Abstract
Osteoclasts (OCs) are large multinucleated cells derived from progenitor cells of the monocyte-macrophage lineage. Signal transduction via the macrophage–colony-stimulating factor (M-CSF) receptor, c-fms, is essential for OC formation. Since we have previously demonstrated inhibition of c-fms by imatinib, we examined the effect of imatinib on OC formation and activity. OC formation was not affected by concentrations of 1.0 μM imatinib and lower, but was reduced by 75% at 3.0 μM imatinib. In contrast, both the area of resorption and the number of resorption lacunae were reduced by 80% at 0.3 μM imatinib, and no resorption was observed at concentrations above 3.0 μM. A dose-dependent decrease in receptor activator of nuclear factor κB (RANK) expression was observed in OCs when cultured in the presence of imatinib, providing a mechanism for the decrease in OC function. In vivo analysis of the effect of imatinib on OC activity in adult mice following 8 weeks of imatinib treatment also demonstrated a decrease in OC activity. These results suggest that imatinib may have therapeutic value as an antiosteolytic agent in diseases such as osteoporosis, metastatic bone disease, and multiple myeloma.
Introduction
Bone remodeling occurs throughout life, to maintain skeletal integrity. This process is carried out by osteoblasts (OBs) and osteoclasts (OCs), which are responsible for the synthesis and resorption of bone respectively. OBs are derived from mesenchymal stem cells, whereas OCs are large multinucleated cells located on endosteal bone surfaces that are derived from monocytic progenitor cells.1
In order for bone homeostasis to be maintained, osteoclastic bone resorption and osteoblastic bone formation must be tightly regulated. Imbalances between OB and OC activity result in the development of skeletal abnormalities including osteoporosis, which occurs due to an increase in bone resorption, and osteopetrosis, which results from defective OC activity.2 Other diseases where OCs are implicated include multiple myeloma (MM) and metastatic bone disease. MM is characterized by focal bone loss throughout the axial and craniofacial skeleton following increased OC activity relative to OB activity.2 Osteolytic metastatic bone disease is commonly observed in patients with advanced breast cancer, and arises from migration of tumor cells from a primary tumor to the bone marrow, where the production of OC-activating factors by the tumor cells results in OC activation and subsequent bone destruction.3
Imatinib (Gleevec, formerly STI-571; Novartis, Basel, Switzerland) is a protein tyrosine kinase inhibitor used to treat chronic myeloid leukemia (CML) and gastrointestinal stromal tumors by inhibiting bcr-abl and stem cell factor receptor (c-kit) tyrosine kinases respectively.4,5 We have also recently demonstrated that imatinib specifically inhibits the growth of cells of the monocyte-macrophage lineage by abrogating signal transduction through c-fms.6 Since OCs are dependent on macrophage–colony-stimulating factor (M-CSF)/c-fms signal transduction for their differentiation from hematopoietic precursor cells,7 inhibition of c-fms may provide a mechanism by which excessive OC differentiation and function observed in various bone diseases can be counteracted. We therefore propose that imatinib may be an effective antiosteolytic agent in skeletal diseases such as osteoporosis, MM, and metastatic bone disease by inhibiting OC differentiation and/or function.
Materials and methods
Isolation of CD14+ cells from peripheral blood mononuclear cells
CD14+ cells were isolated from the peripheral blood of healthy volunteers using a combination of Percoll gradient separation and a MACS negative selection monocyte kit (Miltenyi Biotech, Bergisch Gladbach, Germany), as previously described.8
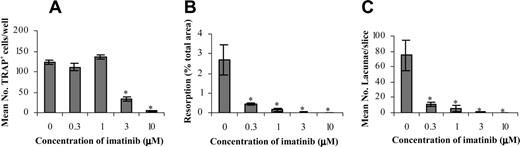
Imatinib decreases the formation and activity of OCs. CD14+ cells from healthy donors were used to establish OC assays to examine the effect of imatinib on bone resorption and the formation of TRAP+ cells. The mean number of TRAP+ cells (A) was significantly reduced in the presence of 3.0 μM imatinib, whereas resorption (B) and lacunae number (C) were affected at 0.3 μM imatinib. Results are representative of 3 individual experiments using different donors. *P < .05. Error bars represent the standard error of the mean (SEM).
Imatinib decreases the formation and activity of OCs. CD14+ cells from healthy donors were used to establish OC assays to examine the effect of imatinib on bone resorption and the formation of TRAP+ cells. The mean number of TRAP+ cells (A) was significantly reduced in the presence of 3.0 μM imatinib, whereas resorption (B) and lacunae number (C) were affected at 0.3 μM imatinib. Results are representative of 3 individual experiments using different donors. *P < .05. Error bars represent the standard error of the mean (SEM).
Osteoclast culture assay
Normal CD14+ peripheral blood mononuclear cells (PBMNCs) were cultured on 150-μm slices of elephant tusk (4 × 104 cells per slice) in 96-well plates as previously described,9 with the inclusion of imatinib at concentrations ranging from 0.3 μM to 10.0 μM. Cultures proceeded for a period of 21 days, after which time the formation of tartrate-resistant acid phosphatase positive (TRAP+) bone-resorbing OCs and the resorption of elephant tusk were assessed.9
Analysis of RANK expression
Total RNA from OCs cultured in the presence of 1.0 μMor3.0 μM imatinib was prepared on days 0, 1, 7, and 14 using Trizol reagent (Life Technologies, Gaithersburg, MD) as per the manufacturer's instructions. RNA was reverse transcribed from up to 1 μg total RNA from each sample, using a cDNA synthesis kit, as per the manufacturer's recommendations (Promega, Madison, WI). Real-time PCR was employed to examine the expression of RANK as previously described.9
Mice
The biologic effect of imatinib on in vivo OC activity was examined by the oral administration of 1.13 mM imatinib through the drinking water to 14-week-old Balb/c mice. The use of mice for this study was approved by the IMVS Animal Ethics Committee (permit number 26a/05). Drinking water consumption was closely monitored to determine the mg/kg per day of imatinib administered to each mouse, which was calculated to be approximately 75 mg/kg per day. Based on published data, this concentration is sufficient to significantly prolong survival in a murine model of CML.10
After 8 weeks of imatinib treatment, mice were humanely killed and the femur was excised and fixed in 10% neutral buffered formalin for 24 hours before decalcification in phosphate buffered 10% EDTA followed by processing into paraffin wax. Slides made from sections 5-μM thick and including some duplicates were assessed without knowledge of the treatment groups by a pathologist experienced in bone morphometry. For each mouse, the number of resorption lacunae occupied or unoccupied by OCs and the number of OCs not located within lacunae were quantified in 10 randomly chosen separate fields of cancellous bone using a 40× objective to provide data for an area totalling 0.22 mm2 in each femur.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA), and differences were considered to be statistically significant when the P value was less than or equal to .05.
Results
Imatinib inhibits the differentiation of CD14+ monocytes to osteoclasts
Since we have previously identified c-fms as a target of imatinib,6 we wished to determine whether imatinib may serve as an effective antiosteolytic agent by inhibiting the differentiation of OCs from CD14+ cells. OC cultures were performed over a period of 21 days using CD14+ cells stimulated with M-CSF in the presence or absence of imatinib. Soluble RANK ligand (RANKL), a potent osteoclastogenic factor,11,12 was included in the growth medium after 7 days of culture.13
Cultures were enumerated for the number of TRAP+ OCs (Figure 1A), with 124 ± 5 (n = 3) TRAP+ cells per well observed in control cultures. The addition of 0.3 μMor1.0 μM imatinib had no effect on the number of TRAP+ OCs, whereas at 3.0 μM and 10.0 μM imatinib, the number of TRAP+ OCs was reduced by 75% and 95% respectively.
Imatinib inhibits the functional capacity of osteoclasts
We next assessed whether the decrease in OC differentiation observed in cultures containing imatinib was accompanied by a decrease in the resorptive capacity of these cells. In the absence of imatinib, 2.7% ± 0.8% of the total area of the bone slice was resorbed by OCs (Figure 1B). Upon addition of 0.3 μM and 1.0 μM imatinib, the area of resorption was reduced by 85% and 95%, respectively, relative to controls. At concentrations of 3.0 μM imatinib and greater, no resorption was observed.
To further quantify the effect of imatinib on OC function, the number of resorption lacunae was assessed (Figure 1C). In control cultures, 75 ± 20 lacunae per bone slice were observed, whereas the addition of 0.3 μM imatinib reduced this by up to 85%. At 1.0 μM imatinib, the number of lacunae was reduced by 95% relative to controls, and at concentrations of 3.0 μM imatinib and greater, no resorption was observed.
Imatinib decreases RANK expression by osteoclasts
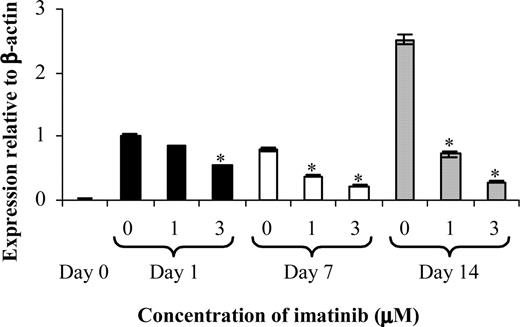
Since OC function was affected by imatinib treatment at lower concentrations than OC differentiation, we next examined whether imatinib was modulating RANK expression in the OC cultures (Figure 2). Previous studies have demonstrated that M-CSF via c-fms regulates the expression of RANK by OC precursors,14 with RANK interacting with RANKL to stimulate OC formation and activity.11,12
Imatinib decreases RANK expression by OCs. CD14+ cells from healthy donors were used to establish OC assays to examine the effect of imatinib on RANK expression on days 0, 1 (▪),7(□), and 14 (▦) of culture. A dose-dependent decrease in RANK expression by OCs was observed in the presence of imatinib across 14 days of culture. Results are representative of 3 individual experiments using different donors. *P < .01 (relative to the 0 μM control for each time point). Error bars represent the SEM.
Imatinib decreases RANK expression by OCs. CD14+ cells from healthy donors were used to establish OC assays to examine the effect of imatinib on RANK expression on days 0, 1 (▪),7(□), and 14 (▦) of culture. A dose-dependent decrease in RANK expression by OCs was observed in the presence of imatinib across 14 days of culture. Results are representative of 3 individual experiments using different donors. *P < .01 (relative to the 0 μM control for each time point). Error bars represent the SEM.
RANK expression was absent on CD14+ cells at the initiation of the OC culture (Figure 2). Following 1 day of culture, a marked up-regulation in RANK expression was observed in the absence of imatinib, and this level of expression was maintained until day 7 of culture. On day 14, a further 2.8-fold ± 0.45-fold increase in RANK expression relative to cells harvested on days 1 and 7 of culture was observed in the absence of imatinib (Figure 2).
The addition of 1.0 μM imatinib had a negligible effect on RANK expression on day 1 of culture, relative to untreated controls. However, culture in the presence of imatinib for 7 or 14 days reduced RANK expression by 55% and 70% respectively, relative to untreated controls. A more profound effect was observed at 3.0 μM imatinib, with a 45% decrease in RANK expression by OCs relative to untreated controls on day 1 of culture, and a 70% and 90% decrease on days 7 and 14 respectively.
Imatinib decreases in vivo osteoclast activity
To confirm the inhibitory effect of imatinib on OCs observed in vitro, adult Balb/c mice were administered imatinib for 8 weeks. Sagittal sections of femur from control and imatinib-treated mice were then analyzed for OC activity with respect to the number of resorptive lacunae present, as well as the number of lacunae associated with or without OCs (Figure 3).
Treatment of mice with imatinib significantly decreased the number of lacunae over 10 fields of view by 36% compared with controls, whereas the number of lacunae containing OCs was reduced by 70% (Figure 3). Imatinib did not affect either the number of lacunae without OCs or the number of OCs not associated with lacunae (Figure 3).
Discussion
OCs are derived from progenitor cells of the monocyte-macrophage lineage1 and become multinucleated following the fusion of mononuclear OCs.15 Studies using osteopetrotic op/op mutant mice, which lack functional M-CSF, have demonstrated the importance of this growth factor in osteoclastogenesis, with these mice displaying a temporary deficiency in OCs.16,17 Since we have previously identified that imatinib inhibits the M-CSF receptor, c-fms, at clinically relevant concentrations,6 and signal transduction via c-fms is important for OC development,7 we examined whether imatinib could inhibit OC formation and activity. Imatinib was found to significantly reduce the mean number of TRAP+ cells at 3.0 μM imatinib, whereas OC resorption and the number of lacunae were affected at 0.3 μM imatinib.
Imatinib decreases lacunae formation and the number of lacunae containing OCs in BALB/c mice. Adult Balb/c mice were administered 75 mg/kg imatinib per day for 8 weeks. Parrafin-embedded sections of mouse femur were enumerated for the number of lacunae over 10 fields of view (Lacunae/10HPF), the number of lacunae containing OCs (Lacunae + OC), the number of lacunae without OCs (Lacunae - OC) and the number of OCs not present in lacunae (OC - Lacunae) in control (▦) and imatinib treated (□) mice. Results are representative of averaged data from 5 mice. *P < .05 (relative to the control). Error bars represent the SEM.
Imatinib decreases lacunae formation and the number of lacunae containing OCs in BALB/c mice. Adult Balb/c mice were administered 75 mg/kg imatinib per day for 8 weeks. Parrafin-embedded sections of mouse femur were enumerated for the number of lacunae over 10 fields of view (Lacunae/10HPF), the number of lacunae containing OCs (Lacunae + OC), the number of lacunae without OCs (Lacunae - OC) and the number of OCs not present in lacunae (OC - Lacunae) in control (▦) and imatinib treated (□) mice. Results are representative of averaged data from 5 mice. *P < .05 (relative to the control). Error bars represent the SEM.
To account for the difference in the sensitivity of OC number and activity to imatinib, the effect of imatinib on RANK expression by OCs was examined over 14 days of culture. In addition to supporting OC differentiation and proliferation,18,19 studies by Arai et al have shown that M-CSF via its receptor, c-fms, regulates the expression of RANK by OC precursors.14 RANK receptor then interacts with RANKL, produced by cells within the bone marrow microenvironment, to stimulate the formation and functional activation of OCs.11,12 A significant decrease in RANK expression by OCs was observed in the presence of 1.0 μM imatinib on days 7 and 14 of culture, indicating that imatinib independently affects OC differentiation and function. At 1.0 μM imatinib, the level of signal transduction through c-fms is sufficient to induce OC differentiation. However, 1.0 μM imatinib significantly decreases RANK expression such that RANKL is less able to stimulate bone resorption.
The in vitro results presented in this study strongly suggest that imatinib may be an effective antiosteolytic agent. However, the observation that OC deficiencies in op/op mice are not permanent and progressively correct with age20 suggest that an alternative mechanism or mechanisms may compensate for the inhibition of M-CSF/c-fms signaling, and preclude the use of imatinib as a viable antiosteolytic therapy. One candidate cytokine is vascular endothelial growth factor (VEGF), with administration of VEGF-A restoring OC formation and activity in op/op mice.21 An important role for VEGF in osteoclastogenesis is further supported by the observation that the introduction of a VEGF receptor-1 tyrosine kinase domain-deficient mutation into op/op mice results in an extensive OC deficiency that does not recover after 24 weeks.22
To determine whether the inhibition of OC activity by imatinib in vitro was reproducible in vivo, adult (14-week-old) Balb/c mice were administered imatinib for 8 weeks. Analysis of hematoxylin-and-eosin–stained femur sections confirmed that imatinib inhibited both OC formation and activity in vivo, further supporting the use of imatinib as an antiosteolytic agent in bone disease. The observation that alternative pathways did not compensate for inhibition of c-fms by imatinib, and restore OC activity, may be due to the fact that imatinib was administered to adult mice that already had an established bone marrow microenvironment and hematopoietic system. In contrast, administration of VEGF-A to op/op mice occurred at day 12, an age where the bone marrow is still being colonized by cells from the liver,23 and where the hematopoietic system may display greater plasticity. In addition, imatinib has been demonstrated to decrease concentrations of VEGF in the bone marrow of patients with CML by decreasing VEGF gene transcription, suggesting that imatinib may also inhibit VEGF-mediated restoration of osteoclastogensis.24
In conclusion, we demonstrate that imatinib inhibits the differentiation and function of OCs at concentrations within the therapeutic dose range, and this effect was reproducible in vivo. The inhibition of OC differentiation is likely to result from an inhibition of c-fms signal transduction, whereas inhibition of OC function occurred indirectly via a decrease in RANK expression. However, we cannot exclude the possibility that imatinib may inhibit the function of other unidentified targets involved in the osteoclastogenic process. Regardless, these results suggest that imatinib may be useful in the treatment of skeletal diseases involving excessive OC activity, including osteoporosis, MM, and metastatic osteolytic bone disease. Studies currently underway are examining the effects of long-term imatinib therapy on bone remodeling in patients with CML, and the ability to inhibit OC resorption in a mouse model of human MM.
Prepublished online as Blood First Edition Paper, January 31, 2006; DOI 10.1182/blood-2005-09-3568.
Supported by grants awarded by the National Health and Medical Research Council of Australia (A.C.W.Z., L.B.T.) and the Cancer Council of South Australia (A.C.W.Z., A.L.D.).
A.L.D. designed research, performed research, analyzed data, and wrote the manuscript; A.N.F. performed research and reviewed the manuscript; M.R.C. performed animal study; L.B.T. provided intellectual input and critical feedback to the manuscript; T.P.H. contributed vital reagents (imatinib [Gleevec]), and provided intellectual input and critical feedback to the manuscript; B.V.-R. analyzed femur sections from in vivo study; and A.C.W.Z. provided intellectual input and designed research, assisted with analysis of data, and provided critical feedback to the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.