 7 days as described in “Materials and methods.” Total RNA was isolated and reverse transcribed using oligo-dT primer. PCR was performed using long VCAM-1 isoform-specific primers (7D), GPI-linked VCAM-1 isoform-specific primers (GPI), and actin-specific primers as internal controls. Note that cultured PB cells express VCAM-1 mRNA only after 3 to 6 days in culture with cytokines, but not before, whereas BM cells express VCAM-1 both before and after culture (BMf/f day 0 and day 7). Of interest, cells from the spleen show expression of the 7 domain (full-length) form of VCAM-1 before culture, likely attributed to myeloid cells in spleen, but not after culture under lymphoid conditions. Lymphoid cells proliferating under the stimulus of IL-7 and IL-2 display only the GPI-anchored form.
VCAM-1 is expressed in proliferating myeloid cells in vitro from BM or PB, but not in proliferating lymphoid cells. Mononuclear cells from the bone marrow or peripheral blood, or cells isolated from the spleen of VCAM-1f/f or VCAM-1Δ/Δ mice were cultured for up to 7 days as described in “Materials and methods.” Total RNA was isolated and reverse transcribed using oligo-dT primer. PCR was performed using long VCAM-1 isoform-specific primers (7D), GPI-linked VCAM-1 isoform-specific primers (GPI), and actin-specific primers as internal controls. Note that cultured PB cells express VCAM-1 mRNA only after 3 to 6 days in culture with cytokines, but not before, whereas BM cells express VCAM-1 both before and after culture (BMf/f day 0 and day 7). Of interest, cells from the spleen show expression of the 7 domain (full-length) form of VCAM-1 before culture, likely attributed to myeloid cells in spleen, but not after culture under lymphoid conditions. Lymphoid cells proliferating under the stimulus of IL-7 and IL-2 display only the GPI-anchored form.
In contrast to VCAM-1 positivity of myeloid cells in BM and spleen, it is of interest that PB cells do not display VCAM-1 (Figure 2B). This is true not only for cells during steady-state hematopoiesis, but also after cytokine-induced mobilization, when significant increases in the numbers of progenitor and mature cells are seen in periphery. To explore the reasons for the absence of VCAM-1 expression by PB cells, we considered the following possibilities: whether VCAM-1– cells were preferentially mobilized, both at steady state and after G-CSF; whether VCAM-1 positivity was masked or VCAM-1 expression was silenced once the cells were in the PB environment; or whether another common characteristic of cells in the PB was responsible for the VCAM-1– phenotype. If preferential mobilization of VCAM-1– cells occurred, one would expect enrichment for VCAM-1+ cells in the BM of controls at the peak of mobilization. Instead, a decrease in VCAM-1 expression was found in BM at the peak of mobilization (Figure 2) in G-CSF and a further decrease in G-CSF plus FL (from 49.6% ± 2.8% to 14.8% ± 0.4%). The possibility of a masking epitope was a viable one; therefore, we examined transcription of VCAM-1 in PB cells. We found that PB cells, from animals both mobilized and at steady state, were not only negative for VCAM-1 protein, but had no detectable levels of VCAM-1 mRNA (Figure 7). Next, we explored whether the known differences in cell cycling between PB and BM46,47 might be responsible for differences in VCAM-1 expression. Indeed, after cytokine-induced proliferation in vitro, mobilized cells displayed a significant expression of VCAM-1 mRNA and protein. However, in vitro culture could have generated new progeny with a VCAM-1–expressing phenotype from VCAM-1– cells, but this was unlikely since VCAM-1 levels were increased prior to increases in cell numbers, suggesting activation of VCAM-1 before cell division. Although these data cannot entirely dissociate activation alone versus cells in cycle under a strong cytokine influence, the fact that proliferating lymphoid cells in vitro do not express VCAM-1 suggests some specificity for myeloid cells, or the activation of molecular pathways leading to VCAM-1 expression only in myeloid cells. Whatever the mechanism, differences in VCAM-1 expression between PB and BM or spleen suggest that VCAM-1 expression is actively maintained by tissue-derived signals, but is down-regulated or silenced in the periphery. This view is consistent with absence of VCAM-1 expression in free cells (macrophages) in peritoneal fluid in thioglycolate-induced aseptic peritonitis.
Role of VCAM-1 in hematopoietic progenitor retention at basal hematopoiesis and in mobilization
Studies of basal hematopoiesis in VCAM-1–ablated mice and their age-matched controls reveal certain notable differences. Among them, the most conspicuous is a modest increase in circulating WBCs, but a significant and sustained increase in circulating hematopoietic progenitors. Although an increased progenitor content is seen in the spleen, its enhancement following splenectomy suggests release from the BM with subsequent sequestration in the spleen. Given the extravascular location of hematopoietic cells in BM, how is the enhanced release of progenitor cells explained? Is it secondary to VCAM-1 absence from hematopoietic cells themselves or to VCAM-1 ablation from endothelial cells? We have already argued that a preferential release of VCAM-1– hematopoietic cells present in BM is an unlikely explanation for this phenomenon. Are VCAM-1– endothelial cells responsible? We believe that absence of VCAM-1 not only from endothelial cells, but especially from BM stromal cells, tested for the first time in the present studies, is likely responsible for this process, suggesting a key role for these cells in the retention of (α4 positive) hematopoietic cells within the BM extravascular space. This ongoing, active retention is disrupted in the VCAM-1Δ/Δ mice and a further disruption of this process can be effected through a proteolytic mechanism in G-CSF–induced mobilization. Indeed, histochemical evaluation of VCAM-1 in BM in prior studies48,49 showed marked reduction in VCAM-1 following G-CSF, and this was attributed to a decrease in nonhematopoietic cells.10,11 However, this decrease, in light of our new data showing lower proportions of VCAM-1+ hematopoietic cells in BM (Figure 2) following G-CSF, needs to be reinterpreted, including both hematopoietic cells, and stroma.48,49
A corollary to the decrease of VCAM-1 in normal BM after G-CSF mobilization is the increase in sVCAM-1 in circulation.48 If VCAM-1 cleavage was critical for G-CSF mobilization, one would have expected that VCAM-1–ablated mice would respond less to such a treatment. Contrary to expectations, our mice responded as well as, or, at suboptimal stimulation, better than controls (Figure 6) by displaying enhanced levels of circulating progenitors. Thus, the proteolytic modulation of VCAM-1 in the bone marrow is not the driving force in G-CSF mobilization, a view consistent with results obtained in protease-deficient mice.49 Furthermore, our data suggest that absence of VCAM-1 may facilitate exit from the bone marrow in response to G-CSF–induced signals. This conclusion is consistent with a sustained hematopoietic progenitor egress from the bone marrow in these mice at baseline, similar to one seen in mice with conditional deletion of α4 integrin,33 the principal ligand of VCAM-1, thereby emphasizing the role of the VLA-4/VCAM-1 pathway in the active retention of progenitor cells and their egress following disruption of this pathway.
In contrast to BM showing decreased VCAM-1 levels, there was an increase in VCAM-1 expression by myeloid cells residing in the spleens of normal animals following G-CSF (Supplemental Figure S3). Therefore, proteolytic conditions for VCAM-1 cleavage were only present in BM, in agreement with prior data.48-50 However, since progenitor cells were also sequestered in the spleens of VCAM-1–ablated mice either before or after G-CSF, VCAM-1 in the spleen may not be responsible for their sequestration or entry into the spleen, in contrast to a previous speculation.48 Nevertheless, since mobilized normal mice accumulated more progenitor cells in their spleens compared with mobilized VCAM-1–ablated mice, one has to postulate that in the absence of VCAM-1, either there is a ceiling in splenic sequestration, or that the proliferative expansion of progenitors within the spleen is limited in the ablated animal. Consistent with this notion is the increased proportion of kit+ cells and increased cycling in the spleens of control animals.
In mobilized mice, levels of soluble VCAM-1 in BM were reduced after mobilization, in contrast to PB. Presence of measurable levels of soluble VCAM-1 in VCAM-1–deficient animals (Table 2) provided confirmatory evidence that VCAM-1 is not completely ablated in the vascular beds of all tissues in the deficient mice, as was independently concluded by our RNA studies (Figure 7). Furthermore, SDF-1 levels in BM were reduced in all mice at the time of peak mobilization (Table 2). Whether these observed SDF-1 changes represent the main cause or just another facilitating pathway in mobilization is difficult to state for our or other murine models.
In summary, we have uncovered new information about the biology of VCAM-1 expression in hematopoietic cells by demonstrating that it is constitutively expressed in myeloid cells confined to tissues. Although an association with their proliferative status was seen in vitro and in vivo, the functional significance of VCAM-1 expressed in hematopoietic cells has yet to be explored. Ablation of VCAM-1, in at least a subset of BM fibroblasts, suggests their developmental origin from a Tie2-expressing (mesenchymal?) progenitor, and further studies to pursue this issue are in progress. Increased egress of BM hematopoietic progenitors in the VCAM-1–ablated mouse validates the role of the VCAM-1/VLA-4 pathway in intramarrow retention of hematopoietic progenitors. Further, our postmobilization data provide further clarification about the role of VCAM-1 in G-CSF–induced mobilization.
Prepublished online as Blood First Edition Paper, March 15, 2005; DOI 10.1182/blood-2004-09-3417.
Supported by National Institutes of Health grants DK46 557, HL58 734 (T.P.), and AI047 379 (P.A.K.).
T.U. and L.M.S. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Devra Batdorf for her assistance with mouse care, husbandry, and mouse splenectomy.

![Figure 2. VCAM-1 expression in BM cells of several lineages and its absence from PB. (A) Dot blots of BM cells from control (f/f) and ablated (Δ/Δ) mice doubly labeled with anti-CD45, anti–c-kit, or lineage-affiliated markers (Gr1, Mac-1, B220, CD3, TER119) and with VCAM-1. Note the presence of VCAM-1 positivity in all lineages in normal BM cells and its virtual absence from VCAM-1–ablated animals. VCAM-1 expression is also seen at the RNA level (reverse transcriptase [RT]–PCR was performed on total RNA isolated from unsorted and sorted Gr1+/VCAM-1+ and Gr1+/VCAM-1– bone marrow cells; see designated boxes). (B) Expression of VCAM-1 in BM or PB kit+ cells before and 5 days after G-CSF mobilization in normal (VCAM-1f/f) mice. BM or PB cells were doubly labeled with anti–c-kit and anti–VCAM-1 and the expression levels of both are depicted in the dot blots. In BM, note the increase in kit+ cells, but the decreased proportion of VCAM-1+ cells after G-CSF–induced mobilization; in PB, note the absence of VCAM-1 positivity both before and after mobilization. Numbers in dot plots indicate the percentage of positive cells. (C) VCAM-1 RNA expression in mobilized and nonmobilized BM cells from a control animal. Total RNA was reverse transcribed and PCR for VCAM-1 was performed on serially diluted cDNA templates. Note the decrease in RNA expression for both 7-domain (VCAM-1 7D) and GPI-linked (VCAM-1/GPI) VCAM-1 isoforms in mobilized (+G/FL) versus nonmobilized (–G/FL) BM cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3417/4/m_zh80130580800002.jpeg?Expires=1767840239&Signature=gsJcqwDh3ZvHnLmN7nmIhV~DeoCzQ2z9esxzGsXBVydDbo-kS42FxH16q3VR0iVnGZ95-dpUmNLY6pX0IMN2Ffjq-6~1BfQh7Q~pgF3PPhrK97S7tHv6GeZ3Cd0AYW5tbmiN9aArfoBfzLh3MZrxSrmcEyO6G5QA7Okeit3Ndl3x-W-wxRvLbpyowbNWEcGAXOcm~SUcee4i-BUDXNht0yr53U1PNOk82-pbCib2DomIKqRmAAYfKYCjz0JqsSg3xnT-VzjyRKMKfGFWGd6aAbfi9Vcm19pKlDtm91wxWyustcW49IFLftDkjYxKmcbJOjQIJDXbPd306a1OdYr6QQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Immunohistochemistry of frozen sections from several tissues. Note the intense staining of myeloid cells in bone marrow (BM) and the red pulp of the spleen (SPL), and in addition, the labeling of blood and lymphatic vessels clearly seen in lymphoid tissues (Peyer patches [PP], thymus and lymph node [LN] stained with an anti–VCAM-1 antibody, as described in “Materials and methods”). Slides were permanently mounted, and photographs were taken using a Nikon COOLPIX 995 digital camera (Nikon USA, Melville, NY) with an adapter that fits into the eyepiece of an Olympus BH-2 microscope (Olympus America, Melville, NY). A 10×/0.25 objective was used for all except the sections from thymus, for which a 20×/0.40 objective was used. These were transferred directly to the computer, and Photoshop 7.0 (Adobe Systems, San Jose, CA) was used to adjust brightness, contrast, and color balance.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3417/4/m_zh80130580800003.jpeg?Expires=1767840239&Signature=CxlHe7fv2GTXRkjISKnSUBMzdXrR1UnVON~Ts8ozdbqtPuM7vLZFxl5CIE8Q161H7tqzQoIScURP~cfz2RAK-NJJGgkAJk1r0xNeJi0v52sW1wf69S5wMl6lA4lMPEf5VyZKhuouQwawbv9xHrc101QKnlzG8cuQbTk8XnUoWZhw-VnP7zb7AoSTsWNHM0p7EoSwX8V5XI2~YUj-2jCYkBAi4iZ6RoSnPqvr20CRZ8ArGphQsFS-iwnEgCfKTndegd5E1lh39KAFfHL6u-5HoQuODo0m7NmAu-lVJ7YLrfiFQ~gaOzbMnzAbkluuOH7QBFMu91erRvgUA5e-X7MmHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Response of VCAM-1Δ/Δ animals and controls to treatments by single or combinations of mobilizing agents. (A) Controls or VCAM-1–ablated mice were treated with cyclophosphamide ([CY]; single dose and tested at day 8 after cyclophosphamide), G-CSF alone (G-5d), Flt-3 ligand alone (FL-7d), or G-CSF+FL (G+FL-7d) and evaluated at the end of treatments. BM values refer to calculated CFU-C content of total bone marrow on the basis of measured femur content and its contribution to total bone marrow.51 PB refers to total circulating CFU-Cs, assuming a blood volume of 2 mL, and spleen CFU-Cs are based on splenic cellularity. The large asterisk indicates P < .05 between f/f and Δ/Δ mice. Note that in the single treatments (G-CSF, FL, CY) circulating PB CFU-Cs were much higher in the ablated group. There was a trend for lower spleen values in ablated mice in all treatments, but statistical significance was seen only in G-CSF (5-day) treatments. A significant proliferative response in BM was seen in both sets of animals with FL treatments and less so in other treatments, but there were no significant differences between the 2 groups. (B) The kinetics of circulating CFU-C disappearance following cessation of G-CSF treatment were studied in one experiment and found to be similar between VCAM-1f/f and VCAM-1Δ/Δ mice. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3417/4/m_zh80130580800006.jpeg?Expires=1767840239&Signature=xo2AlanqXz3g02WbVYCaLirl4rpDsp3VWlxzX9Fsf2ccQeTTeQvndKI8SQ4MR3BaLm8bBhXmKojT6LQBYK8d17HekAjruVIy7Uo~tgt9OYo9075at0uk0IdSMxzNSkd7p4oVV8E~hyARKnsC-vgFlJ-3K73tdajr2DhAs9Su0GONNSMeHSbdttQ2MqxWFLgF~yiiWqWbQu~yW4pfJpEHlFXOTx~OfkaXlJ92JWy7npfccadQvGtg2MymEWr8vFv9zEUl-mm6XbvU2wjKSjl9BX3rGB-29d1dSuc5tYjYBOLGpOMbW-jtLTK~fRHZGh9UgboJjA5zwua88fyMkwIwQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. VCAM-1 expression in BM cells of several lineages and its absence from PB. (A) Dot blots of BM cells from control (f/f) and ablated (Δ/Δ) mice doubly labeled with anti-CD45, anti–c-kit, or lineage-affiliated markers (Gr1, Mac-1, B220, CD3, TER119) and with VCAM-1. Note the presence of VCAM-1 positivity in all lineages in normal BM cells and its virtual absence from VCAM-1–ablated animals. VCAM-1 expression is also seen at the RNA level (reverse transcriptase [RT]–PCR was performed on total RNA isolated from unsorted and sorted Gr1+/VCAM-1+ and Gr1+/VCAM-1– bone marrow cells; see designated boxes). (B) Expression of VCAM-1 in BM or PB kit+ cells before and 5 days after G-CSF mobilization in normal (VCAM-1f/f) mice. BM or PB cells were doubly labeled with anti–c-kit and anti–VCAM-1 and the expression levels of both are depicted in the dot blots. In BM, note the increase in kit+ cells, but the decreased proportion of VCAM-1+ cells after G-CSF–induced mobilization; in PB, note the absence of VCAM-1 positivity both before and after mobilization. Numbers in dot plots indicate the percentage of positive cells. (C) VCAM-1 RNA expression in mobilized and nonmobilized BM cells from a control animal. Total RNA was reverse transcribed and PCR for VCAM-1 was performed on serially diluted cDNA templates. Note the decrease in RNA expression for both 7-domain (VCAM-1 7D) and GPI-linked (VCAM-1/GPI) VCAM-1 isoforms in mobilized (+G/FL) versus nonmobilized (–G/FL) BM cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3417/4/m_zh80130580800002.jpeg?Expires=1768210569&Signature=nd3WlDlQjwoD4gDr1xE8IY0ic-VGvc74UPU3H3k-MCtxKqxlirSPBNgtA3YnL4Ao00jaA-PjMsiLWxBnbmC6jnCriDEDB8t763Hm0tblW4t8Z8A6JklgCJEz0-jxuJSlvD6AZGaxb55LoDQhymfCKT4f4zHqKS-HQVlgo4-K9Ua6S7FxJvEEnHxpBiuMcZwoJ3K8DGVn4zVG1MvF8eUBKwTfWUMjgzLDkKcI1VuUT1~WjwAEK739iAuokgvaKecbUsQZun6g9vg55qOfMs7zqRS42MCnS9hDxTpqpAf-q7VLY1CVIKQiR~6ZjqPW5~~iG0eRgJfPiZlNZcQAeUAMmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Immunohistochemistry of frozen sections from several tissues. Note the intense staining of myeloid cells in bone marrow (BM) and the red pulp of the spleen (SPL), and in addition, the labeling of blood and lymphatic vessels clearly seen in lymphoid tissues (Peyer patches [PP], thymus and lymph node [LN] stained with an anti–VCAM-1 antibody, as described in “Materials and methods”). Slides were permanently mounted, and photographs were taken using a Nikon COOLPIX 995 digital camera (Nikon USA, Melville, NY) with an adapter that fits into the eyepiece of an Olympus BH-2 microscope (Olympus America, Melville, NY). A 10×/0.25 objective was used for all except the sections from thymus, for which a 20×/0.40 objective was used. These were transferred directly to the computer, and Photoshop 7.0 (Adobe Systems, San Jose, CA) was used to adjust brightness, contrast, and color balance.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3417/4/m_zh80130580800003.jpeg?Expires=1768210569&Signature=n5ZS555Pd9wM-I4u48pVP~38mSuP3muMYihWrh7OPmaVrAIwr1Tf2zB4ofD1hoDnDbK-1Ydk6u8do9pjXHCTRyU7g26XOjGoODKay2Ssmz8Ym6~p-PU5xHgZHEPrMWWnxGybz4hYpmCRLOlGTokbanPWMCanghzwkGKsCS1ttdXG-AFVx3hoaKWpOFtXUgMgTpLAtUthoNCH1jHM4qvVoVYBMuLEcAPIUVzWYdMjLyg7tET3GOrrc-jfVycfcETdN0yP7fHxEX29Waixjx9r6QpJiWQkSyiuUZ3MApV8GbCEaqCWK3FROQhaAxpZJmUZ4~nahrsNU-wrYtWkCGXXEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Response of VCAM-1Δ/Δ animals and controls to treatments by single or combinations of mobilizing agents. (A) Controls or VCAM-1–ablated mice were treated with cyclophosphamide ([CY]; single dose and tested at day 8 after cyclophosphamide), G-CSF alone (G-5d), Flt-3 ligand alone (FL-7d), or G-CSF+FL (G+FL-7d) and evaluated at the end of treatments. BM values refer to calculated CFU-C content of total bone marrow on the basis of measured femur content and its contribution to total bone marrow.51 PB refers to total circulating CFU-Cs, assuming a blood volume of 2 mL, and spleen CFU-Cs are based on splenic cellularity. The large asterisk indicates P < .05 between f/f and Δ/Δ mice. Note that in the single treatments (G-CSF, FL, CY) circulating PB CFU-Cs were much higher in the ablated group. There was a trend for lower spleen values in ablated mice in all treatments, but statistical significance was seen only in G-CSF (5-day) treatments. A significant proliferative response in BM was seen in both sets of animals with FL treatments and less so in other treatments, but there were no significant differences between the 2 groups. (B) The kinetics of circulating CFU-C disappearance following cessation of G-CSF treatment were studied in one experiment and found to be similar between VCAM-1f/f and VCAM-1Δ/Δ mice. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3417/4/m_zh80130580800006.jpeg?Expires=1768210569&Signature=kPW5Vc~umciz3ZRdA~JcMooT~ODqzkLt1a4ZOKCQ0zZ4YflF6JuKTzLAVV-3BgV4tWHbSn06eoO1flJ2jHa94QjU92y3qpMo-zm5fD7gHqqIDVByKWao2dUj9PB54~q536yRYRgu9NsOFHiQ0w-v~Tq4l1bm84XbEsn1qjaaE-G4CEjmqfvjb-15yHxTYWjVJJpNYZhPwnWv2X7FKCB3TO72gTSU3wIjCNkgTZmomgIIP-5LSRk2beoxD713XCrPXsmFPUAZ5iHN4C6O8eEQmM9jKtz07xlJGfmMdJhU8DcqktghN4niMwY7ip3Sshf6Qjy6WtD16nztYmkvD7B1WA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)