Abstract
The Polo-like kinases (Plks) are a highly conserved family of protein kinases that function in regulation of cell cycle and DNA damage-induced checkpoints. Evidence of a tumor suppressor function for the Plks in human neoplasia is lacking. Here, we report that Snk/Plk2 is transcriptionally down-regulated in B-cell neoplasms. Silencing occurs with very high frequency in Burkitt lymphoma (BL) but is also detected in B-cell neoplasms of other types and is associated with aberrant cytosine methylation in the CpG island located at the 5′ end of the SNK/PLK2 gene. Silencing is specific to malignant B cells because SNK/PLK2 was unmethylated (and expressed) in primary B lymphocytes, in EBV-immortalized B lymphoblastoid cell lines (LCLs), and in adenocarcinomas (of the breast) and squamous-cell carcinomas (of the head and neck). Expression of Snk/Plk2 in BL cell lines was restored by demethylating agents. The related PLK1 and PLK3 (FNK/PRK) genes were overexpressed in BL cell lines lacking Snk/Plk2 expression, consistent with functional degeneracy among the Plk family. Ectopic expression of Snk/Plk2 in BL cells resulted in apoptosis, a potential mechanistic basis underlying the strong selective pressure for abrogation of Snk/Plk2 function in B-cell neoplasia.
Introduction
Cell-cycle checkpoints slow or arrest the cell cycle in response to stress or damage.1 The Polo-like kinases (Plks) are a family of serine-threonine kinases that occur in organisms from yeasts to humans and participate in cell-cycle regulation and cellular response to stresses such as DNA damage.2 Of these, Plk1 is the best characterized and appears to positively regulate cell progression through G2.3 Plk2 (herein referred to as Snk/Plk2) and Plk3 (herein referred to as Fnk/Prk/Plk3) were both originally identified as immediate-to-early transcripts in mouse fibroblasts,4,5 whereas Plk4 may function in M phase of the cell cycle.2 The precise function of Snk/Plk2 is uncertain. However, from analysis of Plk2-/- mice, it appears that the protein has a role in development and is involved in cell-cycle progression.6 Snk/Plk2 is required for centriole duplication near the G1/S transition although Plk2-/- mice are viable, perhaps because of partial functional degeneracy between Plk2 and other Plk family members.7 There is also evidence that Plk2 functions as a stress response protein. Expression of Plk2 is directly induced by wild-type p53 after DNA damage and in these circumstances activates a G2 checkpoint.8
Methylation-dependent silencing has been described for a number of genes in hematologic malignancies and is likely to be of major importance in the pathogenesis of many such cancers (for a review, see Esteller9 ). Genes known to be subject to methylation-associated silencing include the cyclin-dependent kinase inhibitors (CDKI) p14ARF, p15INK4B, and p16INK4A, the p53 homologue p73, and other genes with putative tumor suppressor functions.10 Finding of additional genes down-regulated by methylation will, therefore, likely identify further genes having important functions as mediators of hematologic neoplasia. In a search for genes that are subject to down-regulation in B-cell lymphomas, we have used subtraction polymerase chain reaction (PCR) methods to identify sequences down-regulated in BL relative to immortalized, nonmalignant B lymphocytes. From these studies, we have identified SNK/PLK2 as a gene subject to methylation-dependent silencing in a high proportion of B-cell lymphoma cell lines and primary lymphomas. Our results imply that loss of Snk/Plk2 expression is one of the most common epigenetic events described in B-cell neoplasia and strongly support the candidacy of SNK/PLK2 as a tissue-selective, human tumor suppressor gene.
Materials and methods
Plasmids
pHA-Plk2 was as described previously.7 pEGFP was obtained from Clontech (Palo Alto, CA).
Cell lines and tumors
BL and EBV-immortalized B LCLs were maintained in RPMI 1640 (Gibco Invitrogen, Carlsbad, CA) medium containing 10% fetal bovine serum. The following cell lines were used in our study: BL: Akata, BJAB, BL2, BL30, BL37, BL40, BL41, DG75, Louckes, Mutu 111, Namalwa, P3HR1, Rael, Raji, and Ramos; mantle-cell lymphoma cell lines: JUM2 and NCEB1; follicular-lymphoma cell line: DOHHC1; diffuse large B-cell lymphoma: LK6; and LCL: CR, FM, IB4, JAC, Odiambo, Otis, PDLCL, and X.50. Primary B cells were isolated from buffy coats of pooled blood donations (North London Blood Transfusion Service) as described previously.11,12 Briefly, buffy coats were centrifuged over Ficoll-Paque-Plus gradients and the mononuclear layer removed; CD19+ B cells removed were immunoselected using anti-CD19 monoclonal antibody (mAb)-coated magnetic beads (M450 pan-B Dynabeads; Dynal, Oslo, Norway). Other cells were removed by repeated washing, the beads released by competition with Detachabeads (Dynal), and the isolated B cells resuspended in 15% (vol/vol) fetal calf serum [FCS]/RPMI 1640 at a density of 106/mL. Flow cytometric analysis was performed using fluorescein isothiocyanate (FITC)-conjugated anti-CD20 mAb (Dako, Carpenteria, CA) to confirm the purity of the isolated population. Purified B cells were incubated for 36 hours after isolation prior to use, after which all cells were passaged in 10% (vol/vol) FCS/RPMI 1640 at 37°C.
For stimulation through CD40, 107 primary B cells were seeded on a feeder layer of 1.5 × 106 irradiated CD40L-L cells13 (exposed to 50 Gy γ radiation) and their growth medium was supplemented with 10 U/mL human recombinant interleukin 4 (IL-4; R&D Systems, Minneapolis, MN). EBV was purified by ultrafiltration from B95.8 cells and used to infect primary B cells as previously described.14 Briefly, B cells were incubated with B95.8 EBV at a density of 107 cells/mL for 2 hours at 37°C. The cells were then diluted to 106/mL in their own conditioned medium to halt infection.15 For B-cell stimulation, phorbol 12-myristate 13-acetate (PMA; Sigma Aldrich, St Louis, MO) was dissolved in DMSO and used at a final concentration of 30 ng/mL.
Primary tumors were obtained as fresh-frozen biopsy samples or as archival specimens. In each case, the diagnosis and presence of adequate tumor representation in the specimen was confirmed by histopathologic analysis. gDNA and RNA were obtained from fresh-frozen material using standard molecular biologic techniques. For archival specimens, gDNA was isolated by extended digestion in proteinase K following xylene treatment of the tissue sections.
Western blotting
Exponentially growing cells were harvested and lysed in lysis buffer (50 mM Tris-HCl, pH 7.5, 250 mM NaCl, 0.1% NP-40, 5 mM EDTA, 50 mM NaF, 1 mM PMSF with protease inhibitor cocktail; Roche, Indianapolis, IN). Cleared lysates were assayed for protein concentration by using the Bio-Rad (Hemel Hempstead, Hertis, United Kingdom) protein assay system and subjected to immunoblotting. Bound primary antibodies were detected with either horseradish peroxidase (HRP)-goat antirabbit antibody, HRP-goat antimouse antibody, or HRP-rabbit antigoat antibody (Dako). Enhanced chemiluminescence Western blotting detection reagents were purchased from Amersham (Freiberg, Germany). The following primary antibodies were used: polyclonal, affinity-purified anti-hPlk2 as described7 and anti-hPlk2 AbVanced panel from Bethyl Labs (Montgomery, TX); polyclonal Plk1 and Plk4, purchased from Abcam (Cambridge, United Kingdom); and polyclonal Plk3 from Transduction Labs (Lexington, KY). PC-10 ascites were used for the detection of proliferating-cell nuclear antigen (PCNA; 1:10 000).
Subtraction PCR
Total cellular RNA was isolated from cell lines by homogenization in RNAzol B according to the manufacturer's protocol (Biogenesis, Kingston, NH). Poly (A+) RNA was prepared using the polyA Spin mRNA isolation kit (New England Biolabs, Beverly, MA). mRNA was obtained from 7 pooled, EBV-transformed B LCLs (JAC, PD LCL, Odiambo, IB4, Otis, CR) and from the DG75 BL cell line. Subtraction suppression PCR16 was performed using the PCR select system (Clontech/BD, Palo Alto, CA), with 2 μg poly (A+) RNA as described previously.17 Forward and reverse subtractions were performed and screened with the PCR select differential cloning system (Clontech/BD). Following 2 rounds of PCR, the subtracted products were ligated into pGemT (Promega, Madison, WI). Inserts were individually sequenced.
Analysis of gene expression
We used reverse transcription PCR (RT-PCR) and Western blotting to analyze expression of the Plk family members. In some experiments we also used real-time PCR to analyze expression of Snk/Plk2. For RT-PCR, cDNA was synthesized from 2 μg total RNA using the Stratagene cDNA synthesis kit. Primers for RT-PCR were: SNK/PLK2 forward, 5′-TCAgCAACCCAgCAAACACAgg-3′ and reverse, 5′-TTTCCAgACATCCCCgAAgAACC-3′ (product = 230 bp).
Reactions were resolved on 1.2% agarose gels and visualized on a transilluminator after staining with ethidium bromide. GAPDH or ARPO was coamplified as a control gene using primers previously described.18
Real-time quantitative PCR was performed in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Weiterstadt, Germany) using the dye SyberGreen (Qiagen, Hilden, Germany). In PCRs, 1.5 mM MgCl2 was used for all primers and the cycling conditions used were 40 cycles 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 40 seconds. Fold inductions were calculated using the formula 2-(ΔΔCt), where ΔΔCt is the ΔCt(SNK)-ΔCt(ARPO) and Ct is the cycle at which the threshold is crossed. The primers used were as follows: GAPDH forward, 5′-TgAAggTCggAgTCAACggATTg-3′ and reverse, 5′-gCCATggAATTTgCCATgCCATgggTgg-3′ (product = 180 bp); ARPO forward, 5′-AgATgCAgCAgATCCgCAT-3′ and reverse, 5′-gTggTgATACCTAAAgCCTg-3′ product = 318 bp); SNK/PLK2, forward 5′-TCAgCAACCCAgCAAACACAgg-3′, and reverse, 5′-TTTCCAgACATCCCCgAAgAACC-3′ (product = 230 bp).
Methylation analysis
gDNA was purified from cell pellets by proteinase K digestion. Analysis of the SNK/PLK2 and FNK/PRK/PLK3 loci identified CpG islands at the 5′ ends of each gene (http://genome.ucsc.edu/cgi-bin/hgGateway?org=human). gDNA (1 μg) was modified by sodium bisulfite as described.19
Primers for MSP were as follows: PLK2 unmethylated: forward, 5′-CACCCCACAACCAACCAAACACACA-3′ and reverse, 5′-ggATggTTTTgAAggTTTTTTTgTggTT-3′ (product = 142 bp); PLK2 methylated: forward, 5′-CCCACgACCgACCgAACgCgCg-3′; reverse, 5′-ACggTTTTgAAggTTTTTTCgCggTC-3′ (product = 137 bp); PLK3 unmethylated: forward, 5′-AgTAAATTTAggTAgTgTTATgTgTggTT-3′ and reverse, 5′-AAACCCAACCAAAAAAACAAACACAACAA-3′ (product = 158 bp); and PLK3 methylated: forward, 5′-AATTTAggTAgCgTTACgCgCggTC-3′ and reverse, 5′-CCgACCgAAAAAACgAACgCgACgA-3′ (product = 150 bp).
Analysis of Snk/Plk2 and p53 sequence
We analyzed the sequence of SNK/PLK2 and of p53 by direct sequencing. Primer sequences for SNK/PLK2 were as follows: SNK 1,5′-gCggACTATCACCTACCAg-3′; Snk 2, 5′-CAATCTgCCTgAggTAgTATCg-3′; SNK 3, 5′-TCACCACCATCACCACCATTCg-3′; SNK 4, 5′-TATCgTTCTCCTTCTgTgTTCC-3′; SNK 5, 5′-ggAACACAgAAggAgAACg-3′; SNK 6, 5′-AACTgTggTggTAggTgg-3′; SNK 7, 5′-ACCTACCACCACAgTTgC-3′; SNK 8, 5′-ACTgAAggAggTAgAgCC-3′; SNK 9, 5′-ACAgTTCACTATTACgCAgAgC-3′; and SNK 10, 5′-AgAACTgTATgCCTTAgCCTgg-3′.
Demethylation treatment
Cells were treated with 5′ deazacytidine (5′AZA; Sigma) for 5 days followed by a combination of 5′AZA and Trichostatin A (TSA; Sigma) for a further 3 days. The cells were split every 2 days with the addition of fresh drug. After drug treatment, cells were harvested for RT-PCR.
Transient transfection and flow cytometry
Cells (107) were resuspended in 400 μL 10% (vol/vol) FCS/RPMI 1640, mixed with either 2 μg pEGFP plus 20 μg pHA-Plk2 or 2 μg pEGFP plus 20 μg pHA vector plasmid. Cells were electroporated at 960 μF and 250 V in a 4-mm cuvette, placed on ice for 1 minute, and then diluted with 600 μL 10% (vol/vol) FCS/RPMI 1640 before being cultured in 4 mL 10% (vol/vol) FCS/RPMI 1640. At 24 hours after transfection cells were sorted for EGFP expression and cultured for a further 24 hours before being processed for analysis by flow cytometry as described previously.20 Briefly, cells were fixed in 90% cold methanol for at least 2 hours, washed twice in PBS, and resuspended in 400 μL propidium iodide (50 μg/mL). The propidium iodide solution contained 100 mg/mL RNase to ensure staining of DNAonly.
Results
Selective down-regulation of Snk/Plk2 expression in BL
To seek genes down-regulated in BL, we performed initial experiments to identify sequences not expressed in the DG75 BL cell line relative to non-transformed B cells. We prepared cDNA from the DG75 BL cell line and subtracted this against cDNA prepared from 7 pooled EBV-immortalized B LCLs. From this screen, we observed that the mRNA of Snk/Plk2 was expressed at high levels in the pooled LCLs relative to DG75 BL cells, in which expression was undetectable by both conventional RT-PCR and real-time analysis using SYBR green (Figure 1A,E). The absence of Snk/Plk2 mRNA in DG75 prompted us to examine expression in other BL cell lines and these studies revealed that Snk/Plk2 mRNA was undetectable in each of 15 BL cell lines analyzed but was abundantly expressed in LCLs (Figure 1B). We wished to verify that the undetectable mRNA was reflected in a corresponding absence of Snk/Plk2 protein. The available antibodies against Snk/Plk2 recognize a doublet of which the lower band is nonspecific and the upper band is Snk/Plk2 as described.7 The nonspecific lower band was detected at a variable level in each of the BL lines, whereas the upper band (Snk/Plk2) was abundantly expressed in each LCL but uniformly absent from each BL line (Figure 1C), consistent with the absence of Snk/Plk2 mRNA. The absence of Snk/Plk2 mRNA and protein in BL prompted us to examine expression of Snk/Plk2 in other B-lymphoma cell lines. Expression of Snk/Plk2 was also reduced or absent in many such lines (Figure 1B-C; Table 1). PLK2-/- cells are viable and recent work suggests that SNK/PLK2 is required for centriole duplication near the G1/S transition in mammalian cells, implying that other Plk family members may perform this function in Snk/Plk2-deficient cells.7 It was therefore of interest to analyze expression of Plk1, Fnk/Prk/Plk3, and Plk4 in each of the cell lines. In contrast to Snk/Plk2, which was invariably undetectable in BL cell lines, each of the other Plk family members was expressed in the BL and other B-lymphoma cell lines (Figure 1B-C). Of note, Fnk/Prk/Plk3 was overexpressed in every BL cell line lacking Snk/Plk2 expression. There was also clear overexpression of Plk1 in many of the lymphoma cell lines relative to LCLs, which would support a possible function for Plk1 in compensating for the absence of Snk/Plk2. However, Plk1 was overexpressed in the LK6 and DOHHC1 cell lines that also express Snk/Plk2. Together, these observations support a mechanistic model in which Fnk/Prk/Plk3 expression can functionally substitute for Snk/Plk2 in the absence of the latter protein.
Expression of Snk/Plk2 is down-regulated in lymphomas. (A) Snk/Plk2 mRNA is down-regulated in DG75 BL cells relative to EBV-immortalized B LCLs. RT-PCR was performed to analyze expression of Snk/Plk2 in DG75 and pooled LCLs as indicated. RT-PCR to detect the mRNA of Snk/Plk2 and GAPDH was performed as described in “Materials and methods.” Mr indicates 100-bp molecular weight markers. (B-C) Expression of Plks in normal and neoplastic B lymphocytes. (B) RT-PCR analysis of Plks 1-4 in BL and LCLs and other B-cell lymphomas as shown. The identity of each cell line is shown above the gels. A loading control (GAPDH) is also shown. RNA was isolated from cell lines and expression of each Plk determined by RT-PCR as described in “Materials and methods.” (C) Western blot analysis of Plks 1-4 in BL, LCL, and other B-cell lymphoma cell lines as indicated. The identity of each cell line is shown above the blots. Expression of loading control gene (PCNA) is also shown. Antibodies against Snk/Plk2 recognize a doublet of which the lower band is nonspecific, as previously reported.7 The upper band of the doublet is Snk/Plk2 as described.7 (D) Snk/Plk2 mRNA is detected in primary B lymphocytes but not in primary BL. RT-PCR was performed as described in “Materials and methods.” Mr indicates 100-bp molecular weight markers; lanes are as shown. (E) Real-time RT-PCR analysis of Snk/Plk2 expression in primary BL and primary B cells. Lanes are as shown. Data shown are relative expression levels normalized against the internal control gene ARPO and bars indicate SDs. The results are derived from 3 independent experiments.
Expression of Snk/Plk2 is down-regulated in lymphomas. (A) Snk/Plk2 mRNA is down-regulated in DG75 BL cells relative to EBV-immortalized B LCLs. RT-PCR was performed to analyze expression of Snk/Plk2 in DG75 and pooled LCLs as indicated. RT-PCR to detect the mRNA of Snk/Plk2 and GAPDH was performed as described in “Materials and methods.” Mr indicates 100-bp molecular weight markers. (B-C) Expression of Plks in normal and neoplastic B lymphocytes. (B) RT-PCR analysis of Plks 1-4 in BL and LCLs and other B-cell lymphomas as shown. The identity of each cell line is shown above the gels. A loading control (GAPDH) is also shown. RNA was isolated from cell lines and expression of each Plk determined by RT-PCR as described in “Materials and methods.” (C) Western blot analysis of Plks 1-4 in BL, LCL, and other B-cell lymphoma cell lines as indicated. The identity of each cell line is shown above the blots. Expression of loading control gene (PCNA) is also shown. Antibodies against Snk/Plk2 recognize a doublet of which the lower band is nonspecific, as previously reported.7 The upper band of the doublet is Snk/Plk2 as described.7 (D) Snk/Plk2 mRNA is detected in primary B lymphocytes but not in primary BL. RT-PCR was performed as described in “Materials and methods.” Mr indicates 100-bp molecular weight markers; lanes are as shown. (E) Real-time RT-PCR analysis of Snk/Plk2 expression in primary BL and primary B cells. Lanes are as shown. Data shown are relative expression levels normalized against the internal control gene ARPO and bars indicate SDs. The results are derived from 3 independent experiments.
We next tested whether expression of Snk/Plk2 was reduced in primary BL relative to normal B lymphocytes purified from the peripheral blood of healthy adults. We isolated mRNA from 10 cases of endemic BL (all shown to be positive for EBV) and performed RT-PCR analysis of Snk/Plk2 expression. Whereas expression was clearly detectable in normal B cells, it was greatly reduced or absent in the primary endemic BL(Figure 1D; Table 1). To confirm the differences in expression between primary B cells and primary BL, we performed real-time PCR analysis, which verified the significantly lower expression in primary BL relative to primary B cells (Figure 1E).
Aberrant CpG methylation of Snk/Plk2 in B-cell lymphoma
Inspection of the upstream regulatory sequences of SNK/PLK2 (http://genome.ucsc.edu/cgi-bin/hgGateway?org=human) revealed the presence of a CpG island in the 5′ sequences of the gene, raising the possibility that expression in lymphomas is subject to epigenetic silencing, via aberrant CpG methylation. To address this, we analyzed a panel of BL cell lines and LCLs by methylation-specific PCR (MSP; Figure 2). The SNK/PLK2 CpG island was fully methylated in all BL cell lines analyzed (Figure 2A; Table 1). In total, we detected methylation in SNK/PLK2 in 15 of 15 BL cell lines and in each case methylation was complete and correlated with undetectable expression. The invariable correlation between methylation of the SNK/PLK2 CpG island and absence of mRNA implies that methylation-dependent transcriptional silencing is the reason for undetectable expression of the gene. In contrast to BL, there was no detectable methylation of the SNK/PLK2 CpG island in any LCL analyzed, consistent with the abundant expression of mRNA and protein (Figure 2A). To exclude the possibility that the frequent methylation of SNK/PLK2 is attributable to nonspecific methylation events, we analyzed CpG methylation of the related gene FNK/PRK/PLK3. We found no evidence of methylation in the CpG island of FNK/PRK/PLK3 in either BL or LCLs (Figure 2B), consistent with expression of Fnk/Prk/Plk3 mRNA and protein (Figure 1B-C). These results imply that in BL, SNK/PLK2 is preferentially subject to methylation-dependent transcriptional silencing in direct contrast to FNK/PRK/PLK3.
Methylation of the CpG island of SNK/PLK2 but not FNK/PRK/PLK3 in B-cell neoplasia. Methylation-specific PCR (MSP) was performed as described in “Materials and methods” and PCR products resolved on 2% agarose gels. (A) The SNK/PLK2 CpG island is fully methylated in BL cell lines that lack expression of the gene, but completely unmethylated in EBV-immortalized LCLs that express Snk/Plk2. U indicates unmethylated; M, methylated. The toppanel is BL cells lines; the bottom panel, LCLs. The identity of each cell line is shown above the gel. Specificity controls are denoted C1 (control unmethylated DNA) and C2 (control methylated DNA). (B) The CpG island in the FNK/PRK/PLK3 promoter is completely unmethylated in BL. The top panel is BL cells lines; the bottom panel, LCLs. The identity of each cell line is shown above the gel. (C) Demethylation of the SNK/PLK2 CpG island reverses transcriptional silencing. BL lines positive (Rael) and negative (DG75) for EBV and lacking expression of Snk/Plk2 mRNA were exposed to demethylating agents and analyzed by RT-PCR as described in “Materials and methods.” Lanes are: 1 and 7, untreated; 2 and 8, 5 μM 5′AZA; 3 and 9, 10 μM 5′AZA; 4 and 10, 300 μM TSA; 5 and 11, 5 μM 5′AZA plus 300 μM TSA; 6 and 12, 10 μM 5′AZA plus 300 μM TSA.
Methylation of the CpG island of SNK/PLK2 but not FNK/PRK/PLK3 in B-cell neoplasia. Methylation-specific PCR (MSP) was performed as described in “Materials and methods” and PCR products resolved on 2% agarose gels. (A) The SNK/PLK2 CpG island is fully methylated in BL cell lines that lack expression of the gene, but completely unmethylated in EBV-immortalized LCLs that express Snk/Plk2. U indicates unmethylated; M, methylated. The toppanel is BL cells lines; the bottom panel, LCLs. The identity of each cell line is shown above the gel. Specificity controls are denoted C1 (control unmethylated DNA) and C2 (control methylated DNA). (B) The CpG island in the FNK/PRK/PLK3 promoter is completely unmethylated in BL. The top panel is BL cells lines; the bottom panel, LCLs. The identity of each cell line is shown above the gel. (C) Demethylation of the SNK/PLK2 CpG island reverses transcriptional silencing. BL lines positive (Rael) and negative (DG75) for EBV and lacking expression of Snk/Plk2 mRNA were exposed to demethylating agents and analyzed by RT-PCR as described in “Materials and methods.” Lanes are: 1 and 7, untreated; 2 and 8, 5 μM 5′AZA; 3 and 9, 10 μM 5′AZA; 4 and 10, 300 μM TSA; 5 and 11, 5 μM 5′AZA plus 300 μM TSA; 6 and 12, 10 μM 5′AZA plus 300 μM TSA.
Demethylation of the Snk/Plk2 CpG island reactivates expression
To further verify the hypothesis that aberrant methylation underlies silencing of SNK/PLK2, we treated 2 BL cell lines in which expression was undetectable: DG75 (EBV-) and Rael (EBV+) with 5′AZA with and without the histone deacetylase inhibitor TSA. RNA was prepared at various times and the levels of Snk/Plk2 and GAPDH mRNA determined by RT-PCR analysis. Whereas Snk/Plk2 was undetectable in untreated cells, there was a clear increase in mRNA following 5′AZA treatment in both cell lines (Figure 2C). Although treatment with TSA alone did not increase expression, the combination of TSA and 5′AZA induced higher expression than 5′AZA alone (Figure 2C).
Aberrant Plk methylation is restricted to PLK2 and to hematologic neoplasms
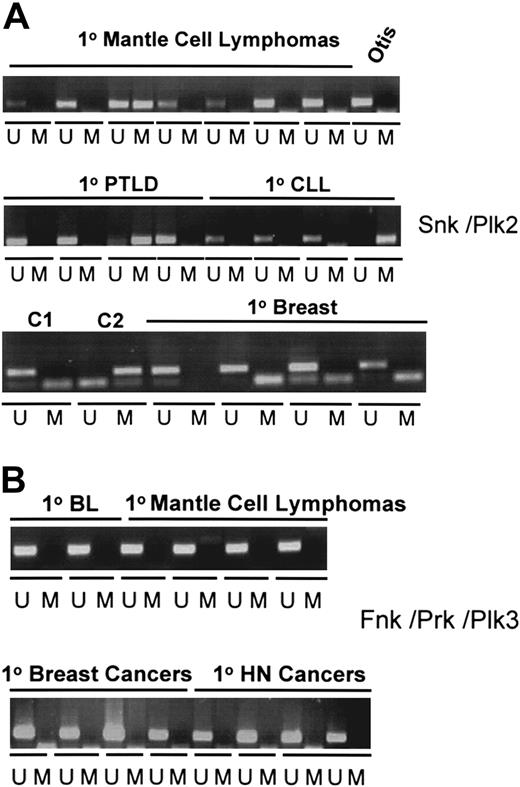
The observation that SNK/PLK2 is subject to silencing in a very high proportion of BL cell lines prompted us to examine methylation in other B-cell lines and in primary lymphomas. We detected SNK/PLK2 methylation in all forms of B-cell neoplasia analyzed, including mantle-cell lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, posttransplantation B-cell lymphoproliferative disorders (PTLDs), and B-cell acute lymphoblastoid leukemia (ALL) and chronic lymphocytic leukemia (CLL) (Figure 3A; summarized in Table 1). In primary BL, methylation was present in a high proportion of cases (9 of 10; Table 1). In contrast, methylation was not detected in T-cell prolymphocytic leukemia (TPLL) or in T-cell ALL (Table 1). To examine the specificity of SNK/PLK2 silencing for hematologic cancers, we performed MSP analysis in a panel of breast carcinomas and squamous carcinomas of the head and neck. No methylation was detected in primary breast cancer (Figure 3A). Similarly, the SNK/PLK2 CpG island was unmethylated in head and neck squamous carcinomas (data not shown). Together these results imply that methylation of SNK/PLK2 occurs primarily in B-cell neoplasia. Consistent with expression analysis, the CpG island of FNK/PRK/PLK3 was unmethylated in all hematologic and solid tumors analyzed (Figure 3B).
SNK/PLK2 silencing occurs in BL mutant and wild type for p53
SNK/PLK2 is a known transcriptional target gene for wild-type p53,8 raising the possibility that silencing of SNK/PLK2 is a mechanism by which cells subvert the function of wild-type p53. This model would imply that methylation is more frequent in cancers retaining wild-type p53. As such, it was of interest to determine whether silencing of SNK/PLK2 was related to p53 status and we therefore determined the sequence of p53 in BL cell lines and primary BL previously analyzed for SNK/PLK2 methylation. These studies revealed that methylation and silencing of Snk/Plk2 expression occurred in cases both wild type and mutant for p53 (Table 2).
SNK/PLK2 is preferentially and selectively methylated in primary hematologic malignancies. (A) Methylation of the CpG island of SNK/PLK2 occurs in primary B-cell lymphomas but not in breast cancer. MSP analysis of the SNK/PLK2 CpG island was performed as described in “Materials and methods.” The figure shows MSPs for unmethylated (U) and methylated (M) DNA. Specificity controls are denoted C1 (control unmethylated DNA) and C2 (control methylated DNA). Examples of methylation are shown for primary mantle-cell lymphomas, posttransplantation lymphoproliferative disease (PTLD), chronic lymphocytic leukemia (CLL), and primary breast cancer. The lymphoblastoid cell line Otis is included as a further specificity control. (B) PLK3 (FNK/PRK/PLK3) is unmethylated in B-cell, breast, and head and neck cancer. MSP to detect methylation in the CpG island of FNK/PRK/PLK3 was performed as described in “Materials and methods.” The figure shows MSPs for unmethylated (U) and methylated (M) DNA. Shown are analysis of primary BL, primary mantle-cell lymphomas, primary breast cancer, and primary squamous carcinomas of the head and neck (HN).
SNK/PLK2 is preferentially and selectively methylated in primary hematologic malignancies. (A) Methylation of the CpG island of SNK/PLK2 occurs in primary B-cell lymphomas but not in breast cancer. MSP analysis of the SNK/PLK2 CpG island was performed as described in “Materials and methods.” The figure shows MSPs for unmethylated (U) and methylated (M) DNA. Specificity controls are denoted C1 (control unmethylated DNA) and C2 (control methylated DNA). Examples of methylation are shown for primary mantle-cell lymphomas, posttransplantation lymphoproliferative disease (PTLD), chronic lymphocytic leukemia (CLL), and primary breast cancer. The lymphoblastoid cell line Otis is included as a further specificity control. (B) PLK3 (FNK/PRK/PLK3) is unmethylated in B-cell, breast, and head and neck cancer. MSP to detect methylation in the CpG island of FNK/PRK/PLK3 was performed as described in “Materials and methods.” The figure shows MSPs for unmethylated (U) and methylated (M) DNA. Shown are analysis of primary BL, primary mantle-cell lymphomas, primary breast cancer, and primary squamous carcinomas of the head and neck (HN).
Expression of Snk/Plk2 is induced by B-cell mitogens
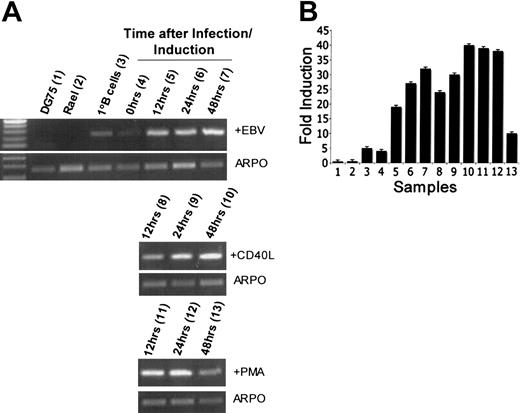
The higher expression of Snk/Plk2 mRNA in EBV-immortalized LCLs relative to primary peripheral-blood B lymphocytes suggests that activation of expression occurs when B lymphocytes are stimulated into proliferation. To address this possibility, we purified B cells from the blood of healthy individuals. The small amount of material available to us precluded Western blot analysis. We therefore performed semiquantitative RT-PCR and real-time PCR after treatment of primary B cells with CD40 ligand plus IL-4, PMA, or infection with immortalizing strains of EBV. In all cases, there was a clear increase in the expression of Snk/Plk2 mRNA with time in the 24 hours following treatment (Figure 4). In the case of CD40 ligand and EBV, the levels of mRNA continued to increase beyond 24 hours (Figure 4). In cells treated with PMA, following the initial increase, the level of Snk/Plk2 mRNA appeared to fall after 48 hours. This is attributable to the reduced cell viability caused by the cytotoxicity of prolonged exposure to PMA.
Induction of Snk/Plk2 expression by EBV raised the possibility that silencing might occur more commonly in lymphomas positive for EBV. However, there was no relationship with EBV status of the BL because Snk/Plk2 silencing occurred in BL cell lines both positive and negative for EBV (Table 2).
Absence of intragenic mutations in Snk/Plk2 in BL
The frequent occurrence of transcriptional silencing of SNK/PLK2 in hematologic malignancies is consistent with a tumor suppressor function. We were, therefore, prompted to examine the possibility that intragenic mutation might cause inactivation of the gene in cases lacking methylation. The presence of inactivating mutations in the coding sequences of SNK/PLK2 was sought by direct sequencing of cDNA from a panel of cell lines and primary malignancies. The only abnormality found was in JUM2 where 2 changes were detected in position 1167 (T>C) and 1200 (G>A; GenBank accession no. NM006622). Neither resulted in amino acid changes. These sequence changes are probable polymorphisms.
Activation of primary B cells leads to SNK/PLK2 gene expression. Primary B cells were treated with CD40 ligand or PMA, or infected with immortalizing strains of EBV as shown. Expression of Snk/Plk2 and the control gene ARPO was analyzed using RT-PCR and real time RT-PCR at different time points after activation/infection as described in “Materials and methods.” (A) RT-PCR showing mRNA expression of Snk/Plk2 and ARPO. Time points are shown above the gel. The top panel shows infection with EBV, the middle panel shows treatment with CD40 ligand, and the bottom panel shows treatment with PMA. (B) Real-time PCR analysis showing expression of Snk/Plk2 mRNA. ARPO was used as an internal control. The figure shows fold induction of Snk/Plk2 corrected for levels of the control gene ARPO (± 1 SD). Values obtained are derived from 3 independent experiments. Lanes are as indicated in brackets in panel A.
Activation of primary B cells leads to SNK/PLK2 gene expression. Primary B cells were treated with CD40 ligand or PMA, or infected with immortalizing strains of EBV as shown. Expression of Snk/Plk2 and the control gene ARPO was analyzed using RT-PCR and real time RT-PCR at different time points after activation/infection as described in “Materials and methods.” (A) RT-PCR showing mRNA expression of Snk/Plk2 and ARPO. Time points are shown above the gel. The top panel shows infection with EBV, the middle panel shows treatment with CD40 ligand, and the bottom panel shows treatment with PMA. (B) Real-time PCR analysis showing expression of Snk/Plk2 mRNA. ARPO was used as an internal control. The figure shows fold induction of Snk/Plk2 corrected for levels of the control gene ARPO (± 1 SD). Values obtained are derived from 3 independent experiments. Lanes are as indicated in brackets in panel A.
Expression of Snk/Plk2 causes apoptosis in BL cells
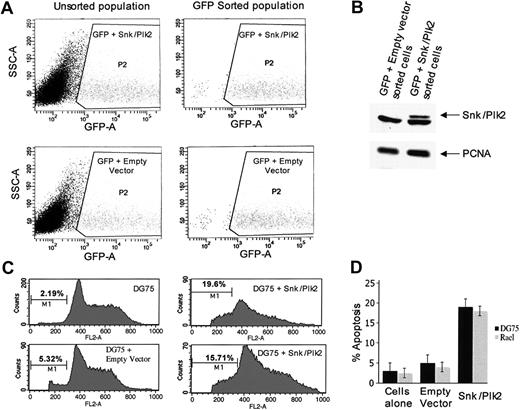
The very high frequency with which SNK/PLK2 is subject to epigenetic inactivation in BL implies the existence of strong selective pressures to abrogate the function of this gene product during lymphomagenesis. To gain insight into the nature of these, we introduced plasmids encoding SNK/PLK2 into 2 independent BL cell lines, DG75 (EBV-) and Rael (EBV+), lacking endogenous expression of Snk/Plk2. Successfully transfected cells were isolated by cell sorting (Figure 5A) and the expression of the transfected sequences confirmed by Western blotting (Figure 5B). Flow cytometric analysis revealed that expression of Snk/Plk2 resulted in a significant increase in the sub-G1 population of cells, compared to cells transfected with vector sequences alone. This was reproducibly observed in at least 3 independent experiments in both cell lines used (Figure 5C-D). These experiments show that expression of Snk/Plk2 in BL cells results in apoptosis and suggests a mechanistic basis for the frequent loss of the gene in this cancer.
Expression of Snk/Plk2 causes apoptosis in BL cells. BL lines DG75 (EBV-) and Rael (EBV+) lacking endogenous Snk expression were transfected with Snk/Plk2 expression plasmids and analyzed by flow cytometry as described in “Materials and methods.” (A) Fluorescence-activated cell sorting (FACS) analysis shows sorting of successfully transfected cells based on GFP expression. (B) Western blot analysis shows expression of Snk/Plk2 protein in the GFP+ fraction but not in the negative fraction of sorted cells. (C) Cell-cycle analysis of GFP-sorted cells shows an increase in apoptosis in cells expressing Snk/Plk2. The figure shows (as indicated) non-transfected cells, cells receiving empty vector only, and 2 typical profiles of cells expressing Snk/Plk2. Essentially similar results were obtained with Rael (data not shown). (D) Histogram summarizes induction of apoptosis by expression of Snk/Plk2. The data shown are percent sub-G1 (apoptotic) cells and are means ± 1 SD of 3 independent experiments.
Expression of Snk/Plk2 causes apoptosis in BL cells. BL lines DG75 (EBV-) and Rael (EBV+) lacking endogenous Snk expression were transfected with Snk/Plk2 expression plasmids and analyzed by flow cytometry as described in “Materials and methods.” (A) Fluorescence-activated cell sorting (FACS) analysis shows sorting of successfully transfected cells based on GFP expression. (B) Western blot analysis shows expression of Snk/Plk2 protein in the GFP+ fraction but not in the negative fraction of sorted cells. (C) Cell-cycle analysis of GFP-sorted cells shows an increase in apoptosis in cells expressing Snk/Plk2. The figure shows (as indicated) non-transfected cells, cells receiving empty vector only, and 2 typical profiles of cells expressing Snk/Plk2. Essentially similar results were obtained with Rael (data not shown). (D) Histogram summarizes induction of apoptosis by expression of Snk/Plk2. The data shown are percent sub-G1 (apoptotic) cells and are means ± 1 SD of 3 independent experiments.
Discussion
Inactivation of tumor suppressor genes (TSGs) in human neoplasia often occurs by mutation or deletion. It is now recognized that epigenetic inactivation by aberrant CpG methylation in the transcriptional regulatory elements of specific TSGs is at least as frequent as these genetic changes. A diversity of epigenetic events has been described previously in hematologic neoplasia; many of the reported silenced genes encode proteins with critical functions in cell growth control and apoptosis.9 In the present study, we show that down-regulation of the Polo-like kinase Snk/Plk2 occurs in a wide range of B-cell neoplasms and is found with particularly high frequency in BL. Our results suggest that methylation-dependent silencing of SNK2/PLK2 is a common epigenetic event predominantly occurring in B-cell neoplasia. Our data are, to the best of our knowledge, the first demonstration of epigenetic inactivation of a Plk family member in human cancer. The importance of transcriptional silencing as a mechanism for inactivating the gene is emphasized by the absence of intragenic mutation shown in the sequence analysis we have performed, which revealed only 2 noncoding polymorphisms in one cell line.
Methylation-dependent silencing of SNK/PLK2 was detected almost exclusively in hematologic neoplasia, and particularly in B-cell cancers. Analysis of breast and head and neck carcinoma cell lines and primary tumors showed that the SNK/PLK2 CpG island was unmethylated and the gene expressed in all cases studied, suggesting that selective pressure for abrogation of Snk/Plk2 function exists predominantly in B-cell tumorigenesis. As such, our results imply an important physiologic function for Snk/Plk2 in regulation of B-cell proliferation/differentiation. It is clearly of interest, therefore, that Snk/Plk2 expression is induced in normal, resting B lymphocytes by treatment with PMA, CD40 ligand plus IL-4, or infection with EBV (each a recognized mitogen in primary B lymphocytes), consistent with a role for Snk/Plk2 in pathways of activation in normal B cells. We note that other genes whose expression is up-regulated during B-cell activation are also candidate tumor suppressors. These include CYCLIN D224 and RUNX3.11 Interestingly, these 2 genes are also subject to transcriptional silencing associated with aberrant hypermethylation in human cancers. SNK/PLK2 was first described as a mitogen-induced early response gene in NIH3T3 cells and human lung fibroblasts.5 These observations complement our observation of up-regulation of Snk/Plk2 mRNA following mitogenic stimulation of primary B lymphocytes. Taken together, these independent studies are consistent with the hypothesis that SNK/PLK2 is an early response gene in multiple tissue types.
Previous studies of genes subject to methylation-associated silencing in BL have identified, among others, p15, p16, p73, and DAPK. The invariable silencing of SNK/PLK2, which we now report, makes it the most frequent target for epigenetic silencing yet described in BL. Studies in other hematologic cancers have identified some correlation between the presence of individual chromosomal translocations and transcriptional silencing of specific genes.9 Translocations between immunoglobulin enhancer loci and c-myc are pathogenic of BL. This raises the possibility of an association between Snk/Plk2 silencing and c-myc deregulation. In this respect, it is of obvious interest that re-expression of Snk/Plk2 in BL cells lacking endogenous expression results in extensive apoptosis, suggesting the interesting hypothesis that the conflicting growth regulatory signals generated by deregulated c-myc (positive) and expression of Snk/Plk2 (negative) result in apoptosis. In any case, induction of apoptosis affords an obvious mechanistic basis for the apparently strong selective pressure to abrogate Snk/Plk2 function during B-cell neoplasia.
Plk2-/- mice are viable,6 yet recent evidence indicates that Snk/Plk2 is required for centriole duplication in mammalian cells,7 suggesting that other Plk family members may functionally complement Snk/Plk2 in the absence of expression of the latter.7 Here, we show that Fnk/Prk/Plk3 (which is also expressed in early phases of the cell cycle) is invariably expressed at high levels in all BL cell lines and also in many other B-cell cancer cell lines, such as mantle cell lymphoma, which lack Snk/Plk2 expression. These observations are consistent with the hypothesis that functional degeneracy between the 2 proteins allows the replication of cells lacking Snk/Plk2. We observed a single lymphoma (DOHHC1, a mantle-cell lymphoma) that did not express Fnk/Prk/Plk3. Interestingly, this cell line did express Snk/Plk2, again consistent with functional degeneracy between the 2 proteins. Together, these results imply that epigenetic inactivation strongly favors Plk2 over Plk3. The reason for this bias is not clear at present. One possibility that we have explored is that the selective silencing of Snk/Plk2 may represent a molecular genetic mechanism by which the functions of wild-type p53 are subverted during tumorigenesis in cells lacking p53 mutation. Our results do not, however, support this possibility because the gene was subject to silencing in BL cell lines and primary BL both mutant and wild type for the p53 sequence. Indeed, the majority of BL cell lines with fully methylated CpG sequences in the SNK/PLK2 promoter express mutant p53 (Table 2). We therefore believe that the most likely explanation for the high frequency of Snk/Plk2 loss of function in BL reflects the ability of Snk/Plk2 to induce apoptosis in cells expressing deregulated c-myc, regardless of the p53 and EBV status of the cell.
Loss of Snk/Plk2 expression increases sensitivity to taxanes and other microtubule inhibitors.8 Our results may therefore have implications for the clinical use of taxanes or other microtubule-disrupting agents in the management of a subset of lymphomas. At the very least, it would be of interest to ascertain whether taxanes demonstrate activity in cases lacking Snk/Plk2 expression.
Prepublished online as Blood First Edition Paper, September 13, 2005; DOI 10.1182/blood-2005-03-1194.
Supported in part by the Ludwig Institute for Cancer Research, of which P.J.F. is an affiliate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.