Abstract
NIP-004 is a novel synthetic compound developed to display human thrombopoietin (TPO) receptor (c-Mpl) agonist activity. NIP-004 displays species specificity, stimulating proliferation or differentiation of human c-Mpl–expressing cells such as UT-7/TPO and human CD34+ cells but not murine c-Mpl–expressing cells or cynomolgus monkey cells. To test the mechanism of its action, we constructed mutant forms of c-Mpl; murine c-MplL490H dis-played a response to NIP-004, whereas human c-MplH499L lost this response, indicating that histidine in the transmembrane domain of c-Mpl is essential for its activity. Because histidine is not present in the c-Mpl transmembrane domain of rats, hamsters, rhesus macaques, and cynomolgus monkeys, we examined the in vivo efficacy of NIP-004 using mice that received xenotransplants. In immunodeficient nonobese diabetic (NOD)/Shi-scid, IL-2Rγnull (NOG) mice receiving transplants of umbilical cord blood–derived CD34+ cells, NIP-004 increased human megakaryoblasts, mature megakaryocytes, and circulating human platelets 6-fold, the latter being morphologically and functionally indistinguishable from normal human platelets. These observations indicate that NIP-004 is a novel human c-Mpl activator and induces human thrombopoiesis.
Introduction
Platelets are important cells for preventing bleeding following injury. Thrombopoietin (TPO), a cytokine produced primarily by the liver and kidney, regulates platelet production by stimulating proliferation and differentiation of hematopoietic stem cells, megakaryocytic progenitor cells, and megakaryocytes via activation of its receptor, c-Mpl.1-5 c-Mpl belongs to the type I cytokine receptor family, and like erythropoietin (EPO) and granulocyte colony-stimulating factor (GCSF) receptors, a major conformational change of the homodimeric receptor ensues upon TPO binding, followed by phosphorylation of the intracellular domain of c-Mpl and various secondary signaling molecules.6 Among the signaling molecules activated by TPO are Janus kinases (Jaks) and signal transducers and activators of transcription (STATs), phosphatidylinositol-3-kinase (PI3K)/Akt, and Ras/mitogen-activated protein kinase (MAPK). Activation of these signaling pathways results in the induction of megakaryopoiesis and thrombopoiesis from hematopoietic stem cells.6 Recombinant human (rh)TPO has been proven to be effective for the treatment of thrombocytopenia in some clinical settings.7 However, adverse events of therapy have been reported, such as the development of neutralizing antibodies to TPO, followed by thrombocytopenia or pancytopenia.8 A nonpeptidyl synthetic compound displaying c-Mpl agonistic activity would therefore offer important medical advantages for the treatment of thrombocytopenia.
Toward this end, we developed a novel human c-Mpl (HuMpl) agonist, NIP-004, that displays species specificity. While NIP-004 stimulated HuMpl-expressing cells such as human bone marrow (BM)–, peripheral blood (PB)–, and umbilical cord blood (CB)–derived CD34+ cells, it did not induce proliferation of murine c-Mpl (MuMpl)–expressing cells or colony formation of megakaryocytes with BM cells from murine or cynomolgus monkey. Using mutants of the c-Mpl receptor, we also found that substitution of histidine (His) for leucine (Leu) (L490H) in the MuMpl transmembrane domain allowed the murine receptor to respond to NIP-004. Conversely, HuMplH499L lost its response to NIP-004, indicating that the His residue is essential for NIP-004 activity. Because this His residue is only present in the HuMpl transmembrane domain, this observation also helps to explain the species specificity of NIP-004. Moreover, this property requires a xenotransplantation model to evaluate the in vivo biologic activities of NIP-004. We used immunodeficient nonobese diabetic (NOD)/Shi-scid, IL-2Rγnull (NOG) mice as recipients. These mice exhibit highly potent reconstitutive activity for human hematopoietic stem cells and can maintain human hematopoiesis,9 including megakaryopoiesis and thrombopoiesis, for at least 6 months after transplantation (Y.M., T.N., Hiroshi Yoshida, M.I., Y.O., and Y.I., manuscript in preparation). Using this model, we demonstrated that NIP-004 possesses HuMpl agonistic activity in vivo, and these data support the in vitro activity of the molecule.
Materials and methods
Reagents
Our search for c-Mpl activators was based on proliferation assays using TPO-responsive cell lines with our chemical library (Nissan Chemical Industries, Chiba, Japan), which comprises approximately 50 000 compounds. This process resulted in the identification of several compounds displaying growth-promoting activities. Lead optimization was performed based on the conversion of scaffold and functional groups to increase the growth-promoting activity of the lead compound. Finally, a novel nonpetptidyl HuMpl activator, NIP-004 (5-[(2-{1-[5-(3,4-dichlorophenyl)-4–hydroxy -3-thienyl]ethylidene}hydrazino)carbonyl]-2-thiophenecarboxylic acid) was chemically constructed at Nissan Chemical Industries. We also synthesized another human TPO mimetic, SB-497115. Its structure was presented in a poster presentation at the 46th annual meeting of the American Society of Hematology.10 Cytokines including rhTPO (PeproTech, Rocky Hill, NJ, and R&D Systems, Minneapolis, MN), rhEPO (Chugai Pharmaceutical, Tokyo, Japan), recombinant murine (rm) interleukin-3 (IL-3) (R&D Systems), rhIL-3 (R&D Systems), and rhGCSF (PeproTech) were obtained as indicated.
Cells
Cells used in this study were human myeloblastic leukemia cell lines UT-7, UT-7/TPO, and UT-7/EPO (kindly donated by Dr Norio Komatsu, University of Yamanashi, Japan), murine pro–B-cell line Ba/F3 (Riken Cell Bank, Tsukuba, Japan), and human embryonic kidney cell line HEK293 (Health Science Research Resources Bank, Osaka, Japan). UT-7/EPO-HuMpl in which the human c-mpl gene was genetically introduced under the control of cytomegalovirus (CMV) promoter was kindly donated by Dr Norio Komatsu. Stable transfectants such as UT-7/EPO-MuMpl, Ba/F3-HuMpl, Ba/F3-MuMpl, and Ba/F3-HuGCSFR were established to transfect human or murine c-mpl or human gcsfr genes under the control of the CMV promoter (pcDNA3 vector; Invitrogen, Carlsbad, CA) using electroporation. UT-7 cells and the variant cell lines were maintained in Iscove modified Dulbecco medium (IMDM) supplemented with 10% fetal bovine serum (FBS) and rhIL-3, rhTPO, or rhEPO. Ba/F3 cells and their transfectants were maintained in RPMI 1640 medium supplemented with 10% FBS and rmIL-3, rhTPO, or rhGCSF. HEK293 cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% FBS. Human primary hematopoietic progenitor cells were BM-, PB-, or CB-derived CD34+ cells (Cambrex, East Rutherford, NJ). Cynomolgus BM-derived CD34+ cells were prepared from BM mononuclear cells (Cambrex) using a CD34 progenitor cell selection system (Dynabeads M-450 CD34; Dynal Biotech, Oslo, Norway). Murine BM cells were prepared from BALB/c mice (Japan SLC, Shizuoka, Japan).
Genes
The cDNAs of human or murine c-mpl or human gcsfr were amplified by reverse transcriptase–polymerase chain reaction (RT-PCR) using a Super-Script First-Strand Synthesis System (Invitrogen) and each specific primer set as follows: human c-mpl primer set (sense, 5′-ATGCCCTCCTGGGCCCTCTT-3′; antisense, 5′-TCAAGGCTGCTGCCAATAGCT-3′), murine c-mpl primer set (sense, 5′-ATGCCCTCTTGGGCCCTCTTCAT-3′; antisense, 5′-TCAGGGCTGCTGCCAATAGCTTAGT-3′), and human gcsfr primer set (sense, 5′-ATGGCAAGGCTGGGAAACTGCA-3′; antisense, 5′-CTAGAAGCTCCCCAGCGCCTCCA-3′). Full-length cDNAs were cloned into pCR vector (Invitrogen) and were subcloned into the EcoRI site of the pcDNA3 vector. In vitro mutagenesis of human or murine c-mpl genes (HuMplH499L or MuMplL490H) was performed using QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and a specific primer set as follows: HuMplH499L primer set (sense, 5′-GGTGACCGCTCTGCTACTAGTGCTGGGCC-3′; antisense, 5′-GGCCCAGCACTAGTAGCAGAGCGGTCACC-3′) and MuMplL490H primer set (sense, 5′-GTGACTGCTCTGCACCTGGTGCTGAGC-3′; antisense, 5′-GCTCAGCACCAGGTGCAGAGCAGTCAC-3′), according to the protocols of the manufacturer. The cDNAs of cynomolgus monkey, rhesus macaque, common marmoset, and squirrel monkey c-mpl homologous genes were amplified by RT-PCR using a human c-mpl primer set and total RNA isolated from whole blood for each nonhuman primate (Hamri, Ibaraki, Japan). A human c-mpl primer set and a nested primer set (sense, 5′-CTTTGGAACCCGATACGTGTG-3′; antisense, 5′-GGAGGATTTCCAGGAGGCTG-3′), designed for high-sequence homology between human and murine c-mpl genes, were used for amplification in Japanese white rabbit (Kitayama Labes, Nagano, Japan), Syrian hamster, Hartley guinea pig (Japan SLC), and Wistar rat (Charles River Japan, Kanagawa, Japan) c-mpl homologous genes. The cDNAs of these animal c-mpl homologous genes were cloned into the pCR vector, and their sequences were identified as accession numbers AB235193, AB235192, AB235194, AB235195, AB235199, AB235198, AB235196, and AB235197 for cynomolgus monkey, rhesus macaque, common marmoset, squirrel monkey, Japanese white rabbit, Syrian hamster, Hartley guinea pig, and Wistar rat, respectively.
Proliferation assay
A total of 2 × 103 to 6 × 103 cells were harvested in IMDM or RPMI 1640 medium containing 10% FBS and cytokines or NIP-004 at the indicated final concentration and incubated in a CO2 incubator (5% CO2, 37°C) for 3 to 4 days, depending on the cell line. Cell proliferation was assayed using WST-8 reagent (Kishida Chemical, Osaka, Japan) according to instructions from the manufacturer. The formazan pigment was detected by measuring absorbance at 450 nm with a 96-well microplate reader, Spectramax 190 (Molecular Devices, Sunnyvale, CA) or Model 550 (Bio-Rad, Hercules, CA).
Immunoprecipitation and Western blotting
A total of 2 × 107 UT-7/EPO-HuMpl cells were starved for 17 hours at 37°C and stimulated with 20 μg/mL NIP-004 or 30 ng/mL hTPO at 37°C for 15 minutes. Cells were solubilized in 1 mL TNE buffer comprising 20 mM Tris-HCl buffer (pH 7.4), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 mM phenyl methane sulfonyl fluoride, 1 mM Na3VO4, and 1:400-diluted protease inhibitor cocktail (Sigma, St Louis, MO). After centrifugation, cleared lysates were incubated with anti-Mpl (IBL, Gunma, Japan), anti-Jak2 (Upstate Biotechnology, Waltham, MA), and anti-STAT5a (Upstate Biotechnology) for 1 hour at 4°C. Immune complexes adsorbed with protein G–Sepharose were resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Transferred polyvinylidene fluoride membrane (Millipore, Bedford, MA) was immunoblotted with monoclonal antiphosphotyrosine antibody (BD PharMingen, San Diego, CA) or anti-Mpl, anti-Jak2, or anti-STAT5a and alkaline phosphatase (AP)–labeled secondary antibody (Invitrogen); then the proteins were visualized using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate reagents (Bio-Rad).
Megakaryocyte colony-forming unit assay
Megakaryocyte colony-forming unit (CFU-MK) assays were performed using MegaCult-C (StemCell, Vancouver, BC, Canada) according to the manufacturer's protocol. A total of 7.5 × 103 human or cynomolgus monkey BM-derived CD34+ cells or 2.5 × 103 human CB- or PB-derived CD34+ cells were cultured with NIP-004 or rhTPO for 11 days in collagen-based, semisolid medium. After fixation, megakaryocytes were visualized with antibody against CD41a using AP staining. Nuclei were stained with Evans blue. Stained colonies were examined under a Nikon Eclipse E800 microscope equipped with a 10 × 0.3 numeric aperture (NA) objective lens (Nikon, Tokyo, Japan). Images were captured using an Olympus Camedia C300 digital camera (Olympus, Tokyo, Japan). The murine CFU-MK assay was performed as follows: 3 × 105 murine BM cells were cultured with NIP-004 or rhTPO for 7 days in agar-based, semisolid medium comprising IMDM, 1% bovine serum albumin, 0.6 mg/mL transferrin, 8 μg/mL cholesterol, and 0.1% agar. After fixation in glutaraldehyde, megakaryocytes were visualized by measuring the internal acetyl cholinesterase activity as previously described.11
Luciferase assay
HEK293 cells were transfected with pcDNA-HuMpl, HuMplH499L, MuMpl, or MuMplL490H in combination with pXM-MGF (STAT5) and acute phase response element–luciferase construct (kindly donated by Dr Akihiko Yoshimura12 ) using the Effectene transfection reagent (Qiagen, Hilden, Germany). Twenty-four hours after transfection, cells were starved for 8 hours, followed by stimulation with rhTPO or NIP-004 at the indicated concentration for 18 hours. Cell extracts were prepared, and luciferase activity was measured using PicaGene reagent (Toyo B-NET, Tokyo, Japan) according to the manufacturer's instructions.
Xenotransplantation assay
NOG mice were developed at the Central Institute for Experimental Animals (Kanagawa, Japan) and were maintained under specific pathogen-free conditions. Shortly before cell transfer, 8- to 10-week-old NOG mice were irradiated with 2.4 Gy x-rays. Frozen 1 × 105 CB-derived CD34+ cells were washed with IMDM medium supplemented with 0.1% BSA and the cells then intravenously inoculated into mice. After transplantation, mice were provided with sterile water containing prophylactic neomycin sulfate (Gibco BRL, Grand Island, NY). Mice received daily subcutaneous administration of NIP-004 for the indicated period at a dosage of 10 or 30 mg/kg (with PBS containing 1.6% PEG400 and 2.4% ethanol vehicle) from 2 to 6 months after transplantation. Whole blood containing EDTA-2Na or 0.38% sodium citrate was prepared recurrently from a mouse. Complete blood counts were obtained using a K-4500 automated analyzer (Sysmex, Hyogo, Japan). BM cells were obtained by flushing the femurs, and cell numbers were counted using a hemocytometer (Erma, Tokyo, Japan) after staining with Turk solution.
Flow cytometry analysis
To detect human cells in murine blood, multicolor flow cytometry was performed using an EPICS-XL flow cytometer (Beckman Coulter, Franklin Lakes, NJ). Samples were incubated with the indicated antibodies for 30 minutes at 4°C and were depleted of erythrocytes by fixation in fluorescence-activated cell sorter (FACS) lysing solution (BD PharMingen). Antibodies used in this study were as follows: anti–human CD33–fluorescein isothiocyanate (FITC), CD3-FITC, CD41a-FITC, CD34-FITC, CD71-FITC, CD34-phycoerythrin (PE), CD41a-PE, CD19-PE, CD45-PE Texas red (ECD), CD38-PE 5-succinimidylester (PC5), anti–murine CD45-FITC, and CD41-FITC. Antibodies conjugated with FITC, PE, or PC5 were purchased from BD PharMingen. Antibodies conjugated with ECD were purchased from Immunotech (Marseilles, France). Platelets were incubated with the indicated antibodies at room temperature and directly applied to the flow cytometer. Human platelets were examined by double staining with anti–murine CD41-FITC antibody and anti–human CD41a-PE antibody, in which platelets were identified by size and gated by forward and side scatter. The actual number of human CD41a+ platelets was calculated as follows: actual human CD41a+ platelet count = (human CD41a+ platelet count/[human CD41a+ platelet count + murine CD41+ platelet count]) × whole platelet count.
To detect human megakaryocyte ploidy, BM cells from NOG mice that received xenotransplants were stained using anti–human CD41a-PE antibody and fixed with 1% paraformaldehyde. Fixed cells were stained using 7-amino-actinomycin D (7-AAD) dye (Immunotech) and examined by 2-color cytometric analysis.
Immunohistochemistry
Femurs of NOG mice that received xenotransplants were removed for histologic examination. Tissue was fixed in neutral buffered formalin and decalcified, followed by paraffin embedding and sectioning. Immunohistochemistry was performed with murine monoclonal antibodies against human CD42b (Chemicon, Temecula, CA) and EnVision+ peroxidase staining kit (Dako, Carpinteria, CA). Stained sections were examined using an Olympus BX51 microscope equipped with a 10 × 0.3 NA objective and connected to an Olympus DP12 camera, using DP12 software.
Electron microscopic analysis of human platelets from mice undergoing xenotransplantation
Immunoelectron microscopy was performed as previously described.13 Whole blood containing 0.38% sodium citrate, 1 μM prostaglandin E1, 2 U/mL apyrase, and 2 mM aspirin (Sigma) was prepared. Platelet-rich plasma (PRP) was fixed with glutaraldehyde and sequentially immersed in 1 M sucrose, 1.84 M sucrose, and 1.84 M sucrose with 20% polyvinylpyrrolidone (MW 10 000; Sigma) in PBS. After freezing platelets in liquid nitrogen, ultrathin frozen sections were cut using an Ultracut ultramicrotome (Reichert, Vienna, Austria) with a cryoattachment (FC-4E; Reichert) at –90°C. Sections were mounted on nickel grids and incubated with anti–human CD41a monoclonal antibody (Immunotech), followed by goat anti–mouse IgG coupled to colloidal gold (10 nm; Amersham, Olen, Belgium). Sections were stained with uranyl acetate and subjected to adsorption staining using a mixture of polyvinyl alcohol and uranyl acetate. Stained sections were examined using a JEM 1200EX electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV.
Functional studies of platelets from NOG mice undergoing xenotransplantation
Functional studies of platelets were performed as previously described.14 After administration of NIP-004 (30 mg/kg/d subcutaneously for 14 days) or vehicle, PRP was obtained, and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)–Tyrode buffer (138 mM NaCl, 0.42 mM NaH2PO4, 2.68 mM KCl, 12 mM NaHCO3, 10 mM HEPES, 5 mM glucose, and 1.7 mM MgCl2, pH 7.4) was added at a 1:9 ratio. To determine the expression of P selectin upon agonist stimulation, platelets were stimulated using 10 μM ADP or vehicle for 15 minutes in the buffer containing anti–human CD62p-PE antibody (BD PharMingen). After fixation with 0.5% paraformaldehyde, cells were washed and incubated with anti–human CD41a-PC5 (BD PharMingen) and anti–murine CD41-FITC antibody. To determine the activation of GPIIb-IIIa, platelets were stimulated with 0 to 10 μM ADP for 15 minutes with PAC-1–FITC (BD Pharmingen), anti–human CD42b-PC5, and anti–murine CD61-PE antibody and then fixed and washed. Stained platelets were analyzed by flow cytometry.
Statistical analysis and ethical considerations
Results are expressed as mean ± SD or mean ± SEM as indicated. Differences between groups were examined for statistical significance using the Student t test. Animal experiments were conducted according to the “Guideline for animal experimentation”15 by the Japanese Association for Laboratory Animal Science. All experimental protocols were approved by the ethics review committees for animal experimentation of Keio University and Nissan Chemical Industries.
Results
NIP-004 is a novel human c-Mpl activator
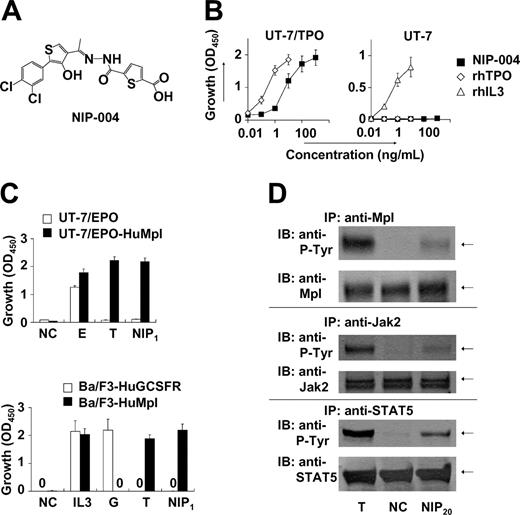
We conducted a search for c-Mpl activators based on proliferation assays with TPO-responsive UT-7/TPO and UT-7/EPO-HuMpl cell lines. Screened agents were obtained from our chemical library, comprising approximately 50 000 compounds. This process resulted in the identification of several compounds displaying growth-promoting activity. Lead optimization was subsequently performed to create c-Mpl activators with greater growth-promoting activity. NIP-004 is a synthetic compound (MW 455; Figure 1A) and stimulated the proliferation of UT-7/TPO,16 a human leukemia cell line endogenously expressing HuMpl, in a dose-dependent manner (Figure 1B). Compared with 10 ng/mL rhTPO, which induces a maximal response in this cell line, the median effective concentration (EC50) of NIP-004 was 23 ng/mL (50 nM) in UT-7/TPO cells (Figure 1B). Proliferation of UT-7 cells, the parental cell line of UT-7/TPO, can be induced by cytokines such as human IL-3, human granulocyte-macrophage colony-stimulating factor (GMCSF), IL-6, stem cell factor, and EPO but not by TPO because UT-7 does not display c-Mpl.16,17 Consistent with its action through c-Mpl, NIP-004 did not induce the growth of UT-7 cells (Figure 1B, right panel). Recombinant human IL-3 induced proliferation of UT-7 cells as a positive control. We also confirmed the roles of HuMpl using other cell lines as follows. When HuMpl was introduced into UT-7/EPO cells,18,19 UT-7/EPO-HuMpl transfectants responded to both rhTPO and NIP-004 (Figure 1C, upper panel). NIP-004 induced proliferation of Ba/F3-HuMpl cells but not Ba/F3-HuGCSFR cells expressing human GCSF receptor, indicating that NIP-004 acts on c-Mpl (Figure 1C, lower panel). Recombinant human GCSF and rhTPO stimulated proliferation of Ba/F3-HuGCSFR and Ba/F3-HuMpl cells as positive controls, respectively (Figure 1C).
NIP-004 is a novel human c-Mpl activator. (A) Chemical structure of NIP-004. (B) Dose-dependent proliferation of UT-7/TPO cells, but not parental UT-7 cells, by NIP-004. Recombinant human TPO and rhIL-3 induced proliferation of UT-7/TPO and parental UT-7 cells, respectively. (C) TPO and NIP-004 induced proliferation of UT-7/EPO-HuMpl but not UT-7/EPO (top panel). EPO stimulated proliferation of UT-7/EPO and UT-7/EPO-HuMpl as a positive control. NIP-004 stimulated proliferation of Ba/F3-HuMpl but not Ba/F3-HuGCSFR (bottom panel). GCSF stimulated Ba/F3-HuGCSFR cells. TPO induced proliferation of Ba/F3-HuMpl cells as a positive control. IL-3 stimulated both Ba/F3-HuGCSFR and Ba/F3-HuMpl. Data in panels B-C are expressed as the mean ± SEM (n = 3 to 4) or mean ± SD (n = 2; Ba/F3-HuGCSFR) from independent duplicate experiments. (D) Induction of tyrosine phosphorylation of c-Mpl, Jak2, and STAT5 in UT-7/EPO-HuMpl cells by TPO and NIP-004. NC indicates negative control; E, 0.1 U/mL rhEPO; IL-3, 0.1 ng/mL rmIL-3; G, 10 ng/mL rhGCSF; T, 10 ng/mL rhTPO; NIP1, 1 μg/mL NIP-004; NIP20, 20 μg/mL NIP-004; P-Tyr, phosphotyrosine.
NIP-004 is a novel human c-Mpl activator. (A) Chemical structure of NIP-004. (B) Dose-dependent proliferation of UT-7/TPO cells, but not parental UT-7 cells, by NIP-004. Recombinant human TPO and rhIL-3 induced proliferation of UT-7/TPO and parental UT-7 cells, respectively. (C) TPO and NIP-004 induced proliferation of UT-7/EPO-HuMpl but not UT-7/EPO (top panel). EPO stimulated proliferation of UT-7/EPO and UT-7/EPO-HuMpl as a positive control. NIP-004 stimulated proliferation of Ba/F3-HuMpl but not Ba/F3-HuGCSFR (bottom panel). GCSF stimulated Ba/F3-HuGCSFR cells. TPO induced proliferation of Ba/F3-HuMpl cells as a positive control. IL-3 stimulated both Ba/F3-HuGCSFR and Ba/F3-HuMpl. Data in panels B-C are expressed as the mean ± SEM (n = 3 to 4) or mean ± SD (n = 2; Ba/F3-HuGCSFR) from independent duplicate experiments. (D) Induction of tyrosine phosphorylation of c-Mpl, Jak2, and STAT5 in UT-7/EPO-HuMpl cells by TPO and NIP-004. NC indicates negative control; E, 0.1 U/mL rhEPO; IL-3, 0.1 ng/mL rmIL-3; G, 10 ng/mL rhGCSF; T, 10 ng/mL rhTPO; NIP1, 1 μg/mL NIP-004; NIP20, 20 μg/mL NIP-004; P-Tyr, phosphotyrosine.
A large body of evidence indicates that like other type I family cytokine receptors, TPO signaling is dependent on Jak-induced phosphorylation of tyrosine residues within the cytoplasmic domain of c-Mpl. Subsequently, secondary signaling molecules, including STAT5, are recruited to the phosphotyrosine residues and are activated by their phosphorylation.6 Administration of 20 μg/mL NIP-004 induced phosphorylation of c-Mpl, Jak2, and STAT5 proteins in UT-7/EPO-HuMpl cells after 15 minutes (Figure 1D). TPO was used as positive control stimulation (Figure 1D). In addition, rhTPO and NIP-004 induced phosphorylation of Akt, an antiapoptotic protein in the PI3K-Akt signaling pathway, via c-Mpl (data not shown).
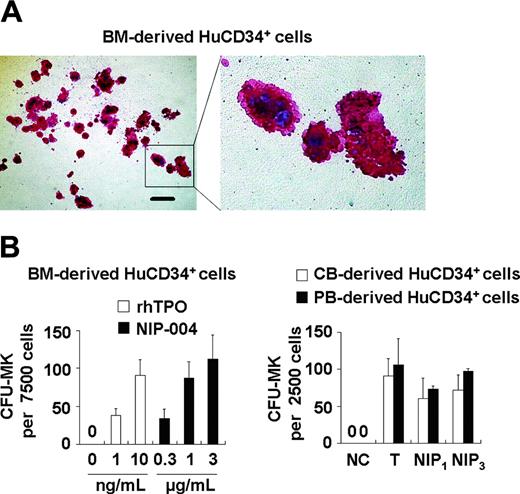
We next examined whether NIP-004 possessed the capacity to induce megakaryocyte differentiation using colony-forming assays and histologic staining with a monoclonal antibody against integrin GPIIb-IIIa (CD41a), a specific marker of the megakaryocyte-platelet lineage.20 NIP-004 1 μg/mL alone stimulated colony formation of CD41a+ megakaryocytes from human BM-derived CD34+ hematopoietic progenitor cells in serumfree, semisolid cultures (Figure 2A). NIP-004 induced maturation of megakaryocytes, since large polyploid CD41a+ cells appeared in the colony (Figure 2A). NIP-004 increased the number of CFU-MKs from human BM-derived CD34+ cells in a dose-dependent manner (Figure 2B, left panel). Furthermore, a number of CFU-MKs developed from human CB- or PB-derived CD34+ cells treated with NIP-004 (Figure 2B). The efficiency of CFU-MK production in CD34+ cells was higher in CB- and PB-derived cells than in BM-derived cells, as previously reported (Figure 2B, right panel).21
NIP-004 displays species-specific activity
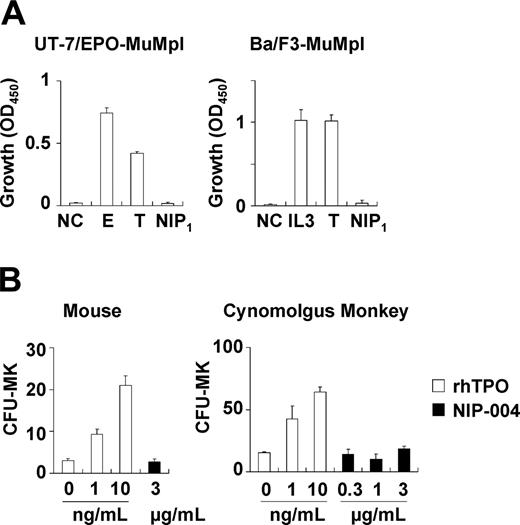
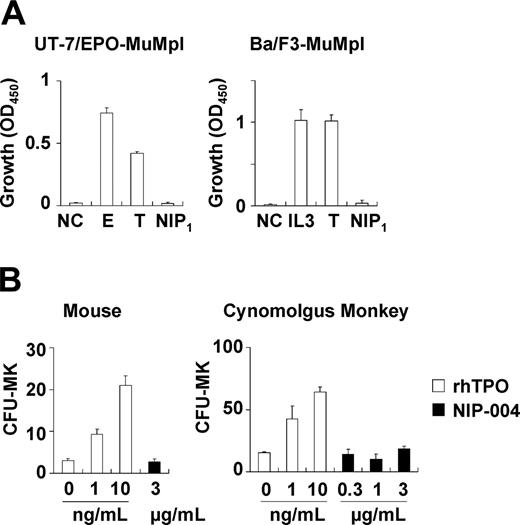
Because NIP-004 was obtained using cells expressing HuMpl, the effects of NIP-004 on c-Mpl from other species were examined. NIP-004 did not induce proliferation of UT-7/EPO-MuMpl or Ba/F3-MuMpl cells, which were engineered to express murine c-Mpl receptor (Figure 3A). Furthermore, NIP-004 did not increase the number of CFU-MK colonies from murine BM cells or cynomolgus monkey BM-derived CD34+ cells within the effective dosage for human cells (Figure 3B). Likewise, rhesus macaque BM-derived CD34+ cells failed to form CFU-MK colonies in the presence of a NIP-004–derived compound (data not shown). These results indicate that NIP-004 displays species specificity.
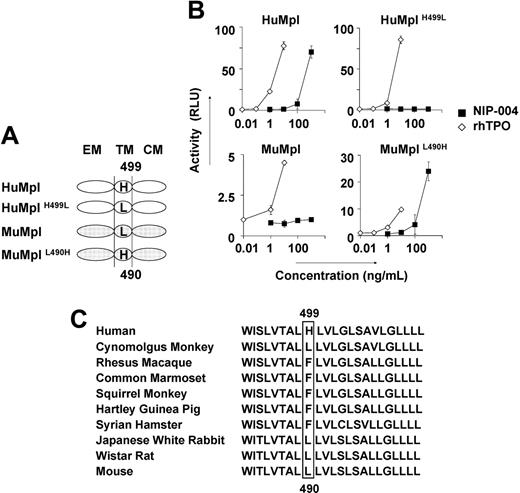
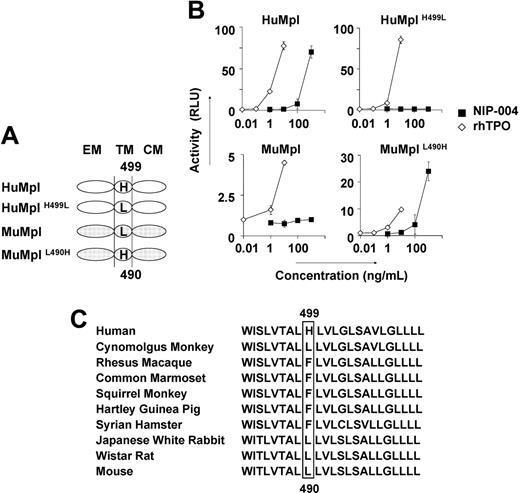
To identify the molecular basis for the species specificity displayed by NIP-004, we analyzed the amino acid sequence of c-Mpl from 2 nonhuman primates, cynomolgus and rhesus monkeys, and compared them with the HuMpl and MuMpl. Cynomolgus c-Mpl displayed the highest level of sequence homology, 96% identical to HuMpl, with only 22 amino acids differing between the 2 species, and 12 of the 22 amino acids of HuMpl differed from rhesus c-Mpl. Comparison of these 12 amino acid residues as either hydrophobic or hydrophilic, and either electrically charged or uncharged, revealed distinguishing features of the amino acid residues. His at position 499 from the N-terminal end in the transmembrane domain of HuMpl is specific for humans. We thus performed site-directed mutagenesis for this residue in HuMpl and MuMpl (Figure 4A) and analyzed STAT5 activation via each receptor in the HEK293 cell line using the STAT-reporter gene assay (Figure 4B). Wild-type HuMpl, but not MuMpl, activated STAT5 after stimulation with NIP-004 in a dose-dependent manner. HuMpl with His499 mutated to Leu failed to induce STAT5 activation following stimulation with NIP-004, although rhTPO was active. Conversely, when MuMpl was engineered to contain His490, as is present in the human receptor, it was then capable of activating STAT5 (Figure 4B). These observations indicate that His in the transmembrane domain of c-Mpl is essential for NIP-004 to function as a c-Mpl activator. We therefore assessed the c-Mpl amino acid sequence in several common experimental animals to find a suitable species in which we could evaluate the in vivo efficacy of NIP-004 for platelet production. Our search revealed no animals with His in the transmembrane domain of c-Mpl among the common marmoset, squirrel monkey, Japanese white rabbit, Syrian hamster, Hartley guinea pig, and Wistar rat (Figure 4C).
Stimulation of megakaryocyte colony formation from human CD34+ cells by NIP-004. (A) Morphology of CFU-MK colonies obtained from human BM-derived CD34+ cells treated with 1 μg/mL NIP-004. CFU-MKs were visualized with AP-labeled antibody against human CD41. Polyploid megakaryocytes are shown in detail in the right panel. Bar, 200 μm. (B) The number of CFU-MKs from human BM-, CB-, and PB-derived CD34+ cells treated with 1 to 3 μg/mL NIP-004 was similar to that with 10 ng/mL rhTPO. Results are expressed as mean ± SEM from 4 independent experiments (BM) or mean ± SD from 2 independent experiments (CB and PB). NC indicates negative control; T, 10 ng/mL rhTPO; NIP1,1 μg/mL NIP-004; NIP3,3 μg/mL NIP-004.
Stimulation of megakaryocyte colony formation from human CD34+ cells by NIP-004. (A) Morphology of CFU-MK colonies obtained from human BM-derived CD34+ cells treated with 1 μg/mL NIP-004. CFU-MKs were visualized with AP-labeled antibody against human CD41. Polyploid megakaryocytes are shown in detail in the right panel. Bar, 200 μm. (B) The number of CFU-MKs from human BM-, CB-, and PB-derived CD34+ cells treated with 1 to 3 μg/mL NIP-004 was similar to that with 10 ng/mL rhTPO. Results are expressed as mean ± SEM from 4 independent experiments (BM) or mean ± SD from 2 independent experiments (CB and PB). NC indicates negative control; T, 10 ng/mL rhTPO; NIP1,1 μg/mL NIP-004; NIP3,3 μg/mL NIP-004.
NIP-004 displays species-specific activity. (A) NIP-004 failed to stimulate proliferation of UT-7/EPO-MuMpl– and Ba/F3-MuMpl–expressing murine Mpl. In contrast, EPO and TPO stimulated induced proliferation of UT-7/EPO-MuMpl as a positive control. IL-3 and TPO enhanced proliferation of Ba/F3-MuMpl as a positive control. Data are expressed as the mean ± SEM from 3 independent experiments. (B) Recombinant human TPO stimulated CFU-MK colony formation from murine and cynomolgus monkey–derived bone marrow cells in a dose-dependent manner. In contrast, NIP-004 failed to induce CFU-MK colonies under the same conditions. Data are expressed as the mean ± SD of duplicate assays. NC indicates negative control; E, 0.1 U/mL rhEPO; IL-3, 0.1 ng/mL rmIL-3; T, 10 ng/mL rhTPO; NIP1, 1μg/mL NIP-004.
NIP-004 displays species-specific activity. (A) NIP-004 failed to stimulate proliferation of UT-7/EPO-MuMpl– and Ba/F3-MuMpl–expressing murine Mpl. In contrast, EPO and TPO stimulated induced proliferation of UT-7/EPO-MuMpl as a positive control. IL-3 and TPO enhanced proliferation of Ba/F3-MuMpl as a positive control. Data are expressed as the mean ± SEM from 3 independent experiments. (B) Recombinant human TPO stimulated CFU-MK colony formation from murine and cynomolgus monkey–derived bone marrow cells in a dose-dependent manner. In contrast, NIP-004 failed to induce CFU-MK colonies under the same conditions. Data are expressed as the mean ± SD of duplicate assays. NC indicates negative control; E, 0.1 U/mL rhEPO; IL-3, 0.1 ng/mL rmIL-3; T, 10 ng/mL rhTPO; NIP1, 1μg/mL NIP-004.
NIP-004 stimulates human megakaryopoiesis in NOG mice undergoing xenotransplantation
Because we were unable to find suitable experimental animals with His in the c-Mpl transmembrane domain to evaluate the effects of NIP-004, we selected a xenotransplantation model to examine in vivo efficacy of the compound for human megakaryopoiesis and thrombopoiesis. We used NOG mice to develop this new experimental animal model of human megakaryopoiesis, because this species displayed high potency for reconstituting human hematopoietic progenitor cells.9
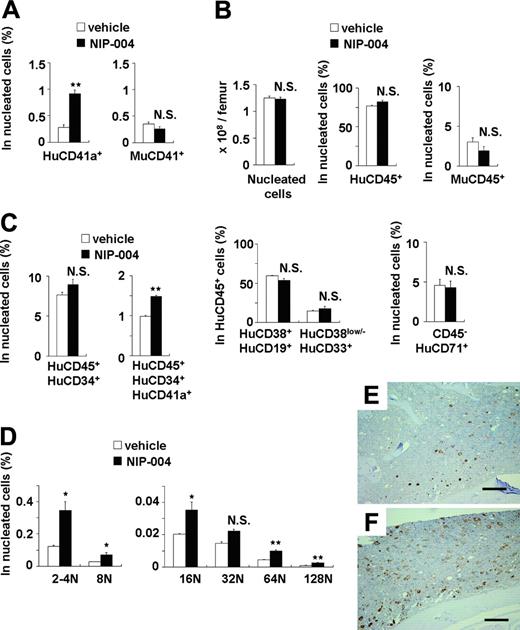
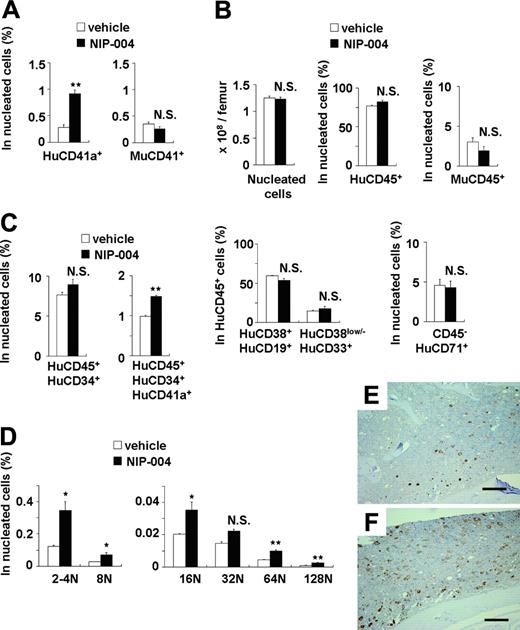
In NOG mice receiving transplants with human CB-derived CD34+ cells, treatment with 30 mg/kg/d NIP-004 for 2 weeks resulted in a 3-fold increase in human (Hu) CD41a+ megakaryocytes in murine BM (0.9% in NIP-004–treated mice versus 0.3% in vehicle-treated mice) but no alteration in the number of murine CD41+ megakaryocytes (Figure 5A). This dosage of NIP-004 achieved a plasma concentration of approximately 0.6 μg/mL at steady state. NIP-004 thus enhanced human megakaryopoiesis at an in vivo dosage comparable to that in the in vitro colony formation study (Figure 2B).
NIP-004 did not influence the total number of nucleated cells, the percentage of human or murine CD45+ leukocytes, the percentage of HuCD38+CD19+ B lymphoid or HuCD38low/–CD33+ myeloid cells in HuCD45+ cells, or the number of HuCD45–CD71+ erythroblasts in murine BM (Figure 5B). Although the total percentage of HuCD45+CD34+ hematopoietic progenitor cells was not altered by NIP-004 administration, we observed a 1.5-fold increase in the percentage of HuCD45+CD34+CD41a+ megakaryoblasts (Figure 5C). This finding indicates that NIP-004 enhanced the number of human megakaryoblasts and megakaryocytes in xenotransplanted murine BM.
We next examined whether NIP-004 induced the maturation of megakaryocytes in vivo. DNA ploidy of HuCD41+ megakaryocytes was analyzed by flow cytometry using anti-HuCD41 antibody and 7-AAD dye. In mice that received transplants, BM cells were prepared after treatment with NIP-004 (30 mg/kg/d for 2 weeks) or vehicle. Each ploidy class of human megakaryocytes was increased in mice treated with NIP-004, with statistical significance in all but the 32N class (Figure 5D). NIP-004 increased the percentage of HuCD41a+ 128N megakaryocytes by a mean 2.7 ± 0.1 times.
Similar results were obtained in immunohistochemistry. BM from mice receiving xenotransplants was stained with monoclonal antibodies against human integrin GPIb (HuCD42b) to specifically visualize human megakaryocytes (Figure 5E-F). Murine megakaryocytes were not stained with this antibody. NIP-004 (30 mg/kg/d for 2 weeks) clearly enhanced human megakaryopoiesis (Figure 5E-F). These findings demonstrate that NIP-004 specifically enhanced human megakaryopoiesis but not other types of human hematopoiesis in mice receiving xenotransplants.
His in the transmembrane domain of c-Mpl is the essential residue for NIP-004. (A) Schema of site-directed mutagenesis in HuMpl and MuMpl. EM indicates extracellular module; TM, transmembrane module; CM, cytoplasmic module. (B) STAT5-reporter gene assay showing induction of STAT activation via HuMpl and MuMplL490H but not HuMplH499L or MuMpl following NIP-004 administration. Data are expressed as the mean ± SD from 2 independent experiments. (C) Comparison of amino acid sequences of c-Mpl transmembrane domain from various animals. His499 only exists in humans and not in other species.
His in the transmembrane domain of c-Mpl is the essential residue for NIP-004. (A) Schema of site-directed mutagenesis in HuMpl and MuMpl. EM indicates extracellular module; TM, transmembrane module; CM, cytoplasmic module. (B) STAT5-reporter gene assay showing induction of STAT activation via HuMpl and MuMplL490H but not HuMplH499L or MuMpl following NIP-004 administration. Data are expressed as the mean ± SD from 2 independent experiments. (C) Comparison of amino acid sequences of c-Mpl transmembrane domain from various animals. His499 only exists in humans and not in other species.
Effects of NIP-004 on human megakaryopoiesis in xenotransplanted murine bone marrow. (A) NIP-004 increased HuCD41a+ megakaryocytes, but not MuCD41+ megakaryocytes, in NOG mice receiving xenotransplants. (B) NIP-004 had no significant effect on the number of total nucleated cells and the percentage of other cell lines in murine BM. (C) NIP-004 increased the number of human megakaryocytic progenitor cells (HuCD45+CD34+CD41a+) but not HuCD45+CD34+ hematopoietic progenitor cells. (D) FACS analysis of DNA ploidy of HuCD41+ megakaryocytes. NIP-004 induced maturation of human megakaryocytes in the BM of NOG mice receiving xenotransplants. Data in panels A-D are expressed as mean ± SEM (n = 3). *P < .05, **P < .01 between NIP-004 and vehicle. N.S. indicates no significant difference compared with vehicle. Similar results were obtained from 2 independent experiments. (E-F) Immunohistochemical staining of xenotransplanted murine BM with a monoclonal antibody against human integrin GPIb (HuCD42b). Sections were obtained from the femurs of mice treated with vehicle (E) or NIP-004 (F). Bars, 200 μm.
Effects of NIP-004 on human megakaryopoiesis in xenotransplanted murine bone marrow. (A) NIP-004 increased HuCD41a+ megakaryocytes, but not MuCD41+ megakaryocytes, in NOG mice receiving xenotransplants. (B) NIP-004 had no significant effect on the number of total nucleated cells and the percentage of other cell lines in murine BM. (C) NIP-004 increased the number of human megakaryocytic progenitor cells (HuCD45+CD34+CD41a+) but not HuCD45+CD34+ hematopoietic progenitor cells. (D) FACS analysis of DNA ploidy of HuCD41+ megakaryocytes. NIP-004 induced maturation of human megakaryocytes in the BM of NOG mice receiving xenotransplants. Data in panels A-D are expressed as mean ± SEM (n = 3). *P < .05, **P < .01 between NIP-004 and vehicle. N.S. indicates no significant difference compared with vehicle. Similar results were obtained from 2 independent experiments. (E-F) Immunohistochemical staining of xenotransplanted murine BM with a monoclonal antibody against human integrin GPIb (HuCD42b). Sections were obtained from the femurs of mice treated with vehicle (E) or NIP-004 (F). Bars, 200 μm.
NIP-004–induced production of human platelets in mice undergoing xenotransplantation
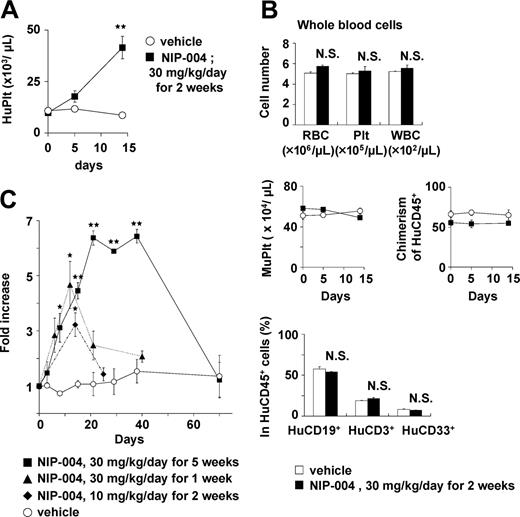
All NOG mice (n = 84) receiving transplants with human hematopoietic cells produced human platelets for at least 1 to 6 months. To clarify whether NIP-004 increased circulating human platelets in mice receiving xenotransplants, several administration protocols were used. NIP-004 30 mg/kg/d subcutaneously for 2 weeks induced a statistically significant 4.4-fold increase in circulating human CD41a+ platelets at day 14 (Figure 6A). NIP-004 did not influence the total number (human and murine) of red blood cells, platelets, and white blood cells (Figure 6B, upper panel). The number of murine CD41+ platelets and chimerisms of HuCD45+ leukocytes was not altered by NIP-004 administration (Figure 6B, middle panel). Furthermore, NIP-004 did not influence the percentage of HuCD19+ B lymphoid, HuCD3+ T lymphoid, or HuCD33+ myeloid cells among the circulating HuCD45+ cells in mice receiving xenotransplants (Figure 6B, lower panel).
Next, the effect of the administration period or dosage was examined. When NOG mice receiving transplants with HuCD34+ cells were treated with NIP-004 30 mg/kg/d for 5 weeks, there was a 6.4-fold increase in human platelets (Figure 6C). In mice receiving transplants, treatment with 10 mg/kg/d NIP-004 for 2 weeks resulted in a 3.2-fold increase in human platelets (Figure 6C). Human platelet counts returned to pretreatment levels after cessation of drug treatment (Figure 6C). When mice were reexposed to NIP-004, the number of human platelets increased again, suggesting human megakaryopoiesis was maintained in NOG mice undergoing xenotransplantation (data not shown).
To elucidate whether the human platelets in NIP-004–treated mice displayed normal morphologic features, immunoelectron microscopic analysis was performed using antibodies against HuCD41 and MuCD41. Platelets were prepared from mice after administration of NIP-004 30 mg/kg/d subcutaneously for 2 weeks, when human platelet chimerism increased from 2% at baseline to approximately 9%. Human platelets were labeled with gold particles to visualize HuCD41 (Figure 7A). Human platelets labeled with gold particles were larger than unlabeled murine platelets. Human platelets exhibited discoid forms similar to normal human peripheral platelets,13 containing some granules, mitochondria, and other organelles. Conversely, when antibody against MuCD41 was used, some platelets were not labeled with gold particles on the surface (data not shown). These observations indicate that NIP-004 administration in mice receiving xenotransplants resulted in the production of human platelets that were morphologically indistinguishable from normal human platelets.
We next studied whether the circulating human platelets in NOG mice were functional. Platelets were obtained from either NIP-004– or vehicle-treated mice. When platelets were stimulated with 10 μM ADP, surface expression of P selectin (CD62p) was observed on human platelets from NIP-004–treated mice at almost the same rate as vehicle-treated mice (Figure 7B), suggesting that α-granules were released.14 In addition, we examined whether the human platelets found in NIP-004–treated mice receiving xenotransplants exhibited a similar response to ADP stimulation as normal platelets (Figure 7C). The active form of fibrinogen receptor (GPIIb-IIIa integrin) on human platelets was recognized by binding of the PAC-1 monoclonal antibody (Figure 7C).14 Human platelet activation by ADP was dose dependent, and the mean concentration of ADP required to produce 50% activation of the human platelets was 0.6 μM in NIP-004–treated and 0.3 μM in vehicle-treated mice (Figure 7C). These observations indicated that NIP-004 had no adverse effects on the function of human platelets.
NIP-004–induced production of human platelets in NOG mice receiving xenotransplants. (A) NIP-004 increased the number of circulating human platelets in NOG mice. (B) NIP-004 did not change the number of murine platelets or chimerism of HuCD45+ cells. NIP-004 had no effect on the percentage of human B (CD19+) cells, human T (CD3+) cells, and human myeloid (CD33+) cells in the peripheral HuCD45+ cells. Data from panels A-B are expressed as the mean ± SEM (n = 3). (C) NIP-004 increased the number of circulating human platelets. The increase was calculated as the number of circulating human platelets at individual time points divided by the pretreatment value (day 0). Data are expressed as the mean ± SEM (n = 3 to 6) or mean ± SD (n = 2). *P < .05, **P < .01 between NIP-004 and vehicle at individual time points. N.S. indicates no significant differences compared with vehicle.
NIP-004–induced production of human platelets in NOG mice receiving xenotransplants. (A) NIP-004 increased the number of circulating human platelets in NOG mice. (B) NIP-004 did not change the number of murine platelets or chimerism of HuCD45+ cells. NIP-004 had no effect on the percentage of human B (CD19+) cells, human T (CD3+) cells, and human myeloid (CD33+) cells in the peripheral HuCD45+ cells. Data from panels A-B are expressed as the mean ± SEM (n = 3). (C) NIP-004 increased the number of circulating human platelets. The increase was calculated as the number of circulating human platelets at individual time points divided by the pretreatment value (day 0). Data are expressed as the mean ± SEM (n = 3 to 6) or mean ± SD (n = 2). *P < .05, **P < .01 between NIP-004 and vehicle at individual time points. N.S. indicates no significant differences compared with vehicle.
Morphologic and functional features of human platelets induced by NIP-004 in NOG mice. (A) Immunoelectron microscopy using antibody against HuCD41a identified human platelets in PRP derived from NIP-004–treated mice. The surface of a platelet located in the center is labeled with gold particles (arrow), indicating that it is of human origin. Bar, 1 μm. (B) P selectin (HuCD62p) expression upon ADP stimulation in human platelets was similarly increased in both vehicle- and NIP-004–treated mice. (C) After stimulation with various concentrations of ADP, there was a similar dose-dependent escalation in the percentage of PAC-1–positive human platelets from vehicle- and NIP-004–treated NOG mice receiving xenotransplants. PAC-1 antibody specifically recognizes the activated form of GPIIbIIIa. Data are expressed as the mean ± SEM (n = 4).
Morphologic and functional features of human platelets induced by NIP-004 in NOG mice. (A) Immunoelectron microscopy using antibody against HuCD41a identified human platelets in PRP derived from NIP-004–treated mice. The surface of a platelet located in the center is labeled with gold particles (arrow), indicating that it is of human origin. Bar, 1 μm. (B) P selectin (HuCD62p) expression upon ADP stimulation in human platelets was similarly increased in both vehicle- and NIP-004–treated mice. (C) After stimulation with various concentrations of ADP, there was a similar dose-dependent escalation in the percentage of PAC-1–positive human platelets from vehicle- and NIP-004–treated NOG mice receiving xenotransplants. PAC-1 antibody specifically recognizes the activated form of GPIIbIIIa. Data are expressed as the mean ± SEM (n = 4).
Discussion
In this study, we identified a nonpeptidyl synthetic compound, NIP-004, as a novel c-Mpl activator. In vitro studies revealed that rhTPO and NIP-004 display a similar potential to activate HuMpl. However, unlike rhTPO, NIP-004 displays species specificity. Recently, nonpeptidyl small molecule compounds such as benzodiazepine derivatives,22 hydrazinonaphthalenes and azonaphthalenes,23,24 and substituted thiazole25 and xanthocillins26 have been reported as possessing TPO-like activity. In some cases, c-Mpl agonists such as SB394725 exhibit species specificity.27 This study confirms that His in the transmembrane domain of c-Mpl is essential for NIP-004 agonist activity (Figure 4B). Although His exists in the transmembrane domain of HuGCSFR and mouse IL-3/GMCSF/IL-5 common β subunit 2 (Muβc2) (ENSP00000342623 and ENSMUSP00000006263 from the Ensembl database, respectively), Ba/F3-HuGCSFR cells expressing Muβc2 and HuGCSFR did not respond to NIP-004 (Figure 1C). Because the position of His in the transmembrane domains of HuGCSFR and Muβc2 does not correspond to HuMpl, we speculate that the His may need to be present in the middle of a transmembrane domain and/or interact with other amino acids of c-Mpl for NIP-004 agonist activity. It has been reported that the point mutation of Leu499 to His in the transmembrane domain of cynomolgus monkey c-Mpl results in the appearance of Mpl agonist activity for SKF-57626, which also possesses HuMpl agonistic activity and does not cause any reactions with cynomolgus monkey c-Mpl,28 supporting the role of a His residue in the c-Mpl transmembraine domain. Onishi et al previously demonstrated that the point mutation of Ser505 to Asn in the HuMpl transmembrane domain creates a constitutively active form of the receptor,29 and patients with familial essential thrombocythemia have recently been shown to possess this mutation.30 Furthermore, mutating Val449 to Gln in the transmembrane domain constitutively activates Huβ 31 c. We speculate that mutation in the transmembraine domain of the cytokine receptors may change their conformation and sensitivity to ligand binding or autophosphorylation. Although EPO receptor and growth hormone receptor have been reported to exist in a dimeric form prior to ligand binding,32,33 no such evidence exists for c-Mpl. Because we have not yet confirmed whether NIP-004 binds directly to HuMpl, further investigation is needed to elucidate its exact mechanisms.
Analysis of human megakaryopoiesis and thrombopoiesis in vivo has been limited to xenotransplantation models, because continual, reproducible reconstruction of human platelet production is difficult using NOD/severe combined immunodeficiency (SCID) or NOD/SCID/β2mnull mice. Perez et al demonstrated that human megakaryopoiesis and thrombopoiesis appeared 1 month after transplantation, and human platelets in these mice were functional using PB-derived CD34+-transplanted NOD/SCID mice.34 Human platelets were hardly detected in NOD/SCID mice receiving transplants of human CB cells.34,35 Reconstitution of human megakaryopoiesis was also limited in NOD/SCID/β2mnull mice.36 We detected human platelets in all animals for 6 months after transplantation with CB-derived CD34+ cells into NOG mice. We therefore believe that the NOG mouse is a suitable animal model for the analysis of human megakaryopoiesis and thrombopoiesis. TPO and c-Mpl are important molecules for megakaryopoiesis and thrombopoiesis. Administration of TPO can induce polyploid megakaryocytes in BM and increase the number of circulating platelets in mice and patients. In contrast, administration of TPO to NOD/SCID mice receiving xenotransplants with human hematopoietic progenitor cells does not alter the number of human platelets.35 The present study demonstrated that NIP-004 increases the number of human platelets in NOG mice by significantly stimulating human megakaryopoiesis. To the best of our knowledge, this is the first report to demonstrate alteration of human megakaryopoiesis and thrombopoiesis in an animal model of xenotransplantation through c-Mpl activation. In our preliminary data, another TPO mimetic, SB-497115, which has been demonstrated to increase the number of platelets in healthy volunteers,10 increased the number of circulating HuCD41a+ platelets in NOG mice receiving xenotransplants (data not shown). In conclusion, we demonstrate that NIP-004 acts as a HuMpl activator to enhance CFU-MK formation in vitro and platelet production in vivo. Our new experimental animal model of human megakaryopoiesis may be a useful tool to study megakaryopoiesis in vivo.
Prepublished online as Blood First Edition Paper, February 16, 2006; DOI 10.1182/blood-2005-11-4433.
Several of the authors (T.N., A.M., N.I., and N.T.) are employed by a company (Nissan Chemical Industries) whose product was studied in the present work. T.N., Y.M., and N.I. designed the study; T.N., A.M., A.Y., and H.S. carried out the research; M.I. and Y.O. contributed live mice; T.N., Y.M., A.M., and H.S. analyzed the data; N.I., Y.I., and N.T. controlled the data; T.N. and Y.M. wrote the paper; and all authors checked the final version of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Kenneth Kaushansky for his critical review, K. Miyaji for chemical construction of the compound, Y. Hirai for construction of HuGCSFR, and A. Ikejima for her technical assistance.