Abstract
Myelotoxic injury in the bone marrow (BM) as a consequence of total body irradiation (TBI) or granulocyte colony-stimulating factor (G-CSF) mobilization results in the deposition of iC3b on BM stroma (stroma-iC3b). In the present study, we have examined how stroma-iC3b interacts with hematopoietic progenitor cells (HPCs) and the role of complement (C) and complement receptor 3 (CR3) in BM injury/repair. We demonstrate here that stroma-iC3b tethers HPCs via the inserted (I) domain of HPC complement receptor 3 (CR3, CD11b/CD18, Mac-1). Following irradiation, stroma-iC3b was observed in the presence of purified IgM and normal mouse serum (NMS), but not serum from Rag-2-/- mice, implicating a role for antibody (Ab) and the classic pathway of C activation. Furthermore, a novel role for soluble yeast β-glucan, a ligand for the CR3 lectin-like domain (LLD), in the priming of CR3+ HPC is suggested. Soluble yeast β-glucan could enhance the proliferation of tethered HPCs, promote leukocyte recovery following sublethal irradiation, and increase the survival of lethally irradiated animals following allogeneic HPC transplantation in a CR3-dependent manner. Taken together, these observations suggest a novel role for C, CR3, and β-glucan in the restoration of hematopoiesis following injury. (Blood. 2006;107:835-840)
Introduction
The complement (C) system is a major component of innate immunity and plays a critical role in host defense, but also enhances adaptive immunity through the regulation of both T- and B-cell responses.1,2 New roles for C are emerging in the context of tissue injury and repair following particular insults.3,4 For example, the soluble anaphylatoxin C3a, derived from the cleavage of C3, has been shown to prime granulocyte colony-stimulating factor (G-CSF)-mobilized CXCR4+ hematopoietic progenitor cells (HPCs) for chemotaxis to otherwise subthreshold gradients of stromal derived factor-1 (SDF-1, CXCL-12) in a C3a receptor (C3aR)-dependent manner.4 Also, wild-type (WT) animals ablated with total body irradiation (TBI) and rescued with bone marrow (BM) from C3aR-/- animals exhibited subsequent deficiencies in normal erythropoiesis.4 In addition, C3-deficient (C3-/-) animals demonstrated slower spontaneous recovery of leukocytes and thrombocytes following sublethal irradiation.5 CR3 (CD11b/CD18, Mac-1, αmβ2 integrin) has recently been identified as a putative marker of HPCs.6 An anti-CR3 monoclonal antibody (mAb) directed against the inserted (I) domain of the CD11b subunit has been reported to enhance mobilization of HPCs by abrogating interactions between CR3 and adhesion molecules, including ICAM-1.7 In addition, deposition of iC3b has also been observed on cardiac myocardium and gut smooth muscle following experimental ischemia/reperfusion (I/R) injuries and on liver parenchyma following chemical injury.8-10 The iC3b deposition in the murine gut I/R models was shown to be dependent on a particular clone of natural IgM Ab produced by peritoneal B-1 cells that was later found to recognize a neoepitope of muscle tissue that was only exposed following injury.11 While these studies demonstrate the critical role of C in the tissue injury, recent experimental evidence has also indicated that the C system may have an in situ role for the restoration of normal hematopoiesis following tissue injury, and that therapeutic manipulation of the C system via CR3 may enhance the number and function of HPCs, and possibly other tissue-specific progenitor cells, following injury.
Our previous studies have shown that the binding site of low-molecular-weight soluble β-glucans to neutrophil CR3 was mapped to the lectin-like domain (LLD) of CD11b-located C terminal with respect to the iC3b- and ICAM-1-binding I domain.12 β-glucans are polymers of D-glucose derived from the cell walls of some yeast, fungi, cereal grains, bacteria, and algae and belong to the family of biologic response modifiers (BRMs). Many β-glucans have been observed to demonstrate significant bioactivity in vivo including antitumor efficacy and stimulation of hematopoiesis.13-22 For example, a good-manufacturing process (GMP)-produced β-glucan, poly-(1,6)-β-D-glucopyranosyl-(1,3)-β-D-glucopyranose (β-glucan PGG), was shown in in vivo and ex vivo models to enhance murine and primate myelopoiesis.23 In mice and cynomolgus monkeys, prophylactic administration of Betafectin PGG (Alpha-Beta Technologies, Worcester, MA) was shown to accelerate the recovery of peripheral blood leukocytes, with particular influence on the absolute neutrophil count (ANC), in animals treated with either myelosuppressive or myeloablative doses of cyclophosphamide.23 The use of β-glucan PGG in an ex vivo model increased the short-term colonogenic potential in methylcellulose culture of human CD34+ bone marrow mononuclear cells (BMMCs) treated with subtherapeutic concentrations of granulocyte-macrophage (GM)-CSF or G-CSF.24 Interestingly, the mechanism of action of β-glucan PGG on promoting human myelopoiesis was observed to be independent of cytokine elicitation by BMMCs. Thus, it is proposed that soluble β-glucan enhances hematopoiesis by a novel, but previously uncharacterized, mechanism.
In this study, we tested the hypothesis that stroma-iC3b was the consequence of the activation of the classic pathway of C by a natural Ab binding to a noncharacterized neoepitope of BM stroma that was exposed following injury. Stroma-iC3b is observed following TBI as well as G-CSF mobilization. It is hypothesized that proteases elaborated from injured stroma and BM parenchyma may induce or expose a neoepitope on BM stroma that can be recognized by natural Ab in the serum, resulting in C activation. Stroma-iC3b could possibly tether CR3+ HPCs that had homed to the BM following injury in response to gradients of SDF-1. β-glucan, as a CR3 LLD agonist, may influence HPCs bound to stroma-iC3b in a manner resulting in the proliferation of the HPCs. The experimental observations suggest a possible mechanism of action for the observed in vivo and in vitro efficacy of β-glucans in models of hematopoietic recovery. In addition, they establish a comparable role for orally administered whole glucan particles (WGPs) with respect to β-glucan PGG in in vivo models of leukocyte recovery and allogeneic transplantation. The bioactive moiety derived from WGPs or β-glucan PGG primed HPC CR3 for proliferation, differentiation, and reconstitution of peripheral blood leukocytes. Thus, these studies suggest a novel role for C in BM repair following injury and suggest roles for β-glucan as a radioprotectant and therapeutic agent.
Materials and methods
Experimental animals
All experimental protocols using animals or biologic animal specimens were conducted in accordance with all relevant federal guidelines and had received approval from the University of Louisville Institutional Animal Care and Use Committee (IACUC). Wild-type C57BL/6 mice were obtained from the Frederick Campus of the National Cancer Institute (NCI-Frederick; Frederick, MD). CR3-/- mice on the C57BL/6 background were described formerly and were bred in the colony of J.Y.25 Rag-2-/- mice and C3H/HeJ mice were purchased from NCI-Frederick. All animals were given access to food and water ad libitum and were maintained in the University's specific pathogen-free (SPF) animal facility.
Therapeutic β(1,3)glucans
WGPs (Biothera, Eagan, MN) were purified from baker's yeast through a series of alkaline and acid extractions to yield hollow yeast cell-wall ghosts composed primarily of β(1,3;1,6)glucan. WGPs were hydrated by addition of distilled water and sonicated to produce a single-particle suspension. The soluble low-molecular-weight yeast β(1,3;1,6)glucan derived from in vitro J774 murine macrophage cell-line cultures was described previously and its ability to bind to and prime CR3 was defined.26
Stromal cells
Primary stromal cultures were obtained from BMMCs and cultured in Iscoves media (Gibco; Carlsbad, CA) with 10% fetal calf serum (FCS), 5% horse serum, 2 mM glutamine, and 25 mM Na HCO3 at 5% CO2 and 37°C for 1 week. The adherent cells were then cultured in Iscoves with 10% FCS, 10% horse serum, 2 mM glutamine, 25 mM Na HCO3, and 1 μM hydrocortisone for 1 to 2 weeks until an 80% confluent monolayer of stromal cells was formed.
Serum collection and IgM purification
Whole blood was collected from mice on the day it was used and kept on ice. Serum was separated from other blood components by centrifugation at 3500g for 15 minutes at 4°C. IgM was purified from normal mouse serum using anti-mouse IgM magnetic spheres (Novogen; Stamford, CT). The IgM was removed from the beads by filtration then quantified using enzyme-linked immunosorbent assay (ELISA).
Myelotoxic injury of stromal cells and detection of iC3b, IgM, or IgG deposition
Stromal cells were exposed to 1750 cGy of γ-irradiation from a cesium-137 source (Nordion Systems; Ohawa, ON, Canada). Twelve hours following irradiation, the cells were incubated with serum or purified IgM for 20 minutes at 4°C to allow for the binding of Ab. The cells were then washed and fresh serum from RAG-2-/- mice as a C source was added for 30 minutes at 37°C. The cells were immediately stained with FITC-labeled affinity-purified anti-mouse C3 and analyzed by flow cytometry. The affinity purified anti-mouse C3 was generated from the IgG fraction of goat antiserum to mouse C3 (Immunology Consultants Laboratory, Newberg, OR) that was isolated by Mono-Q anion exchange chromatography (Amersham Pharmacia Biotech, Piscataway, NJ) followed by absorption and elution from mouse iC3b-zymosan particles as described previously.3 To detect IgM or IgG deposition on irradiated BM stromal cells, stromal cells were incubated with NMS and then stained with goat anti-mouse IgG-FITC or anti-mouse IgM-FITC (Southern Biotechnology Associates, Birmingham, AL).
Method for cell sorting
Bone marrow cells were prepared and stained with the following fluorochrome-conjugated mAbs: anti-Class I and -Class II, CD8, αβTCR, γδTCR, Sca-1, C-Kit, CD11b, CD11c, B220, and GR-1 (BD-Pharmingen, San Diego, CA). The lymphocyte region was gated based on light scatter, and a second region negatively gated based on a mAb cocktail of lineage (lin) markers consisting of CD8, αβTCR, γδTCR CD11b, CD11c, B220, and GR1 was established. Lin- lymphocytes were then gated for double-positive Sca1 and C-Kit expression. Isotype controls were performed to assist in establishing the gate for HPC sorting. Cell sorts were performed using MoFlo (Cytomation, Ft Collins, CO) and the purity of all sorts was greater than 95%.
Cobblestone and LTC-IC cultures
Primary bone marrow stromal cultures were irradiated with 2000 cGy to remove any residual HPCs. Fresh NMS as a C source was added to 1.5 × 105 stromal cells for 1 hour, and was then removed by washing with serum-free medium. Purified HPCs from wild-type (WT) or CR3-deficient (CR3-/-) mice were added to the stromal layer in the presence or absence of soluble yeast β-glucan in the wells of a 96-well flat-bottom plate. Half of the media was changed weekly. Cobblestone formation by tethered HPCs was evaluated at day 10. On day 35, to evaluate the long-term culture-initiating culture (LTC-IC), the media was replaced with 100 μL methylcellulose (GF M3434 MethoCult; Stem Cell Technologies, Vancouver, BC, Canada).
Stem cell transplantation
C3H/HeJ (H-2Kk) mice served as recipient animals and were given transplants of HPCs isolated from either WT or CR3-/- animals on the C57BL/6 (H-2Kb) background. The major alleleic variant at the H-2K locus also served as a reporter to detect successful donor engraftment. All recipient mice were preconditioned with a fully myeloablating dose of 950 cGy of γ-irradiation by exposure to a cesium-137 source (Nordion Systems). Up to 5 mice were restrained in a small mouse holder that was inserted into the larger irradiation device. The recipient mice were placed in a small cage, which was then placed under a heat lamp for 1 to 2 minutes in order to dilate the lateral tail veins. Infusion of HPCs was given by IV injection in 0.5 mL or less of M199 (Gibco) media with 20 μg/mL gentamicin. Following HPC transplantation, the mice were still maintained in the University's SPF facility and were given ad libitum access to food and water containing 0.5 mg/mL tetracycline as antibiotic prophylaxis.
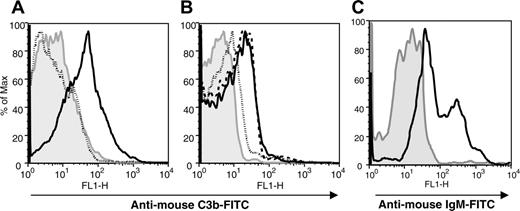
The deposition of iC3b on injured BM stroma requires IgM. Macrophage-depleted BM stroma cultures were irradiated with 1750 cGy from a cesium-137 source. The gray regions in all panels represent irradiated BM stroma in the absence of serum that were stained with the respective secondary Abs as controls for specificity. (A) Irradiated BM stromal cells were incubated with fresh C57BL/6 NMS (solid line) or heat-inactivated NMS (dotted line) and stained with affinity-purified FITC-conjugated anti-C3 Ab. (B) Fresh Rag-2-/- serum (dotted line), purified IgM (dashed line), or heat-inactivated NMS (solid line) were incubated with irradiated stromal cells and then incubated with Rag-2-/- serum as a C source. The cells were then stained with anti-C3Ab to detect iC3b deposition. (C) Following incubation with NMS, irradiated BM stromal cells were stained with goat anti-mouse IgM (solid line) or anti-mouse IgG (not shown). The data are representative of 1 of 3 separate experiments.
The deposition of iC3b on injured BM stroma requires IgM. Macrophage-depleted BM stroma cultures were irradiated with 1750 cGy from a cesium-137 source. The gray regions in all panels represent irradiated BM stroma in the absence of serum that were stained with the respective secondary Abs as controls for specificity. (A) Irradiated BM stromal cells were incubated with fresh C57BL/6 NMS (solid line) or heat-inactivated NMS (dotted line) and stained with affinity-purified FITC-conjugated anti-C3 Ab. (B) Fresh Rag-2-/- serum (dotted line), purified IgM (dashed line), or heat-inactivated NMS (solid line) were incubated with irradiated stromal cells and then incubated with Rag-2-/- serum as a C source. The cells were then stained with anti-C3Ab to detect iC3b deposition. (C) Following incubation with NMS, irradiated BM stromal cells were stained with goat anti-mouse IgM (solid line) or anti-mouse IgG (not shown). The data are representative of 1 of 3 separate experiments.
Measurement of engraftment and complete blood counts
Successful engraftment was measured by survival and chimerism. Thirty days following HPC injection, the surviving animals were characterized for engraftment using flow cytometry to determine the percentage of peripheral blood lymphocytes (PBLs) bearing the donor H-2Kb haplotype using flurochrome-conjugated mAbs. At the same time as the analysis of chimerism, a complete blood count (CBC) with automated differential analysis was performed on all surviving animals with a Hemavet 840 (Drew Scientific; Oxford, CT).
Graphing and statistical analysis of data
Data were entered into Prism 4.0 (Graph Pad Software, San Diego, CA) to generate graphs of colony-forming units (CFUs) and leukocyte recovery and survival curve, and the Student t test was employed from within the program to determine the significance of differences between 2 data sets. Survival curves were created using the Kaplan-Meier method and statistical analyses of survival curves used a log-rank test.
Results
iC3b is deposited on BM stroma after irradiation and is dependent on IgM
The activation of C and opsonization of targets with iC3b not only play a role in the clearance of pathogenic microbes or circulating immune complexes, but also are critical in facilitating the repair of injured tissues. To evaluate the role of natural Ab in the opsonization of stroma with iC3b, isolated BM stroma was irradiated and incubated with serum from Ab- and C-sufficient and -deficient sources and analyzed for the presence of stroma-iC3b. Deposition of iC3b was observed on macrophage-depleted BM stroma 24 hours following 1750 cGy γ-irradiation in the presence of NMS, but not heat-inactivated NMS (Figure 1A). Abrogation of iC3b deposition on irradiated BM stroma in the presence of serum from Rag-2-/- mice, with respect to NMS or purified IgM from NMS, implied a role for Ab, and therefore the classic pathway, in the mechanism of C activation (Figure 1B). IgM (Figure 1C), but not IgG (not shown), could be detected on the surface of stroma-iC3b, and may implicate natural IgM Ab in the pathogenesis of this injury.
A subset of murine HPCs expresses CR3 and proliferates in response to stroma-iC3b and soluble β-glucan
HPCs express a variety of adhesion molecules that mediate homing and engraftment, as well as adhesion to stroma during steady-state conditions. Despite the routine removal of CD11bhigh cells as lineage-committed during the isolation of HPCs from BM or mobilized peripheral blood, a population of CD11bintermediate cells can be observed to persist that accounts for approximately 25% of the HPC pool. Indeed, a CR3+ population of HPCs derived from WT C57BL/6 mice, accounting for approximately 25% of all HPCs, was detected and is consistent with other published reports (Figure 2A).6 To further examine whether these CR3+ HPCs tether to stroma-iC3b and investigate whether soluble β-glucan, as a CR3LLD agonist, can stimulate the tethered HPCs to proliferate in vitro, the short-term proliferation of HPCs and LTC-ICs were performed. HPCs isolated from WT, but not CR3-/-, mice proliferated in short-term liquid culture, as measured by the number of CFUs, in the presence of stroma-iC3b and soluble β-glucan with respect to non-β-glucan-treated controls (Figure 2B). Interestingly, in the absence of serum (no C) and β-glucan, WT HPCs were observed to form significantly more colonies than their CR3-/- counterparts (P < .05). The relative deficit in long-term proliferative potential demonstrated by CR3-/- HPCs with respect to their WT counterparts implied an important role for CR3-mediated adhesion to stroma. Comparison of representative micrographs from methylcellulose cultures of control and β-glucan-treated WT HPCs (Figure 2C-D, respectively) demonstrated that β-glucan-treated HPCs formed many more colonies (arrows) compared with nontreated HPCs. However, short-term proliferation of HPCs does not adequately predict or describe the ability of HPCs to repopulate the BM following myelotoxic insults and to restore physiologic hematopoiesis. To examine if β-glucan also affects more primitive HPCs such as LTC-IC, WT, or CR3-/-, HPCs were cocultured with irradiated stroma in the presence or absence of serum (source of iC3b). After day 35, cells from LTC-IC were replated in methylcellulose culture to grow CFU-C colonies (Figure 2E). We found that CFU-C colony formation in LTC-IC of WT HPCs was enhanced by soluble β-glucan, and therefore indicated the capacity of soluble β-glucan to stimulate CR3+ HPCs to proliferate and potentially to repopulate the BM (Figure 2E).
Orally administered WGPs enhance the recovery of peripheral blood leukocytes following sublethal irradiation in a CR3-dependent manner
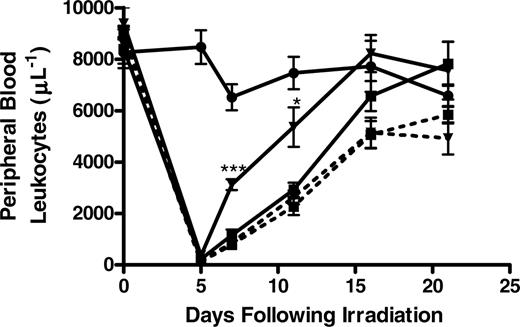
Having demonstrated that stroma-iC3b could potentially tether CR3+ HPCs and that soluble β-glucan could enhance the proliferation of HPCs in a CR3-dependent manner, the ability of orally administered WGPs to enhance leukopoiesis in sublethally irradiated (500 cGy) WT or CR3-/- mice was explored. Oral WGPs had previously been shown to be digested by gastrointestinal macro-phages and released as a low-molecular-weight moiety that was capable of priming CR3 for bioactivity.26 To that end, WT or CR3-/- mice were treated with daily oral WGPs 1 day prior to and for 3 weeks following sublethal irradiation. The leukocyte nadir was observed 5 days following irradiation. WT, but not CR3-/-, mice treated with daily oral WGPs were observed to have a significantly accelerated recovery of leukocytes in the peripheral blood compared with control animals receiving oral saline at 7 and 9 days following irradiation (Figure 3). While the peripheral blood leukocyte counts were equivalent in WT and CR3-/- animals approximately 2 weeks following irradiation, the acceleration of leukocyte recovery in the time immediately after the nadir could potentially prevent significant morbidity and mortality secondary to opportunistic infections in irradiated hosts.
A CR3+population of HPCs tethered to stroma-iC3b and can be observed to proliferate in response to β-glucan in a CR3-dependent manner. (A) Sorted Sca1+, c-Kit+, lin- HPCs were stained with anti-CD11b mAb and analyzed by flow cytometry. Filled curve represents isotype control for anti-CD11b; open curve shows HPCs gated for anti-CD11b staining. (B) A short-term cobblestone assay using WT (▪) or CR3-/- HPCs (□) was analyzed after 12 days of culture in the presence of stroma-iC3b in response to β-glucan (10 μg/mL) compared with their counterparts incubated with stroma-iC3b only. The CFU was also compared between WT and CR3-/- HPCs in the absence of serum (no serum) and β-glucan. (C-D) Representative micrographs captured from WT HPCs grown in the presence of serum and serum and β-glucan, respectively. Arrows indicate colonies. (E) Long-term culture-initiating culture (LTC-IC), established 35 days after coculture of WT (▪) or CR3-/- HPCs (□) and stroma in the presence of stroma-iC3b and β-glucan and analyzed 35 days later in methylcellulose culture compared with their counterparts incubated with stroma-iC3b only. Data in panels B and E represent the mean ± SEM number of CFUs observed in triplicate wells in experiments that were repeated twice (*P < .05 and **P < .005). HPCs were sorted from 3 WT or CR3-/- mice and then plated with stromal cells. The data shown here are 1 representative experiment of 3 separate experiments (total n = 9 in each group).
A CR3+population of HPCs tethered to stroma-iC3b and can be observed to proliferate in response to β-glucan in a CR3-dependent manner. (A) Sorted Sca1+, c-Kit+, lin- HPCs were stained with anti-CD11b mAb and analyzed by flow cytometry. Filled curve represents isotype control for anti-CD11b; open curve shows HPCs gated for anti-CD11b staining. (B) A short-term cobblestone assay using WT (▪) or CR3-/- HPCs (□) was analyzed after 12 days of culture in the presence of stroma-iC3b in response to β-glucan (10 μg/mL) compared with their counterparts incubated with stroma-iC3b only. The CFU was also compared between WT and CR3-/- HPCs in the absence of serum (no serum) and β-glucan. (C-D) Representative micrographs captured from WT HPCs grown in the presence of serum and serum and β-glucan, respectively. Arrows indicate colonies. (E) Long-term culture-initiating culture (LTC-IC), established 35 days after coculture of WT (▪) or CR3-/- HPCs (□) and stroma in the presence of stroma-iC3b and β-glucan and analyzed 35 days later in methylcellulose culture compared with their counterparts incubated with stroma-iC3b only. Data in panels B and E represent the mean ± SEM number of CFUs observed in triplicate wells in experiments that were repeated twice (*P < .05 and **P < .005). HPCs were sorted from 3 WT or CR3-/- mice and then plated with stromal cells. The data shown here are 1 representative experiment of 3 separate experiments (total n = 9 in each group).
Daily administration of oral WGP β-glucan significantly enhances the recovery of peripheral blood leukocytes following sublethal irradiation in a CR3-dependent manner. Groups of 6 WT (—) or CR3-/- (- - -) were sublethally irradiated with 500 cGy of TBI from a cesium-137 source, and the leukocyte nadir was observed 5 days following irradiation. One day prior to irradiation, the mice were divided to receive no treatment (•), 0.1 mL oral phosphate-buffered saline (PBS; ▪), or 0.1 mL oral WGP β-glucan (0.8 mg/mL; ▾). Following irradiation, mice were treated as indicated daily for 3 weeks, during which time peripheral blood was collected by retro-orbital venipuncture at the indicated intervals for analysis by manual counting by investigators who were blinded to the treatment arms. WT, but not CR3-/-, mice receiving oral WGPs were observed to have a significantly accelerated recovery of peripheral blood leukocytes 7 and 11 days following irradiation compared with their counterparts receiving PBS (*P < .05; ***P < .001). All animals had normal peripheral blood leukocyte counts 3 weeks following irradiation. The data shown here are 1 representative experiment (6 mice in each group) of 3 separate experiments.
Daily administration of oral WGP β-glucan significantly enhances the recovery of peripheral blood leukocytes following sublethal irradiation in a CR3-dependent manner. Groups of 6 WT (—) or CR3-/- (- - -) were sublethally irradiated with 500 cGy of TBI from a cesium-137 source, and the leukocyte nadir was observed 5 days following irradiation. One day prior to irradiation, the mice were divided to receive no treatment (•), 0.1 mL oral phosphate-buffered saline (PBS; ▪), or 0.1 mL oral WGP β-glucan (0.8 mg/mL; ▾). Following irradiation, mice were treated as indicated daily for 3 weeks, during which time peripheral blood was collected by retro-orbital venipuncture at the indicated intervals for analysis by manual counting by investigators who were blinded to the treatment arms. WT, but not CR3-/-, mice receiving oral WGPs were observed to have a significantly accelerated recovery of peripheral blood leukocytes 7 and 11 days following irradiation compared with their counterparts receiving PBS (*P < .05; ***P < .001). All animals had normal peripheral blood leukocyte counts 3 weeks following irradiation. The data shown here are 1 representative experiment (6 mice in each group) of 3 separate experiments.
The survival of lethally irradiated animals receiving allogeneic HPC transplantation is enhanced in a β-glucan- and CR3-dependent manner
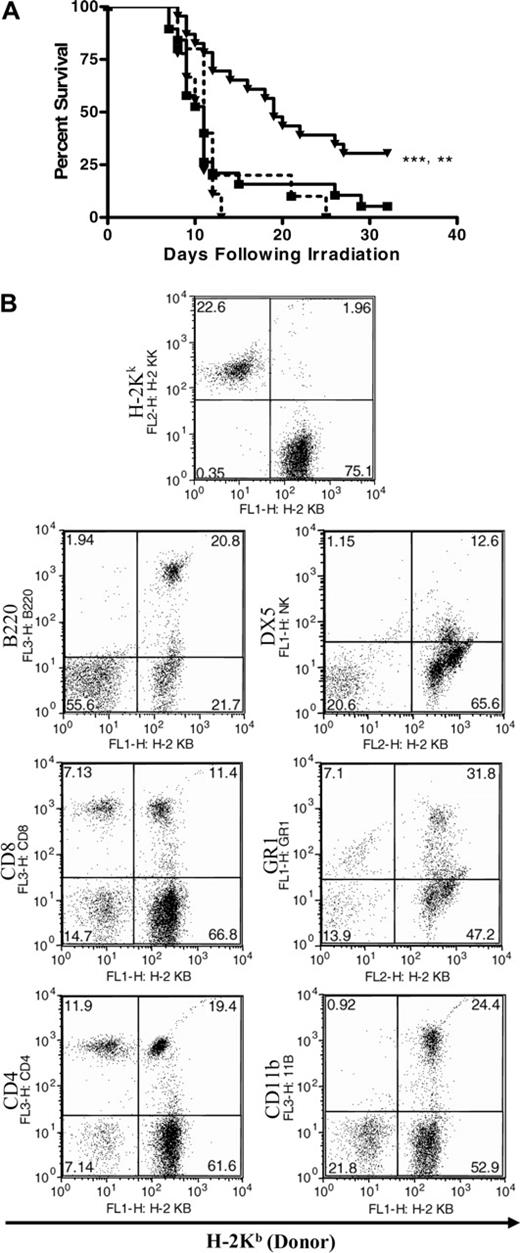
The ability of oral WGPs to enhance the repopulation of the BM in a lethally irradiated host following allogeneic HPC transplantation was examined. C3H/HeJ mice (H-2Kk) were conditioned with 950 cGy γ-irradiation. Eight hours following irradiation, animals were rescued with 2000 HPCs isolated from either WT or CR3-/- mice on a C57Bl/6 background (H-2Kb). The recipients were treated, or not, with daily oral WGPs for 10 days following HPC transplantation. The recipients were monitored for survival to a fixed end point of 40 days in an SPF animal facility, at which time they were also assayed by flow cytometry for lymphocyte donor chimerism, and complete blood counts with differentials were performed. Animals receiving WT HPCs were observed to have a nonsignificant survival advantage compared with animals receiving CR3-/- HPCs (Figure 4A). However, animals receiving daily oral WGPs exhibited significantly enhanced survival in a CR3-dependent manner, and animals receiving oral WGPs and WT HPCs were observed to have enhanced survival compared with their non-treated counterparts receiving only WT HPCs (P < .005; Figure 4A). WT, compared with CR3-/-, HPCs also conferred a survival advantage in animals not receiving WGPs. At the 30-day end point, animals receiving WT HPCs and WGPs had normal peripheral blood hematologic parameters as measured by a complete blood count with differential. In addition, recipient animals displayed between 20% and 50% donor chimerism (Figure 4B). To further delineate the role of β-glucan in the engraftment of HPCs, day-12 CFU-S (spleen) colony formation in lethally irradiated mice was performed. The mice that were treated with WGP β-glucan after WT HPC transplantation had approximately 40% more day-12 CFU-S colonies in the spleen compared with mice without β-glucan treatment receiving WT HPCs or mice treated with WGP β-glucan receiving CR3-/- HPCs (data not shown). These observations suggest that CR3 may indeed contribute to the early engraftment of HPCs, particularly in the presence of stroma-iC3b.
Discussion
The conventional wisdom regarding the C system has largely revolved around the role of C in innate host defense. A significant role for C in host defense against yeast has been recognized, and this paradigm has ultimately contributed to the hypotheses tested in this work. An important means of clearance of pathogenic yeast and fungi is CR3-dependent cellular cytotoxicity (CR3-DCC).27 The killing of iC3b-opsonized yeast or fungi by CR3-DCC is dependent on the priming of CR3 by endogenous β-glucans that are components of many fungal cell walls.27 The work presented here suggests a novel role for C in mediating tissue repair and engraftment of HPCs following myelotoxic insult via a mechanism akin to the host defense and antitumor immunotherapy paradigms. However, the outcome of priming CR3+ HPCs with β-glucan was not observed to be cytotoxicity of an iC3b-opsonized target, but rather proliferation and differentiation of the HPCs, which may suggest a unique signaling capacity for HPC CR3.
Oral WGP β-glucan significantly enhances the survival of lethally irradiated mice that are rescued with an allogeneic WT, but not CR3-/-, HPC transplantation. Two thousand WT or CR3-/- C57BL/6 (H-2Kb) HPCs were transplanted into lethally irradiated (950 cGy) C3H/HeJ (H-2Kk) recipients. One group of recipients (n = 23) received daily oral WGP β-glucan (80 mg/kg) on days 1 to 10 after transplantation, and another group (n = 19) received saline. Groups of 10 recipients receiving CR3-/- HPCs were treated similarly. (A) The treatment of recipient mice with oral WGP β-glucan (▾) resulted in a significant increase in survival 35 days after transplantation in mice receiving WT (—), but not CR3-/- (- - -), HPCs (▪ indicates mice receiving HPCs but not WGP β-glucan). In addition, recipients receiving WT HPCs and oral WGP β-glucan were observed to have significantly enhanced survival with respect to recipients receiving only WT HPCs (**P < .005; ***P < .001). (B) Representative experiment of multilineage engraftment from a total of 7 mice. The data are 1 representative experiment of 2 separate experiments.
Oral WGP β-glucan significantly enhances the survival of lethally irradiated mice that are rescued with an allogeneic WT, but not CR3-/-, HPC transplantation. Two thousand WT or CR3-/- C57BL/6 (H-2Kb) HPCs were transplanted into lethally irradiated (950 cGy) C3H/HeJ (H-2Kk) recipients. One group of recipients (n = 23) received daily oral WGP β-glucan (80 mg/kg) on days 1 to 10 after transplantation, and another group (n = 19) received saline. Groups of 10 recipients receiving CR3-/- HPCs were treated similarly. (A) The treatment of recipient mice with oral WGP β-glucan (▾) resulted in a significant increase in survival 35 days after transplantation in mice receiving WT (—), but not CR3-/- (- - -), HPCs (▪ indicates mice receiving HPCs but not WGP β-glucan). In addition, recipients receiving WT HPCs and oral WGP β-glucan were observed to have significantly enhanced survival with respect to recipients receiving only WT HPCs (**P < .005; ***P < .001). (B) Representative experiment of multilineage engraftment from a total of 7 mice. The data are 1 representative experiment of 2 separate experiments.
IgM was required to yield stroma-iC3b following irradiation of BM stroma. A similar result was reported in a murine model of gut I/R injury in which natural IgM Ab, produced by a single clone of peritoneal B-1 cells, could reconstitute iC3b deposition in otherwise resistant severe combined immunodeficiency (SCID) mice. The natural IgM Ab was subsequently shown to bind a neoepitope of gut smooth muscle that was exposed after I/R.11 The fate of iC3b-opsonized tissues following injury is controversial and may be tissue specific. For example, a published model of chemically induced liver injury demonstrated that C3a and C5a recruit phagocytes for the clearance of damaged iC3b-opsonized tissues.9,28 The observations regarding C in the gut I/R and hepatic injury models recall host defense. The anaphylatoxins C3a and C5a induce inflammation and the recruitment of phagocytes responsible for the clearance of iC3b-opsonized targets, injured hepatocytes, in a manner analogous to the clearance of an infection. However, in models of BM injury, C3a was shown to prime mobilized CXCR4+ HPCs to gradients of SDF-1, thus facilitating their return to the bone marrow via a process called “reverse mobilization.”3-5 Experimental evidence presented here further suggests that a subset of HPCs that returned to the BM and that express CR3 may tether to stroma-iC3b. This notion is also supported by the recent publication indicating that CD11b-deficient progenitor cells were preferentially mobilized over these CD11b-intact progenitor cells.29
In short-term assays, CR3+ HPCs proliferated more in the presence of stroma-iC3b than CR3+ HPC counterparts that were incubated with stroma in the absence of C. Uniformly, CR3-/- HPCs proliferated less in the presence of stroma-iC3b than their WT counterparts despite treatment with soluble β-glucan. Thus, the role of C in the BM injury model also recalls host defense, but with a novel outcome, host repair. Activation products of C recruit HPCs to home to the injured BM, where they tether to stroma-iC3b. Interactions between CR3+ HPCs and stroma-iC3b do not result in the clearance of the stromal cells, but rather in the proliferation of the HPCs. Although the phenotypic features and their biologic functions of CR3+ HPCs have not been extensively characterized, it is worth noting that CR3+ HPCs up-regulate very-late antigen (VLA)-4 expression compared with CR3- HPC counterparts (D.E.C., B.L., J.M., M.Z.R., and J.Y., unpublished observation, May 2005). VLA-4 has been demonstrated to play a critical role in the maintenance of human hematopoiesis.30,31 Moreover, soluble β-glucan, a microbe-derived product that normally results in the CR3-DCC of iC3b-opsonized yeast or tumors, instead further enhances the short- and long-term proliferation of the tethered HPCs. These observations implicate CR3 as a possible therapeutic target for enhancing HPC proliferation following a myelotoxic insult. These results may suggest a potential role for CR3-mediated outside-in signaling, resulting in the proliferation of HPCs. The disparate functions resulting from the priming of CR3 on HPCs and neutrophils obviates the necessity of carefully dissecting the signaling pathways that are activated via CR3 and that may be unique to particular cell types.
The use of highly purified, orally administered yeast β-glucan (WGPs) accelerated the early recovery of peripheral blood leukocytes following sublethal irradiation in a CR3-dependent manner. Moreover, conditioned C3H/HeJ mice survived significantly longer when rescued with WT, but not CR3-/-, allogeneic C57Bl/6 HPCs and treated with oral WGPs. Mice that had survived to the 40-day endpoint demonstrated between 20% and 50% donor chimerism as well as a normal peripheral blood hematologic profile. Furthermore, β-glucan also enhanced CR3+ HPC day-12 colony formation in spleen. These in vivo observations affirm the in vitro observations regarding the ability of β-glucan to enhance the proliferation of CR3+ HPCs. In addition, these observations suggest the efficacy of using yeast β-glucans for the purpose of accelerating the repopulation of the BM with HPCs that are capable of restoring normal hematopoiesis in a compromised host. Therefore, yeast β-glucan may have utility as a novel therapeutic agent in the recovery of peripheral blood leukocytes in leukopenic cancer patients undergoing myelotoxic chemotherapy or radiation therapy or in the victims of hostile or accidental exposure to ionizing radiation. Similarly, yeast β-glucan may assist in the engraftment of HPCs and the acceleration of hematopoietic function following allogeneic HPC transplantation.
These observations describe a novel role for the C system in tissue repair via a mechanism that can be reconciled with the traditional understanding of the role of C in host defense. A unique function for CR3 expressed by HPCs is proposed that is disparate compared with the role of CR3 as a receptor mediating phagocytosis and CR3-DCC of iC3b-opsonized targets. Moreover, a role for yeast β-glucan as a therapeutic for the acceleration of HPC engraftment and leukocyte recovery is described that further contributes to this novel role for CR3 and the C system in mediating tissue repair. These observations therefore justify the exploration of the C system as not only a means of host defense, but as a means of host repair through similar mechanisms, and may indicate a heretofore unrecognized means of tissue regeneration that may not be unique to only the BM.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-07-2705.
Supported by research funding from National Institutes of Health (NIH) grant no. RO1 CA86412, US Army Breast Cancer Research Program grant no. DAMD17-02-01-0445, the Kentucky Lung Cancer Research Board, and a gift fund from Biothera, Eagan, MN. J.Y. is a recipient of an American College of Rheumatology and Arthritis Foundation Investigator Award.
One of the authors (J.Y.) has declared a financial interest in Biothera, whose product was studied in the present work.
D.E.C. and D.J.A. performed research and wrote the paper; J.T.B., R.H., J.M., and B.L. performed research; J.R. and M.Z.R. designed research; and J.Y. designed research and wrote the paper.
D.E.C. and D.J.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Suzanne T. Ildstad for her critical reading of this manuscript.