Abstract
Activated protein C (APC) anticoagulant activity and the ability to be inhibited by auto-antibodies associated with thrombosis are strongly augmented by the presence of phosphatidylethanolamine (PE) and phospholipid oxidation. β2-glycoprotein I (β2-GPI) is a major antigen for antiphospholipid antibodies present in patients with the antiphospholipid syndrome. We therefore investigated whether anti–β2-GPI monoclonal antibodies (mAbs) could inhibit APC with similar membrane specificity. Five mouse mAbs that reacted with different epitopes on β2-GPI were examined. Each inhibited the PE-, phospholipid oxidation–dependent enhancement of APC anticoagulant activity and required antibody divalency. A chimeric APC that retains anticoagulant activity but is relatively unaffected by protein S, PE, or oxidation was not inhibited by the antibodies. In purified systems, anti–β2-GPI mAb inhibition of factor Va inactivation was greater in the presence of protein S and required β2-GPI. Surprisingly, although the mAbs did increase β2-GPI affinity for membranes, PE and oxidation had little influence on the affinity of the β2-GPI antibody complex for the membrane vesicles. We conclude that antibodies to β2-GPI inhibit APC function specifically and contribute to a hypercoaguable state by disrupting specific protein-protein interactions induced by oxidation of PE-containing membranes.
Introduction
Antiphospholipid antibodies (APAs) are a family of autoantibodies that are often associated with an increased risk of thrombotic disease. This clinical association has been called the “antiphospholipid syndrome.” These antibodies are defined by their ability to bind to negatively charged phospholipid—usually cardiolipin in a protein-cofactor–dependent fashion—in enzyme-linked immunosorbent assay (ELISA) (APAs) or by their ability to inhibit phospholipid-dependent coagulation assays (lupus anticoagulants [LAs]). Suggestions of possible mechanisms by which these antibodies might contribute to hypercoaguability and thrombotic risk have been varied, as have the potential molecular targets of the antibodies (Levine et al1 and Inanc et al2 and references therein). We3,4 and others5-7 have proposed that antibodies that inhibit the protein C anticoagulant pathway would be likely candidates as pathogenic antibodies. The protein C pathway serves as a major regulatory loop that limits thrombin generation through the inactivation of the cofactors, factor VIIIa and factor Va. As little as a 50% inhibition of its activity, either genetic or acquired,8 has been found to be associated with an increased risk of thrombosis. Impairment of this pathway has been correlated with both venous and arterial disease, although the data for the latter are not as clear.9,10
Many APAs are now recognized to be directed toward β2-glycoprotein I (β2-GPI), either alone or in complex with negatively charged phospholipid.11 Such antibodies have also been associated with thrombotic risk in some studies.12-15 β2-GPI is a 50-kDa plasma glycoprotein of unknown function that is capable of binding to anionic phospholipids. The coagulation reactions require negative surfaces to function,16 and it can be envisioned that phospholipid binding proteins could inhibit these reactions. Although there are some reports of direct β2-GPI inhibition of activated protein C (APC) anticoagulant activity,17,18 in general, direct effects of β2-GPI on the coagulation reactions are small,18,19 probably resulting from the low affinity of β2-GPI for phospholipids in the presence of calcium.18,20 Antibody β2-GPI complexes, however, have been found to have higher affinity for membrane than β2-GPI alone.19,21 This may contribute to LA19 and anti-APC activity.12,22-24 However, it is unclear how such antibodies would result in a net procoagulant state. Masking the membrane surface by antibody complexes would be predicted to mimic the effect of oral anticoagulants where all of the vitamin K–dependent proteins lose affinity for membrane surfaces, leading to a net anticoagulant rather than a procoagulant state.
Recent investigations of the membrane requirements of the procoagulant versus anticoagulant complexes have revealed a possible basis for specificity toward the anticoagulant complexes. We have reported that unlike the procoagulant complexes, optimal function of the APC anticoagulant complex requires the presence of phosphatidylethanolamine (PE), polyunsaturated fatty acids,16,25 and phospholipid oxidation.4 However, this membrane composition has little, if any, effect on the procoagulant reactions.4 PE26-28 and oxidation29,30 increase the binding of many APAs and the ability of at least some APAs to inhibit the APC complex specifically.3,4,16,31 Indeed, immunoglobulin G (IgG) purified from several antiphospholipid syndrome patients inhibited only the lipid oxidation enhancement of APC activity, whereas IgG from non-thrombotic patients with APAs or LAs did not.31 The potential clinical relevance of enhanced inhibition of APC function is suggested by the presence of increased oxidized lipids in the antiphospholipid syndrome,30 which we presume would normally enhance APC function. The presence of APAs would block this protective effect of phospholipid oxidation.
Phospholipid oxidation has also been implicated in the ability of APAs to recognize β2-GPI when it is bound to the phospholipid surface.29,32 However, the impact of phospholipid oxidation on inhibition of APC activity by anti–β2-GPI antibodies has not been reported. We have now used mouse monoclonal antibodies (mAbs) raised to purified β2-GPI as a model for antibodies likely to be present in thrombotic antiphospholipid syndrome patients. We demonstrate that antibodies directed toward several domains of β2-GPI share the ability to specifically block the phospholipid oxidation–dependent enhancement of APC activity.
Materials and methods
Proteins and reagents
Human β2-GPI was purified using ion exchange and heparin agarose chromatography as described previously.33 This method does not entail the use of acidic conditions that may alter the structure of the β2-GPI. Human APC; prothrombin; factors Xa, V, and Va; protein S; the protein C–prothrombin gla-domain chimera (PC-PtGla) and the protein C chimera containing the first 22 amino acids of prothrombin (PC-Pt1-22 ); and the factor X activator from Russell viper venom (X-CP) were all prepared as described previously.34 Papain, bovine serum albumin (BSA), gelatin, and all chemicals were purchased from Sigma (St Louis, MO). MEP Hyper Cel was from Life Technologies (Bethesda, MD), Chelex 100 resin was from Bio-Rad (Hercules, CA), and Spectrozyme-TH was from American Diagnostica (Stamford, CT). All phospholipids were from Avanti Polar Lipids (Birmingham, AL). Phospholipids derived from bovine brain were utilized. N-hydroxysulfosuccinimide (Sulfo-NHS)–LC-Biotin and Immuno-Pure alkaline phosphatase–conjugated streptavidin were from Pierce (Rockford, IL).
Liposome preparation
Vesicles were prepared by extrusion as described25 using a 100-nm nucleopore membrane. PS/PC vesicles were 20% phosphatidylserine (PS) and 80% phosphatidylcholine (PC), and PE/PS/PC vesicles were 40% phosphatidylethanolamine (PE), 20% PS, and 40% PC.
Phospholipid oxidation
Liposomes were oxidized with copper sulfate as previously described.4 Briefly, to 1 mL of liposomes (200 μg/mL) was added 1 μL of 10 mM CuSO4 in a glass tube. Suspensions were vortexed to introduce air, and the liposomes were incubated at 37°C. Standard oxidized liposomes were composed of brain-derived phospholipids oxidized for 20 hours.
Preparation of monoclonal β2-GPI antibodies
Five mAbs (1519, 1522, 1527, 1528, and 1529) were obtained from BALB/c mice immunized with human β2-GPI by standard techniques.35 Hybridomas were screened versus purified β2-GPI on non–γ-irradiated polyvinylchloride plates (Falcon, Billerica, Spain). Antibodies for large-scale production were produced using serum-free media (Invitrogen Hybridoma-SFM, Carlsbad, CA). IgG from conditioned media was purified using MEP Hyper Cel chromatography according to the manufacturer's instructions. All mAbs studied were IgG1κ. The purified mAbs recognized β2-GPI in solution, adsorbed on both plain or oxygenated polystyrene plates, and in Western blots under nonreducing conditions. None detected β2-GPI under reduced conditions. For some experiments, purified IgG was biotinylated using the Pierce reagents according to the manufacturer's directions.
Preparation of Fab fragments
Fab fragments were prepared by digestion with papain. Purified IgG (6 mg/mL) was incubated with 30 μg/mL active papain (Sigma) in 0.15 M NaCl, 20 mM Tris (tris(hydroxymethyl)aminomethane) HCl, pH 7.5 (Tris-buffered saline [TBS]), 2 mM EDTA (ethylenediaminetetraacetic acid), and 2 mM dithiothreitol (DTT) for 1 hour at 37°C. The reaction was stopped with 10 mM iodacetamide followed by dialysis against TBS. The reaction mixture was applied to a MonoQ column (Amersham Biosciences, Piscataway, NJ) in TBS EDTA buffer without DTT. The column retained the Fc fragment and undigested IgG but allowed the Fab fragments to pass through. The Fabs were dialyzed against TBS and concentrated.
Characterization of mAb binding to β2-GPI
Standard ELISAs were used to estimate the binding affinity (K1/2) for purified antibody binding to β2-GPI. Briefly, non–γ-irradiated microtiter plates (Falcon) were coated with 100 μL of 1 μg/mL β2-GPI in 0.15 M NaCl, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5 (HEPES-buffered saline [HBS]), overnight at 4°C. After blocking with 5% BSA in HBS, 100 μL of serial dilutions of purified mAbs were added. After 1 hour of incubation, wells were washed and developed with horseradish peroxidase–labeled goat anti–mouse IgG followed by chromogenic substrate. All washes were with HBS containing 0.05% Tween. Of the 5 mAbs studied, 4 had K1/2's for binding between 50 and 150 ng/mL (0.3 to 1 nM) and a fifth (no. 1528) had a K1/2 for binding of more than 10 μg/mL (more than 60 nM) by this method.
The domain specificity of the antibodies was determined by competitive inhibition studies using β2-GPI domain deletion mutants as inhibitors of binding to full-length protein essentially as described.36 The β2-GPI deletion mutants used were kindly provided by Dr G. Michael Iverson, La Jolla Pharmaceutical, San Diego, CA. Briefly, β2-GPI was adsorbed onto Maxisorb plates (Nalge-Nunc, Rochester, NY) and the plates blocked with HBS containing 2% nonfat dry milk. Dilutions of the β2-GPI deletion mutants in HBS–2% milk followed by a fixed, appropriate dilution of biotinylated antibody were then added. After 1 hour of incubation at room temperature, wells were washed with HBS-milk and incubated with alkaline phosphatase–streptavidin followed by alkaline phosphatase chromogenic substrate. Absorbance at 450 nm was measured in a Vmax Microplate Reader (Molecular Devices, Sunnyvale, CA). Controls included full-length β2-GPI as inhibitor of binding and TBS-milk alone.
Coagulation assays
Clotting times were determined by a modified factor Xa one-stage clotting assay using X-CP to activate factor X and an ST4 coagulometer (Diagnostica Stago, Parsippany, NJ) as described previously.4 In the standard assay, normal pooled plasma (50 μL) was mixed with X-CP, phospholipid (10 μg/mL), in the presence or absence of APC (0.2 μg/mL) in a total volume of 150 μL. When present, IgG was at the final concentrations indicated. After 1 minute of incubation at 37°C, 50 μL of 25 mM CaCl2 was added to initiate clotting. X-CP was adjusted to obtain a 30-second clotting time in the absence of APC. Because the assay in the absence of APC is similar but not equivalent to those used clinically to define LA activity, anticoagulant activity observed under these conditions is considered “LA-like” rather than LA.
Measurement of APC activity in purified systems
Factor Va (50 nM) was reacted with 2.5 pM APC, 20 μg/mL oxidized or nonoxidized PE/PS/PC vesicles, in the presence or absence of 20 nM protein S, 1 μM β2-GPI, and/or 1 μM IgG at 37°C. At various times, the APC was inhibited by the addition of benzamidine HCl to 10 mM. After dilution, residual factor Va was determined by standard one-stage clotting assays using factor V–deficient plasma or by its activity in the prothrombinase complex as described previously.25,34
Liposome-protein interactions measured by right-angle light scattering
Right-angle light scattering was performed as described previously34 on an SLM 8000 fluorimeter (SLM Instruments, Urbana, IL) with the wavelength set at 320 nm. The liposome concentration was 10 μg/mL. Binding experiments were performed in HBS with or without 2 mM CaCl2 Similar profiles were obtained in experiments performed on at least 2 separate days.
Results
Effect of anti–β2-GPI monoclonal antibodies on APC anticoagulant activity
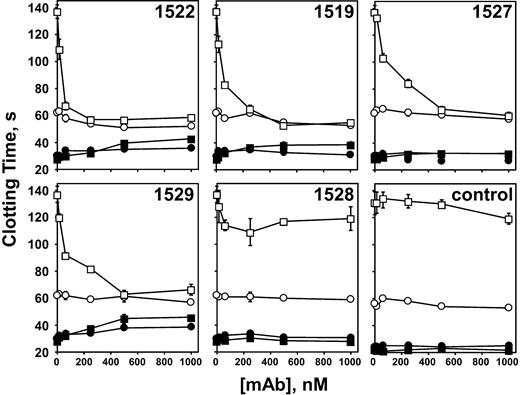
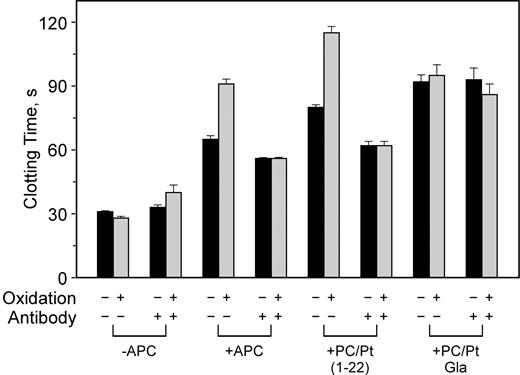
To test the functional ramifications of antibodies to β2-GPI, we generated 5 mouse mAbs and tested their ability to inhibit APC anticoagulant activity preferentially on oxidized PE containing phospholipids. When nonoxidized phospholipid vesicles were used, none of the anti–β2-GPI mAbs had a significant effect on APC activity (Figure 1, open circles). In contrast, 4 of the mAbs completely inhibited the oxidation-dependent augmentation of APC anticoagulant activity, reducing the anticoagulant activity to that observed in the absence of lipid oxidation (Figure 1, open squares). In the absence of APC, the effect of the antibodies was variable. Although mAb nos. 1522 and 1529 exhibited LA-like activity at higher antibody concentrations—lengthening the clotting time at least 2-fold when oxidized phospholipid was used—mAb no. 1527 showed no LA-like activity whether or not the phospholipid was oxidized. Monoclonal antibody no. 1519 showed minimal LA-like activity on oxidized vesicles. Monoclonal antibody no. 1528 showed 25% or less inhibition on oxidized phospholipid and was not studied further. That both PE and oxidation are required for inhibitory activity of the anti–β2-GPI is shown in Figure 2, where antibody no. 1522 had no effect on APC activity on oxidized PS/PC vesicles.
Anti–β2-GPI mAbs inhibit APC anticoagulant activity in a phospholipid oxidation–dependent manner. Plasma clotting was initiated with X-CP as described in “Materials and methods,” in the presence (□, ○) or absence (▪, •) of 0.2 μg/mL APC. Monoclonal IgG was incorporated in the clotting assays at the final concentrations indicated. Nonoxidized phospholipid (○, •); oxidized phospholipid (□, ▪). “Control” indicates addition of an irrelevant mAb IgG at the concentrations indicated. The normal range of the clotting time of pooled normal plasma using unoxidized PE-containing liposomes was 28.9±1.1 seconds SD (n = 15) in the absence of APC and 64.2±2.4 seconds (n = 9) in the presence of APC. The data represent 3 experiments run in duplicate.
Anti–β2-GPI mAbs inhibit APC anticoagulant activity in a phospholipid oxidation–dependent manner. Plasma clotting was initiated with X-CP as described in “Materials and methods,” in the presence (□, ○) or absence (▪, •) of 0.2 μg/mL APC. Monoclonal IgG was incorporated in the clotting assays at the final concentrations indicated. Nonoxidized phospholipid (○, •); oxidized phospholipid (□, ▪). “Control” indicates addition of an irrelevant mAb IgG at the concentrations indicated. The normal range of the clotting time of pooled normal plasma using unoxidized PE-containing liposomes was 28.9±1.1 seconds SD (n = 15) in the absence of APC and 64.2±2.4 seconds (n = 9) in the presence of APC. The data represent 3 experiments run in duplicate.
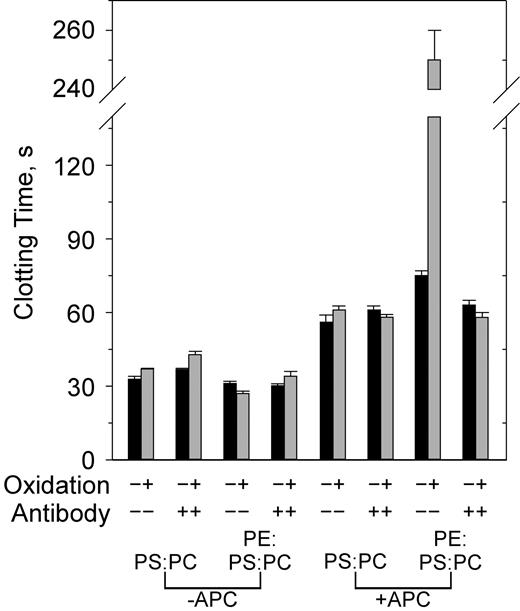
Both PE and oxidation are required to observe inhibition of APC activity by anti–β2-GPI. PS/PC and PE/PS/PC vesicles were either not oxidized (▪) or oxidized (▦) in the presence or absence of anti-β2 mAb no. 1522 (120 nM) and APC (0.4 μg/mL) as indicated on the x-axis. Similar results were obtained with the other antibodies. Error bars indicate the SE of 2 or 3 determinations run in duplicate.
Both PE and oxidation are required to observe inhibition of APC activity by anti–β2-GPI. PS/PC and PE/PS/PC vesicles were either not oxidized (▪) or oxidized (▦) in the presence or absence of anti-β2 mAb no. 1522 (120 nM) and APC (0.4 μg/mL) as indicated on the x-axis. Similar results were obtained with the other antibodies. Error bars indicate the SE of 2 or 3 determinations run in duplicate.
It has been reported that to have APA or LA activity, anti–β2-GPI antibodies must be divalent 19-21,37 However, it has also been proposed that β2-GPI exists in multiple conformations, one of which is stabilized by interaction with oxidized lipid, leading to exposure of the pertinent antibody binding site(s).29,32,38,39 This would imply that antibody binding would also stabilize the lipid binding conformer of β2-GPI, leading to enhanced binding even in the absence of antibody divalency. To distinguish these possibilities, and because the membrane requirements for APC inhibition by APAs differ from those for LA activity,3 Fab fragments of mAb no. 1522 were tested for their ability to inhibit APC function. Although the fragments were able to bind β2-GPI with the expected affinity, they were unable to directly inhibit APC function on PE-containing liposomes, whether or not they were oxidized (data not shown). Thus, the effectiveness of anti–β2-GPI antibodies on APC activity also depends on the bivalency conferred upon β2-GPI by the antibody.
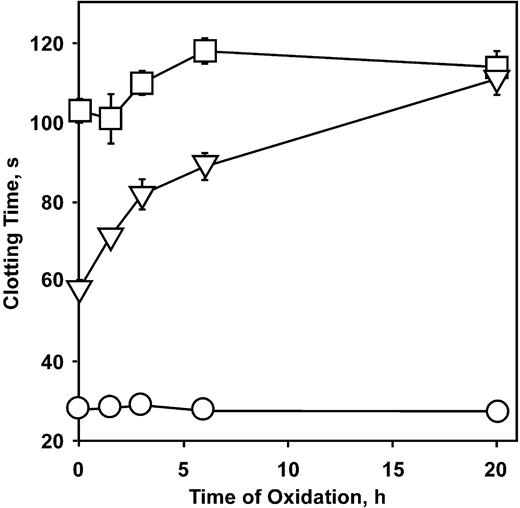
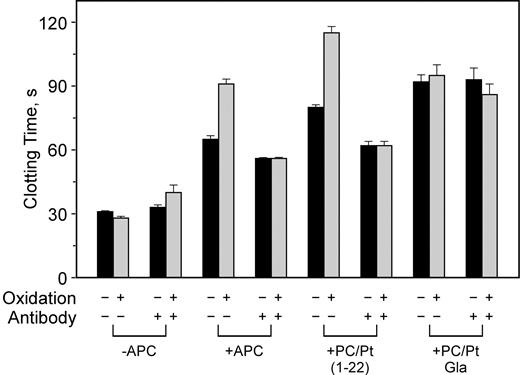
To determine what structures on APC may be necessary to be effectively inhibited by the anti–β2-GPI antibodies, we used chimeric forms of APC that have proven useful in past structure-function studies.34 We have previously shown that the activated PC-PtGla chimera, in which the gla domain of protein C is replaced with that of prothrombin, no longer requires the presence of protein S or PE for optimal activity.34 Oxidation of PE-containing vesicles also has only a minimal effect on the anticoagulant activity (Figure 3). In addition, this enzyme is only minimally affected by patient-derived APAs,31 suggesting it may be a useful therapeutic agent. The chimera in which only the first 22 amino acids have been replaced by the homologous region of prothrombin (PC/Pt1-22 ) retains PE and protein S dependence.34 The effect of mAb no. 1522 on these enzymes is illustrated in Figure 4. The phospholipid oxidation–dependent anticoagulant activity of APC and the PC/Pt1-22 chimera was blocked by this antibody whereas the APC-PtGla was only slightly affected (Figure 4). Similar inhibition profiles of APC and APC-PtGla were observed with all other mAbs (data not shown).
Epitope specificity of the anti–β2-GPI mAbs
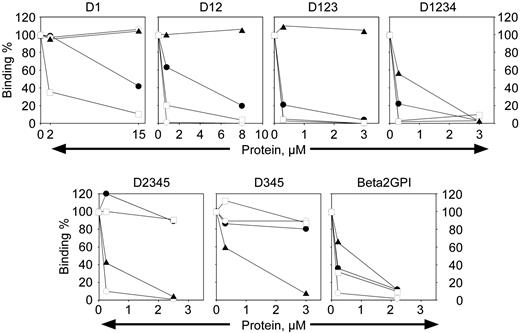
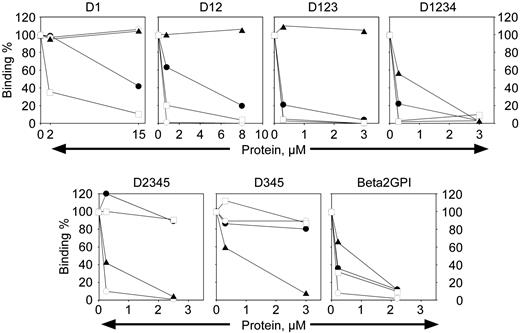
β2-GPI is composed of 4 homologous “sushi” domains and a fifth noncanonic sushi domain where the phospholipid binding site is located.40-42 It has been suggested that antibodies directed to β2-GPI with pathologic function may be limited in their epitope specificity to a particular domain(s), although it is unclear which domain may be of functional significance (de Laat et al,15 Iverson et al,36 and de Laat et al43 and references therein). To determine the domain specificity of anti–β2-GPI mAbs that inhibit the oxidation-dependent activity of APC, deletion mutants of β2-GPI in the soluble phase were used as a soluble competitor with the intact β2-GPI adsorbed to an ELISA plate for binding to each mAb (Figure 5). The name of each construct indicates the domains of β2-GPI that are present in the competing protein fragment. Each biotinylated antibody was present at 5 nM (0.7 μg/mL), a concentration that resulted in 40% to 60% maximal binding depending on the antibody (the K1/2 for 50% binding of directly biotinylated mAbs was not equivalent to that observed using a secondary antibody for development of the ELISAs indicated in “Materials and methods”). It is evident that mAb no. 1529 (Figure 5, open squares) is capable of binding to isolated domain 1 of β2-GPI, because binding to the plate is abrogated by the presence of domain 1 in the soluble phase. Similarly, mAb no. 1522 (Figure 5, open circles) shows competition for binding to β2-GPI by any construct that contains domain 2 in the fragment. Monoclonal antibody no. 1527 (Figure 5, solid triangles) clearly reacts with domain 4. Although mAb no. 1519 (Figure 5, solid circles) binds isolated domain 1 (D1), the affinity is increased at least 10-fold when domain 2 is also present (data not shown). This may indicate an extended epitope that bridges domain 1 and 2, or mAb no. 1519 binds to an epitope whose conformation is greatly affected by the presence of the rest of the molecule. Thus, it is apparent that anti–β2-GPI antibodies that can inhibit the phospholipid oxidation–dependent anticoagulant activity of APC are not restricted with respect to epitope specificity.
Oxidation does not enhance PC-PtGla activity. PE/PS/PC phospholipid liposomes were oxidized with copper sulfate as described in “Materials and methods,” and plasma clotting was measured. Error bars indicate the SE of 3 determinations. □, 0.2 μg/mL APC-PtGla added; □, 0.2 μg/mL APC added; ○, no additions.
Oxidation does not enhance PC-PtGla activity. PE/PS/PC phospholipid liposomes were oxidized with copper sulfate as described in “Materials and methods,” and plasma clotting was measured. Error bars indicate the SE of 3 determinations. □, 0.2 μg/mL APC-PtGla added; □, 0.2 μg/mL APC added; ○, no additions.
Anti–β2-GPI does not affect APC-Pt z-carboxyglutamic acid (Gla) activity but does inhibit APC/Pt1-22 activity. PE/PS/PC vesicles were either not oxidized (▪) or oxidized (▦) and used in clotting assays in the presence or absence of 250 nM anti–β2-GPI mAb no. 1522 and anticoagulant enzyme as indicated on the x-axis. Similar results were obtained with APC and APC-PtGla with the other antibodies. Error bars indicate the SE of 2 determinations run in duplicate.
Anti–β2-GPI does not affect APC-Pt z-carboxyglutamic acid (Gla) activity but does inhibit APC/Pt1-22 activity. PE/PS/PC vesicles were either not oxidized (▪) or oxidized (▦) and used in clotting assays in the presence or absence of 250 nM anti–β2-GPI mAb no. 1522 and anticoagulant enzyme as indicated on the x-axis. Similar results were obtained with APC and APC-PtGla with the other antibodies. Error bars indicate the SE of 2 determinations run in duplicate.
Domain mapping of the anti–β2-GPI mAbs. Monoclonal antibody (5 nM) was mixed with varying concentrations of inhibitor as indicated and tested for binding to intact β2-GPI in ELISA as described in “Materials and methods.” The names over the panels indicate the domains of β2-GPI present in the inhibitor being tested. The percent binding remaining is indicated on the y-axis. □, mAb no. 1529; •, mAb no. 1519; ○, mAb no. 1522; ▴, mAb no. 1527.
Domain mapping of the anti–β2-GPI mAbs. Monoclonal antibody (5 nM) was mixed with varying concentrations of inhibitor as indicated and tested for binding to intact β2-GPI in ELISA as described in “Materials and methods.” The names over the panels indicate the domains of β2-GPI present in the inhibitor being tested. The percent binding remaining is indicated on the y-axis. □, mAb no. 1529; •, mAb no. 1519; ○, mAb no. 1522; ▴, mAb no. 1527.
Effect of monoclonal antibody on APC activity in purified systems
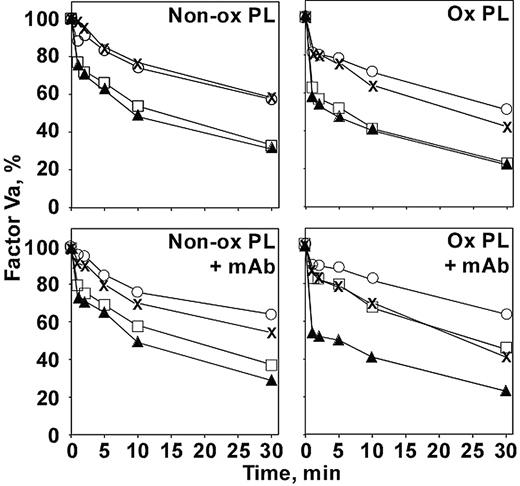
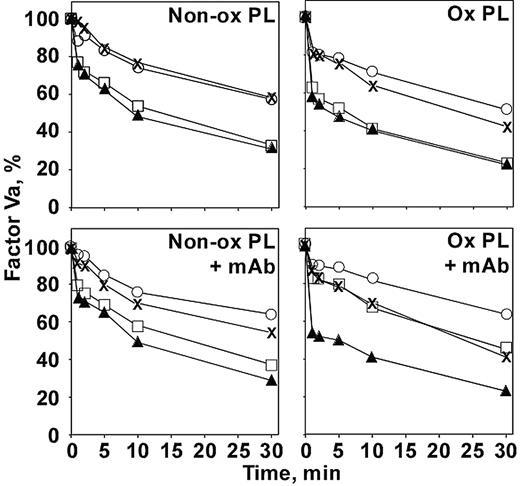
To be certain that the anti–β2-GPI was affecting the APC complex directly and not acting through another plasma component, the effect on factor Va inactivation by APC was investigated in a purified system (Figure 6). Under our conditions, β2-GPI at 1 μM (the approximate concentration present in the plasma clotting assays) did not affect factor Va inactivation in the absence of antibody (Figure 6, upper panels). This is true whether or not protein S was present or whether the phospholipid was oxidized. When nonoxidized phospholipid was used as the membrane surface (Figure 6, left panels), the addition of antibody (Figure 6, lower panels) also did not affect factor Va inactivation under any conditions. When oxidized phospholipid was used (Figure 6, right panels), a different picture emerged when antibody was added (Figure 6, lower right panel). In the absence of β2-GPI (Figure 6, × [–protein S] and solid triangles [+ protein S]), the antibody did not affect the reaction. However, in the presence of β2-GPI (Figure 6, open circles [–protein S] and open squares [+ protein S]), inhibition was observed. The presence of the β2-GPI–antibody complex had its strongest effect when protein S was included (Figure 6, open squares). The protein S enhancement of APC activity was essentially negated on the oxidized phospholipid surface by the antibody complex, returning the rate of factor Va inactivation to that observed in the absence of protein S (Figure 6, ×). Notably, the protein S effect was not abrogated by the antibody on nonoxidized phospholipid (Figure 6, lower left panel, open squares).
Inhibition of APC inactivation of factor Va by anti–β2-GPI requires oxidized phospholipid in purified systems. Factor Va (50 nM) was reacted with APC (2.5 pM) on nonoxidized (left panels) or oxidized (right panels) phospholipid vesicles in the absence (top panels) or presence (bottom panels) of anti–β2-GPI no. 1522 (1 μM) for the times indicated. Factor Va remaining (y-axis) was determined by clotting assay in factor V–deficient plasma as described in “Materials and methods.”× indicates no other additions; ▴, + protein S; ○, +β2-GPI; □, + protein S +β2-GPI. Similar results were obtained with mAbs 1519 and 1529.
Inhibition of APC inactivation of factor Va by anti–β2-GPI requires oxidized phospholipid in purified systems. Factor Va (50 nM) was reacted with APC (2.5 pM) on nonoxidized (left panels) or oxidized (right panels) phospholipid vesicles in the absence (top panels) or presence (bottom panels) of anti–β2-GPI no. 1522 (1 μM) for the times indicated. Factor Va remaining (y-axis) was determined by clotting assay in factor V–deficient plasma as described in “Materials and methods.”× indicates no other additions; ▴, + protein S; ○, +β2-GPI; □, + protein S +β2-GPI. Similar results were obtained with mAbs 1519 and 1529.
Effect of phospholipid composition and oxidation on β2-GPI binding to vesicles
To determine whether the observed functional effect of the β2-GPI–antibody complex was due to enhanced binding of β2-GPI to the oxidized PE-containing liposomes, light-scattering studies were performed. In agreement with previous reports,37 in the absence of calcium, β2-GPI alone had moderately low affinity (about 1.5 μM), which could be improved in the presence of oxidized PE to about 300 nM. However, when 2 mM calcium was added, although somewhat increased on oxidized PE-containing vesicles, the binding was barely detectable up to 4 μM β2-GPI (data not shown). In agreement with the studies of Harper et al,18 it seems unlikely that β2-GPI would be a significant effector of coagulation reactions in the absence of antibody.
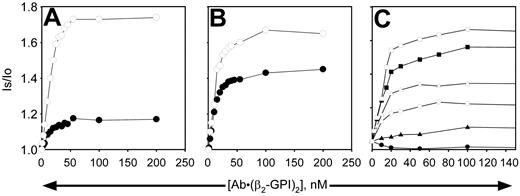
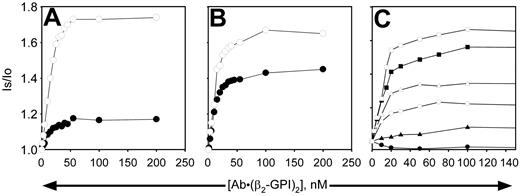
When β2-GPI was complexed to antibody, binding to the vesicles was dramatically enhanced on all phospholipid vesicles, whether or not PE was present and whether or not the phospholipids were oxidized (Figure 7). Due to the tight nature of the binding on all phospholipid vesicles tested, reliable dissociation constants (Kd's) could not be determined, because virtually all of the added protein was bound at low concentrations. Estimates determined by deleting the values observed at the lower antibody–β2-GPI complex concentrations indicated Kd's in the range of 5 to 12 nM, with oxidation having little, if any, effect. However, the number of binding sites increased substantially when the phospholipids were oxidized, particularly when the PS content was low (Figure 7C). It is apparent that even under conditions where the antibody–β2-GPI complex binds tightly (such as Figure 7A or the solid squares in Figure 7C), the complexes are unable to inhibit APC activity unless oxidized phospholipid and PE are present (Figure 2). Fab fragments of mAb no. 1522 had no effect on the binding of β2-GPI to the vesicles under any conditions (data not shown).
Binding of β2-GPI-mAb complex to liposomes does not necessarily require PE or oxidation. Nonoxidized (▪, •, ▴) or oxidized (□, ○, ▵) liposomes (10 μg/mL) were used to study the binding of β2-GPI–anti-β2-GPI complexes in the presence of 2 mM calcium. Complexes were formed by mixing the mAb no. 1522 with 2.25 times its concentration of β2-GPI to ensure saturation of the mAb before addition to the liposomes. Concentrations indicated on the x-axis refer to those of the mAb. (A) PS/PC vesicles; (B) PE/PS/PC vesicles; (C) vesicles contain 40% PE plus 2% PS (○, •), 5% PS (▵, ▴), or 20% PS (□, ▪), with the remainder of vesicles comprised of PC. Binding isotherms of panel C were performed with a different set of liposomes than panels A and B, resulting in slightly different maximum binding values for the 20%PS/PE/PC vesicles (□, ▪) than observed in panel B. Similar results were obtained with the other mAbs. Is/lo is the ratio of signal intensity after reagent addition (Is) to the baseline value (lo).
Binding of β2-GPI-mAb complex to liposomes does not necessarily require PE or oxidation. Nonoxidized (▪, •, ▴) or oxidized (□, ○, ▵) liposomes (10 μg/mL) were used to study the binding of β2-GPI–anti-β2-GPI complexes in the presence of 2 mM calcium. Complexes were formed by mixing the mAb no. 1522 with 2.25 times its concentration of β2-GPI to ensure saturation of the mAb before addition to the liposomes. Concentrations indicated on the x-axis refer to those of the mAb. (A) PS/PC vesicles; (B) PE/PS/PC vesicles; (C) vesicles contain 40% PE plus 2% PS (○, •), 5% PS (▵, ▴), or 20% PS (□, ▪), with the remainder of vesicles comprised of PC. Binding isotherms of panel C were performed with a different set of liposomes than panels A and B, resulting in slightly different maximum binding values for the 20%PS/PE/PC vesicles (□, ▪) than observed in panel B. Similar results were obtained with the other mAbs. Is/lo is the ratio of signal intensity after reagent addition (Is) to the baseline value (lo).
Discussion
The studies presented here, based on the membrane requirements of the anticoagulant complex, may help explain how apparently nonspecific membrane or phospholipid binding of antibody–β2-GPI complexes can have specific functional consequences. Several investigators have noted a correlation of antibodies to β2-GPI and the development of resistance to APC 23 and/or thrombosis.12,15 Because of a growing belief that antibodies to β2-GPI with LA activity are more strongly associated with thrombosis that those of other specificities, assays have recently been developed to better identify this population.44,45 However, no mechanism is proposed to account for these in vitro anticoagulant populations becoming procoagulant in vivo. In addition, as seen in Figure 1, anti–β2-GPI mAbs with little or no LA-like activity in the absence of APC (our assay most closely resembles the dilute Russell viper venom time [dRVVT] clotting assay) can have significant activity when APC activity is monitored. Previous studies of β2-GPI or β2-GPI–antibody complex binding have been predicated on the assumption that β2-GPI and the coagulant and anticoagulant proteins shared similar phospholipid specificity.18-20,46,47 The impact of PE and/or phospholipid oxidation under physiologic conditions of calcium and ionic strength were not tested. Other studies7,12,17,24,48 have investigated the role of anti–β2-GPI on APC anticoagulant activity directly. However, these studies were compromised due to the choices of lipid (PS/PC) or complex phospholipid mixtures (thromboplastins or platelin) and/or the lack of control for oxidation. Furthermore, these studies could not explain the specificity that could lead to thrombosis.
In previous studies, IgG purified from thrombotic patients exhibited oxidation-, PE-dependent inhibition of APC anticoagulant function.4,31 Further studies have indicated that most of these antibodies are β2-GPI dependent (data not shown). The mouse mAbs described here, raised to purified β2-GPI, exhibited similar properties. That is, inhibition of APC function required the presence of PE, oxidation of the phospholipid and could be observed at concentrations below that necessary for significant LA-like activity. Very recent studies of de Laat et al43 indicate that β2-GPI antibodies directed to a specific epitope on domain 1 involving Gly40 and Arg43 are most strongly associated with thrombosis. We have not observed a requirement for domain specificity in the mAbs with specific APC inhibitory activity. Some patients with thrombosis (with or without LA activity) did not have specificity for this epitope in the report by de Laat et al.43 It is possible these patients may have antibodies that inhibit APC in an oxidation-, PE-dependent manner similar to the monoclonals described here. It is also not necessary to invoke lattice formation by heterogeneous anti–β2-GPI antibodies in patient samples to explain inhibition of the anticoagulant complex. It would appear that divalency, rather than specific orientation of β2-GPI on the membrane, is sufficient for APC inhibitory activity. In contrast, specific orientation such as that observed by de Laat et al43 seems to be important for LA activity.
The arguments described in the previous paragraph would favor the concept that any antibody to β2-GPI should inhibit APC function and hence be prothrombotic. This may not be the case as illustrated by mAb no. 1528. Although it binds with high affinity based on the functional assay (although inhibition is partial, the plateau is reached at low concentration; Figure 1), the inhibition of APC attained is minimal, possibly due to direct binding to one or more complex constituents but incomplete steric hindrance of the APC complex.
A potential hypothesis to explain the selective inhibition of APC activity would be that PE and phospholipid oxidation increase the membrane binding affinity of the antibody–β2-GPI complex. Because the APC anticoagulant complexes benefit selectively from the PE and oxidation effects, increased antibody–β2-GPI complex affinity for the shared sites would lead to selective inhibition of the APC anticoagulant complexes. However, this does not appear to be the case. The antibody complex bound to the liposomes with similar high affinity independent of PE or lipid oxidation. The number of available sites was increased, however, by oxidation. This would suggest formation of new domains capable of binding the antibody complex. These same PE- and oxidation-dependent domains make major contributions to APC anticoagulant activity, hence allowing for selective inhibition of the anticoagulant complexes.
It has been suggested that β2-GPI interacts directly with protein S.49 Although those authors propose a different mechanism for the prothrombotic effect of anti–β2-GPI antibodies, such molecules might also interfere with protein S–APC interaction, as suggested here in the purified system (Figure 6). However, the β2-GPI–protein S interaction was not oxidation, PE, or phospholipid dependent. Unlike the purified system, which is only minimally dependent on protein S, in plasma the anticoagulant activity of APC is almost totally dependent on protein S stimulation.34 Even so, although the prolongation in protein S–deficient plasma by high concentrations of APC was slight, the addition of mAb shortened the clotting time (data not shown). This would be expected if the antibody was not enhancing a direct β2–GPI–protein S interaction.
At this time, it is not known whether the β2-GPI–anti-β2-GPI complexes physically compete with the APC or another component of the complex for the membrane surface or disrupt an interaction within the complex. We favor the concept that oxidation of PE-containing membranes induces specific interactions between the proteins of the anticoagulant complex. Disruption of these specific protein-protein interactions by the antibody–β2-GPI complexes would lead to specific inhibition. In the purified systems, APC function is significantly more inhibited in the presence of protein S than in its absence (Figure 6), even though the affinity of APC for factor Va phospholipid is tighter in the presence of protein S than its absence.34 The PC-PtGla chimera was not affected by the antibody complex, even though the chimera and native APC have similar affinities for these membranes.34 It is unlikely the antibody–β2-GPI complexes would compete for the membrane surface with one but not the other.
We have previously shown that the titer of LA-like activity and the APC inhibitory activity described here do not necessarily correlate.3 In the case of an individual with anti–β2-GPI, or possibly antibodies with other specificities, these complexes will bind to the entire membrane more or less equivalently. However, even if some competition occurs with the procoagulant reactions in the non–PE-containing regions, significant procoagulant activity would remain due to the high concentrations and membrane affinities of these proteins. It is well documented that a 50% decrease in APC activity can result in a hypercoaguable state.8 Therefore, by blocking the oxidation-dependent enhancement of APC activity even though the nonoxidation-dependent activity remains mostly intact, the balance can be shifted back toward a hypercoaguable state.
In terms of overall regulation of coagulation, we envision that cells become activated by strong agonists and expose procoagulant membrane phospholipids. Leukocytes are recruited to the injury site and release a variety of potent oxidants, eventually transforming the nature of the membrane surface, creating sites that optimize APC anticoagulant activity. Normally, this serves to dampen the coagulant response and limit thrombus growth. When antibody–β2-GPI complexes are present, they also interact within these domains and, through protein-protein interactions with components of the APC anticoagulant complex, eliminate the selective enhancement of the anticoagulant function. If there has not been an injury or inflammatory reaction resulting in an oxidized surface, such antibody complexes may not be prothrombotic, possibly accounting for the episodic nature of the thrombotic complications.
There is some support for the overall scheme outlined in the previous paragraph. In mice deficient in P-selectin and hence with impaired leukocyte recruitment, thrombus formation in response to deep arterial injury is increased relative to controls despite having the opposite effect on the vascular proliferative response following the injury.50 This would be consistent with an anticoagulant effect elicited by leukocyte recruitment. Obviously, in such complex models alternative mechanisms could account for the observed response. Also lacking in this system is any indication of whether antibody–β2-GPI complexes inhibit the apparent leukocyte-dependent dampening of the thrombotic response.
Prepublished online as Blood First Edition Paper, May 12, 2005; DOI 10.1182/blood-2005-01-0404.
Supported by a Specialized Center on Thrombosis grant awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant no. P50 HL54502; N.L.E.). C.T.E. is an investigator of the Howard Hughes Medical Institute and holds the Lloyd Noble Chair in Cardiovascular Research at the Oklahoma Medical Research Foundation.
O.S. performed the research; all authors contributed to the design and interpretation of the research and writing of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.