Abstract
The nonobese diabetic/severe combined immune deficiency (NOD/SCID) xenotransplantation model has emerged as a widely used assay for human hematopoietic stem cells; however, barriers still exist that limit engraftment. We previously identified a short-term SCID-repopulating cell (SRC) following direct intrafemoral injection into NOD/SCID mice, whereas others characterized similar SRCs using NOD/SCID mice depleted of natural killer (NK) cell activity. To determine the model that most efficiently detects short-term SRCs, we compared human engraftment in 6 different xenotransplantation models: NOD/SCID-β2-microglobulin-null mice, anti-CD122 (interleukin-2 receptor β [IL-2Rβ])–treated or unmanipulated NOD/SCID mice, each given transplants by intravenous or intrafemoral injection. Human cell engraftment was highest in intrafemorally injected anti-CD122–treated NOD/SCID mice compared to all other groups at 2 and 6 weeks after transplantation. These modifications to the SRC assay provide improved detection of human stem cells and demonstrate that CD122+ cells provide barriers to stem cell engraftment, a finding with potential clinical relevance.
Introduction
The conventional nonobese diabetic/severe combined immune deficiency (NOD/SCID) xenotransplant model provides a powerful tool to characterize human hematopoietic stem cells (HSCs). This system relies on intravenous injection of transplanted cells, with subsequent circulation through the blood prior to homing to appropriate niches in the bone marrow.1,2 Two major limitations of this model are the presence of residual host factors that resist engraftment and the inability to detect stem cells that are incapable of homing. Therefore, due to these limitations of the conventional NOD/SCID xenotransplantation model, some scid-repopulating cells (SRCs) may go undetected.
NOD/SCID-β2-microglobulin-null (NOD/SCID-β2m–/–) mice, in which natural killer (NK) cells are genetically depleted, show a 10-fold increase in efficiency of SRC engraftment.3 Subsequent analysis showed that this model enables detection of a population of CD34+CD38+ short-term repopulating cells undetected in NOD/SCID mice, indicating that immune recognition is an important determinant of host resistance to xenotransplantation of this SRC class.4,5 We previously showed that injection of cells directly into the BM cavity of the femur enabled identification of rapid-SRC (R-SRC) within the Lin–CD34+CD38+/lo subpopulation that generated a robust myeloerythroid graft at 2 weeks after transplantation.6 This indicates that homing and migration factors may also limit SRC engraftment. R-SRCs are critical for stem cell therapies that require rapid engraftment and their characterization necessitates an efficient assay. Thus, in the present paper we evaluate human engraftment in various NOD/SCID models in combination with the intrafemoral and intravenous transplantation routes to determine the most effective assay for characterizing R-SRCs. Because NK cells were previously shown to be an important factor for resisting early engraftment,4,5,7,8 we used NOD/SCID-β2m–/– mice. Additionally, the anti-CD122 antibody directed against the IL-2Rβ chain was injected into NOD/SCID mice because it targets several mature hematopoietic cell populations including NK cells and macrophages.7 Mice were evaluated for the level of human cell engraftment at 2 weeks and 6 weeks to determine the optimal NOD/SCID xenotransplantation model. These experiments showed that anti-CD122–treated NOD/SCID mice given intrafemoral transplants generated the highest engraftment in the injected femur as well as other hematopoietic territories. Therefore, the intrafemoral strategy provides a novel approach to examine the in vivo migratory properties of the injected stem cells as they migrate from the site of injection to other bone marrow niches.
Study design
NOD/SCID mouse repopulation
The NOD/SCID repopulation assay by intravenous or intrafemoral injection was performed as previously described.6 Briefly, the NOD/SCID mice and the NOD/SCID-β2m–/– mice were irradiated at 3.5 Gy and 3.4 Gy, respectively, 24 hours prior to transplantation. Mice treated with anti-CD122 antibody were given injections of 200 μg purified antibody into the intraperitoneal cavity immediately following irradiation. The anti-CD122 monoclonal antibody generated from the hybridoma cell line, TM-β1 (the gift of Dr T. Tanaka, Osaka University Medical Center, Osaka, Japan),9 was purified using the High Trap Protein G Column (Amersham Pharmacia, Piscataway, NJ).
Human cells
Samples of human cord blood were processed and sorted as previously described.6 Lin–D34+CD38+/lo (4-5 × 104) cells were used for the transplants. Human engraftment was evaluated by flow cytometry in the bone marrow of the injected right femur (RF) and noninjected bones (noninjected left femur, 2 tibiae, and the pelvis were designated as bone marrow; BM) for cells transplanted intrafemorally and also in the RF and BM of cells transplanted intravenously. The number of human cells generated in each mouse was calculated by combining the values obtained using a hemocytometer for the RF and BM and multiplied by the human engraftment in each tissue.
Statistical analysis
Data are presented as the mean plus or minusSEM. The significance of the differences between groups was determined by using Student t test and analysis of variance (ANOVA) (SigmaStat software; Jandel, Chicago, IL).
Results and discussion
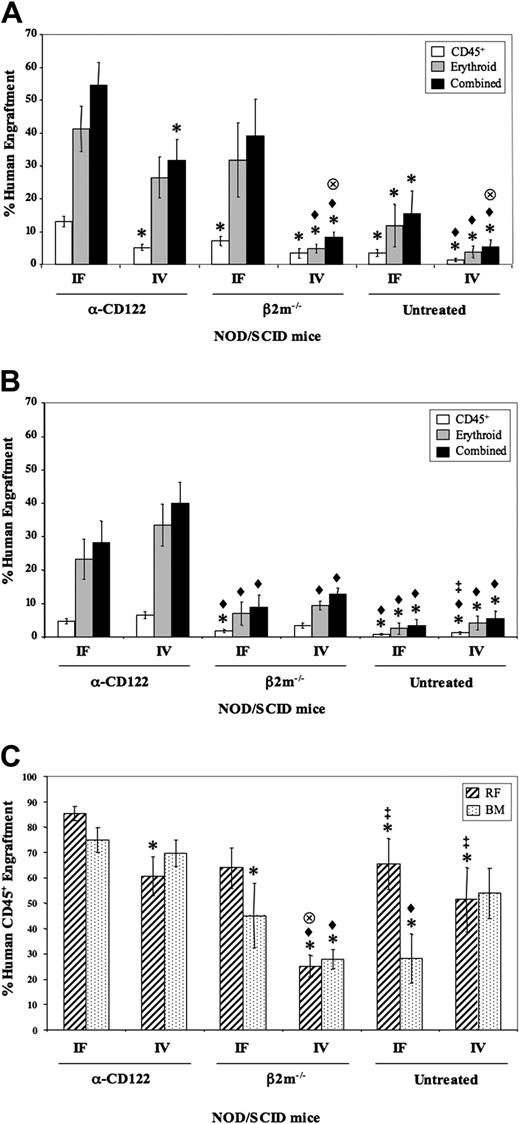
Six NOD/SCID xenotransplant models were examined for human cell engraftment: NOD/SCID-β2m–/– mice, anti-CD122 treated, or untreated NOD/SCID mice that were given transplants of 4-5 × 104 Lin–CD34+CD38+/lo cells intrafemorally or intravenously. The engraftment of myelolymphoid (CD45+) or erythroid (CD45–CD36+GlyA+)6,10 cells, as well as the combined total, was determined for both the injected RF (Figure 1A) and the noninjected bones (BM; Figure 1B) at 2 weeks after transplantation. Those mice given transplants with cells via the intrafemoral procedure and treated with anti-CD122 antibody had significantly higher (P < .05) myelolymphoid engraftment in the RF than all other groups assayed (Figure 1A) with a trend to overall higher total human cell engraftment. Because NOD/SCID-β2m–/– mice having intrafemoral injections had the next highest level of combined human engraftment, these data indicate that R-SRCs are very sensitive to NK cell killing. SRCs within the Lin–CD34+CD38+/lo have engraftment potential at 6 weeks after transplantation. We determined whether depletion of CD122+ cells enhanced human engraftment at this later time point. Similar to the 2-week data, those mice depleted of CD122+ cells had significantly higher (P < .05) human engraftment levels in the RF than all other groups, with a trend toward higher engraftment in the NOD/SCID-β2m–/– mice (Figure 1C).
When only groups given intravenous transplants are considered, the mice depleted of CD122+ cells had significantly higher (P < .05) erythroid and combined total human engraftment in both the RF and BM than all groups including NOD/SCID-β2m–/– mice (Figure 1A-B). Following intravenous transplantation, SRCs must circulate through the blood system prior to homing to appropriate hematopoietic niches; thus, any host resistance factor preventing the cells from reaching their destination within the BM would limit the human graft generated. Therefore, the fact that mice treated with anti-CD122 had higher engraftment compared to NOD/SCID-β2m–/– mice (depleted of NK cells alone) strongly suggests that other CD122+ cells, likely macrophages or monocytes, present in the circulation or the bone marrow mediate a negative effect on human engraftment.
Murine studies have indicated that an important property of HSCs, in steady state, is their exit from the bone marrow, entry to the circulation, and reseeding of other bone marrow niches.11 The intrafemoral method provides a novel approach to examine such in vivo migratory properties of injected SRCs from the site of injection to noninjected bones. We determined that the level of 6-week engraftment was significantly higher (P < .05) in the BM of the CD122-depleted group compared to the other 2 intrafemoral groups, thus providing further support for the concept that CD122+ cells markedly affected R-SRC migration (Figure 1C). The lower engraftment from NOD/SCID-β2m–/– mice again highlights the role of other CD122+ cells apart from NK cells.
Two-week and 6-week human engraftment from Lin–CD34+CD38+/lo SRCs in the RF and BM of various NOD/SCID mouse models. Lin–CD34+CD38+/lo cells were transplanted into 6 different xenotransplantation models: NOD/SCID-β2m–/– mice (β2m–/–), anti-CD122 (α-CD122)–treated or untreated NOD/SCID mice, each intravenous (IV) or intrafemoral (IF) injection. Percentage of human cell engraftment in the RF (A) or BM (noninjected left femur, 2 tibiae, and pelvis BM; B) of 2-week engrafted mice was determined by flow cytometry. Bars represent myelolymphoid (CD45+; □), erythroid (CD45–CD36+ glycophorin A+; ▦), and the combined total (▪) of the human graft (n = 76 mice from 5 experiments). (C) Percentage of human CD45+ cell engraftment in the RF (▨) and BM (▦) of the 6 xenotransplantation models evaluated at 6 weeks after transplantation (n = 34 mice from 3 experiments). *P < .05 versus α-CD122 IF; ♦, P < .05 versus α-CD122 IV;  , P < .05 versus β2m–/–IF; ‡P < .05 versus β2m–/–IV. Error bars represent SEM.
, P < .05 versus β2m–/–IF; ‡P < .05 versus β2m–/–IV. Error bars represent SEM.
Two-week and 6-week human engraftment from Lin–CD34+CD38+/lo SRCs in the RF and BM of various NOD/SCID mouse models. Lin–CD34+CD38+/lo cells were transplanted into 6 different xenotransplantation models: NOD/SCID-β2m–/– mice (β2m–/–), anti-CD122 (α-CD122)–treated or untreated NOD/SCID mice, each intravenous (IV) or intrafemoral (IF) injection. Percentage of human cell engraftment in the RF (A) or BM (noninjected left femur, 2 tibiae, and pelvis BM; B) of 2-week engrafted mice was determined by flow cytometry. Bars represent myelolymphoid (CD45+; □), erythroid (CD45–CD36+ glycophorin A+; ▦), and the combined total (▪) of the human graft (n = 76 mice from 5 experiments). (C) Percentage of human CD45+ cell engraftment in the RF (▨) and BM (▦) of the 6 xenotransplantation models evaluated at 6 weeks after transplantation (n = 34 mice from 3 experiments). *P < .05 versus α-CD122 IF; ♦, P < .05 versus α-CD122 IV;  , P < .05 versus β2m–/–IF; ‡P < .05 versus β2m–/–IV. Error bars represent SEM.
, P < .05 versus β2m–/–IF; ‡P < .05 versus β2m–/–IV. Error bars represent SEM.
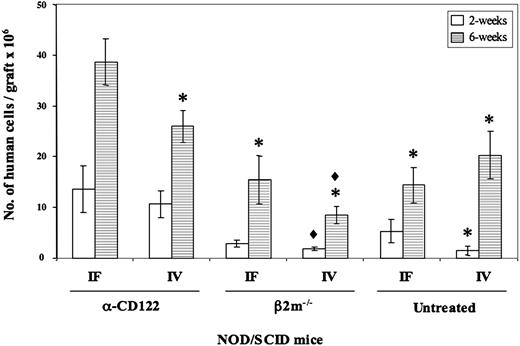
The data presented in Figure 1 reflect the relative level of human engraftment; however, it is important to determine the total proliferative potential of the injected SRCs because robust generation of stem cell–derived progeny would enhance the utility of the xenotransplantation assay. The number of human cells generated from SRCs within the RF and BM of mice given cells intrafemorally and treated with the anti-CD122 antibody showed a trend toward higher levels at 2 weeks that became significant (P < .05) at 6 weeks after transplantation compared to all other groups (Figure 2). This enhanced human repopulation provides a setting for improved analysis of human HSC self-renewal. Serial transplantation is the only method to reliably measure self-renewal, which in the past was often hampered by the low level of engraftment in secondary mice. We have found that the combination of intrafemoral transplantation and anti-CD122 results in enhanced human engraftment in secondary recipients, which when coupled with clonal tracking,12,13 enables fundamental biologic questions of human HSC self-renewal to be addressed.14
Number of human cells generated in the various NOD/SCID mouse models evaluated at 2 weeks and 6 weeks after transplantation. The number of human cells generated in each mouse model was calculated from the RF plus BM at 2 weeks (□) and 6 weeks (▦) after transplantation. *P < .05 versus α-CD122 IF; ♦, P < .05 versus α-CD122 IV. Error bars represent ± SEM.
Number of human cells generated in the various NOD/SCID mouse models evaluated at 2 weeks and 6 weeks after transplantation. The number of human cells generated in each mouse model was calculated from the RF plus BM at 2 weeks (□) and 6 weeks (▦) after transplantation. *P < .05 versus α-CD122 IF; ♦, P < .05 versus α-CD122 IV. Error bars represent ± SEM.
Our data establish that NOD/SCID xenotransplantation models using the intrafemoral transplantation procedure and eradication of CD122+ cells represents the most sensitive assay for SRCs. These modifications to the standard NOD/SCID assay provide a powerful tool to identify novel populations of stem cells and to better characterize their self-renewal potential, thereby giving insight into fundamentally important properties of stem cell biology and transplantation. These data also predict that therapeutic strategies to deplete NK cells and certain subpopulations of monocytes and macrophages in transplant recipients might improve human stem cell transplantation, especially when stem cell numbers are limiting as is the case for cord blood transplantation into adults.
Prepublished online as Blood First Edition Paper, May 5, 2005; DOI 10.1182/blood-2005-03-1081.
Supported by grants from the Stem Cell Network of National Centres of Excellence, the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society, the Canadian Institutes for Health Research, and a Canada Research Chair.
J.L.M. and O.I.G. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank P. Scheufler, P. Savage, and the entire obstetrics unit (Trillium Hospital) for providing cord blood samples; S. Zhao (Hospital for Sick Children) and C. Cantin (Ontario Cancer Institute) for sorting; and T. Tanaka (Osaka University Medical Center) for supplying the TM-β1 hybridoma cell line.




 , P < .05 versus β2m–/–IF; ‡P < .05 versus β2m–/–IV. Error bars represent SEM.
, P < .05 versus β2m–/–IF; ‡P < .05 versus β2m–/–IV. Error bars represent SEM.