Relapse is a major obstacle in the cure of acute myeloid leukemia (AML). The Pediatric Oncology Group AML Study 9421 tested 2 different strategies to improve event-free survival (EFS) and overall survival (OS). Patients were randomized to receive standard-dose DAT (daunorubicin, cytarabine, and thioguanine) or high-dose DAT during induction. To interfere with P-glycoprotein (P-gp)-dependent drug efflux, the second randomization tested the benefit of cyclosporine (CsA) added to consolidation chemotherapy. Of the 282 children randomly assigned to receive standard DAT induction, 248 (87.9%) achieved remission compared to 253 (91%) of the 278 receiving high-dose DAT (P = ns). Children with HLA-identical sibling donors who achieved a complete remission received an allogeneic bone marrow transplant as consolidation. For the 83 patients receiving a matched related donor bone marrow transplantation (BMT), the 3-year disease-free survival (DFS) is 67%. Of the 418 children who achieved remission and went on to consolidation with and without CsA, the DFS was 40.6% and 33.9%, respectively (P = .24). Overexpression of P-gp was infrequent (14%) in this pediatric population. In this study, intensifying induction with high-dose DAT and the addition of CsA to consolidation chemotherapy did not prolong the durations of remission or improve overall survival for children with AML.
Introduction
Despite intensive induction and postremission chemotherapy, 5-year event-free survival (EFS) of children with acute myeloid leukemia (AML) is only 40% to 50%.1 Bone marrow transplantation (BMT) from HLA-matched sibling donors is associated with lower relapse risk and superior overall survival (OS) compared with other types of postremission therapy, including consolidation therapy, maintenance chemotherapy, and autologous BMT.2,3 Since only 15% to 20% of children with AML have suitable related donors, other strategies are necessary to improve overall survival.4,5 Several adult and pediatric studies explored the benefits of modifying induction therapy in AML. Some included substituting idarubicin or mitoxantrone for daunorubicin,6-9 increasing the dose or duration of cytarabine,10-12 and administering the second course of induction chemotherapy on days 10 to 14.13,14 The latter approach is referred to as timed sequential therapy and theoretically may recruit residual leukemic cells into cell cycle, making them more susceptible to cytarabine and other cell-cycle-specific agents. High cytarabine dosage (> 1 g/m2/dose) during induction and timed sequential therapy have increased remission duration but have not improved complete remission rate.10,11 Optimal cytarabine dose has not been determined, and a number of nonrandomized studies suggest that dose escalation improves efficacy.2 Other recent approaches have focused on preventing emergence of drug-resistant leukemia cells.
Drug resistance is a major obstacle to effective AML treatment. Resistance of AML and many other human cancers to certain chemotherapeutic agents is multifactorial, but one mechanism may be overexpression of multidrug transporters that contain an adenosine triphosphate-binding cassette (ABC transporters).15,16 Of these multidrug transporters, P-glycoprotein (P-gp), which is encoded by multidrug resistance (MDR1, ABCB1) gene (localized to 7q21), is a mediator of anthracycline efflux and has been investigated most extensively in patients with AML. Increased P-gp may cause initial and acquired drug resistance. CsA is a potent inhibitor of P-gp-mediated drug efflux in vitro. CsA concentrations of 1000 to 2000 ng/mL, which reverse the effects of P-gp in vitro, are readily achievable with short-term intravenous administration in adult patients and cause acceptable toxicity.17
Overexpression of P-gp is relatively frequent in adults with AML at diagnosis and especially at relapse.18,19 In several studies of AML in adults, complete remission (CR) rates were significantly lower among patients with overexpression of MDR1/P-gp than those without. However, interpretation of clinical data has been hampered by variation in methods of measuring relative levels of P-gp expression and lack of concurrent assays of drug efflux. Preliminary evaluations of P-gp overexpression in children with AML have revealed that incidence is lower in children than in adults.20
In a phase 2 study, the Pediatric Oncology Group (POG, now part of Children's Oncology Group [COG]) evaluated the toxicity of the MDR1 modulator CsA administered in conjunction with mitoxantrone and etoposide.21 This phase 2 study demonstrated that simultaneous treatment with mitoxantrone, etoposide, and high doses of CsA can be incorporated into antileukemia therapy with manageable nonhematologic toxicity.
In 1994, POG initiated the first prospective randomized study in children with AML (POG 9421) to determine whether EFS improved with intensification of induction therapy with high-dose Ara-C and addition of CsA, an MDR1-modulating agent, in consolidation therapy. In this manuscript we describe the primary results of the POG 9421 AML study.
Patients and methods
Patient eligibility
POG 9421 was open for enrollment from February 15, 1995, until August 15, 1999. Eligibility criteria included age younger than 21 years and de novo AML confirmed by analysis of bone marrow aspirates, biopsy, or both. De novo AML was characterized by increased nonlymphoid bone marrow blasts (> 30%). Patients with more than 5% but less than 30% marrow blasts were eligible if they had karyotypic abnormalities typical of AML, for example, t(8;21) or inv(16) or myelofibrosis with unequivocal megakaryoblasts (French-American British [FAB] M7). Patients were ineligible if they had therapy-related AML, a prior diagnosis of myelodysplastic syndrome, or acute promyelocytic leukemia (FAB M3) diagnosed on the basis of morphology, or were subsequently found to have a t(15;17) translocation detected by cytogenetic methods. Patients with AML of any other FAB subtype (M0 to M7) were eligible. Patients with AML and Down syndrome (DS) were required to be registered on POG 9481 (a study of AML biology in patients with DS) to be eligible for 9421.
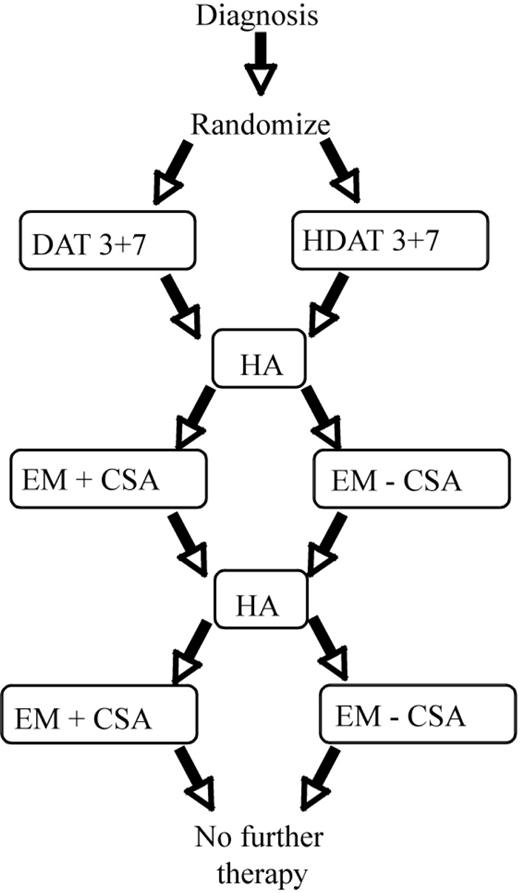
Chemotherapy regimens. DAT 3 + 7 indicates daunorubicin 45 mg/m2 intravenous push (over 15 minutes) on days 1, 2, and 3; Ara-C 100 mg/m2 continuous 24-hour intravenous infusion on days 1-7 (168 hours); and thioguanine 100 mg/m2 by mouth, single daily dose on days 1-7. HDAT 3 + 7 indicates daunorubicin 45 mg/m2 intravenous push (over 15 minutes) on days 1, 2, and 3; Ara-C 1 g/m2 intravenous 1-hour infusion every 12 hours for 7 days (14 doses); and thioguanine 100 mg/m2 by mouth, single daily dose on days 1-7. HA indicates Ara-C 1 g/m2 intravenous 1-hour infusion every 12 hours for 5 days (10 doses). EM - CSA indicates etoposide 100 mg/m2 intravenous 45 minutes-1 hour infusion on days 1-5 and mitoxantrone 10 mg/m2 intravenous 30-minute infusion on days 1-4. EM + CSA indicates etoposide 100 mg/m2 intravenous 45 minutes-1 hour infusion on days 1-5; mitoxantrone 10 mg/m2 intravenous 30-minute infusion on days 1-4; and CsA 10 mg/kg intravenous 2-hour loading dose followed by 30 mg/kg continuous infusion for 98 hours (total 100 hours).
Chemotherapy regimens. DAT 3 + 7 indicates daunorubicin 45 mg/m2 intravenous push (over 15 minutes) on days 1, 2, and 3; Ara-C 100 mg/m2 continuous 24-hour intravenous infusion on days 1-7 (168 hours); and thioguanine 100 mg/m2 by mouth, single daily dose on days 1-7. HDAT 3 + 7 indicates daunorubicin 45 mg/m2 intravenous push (over 15 minutes) on days 1, 2, and 3; Ara-C 1 g/m2 intravenous 1-hour infusion every 12 hours for 7 days (14 doses); and thioguanine 100 mg/m2 by mouth, single daily dose on days 1-7. HA indicates Ara-C 1 g/m2 intravenous 1-hour infusion every 12 hours for 5 days (10 doses). EM - CSA indicates etoposide 100 mg/m2 intravenous 45 minutes-1 hour infusion on days 1-5 and mitoxantrone 10 mg/m2 intravenous 30-minute infusion on days 1-4. EM + CSA indicates etoposide 100 mg/m2 intravenous 45 minutes-1 hour infusion on days 1-5; mitoxantrone 10 mg/m2 intravenous 30-minute infusion on days 1-4; and CsA 10 mg/kg intravenous 2-hour loading dose followed by 30 mg/kg continuous infusion for 98 hours (total 100 hours).
Patients, family members, or both, as appropriate, gave written informed consent before enrollment. The protocol was approved at each participating center by its institutional review board. The study was evaluated at regular intervals by a data-monitoring committee.
Registration and randomization
Patients were registered by telephone or computer before therapy began. Exceptions were patients who received emergency local irradiation for symptomatic central nervous system (CNS) disease or underwent apheresis for hyperleukocytosis (> 100 × 109 white blood cells [WBCs]/L). Patients were assigned based on FAB subtype to stratified treatment groups. Treatment regimens for induction and consolidation are shown in Figure 1.
At registration, assignments were randomized for induction treatment (standard-dose cytarabine [Ara-C]) combined with daunomycin and thioguanine (DAT) versus high-dose Ara-C and daunomycin and thioguanine (HDAT) and consolidation therapy (with or without CsA). Patients with DS were assigned to the group that did not receive CsA during consolidation (treatment 5). Treatment arms are defined in Table 1.
Treatment plan
Induction therapy. Patients who were assigned randomly to standard DAT therapy (treatments 1, 2, and 5) received Ara-C (100 mg/m2 per day) by continuous infusion for 7 consecutive days (total, 168 hours); daunorubicin (45 mg/m2 per day) by intravenous infusion over 15 minutes on days 1 through 3; and thioguanine (100 mg/m2 per day) orally for 7 days. Patients assigned randomly to HDAT group (treatments 3 and 4) received identical doses of daunorubicin and thioguanine, but Ara-C (1 g/m2 per dose) was administered as a 1-hour infusion every 12 hours for 7 consecutive days. Dexamethasone eye drops were given 4 times daily for 10 days during HDAT induction.
All patients underwent bone marrow aspiration and biopsy on day 15 of initial induction therapy. For patients with hypoplastic marrow (≤ 20% cellularity) and evidence of clearance of leukemic cells (≤ 10% blasts), the second course of induction was delayed until there was recovery of peripheral blood cell counts (absolute neutrophil counts [ANCs] > 0.5 × 109/L [500/μL], platelet counts > 75 × 109/L [75 000/μL]). If recovery was delayed, BM aspiration was repeated at day 28 and weekly until there was recovery of peripheral blood cell counts or evidence of residual leukemia.
Patients with evidence of residual leukemia at day 15 (> 20% cellularity detected by biopsy, > 10% blasts) began the second course of induction therapy at that time.
Induction 2. All patients were given identical second induction courses (treatments 1-5): Ara-C (1 g/m2) was administered as a 1-hour infusion every 12 hours for 5 days, and dexamethasone eye drops also were given. All patients underwent BM aspiration and biopsy on day 22 of induction 2. Patients whose AML was in remission (≤ 5% blasts or M2A marrow [ie, 5%-15% blasts]) proceeded to consolidation therapy or BMT. Those with residual leukemia (> 25% blasts) were considered induction failures and removed from the study. Patients with hypocellular bone marrow were evaluated weekly until they attained remission status or showed signs of residual leukemia. Patients and family members underwent HLA typing during induction therapy. Patients without DS and with a related donor who matched for 5 of 6 or 6 of 6 antigens were to undergo BMT by protocol. All other patients whose AML was in remission proceeded to consolidation therapy selected during initial randomization.
CNS therapy during induction
All patients underwent diagnostic lumbar puncture at diagnosis, and those who received standard DAT therapy received Ara-C (40 mg/m2) intrathecally on day 1 of induction 1. Patients assigned to HDAT therapy did not receive intrathecal Ara-C during inductions 1 and 2 because of concern for CNS toxicity. All patients with CNS disease (cerebrospinal fluid [CSF] WBCs > 5/μL, blasts on cytospin) received intrathecal Ara-C (40 mg/m2)7 days after completion of inductions 1 and 2, regardless of absolute neutrophil count (ANC). Patients with symptomatic CNS disease were eligible for emergency radiation therapy concurrent with start of induction 1, in addition to intrathecal therapy as described.
Infant dosing correction
Infants younger than 12 months had their chemotherapy doses modified throughout all phases of the protocol, but CsA doses were not modified. The following formula was used to convert doses based on body surface area to those based on mass in kilograms: (mass × dose based on body surface area) ÷ 30 = infant dose.
Consolidation therapy
Patients without matched-related donors received 3 courses of consolidation chemotherapy. Assignment to consolidation (consolidation 1 and 3, respectively) was accomplished by 2 × 2 randomization at initial diagnosis, with stratified randomization between standard and CsA-containing arms. Consolidation course 2 was the same for all patients. Each consolidation course began when ANC was more than 0.5 × 109/L (500/μL) and the platelet count was more than 75 × 109/L (75 000/μL).
Consolidations 1 and 3
Patients assigned randomly to treatment arms 1, 3, or 5 received etoposide (100 mg/m2) as a daily 1-hour infusion for 5 days, mitoxantrone (10 mg/m2) as a daily 30-minute infusion on days 1 through 4, and Ara-C (40 mg/m2) intrathecally on day 1. With appropriate hydration and antiemetic therapy, this treatment was administered on an outpatient basis in some cases.
Patients assigned randomly to treatment arms 2 or 4 received continuous-infusion CsA with modified doses of etoposide (60 mg/m2 per day) given by 1-hour infusion for 5 days and mitoxantrone (6 mg/m2 daily) as daily 30-minute infusion on days 1 through 4. These doses were 60% of the standard dose based on pharmacokinetic data that demonstrated clearance of etoposide and mitoxantrone was delayed by 40% when both agents were administered with high doses of CsA. Administration of etoposide and mitoxantrone at 60% of standard doses with CsA was expected to result in drug exposure and myelotoxicity similar to that observed when standard doses of the 2 are given without an MDR1-modulating agent.22,23
Patients assigned randomly to treatment arms 2 or 4 (CsA therapy) required hospitalization. CsA administration was initiated 2 hours before first chemotherapy dose. This bolus loading dose of CsA was 10 mg/kg given over 2 hours, and a subsequent continuous infusion of 30 mg/kg per day for a total of 98 hours followed (total, 100 hours of CsA administration). The goal was to achieve a steady-state serum or whole-blood concentration that was 2.5 to 4.0 μM CsA (3000-5000 ng/mL). Blood for use in analyzing CsA concentration was collected from a site that was distant from that used for infusion at 14, 26, 38, 50, 74, and 98 hours after the start of CsA infusion. Doses of CsA were modified on the basis of concentrations in serum or whole blood. For levels more than 5000 ng/mL, infusion was discontinued until the concentration was less than 5000 ng/mL and then re-started at a 25% reduction in dose; if CsA concentration was less than 3000 ng/mL, infusion dose was increased by 25% increments until concentration was between 3000 and 5000 ng/mL. Chemotherapy administration was not delayed because of variation in CsA concentrations.
Consolidation 2
All patients received consolidation 2, which was identical to induction 2.
Allogeneic BMT
As described in the protocol, patients with HLA-matched (6 of 6 antigens or 5 of 6 antigens) related donors were allocated to allogeneic BMT. The conditioning regimen included total body irradiation (TBI) and etoposide. TBI was administered on days - 7 to - 4 (day 0 was the day of bone marrow infusion). TBI was to be given in 8 fractions of 150 cGy per fraction (total dose, 1200 cGy). Etoposide (60 mg/kg) was administered as a single 4-hour infusion on day - 3. To prevent graft-versus-host disease (GVHD), CsA was begun on day - 2, and levels were measured to maintain trough levels of 250 to 300 ng/mL. Four doses of intravenous methotrexate 15 mg/m2 on day +1 and 10 mg/m2 on days 3, 6 and 11 were administered in a standard fashion to all transplant recipients.
Required observations
Bone marrow aspirations were studied centrally to assess cell morphology, immunophenotype, and cytogenetic features (D.H., F.B., and S.R., respectively; St Jude Children's Research Hospital, Memphis, TN). Data pertaining to treatment toxicity were collected prospectively. Patients with CNS toxicity higher than grade 3 from treatment with high-dose Ara-C were removed from study.
Bone marrow samples, peripheral blood samples, or both were evaluated to determine the drug-resistance phenotype. After informed consent was obtained from participants, samples were collected at local institutions, diluted in an equal volume of RPMI-1640 medium, and transported at room temperature by overnight courier to the MDR1/drug-resistance reference laboratory (Y.R.; Wayne State University, Detroit, MI). Mononuclear cells were separated from other cells by centrifugation on ficoll-hypaque gradients and resuspended in RPMI-1640 medium containing 20% dialyzed fetal bovine serum and insulin, transferrin, and selenite. Surface expression of P-gp was measured by flow cytometry using monoclonal antibodies to this protein (MRK16 and 4E3, the latter a gift from R.J.A., Johns Hopkins University, Baltimore, MD). Early in the study, incubation with the MRK16 and 4E3 antibodies was done at 0°C. In early 1996, it became apparent that antibody-binding affinity was better at room temperature.24 This change in protocol was adapted in April 1996. Other changes in the protocol included the use of phycoerythrin instead of fluorescein isothiocyanate in the P-gp expression studies using MRK16.25 The samples that were initially tested at 0°C with fluorescein isothiocyanate-conjugated antibody were retested with phycoerythrin-conjugated antibodies at room temperature. Functional MDR was measured by flow cytometry analyzing rhodamine123 (R123) and daunorubicin uptake and accumulation in the presence or absence of CsA.24-26 For this report P-gp positivity was defined as the presence of more than 5% MRK16-stained cells (“Discussion” under “MDR1 expression and functional drug efflux”).
Statistical analysis
EFS is defined as the time from randomization to treatment failure (relapse, second malignancy, or remission failure) or death. DFS is defined as the time from the date that remission was first observed to treatment failure or death.
It was necessary to randomly assign to arms 1 to 4 at least 560 patients without DS to achieve a power of 80% at the 1-sided significance level of 0.05, to detect a difference of 10% (35% versus 45%) in EFS probability 2 years after the random assignment of patients to HDAT induction therapy and to standard-dose DAT induction therapy. With the designated sample size of 560 and a power of 80% at the 1-sided significance level of 0.05, we were able to detect a difference of 13% (45% vs 58%) in DFS at 2 years after the start of remission between patients who received consolidation with CsA and patients who received no CsA. Also, the sample of 560 patients allowed us, with more than 80% power at the 1-sided 0.05 significance level, to detect a difference of 8% (80% versus 88%) in remission rate between patients who received HDAT and patients who received standard-dose DAT.
Actuarial curves showing EFS probability, DFS, and OS probability were constructed according to the Kaplan-Meier method.27 Findings with respect to EFS probability, DFS, and OS probability were tested for significance by the log-rank test.28 The difference in remission rate was tested by the chi-square test. All reported P values are 2-sided.
Results
Patient characteristics
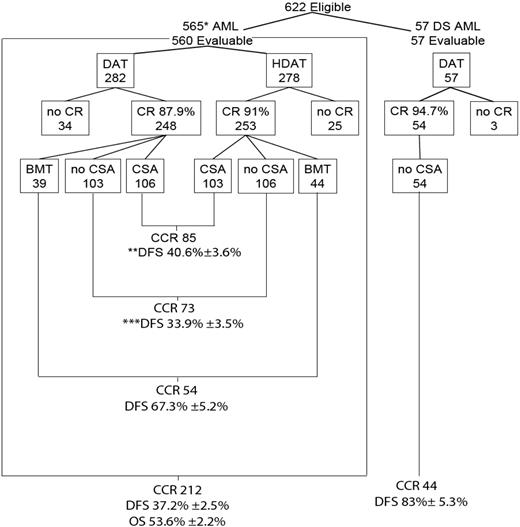
Over 4.5 years (February 15, 1995, to August 15, 1999), 654 patients with AML were registered. Thirty-two were ineligible: 20 had incorrect diagnoses, 4 DS patients were mistakenly assigned to treatment arms 1 through 4, 4 DS patients were not registered on the required companion biology study, POG 9481, 3 were registered incorrectly or were not included because of an administrative error, and one received hydroxyurea therapy before the protocol treatment was given. Of 622 eligible patients, 57 with DS were assigned nonrandomly to treatment 5. At registration, 565 AML patients without DS were assigned randomly to 4 treatment arms: 145 to treatment 1; 139 to treatment 2; 138 to treatment 3; and 143 to treatment 4 (Figure 2). The 4 randomized groups had similar distributions of clinical, racial or ethnic, morphologic, and cytogenetic features (Table 2), except for a slight difference in WBC count.
Of 565 eligible, randomly assigned patients, 361 (64%) were white, 81 (14%) were black, 86 (15%) were Hispanic, and 37 (7%) were from other racial or ethnic groups. Eighty-three patients without DS but with HLA-matched related donors were allocated to BMT. Additionally, 19 patients randomly assigned to chemotherapy only received a “nonprotocol” BMT, which was on a local institutional protocol or one in which the source was a matched unrelated or cord blood donor.
MDR1 expression and functional drug efflux
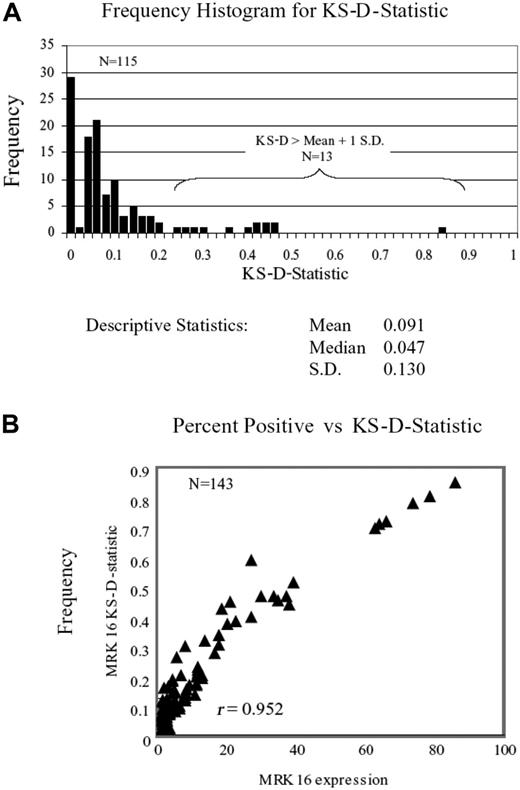
Of 565 eligible patients without DS, expression of MDR1 was tested in 447 (79%) and not tested in 118 (21%). These 2 groups of patients had balanced race, sex, and cytogenetic abnormalities. However, the MDR1-tested patients were imbalanced in age (tended to be older, P = .01), WBCs (tended to have higher WBC count, P < .001), and slightly FAB imbalanced, (P = .042). Comparisons of results in samples evaluated at 40°C and at room temperature revealed more intense staining with phycoerythrin at room temperature. Comparison of MRK16 and 4E3 expression versus functional MDR1 (rhodamine efflux) showed that MRK16 expression correlated better with efflux (Pearson correlation coefficient for MRK 16 was 0.403 vs 0.228 for 4E3, P = .01). Thus only MRK16 data tested at room temperature was included for this analysis. Comparison of staining intensity by the Kolmogorov-Smirnov D statistic29 (KS-D) was not part of our initial protocol so that this parameter was not prospectively evaluated in all samples. However, KS-D value was retrospectively calculated in 143 samples, which included all samples with more than 5% expression of MRK16 and as well 100 other randomly selected patients. No case was found where the MRK16 expression curve was completely separate and nonoverlapping with isotype control, for instance, a KS-D value of 1. A frequency distribution of the KS-D value is shown in Figure 3A, and when compared with percent expression of MRK16, a tight correlation was noted (r = 0.952; Figure 3B), consistent with the earlier observations of Leith et al.30 Therefore, MDR1 is reported here as percent cells expressing MRK16; given the low frequency of surface expression of MDR1 noted in this study, we elected to describe the results in increments of 5 percentage points31 instead of using the traditional 20% level to define positivity in defining immunophenotype determinations. Expression of MDR1 was low (0% to 4.9% of cells expressed MDR1) in 385 (86%) of 447 samples, and 62 (14%) of 447 samples showed at least 5% positivity (medium [5% to 9.9% of cells expressed MDR1] in 48 samples [10.7%], high [10% to 19.9% of cells] in 7 samples [1.7%], and very high [> 20% of cells] in 7 samples [1.7%]). Thus, high expression of MDR1 (> 10%) was infrequent in the population studied. For meaningful comparisons of frequencies between treatment arms any expression higher than 5% was taken as positive.
Distribution and outcome of patients.*5 patients withdrawn in less than 14 days of treatment; **12 patients received nonprotocol BMT; ***7 patients received nonprotocol BMT.
Distribution and outcome of patients.*5 patients withdrawn in less than 14 days of treatment; **12 patients received nonprotocol BMT; ***7 patients received nonprotocol BMT.
Induction response
Of 622 eligible patients, responses of 560 patients to induction therapy were evaluable (excluding 57 with DS and 5 who withdrew during induction; Figure 2). Of 282 evaluable patients randomly assigned to and who received standard-dose DAT, 248 (87.9%) experienced remission, and 253 (91.0%) of 278 evaluable patients randomly assigned to and who received HDAT experienced remission (P = .23). Fifty-nine children (10.5%) did not achieve remission: 14 (2.5%) with early death (< 2 weeks from start of therapy) from complications of leukemia or therapy (infection or hemorrhage), and 45 (8.0%) secondary to persistent leukemia. There was no significant difference in early death rate between induction regimens. Of 501 patients who achieved remission and went on to their randomly assigned consolidation therapy (Table 3), 26 had M2A marrow (between 5% and 15% blasts), and the remainder had M1 (< 5% blasts). Overall, 71% of patients who attained remission did so after Induction 1 in either the standard-dose DAT or the HDAT groups.
Toxicity associated with induction therapy
The predominant toxicity during induction therapy was myelosuppression. More than 90% of patients who received either induction regimen experienced grade 4 neutropenia and required continuous hospitalization throughout induction therapy or readmission for fever and presumed infection associated with neutropenia. Bacteremia or sepsis was documented for 62 (22%) of the 282 patients who received standard-dose DAT and for 78 (28%) of the 278 who received HDAT (P = .10). There were 14 patients (5%) with documented fungal infection in each arm. Seven patients in each arm had grade 2 to 4 CNS toxicity. There was only one report of cerebellar ataxia associated with HDAT induction.
Postremission phase
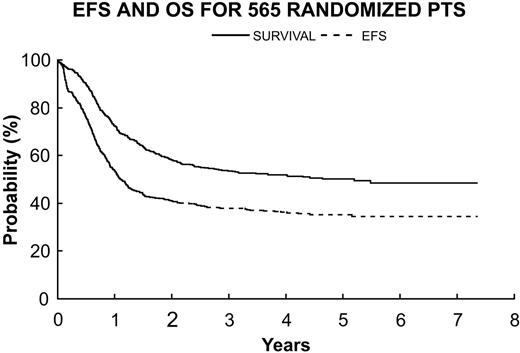
For all eligible patients without DS (n = 565), EFS and OS estimates at 3 years after randomization were (36.3% ± 2.2%) and (53.6% ± 2.2%), respectively (Figure 4). The estimates of 3-year EFS for patients randomly assigned to receive standard-dose DAT (n = 284) and for patients randomly assigned to receive HDAT (n = 281) were 35.2% ± 3.0% and 40.1% ± 3.2%, respectively (Figure 5). The difference was not statistically significant (P = .28). When the 83 patients who underwent protocol-directed BMTs were excluded, DFS estimates 3 years after remission were 33.9% ± 3.5% for patients randomly assigned to receive no CsA (n = 209) and 40.6% ± 3.6% for those randomly assigned to receive CsA (n = 209, P = .24) (Figure 6). Of interest, 19 patients received a nonprotocol BMT, 7 of the 209 assigned to no CsA and 12 among the 209 assigned to CsA.
The 4 randomized treatment arms were analyzed individually. Excluding patients who underwent protocol-specified allogeneic BMT, the 3-year EFS estimate for patients assigned to treatment arms 1 to 4 was 22% ± 3.8%, 36.9% ± 4.8%, 35.4% ± 4.6%, and 36.2% ± 4.7%, respectively. When the EFS in arm 4 (HDAT, CsA) was compared to that of arm 1 (DAT, no CsA), the P value = .08 (Figure 7). The 3-year DFS estimate for patients in treatment arm 1 was 27.2% ± 4.6%, but estimates for patients in treatment arms 2, 3, and 4 were 41.1% ± 5.2%, 40.5% ± 5.1%, and 40.2% ± 5%, respectively. The 3-year OS estimates were 53.6% ± 2.2% for all patients who underwent randomized assignment, and 67.3% ± 5.2% for those who underwent protocol-specified allogeneic BMT. The 3-year OS estimated by randomized treatment regimen is seen in Figure 8.
Patients with AML and DS treated with DAT and consolidation without CsA had an excellent outcome with a 3-year EFS of 78.7% ± 5.6% and DFS of 83% ± 5.3%.
Panel A shows the frequency distribution of the KS-D value, and panel B shows the comparison with percent expression of MRK16, which showed tight correlation (r = 0.952).
Panel A shows the frequency distribution of the KS-D value, and panel B shows the comparison with percent expression of MRK16, which showed tight correlation (r = 0.952).
Toxicity associated with consolidation therapy
Myelosuppression was severe: more than 90% of evaluable patients randomly assigned to receive either consolidation regimen experienced grade 4 neutropenia and thrombocytopenia. Of 173 in treatment arms 1 and 3 (no CsA), 76 (44%) experienced bacteremia or sepsis; 63 (38%) of 164 patients in treatment arms 2 and 4 (with CsA) also experienced this effect. However, CsA therapy was associated more frequently with hyperbilirubinemia, stomatitis, renal impairment, and hypertension. Hyperbilirubinemia was temporally related to CsA administration and resolved once CsA infusions stopped in all cases. Six patients assigned to receive CsA did not receive the drug with their final consolidation treatment because of persistent renal insufficiency (n = 2) or allergic reaction (n = 4) associated with their initial course of CsA, etoposide, and mitoxantrone. These 6 patients received the unmodified doses of chemotherapy described in the non-CsA arm of the protocol, and their data were analyzed with respect to their initial randomized treatment.
Cyclosporine concentration
CsA concentrations were monitored at regular intervals during drug administration, and infusion rate was modified when necessary to maintain the CsA concentration between 3000 and 5000 ng/mL. There was a trend toward an age-dependent response to CsA infusion: infants and young children frequently required 1 to 3 increments of 25% in their CsA infusion doses to achieve concentrations higher than 3000 ng/μL, whereas teenagers were more likely to require temporary cessation of CsA infusion and resumption at a 25% to 50% lower rate.
Pharmacokinetic analysis
To compare mitoxantrone and etoposide clearance and area under the curve (AUC) between groups, we measured serial concentrations of the 2 drugs in 14 patients assigned to treatment arms 1 or 3 (no CsA) and in 22 patients assigned to treatment arms 2 or 4. The 40% reduction in dose of mitoxantrone and etoposide combined with CsA resulted in a 47% increase in mean AUC for etoposide and 12% increase for mitoxantrone.31 The proportions of patients who had stomatitis or infection did not significantly differ between the group treated with CsA and the group that received no CsA. Thus, the variability in clearance combined with empiric 40% reduction in dose resulted in statistically similar systemic exposure and toxicity.
Bone marrow transplantation
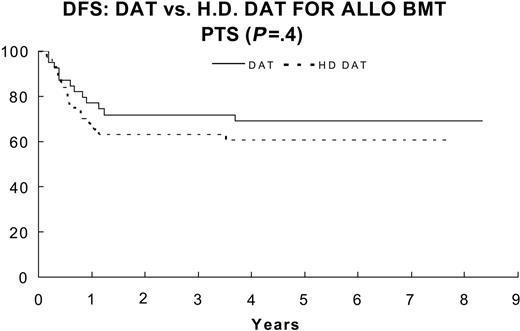
Patients with an HLA-matched related donor underwent allogeneic BMT with protocol-directed therapy after they had recovered from induction 2 and before they received consolidation chemotherapy. Eighty-three patients underwent allogeneic BMT prescribed by the protocol. The 3-year remission duration estimate for patients who underwent BMT was 67.3% ± 5.2%, whereas that for 418 other patients who received consolidation with chemotherapy was only 37.2% ± 2.5% (P < .001). There was no significant effect of induction on outcome of BMT (P = .40) when DFS of BMT patients who received DAT (n = 39) was compared to that of BMT patients who received HDAT (n = 44); P = .38 for OS (Figure 9).
Of 83 BMT patients, 29 had adverse events: 10 (12%) died during remission (6 within 100 days after BMT), 18 (21%) had recurrence of leukemia, and one had a second malignancy (non-Hodgkin lymphoma).
Of the 209 patients who achieved remission after standard-dose DAT as induction therapy and did not undergo protocol BMT (although 12 nonprotocol BMTs were performed), 101 died, 37 relapsed, and 71 were in continuous complete remission (CCR). Similarly, of the 209 patients who achieved remission after HDAT induction therapy and did not undergo protocol-directed BMT, 99 died (7 had nonprotocol BMT), 23 relapsed, and 87 were in CCR. The 19 patients who received nonprotocol BMT were included for analysis by the intent-to-treat principle.
Discussion
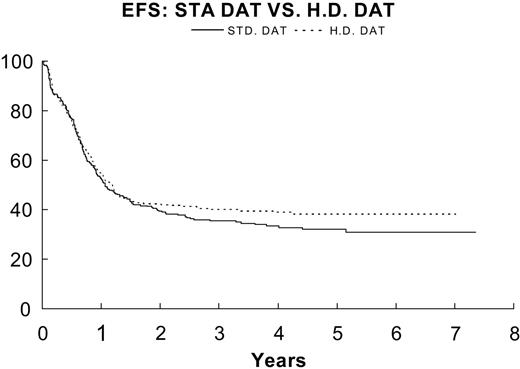
Overall survival among several large pediatric AML studies of this era (CCG-2891,5 54%; BFM 93,7 60%, and MRC AML 10,32 56%) find as in this study that more than 50% of AML patients can be cured. It is noteworthy that in our study the overall survival does not include children with acute promyelocytic leukemia or Down syndrome, patients known to have a favorable outcome. In this study, a 2-by-2 factorial design was used to determine whether intensifying initial induction by using high-dose cytarabine could increase remission rates and EFS and whether remission duration could be increased by addition of CsA to consolidation. The decision to test P-gp modulation during consolidation rather than during induction was based on the prevailing notion at the time that emergence of resistance to anthracyclines may be a risk factor for relapse and the lack of published adult trials of safety and efficacy of the use of inhibitors of P-gp during induction (especially in combination with high-dose Ara-C). In this study the doses of chemotherapy were reduced in the experimental arm due to anticipated delayed elimination of the targeted drugs and the desire to keep AUCs equivalent in both arms. The potential advantage of CsA was hypothesized to be sensitization of the subpopulation of P-gp-positive leukemic blasts. Only 14% of the study population was identified as P-gp positive even using the liberal criterion of 5% MRK16 positivity. There was no significant difference in outcome for the P-gp-positive patients among the 4 treatment arms. Enrollment was set to detect a 10% difference in 2-year EFS from intensification of induction and a 13% difference in 2-year DFS from MDR1 modulation during consolidation. Randomization was not dependent on pretreatment MDR1 expression. Patients in both induction arms achieved excellent remission rates. The CR rate after course 2 of induction was 87.9% for DAT and 91.0% for HDAT (P = .23). No significant differences were noted in CR rate after induction course 1 with standard or high-dose Ara-C. The early death rate of 2.5% was low compared with other recent trials.5 The EFS of patients who received HDAT was slightly better than that of DAT, but the difference was not statistically significant (3-year EFS, 40.1% for HDAT, 35.2% for DAT, P = .28). It is possible that Ara-C at a dosage of 1 g/m2 every 12 hours for 5 days in the second induction cycle for all patients may have minimized potential differences in response rates and EFS of the 2 groups or that 1 gm/m2 of Ara-C does not provide a therapeutic advantage over standard doses of Ara-C in AML.
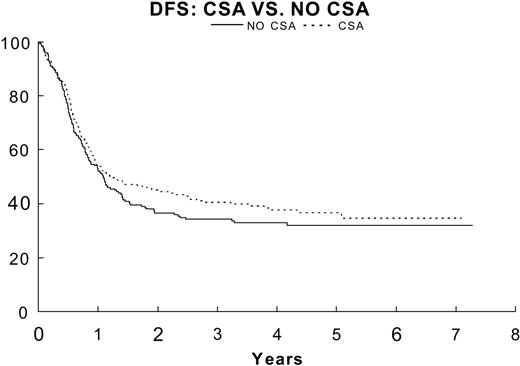
Resistance to drug therapy, particularly that mediated by P-gp, is considered one of the obstacles to effective AML therapy. Although MDR1-mediated drug resistance has been well defined in adults, its incidence and impact on therapy had not been studied in a large cohort of untreated de novo childhood AML patients. Therefore, we prospectively evaluated the effect of a noncytotoxic MDR-reversal agent, CsA, to determine its impact on the DFS of patients who had remissions. Patients who received CsA had a 3-year DFS estimate of 40.6% ± 3.6%, compared with 33.9% ± 3.5% for those who did not, but this trend did not achieve the designed statistical significance (P = .24). There are several potential reasons for the lack of a significant effect of MDR1 modulation in this study: (1) patients were not selected for randomization based on their MDR1 expression or functional efflux; (2) the study was powered to detect a 13% difference; thus, the sample is too small to permit detection of the 6% difference observed; (3) the MDR modulating drug was not started early enough (ie, during induction); (4) emergence of P-gp-overexpressing clones after remission induction is not a major mechanism for relapse24,33 ; and (5) MDR1 overexpression in childhood AML is too low to be clinically relevant for the overall population of patients. Expression of MDR as measured by flow cytometry or by drug efflux studies was relatively uncommon in our population (< 15% of patients when any expression over 5% is considered positive) compared with adult AML patients in whom MDR1 expression has been reported to vary from 30% to 65% of cases at diagnosis18,19 ; (6) The dose of CsA and the in vitro concentrations of CsA achieved were not sufficient to completely block drug efflux. There are also other ABC transporters, in particular the multidrug-resistance-related proteins (MRPs, ABCCs)34 and the recently described breast-cancer-resistance protein (BCRP, ABCG)35-37 that may also be more important in conferring resistance in AML; (7) Mandated chemotherapy dose reductions in the +CsA group may have negatively effected the failure rate. Recent studies suggest that inhibition of MDR1 and MRP1 is not sufficient to suppress efflux of mitoxantrone and that suppression of BCRP38,39 is necessary to overcome mitoxantrone resistance in cell lines. Thus, MDR1 modulation may have only a modest effect on cellular efflux of this drug. It is also important to note that the nonsignificant but modest response to CsA treatment could be unrelated to drug efflux, as observed in a recent trial using CsA during induction therapy for adult patients.40
565 randomized patients excluding 57 with DS. OS (—) 53.6% ± 2.2% versus EFS (- - -) 36.3% ± 2.2%.
565 randomized patients excluding 57 with DS. OS (—) 53.6% ± 2.2% versus EFS (- - -) 36.3% ± 2.2%.
EFS for DAT (—; n = 284) 35.2% ± 3% versus HDAT (- - -; n = 281) 40.1% ± 3.2% P = .28.
EFS for DAT (—; n = 284) 35.2% ± 3% versus HDAT (- - -; n = 281) 40.1% ± 3.2% P = .28.
DFS for patients in remission randomized to no CsA (—; n = 209) 33.9% ± 3.5%, CsA (- - -; (n = 209) 40.6% ± 3.6% (P = .24).
DFS for patients in remission randomized to no CsA (—; n = 209) 33.9% ± 3.5%, CsA (- - -; (n = 209) 40.6% ± 3.6% (P = .24).
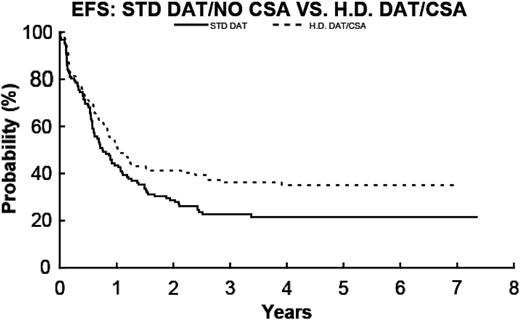
EFS for DAT/no CsA (—; n = 103) 22.7% ± 3.8% and for HDAT/CsA (- - -; n = 103) 36% ± 5.7% (P = .08).
EFS for DAT/no CsA (—; n = 103) 22.7% ± 3.8% and for HDAT/CsA (- - -; n = 103) 36% ± 5.7% (P = .08).
In our study, CsA infusions sufficient to block MDR1 with prolonged serum or whole-blood concentrations of 3000 to 5000 ng/mL were relatively well tolerated.30 Reversible hyperbilirubinemia occurred in most patients treated with CsA, but none suffered significant hepatic injury or veno-occlusive disease. Pharmacokinetic studies demonstrated the expected reduced clearance of mitoxantrone and etoposide in patients who received CsA, but the 40% reduction in drug dose led to no significant differences in the mean AUC for either drug between groups. In fact, there were no significant differences in myelosuppression or the rate of invasive bacterial infection between CsA and standard patients.
This study was originally designed to identify the effect on EFS of either intensive HDAT induction or P-gp modulation by CsA in consolidation. Excluding patients who underwent allogeneic BMT, the combination of experimental interventions (HDAT induction with CsA consolidation, treatment 4) (n = 103) demonstrated a nonstatistically significant advantage in EFS compared to treatment 1 therapy (n = 103), 40.4% ± 5.6% versus 28.0% ± 5.2% (P = .03). A multiple testing procedure has been used, and the P value of .036 does not reach the level of statistical significance. Caution in overinterpreting this difference is also supported by a secular trend in the remission induction rates between treatments 1 and 2. Although both arms used standard-dose DAT, the CR rate for treatment 1 (84%) was less than that of treatment 2 (92%). In contrast to adult studies, where up to 71% of AML samples are positive for MDR1/P-gp in patients older than 60, only 2% of our pediatric AML specimens were strongly positive for P-gp expression, and another 12% showed low expression. Thus, P-gp expression is not a major factor for therapeutic response in the large majority of cases of pediatric AML. Modulation of P-gp function by CsA was of no apparent benefit in our AML patient population as a whole or for those patients who overexpressed P-gp. Intensified induction strategies and the concept of reversing chemotherapy resistance mediated by MDR1/P-gp remain reasonable questions for future trials in both children and adults with AML.
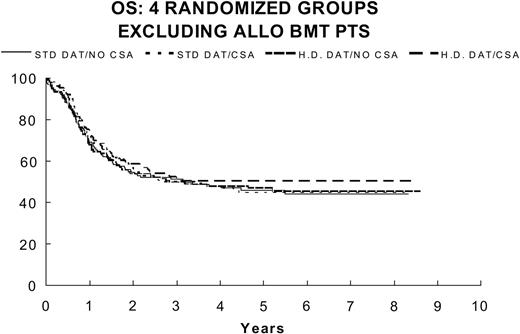
OS for 4 randomized groups excluding BMT patients: DAT/no CsA (n = 127) 51.2% ± 4.5%; DAT/CsA (n = 118) 50.2% ± 4.9%; for HDAT/no CsA (n = 122) 49.9% ± 4.6%; and for HDAT/CsA (n = 115) 51.6% ± 4.8%.
OS for 4 randomized groups excluding BMT patients: DAT/no CsA (n = 127) 51.2% ± 4.5%; DAT/CsA (n = 118) 50.2% ± 4.9%; for HDAT/no CsA (n = 122) 49.9% ± 4.6%; and for HDAT/CsA (n = 115) 51.6% ± 4.8%.
DFS for DAT/no CsA (—; n = 39) 69% ± 38.4% versus HDAT/CsA (- - -; n = 44) 61% ± 22% (P = .4).
DFS for DAT/no CsA (—; n = 39) 69% ± 38.4% versus HDAT/CsA (- - -; n = 44) 61% ± 22% (P = .4).
Appendix
Sharad Salvi, Advocate Hope Children's Hospital, Oak Lawn, IL; Douglas Strother, Alberta Children's Hospital, Calgary, Canada; Jerry Barbosa, All Children's Hospital, St. Petersburg, FL; Doured Daghistani, Baptist Children's Hospital, Miami, FL; Cynthia Kretschmar, Boston Floating Hospital for Infants & Children, Boston, MA; Rudolph Roskos, Broward General Medical Center, Ft. Lauderdale, FL; Robert Wilkinson, Cancer Research Center of Hawaii, Honolulu, HI; William Ferguson, Cardinal Glennon Children's Hospital, St. Louis, MO; Joan Fisher, Carilion Medical Center for Children at Roanoke Community Hospital, Roanoke, VA; Daniel McMahon, Carolinas Medical Center, Charlotte, NC; Yvan Samson, Centre Hospitalier Universitaire de Quebec, Ste-Foy, Canada; Josee Brossard, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Canada; Howard Katzenstein, Children's Healthcare of Atlanta, Emory University, Atlanta, GA; Jacqueline Halton, Children's Hospital of Eastern Ontario, Ottawa, Canada; Yaddanapudi Ravindranath, Children's Hospital of Michigan, Detroit, MI; Cary Stroud, Children's Hospital of the Greenville Hospital System, Greenville, SC; Richard Kadota, Children's Hospital San Diego, San Diego, CA; Susan Cohn, Children's Memorial Medical Center at Chicago, Chicago, IL; Lolie Yu, Children's of New Orleans/LSUMC CCOP, New Orleans, LA; Timothy Griffin, Cook Children's Medical Center, Fort Worth, TX; Holcombe Grier, Dana-Farber Cancer Institute and Children's Hospital, Boston, MA; Sara Chaffee, Dartmouth-Hitchcock Medical Center, Lebanon, NH; Judith Mullins, Driscoll Children's Hospital, Corpus Christi, TX; Susan Kreissman, Duke University Medical Center, Durham, NC; Charles Daeschner, East Carolina University, Greenville, NC; David Kalwinsky, East Tennessee State University, Johnson City, TN; Judith Allen, Eastern Maine Medical Center, Bangor, ME; Janice Olson, Emanuel Hospital-Health Center, Portland, OR; Clifford Selsky, Florida Hospital Cancer Institute, Orlando, FL; Burton Appel, Hackensack University Medical Center, Hackensack, NJ; Albert Moghrabi, Hopital Sainte-Justine, Montreal, Canada; Alberto Pappo, Hospital for Sick Children, Toronto, Canada; Susumu Inoue, Hurley Medical Center, Flint, MI; Jay Greenberg, Inova Fairfax Hospital, Fairfax, VA; Iftikhar Hanif, Joe DiMaggio Children's Hospital at Memorial Hollywood, FL; Robert Arceci, Johns Hopkins Hospital, Baltimore, MD; Wanda Salzer, Madigan Army Medical Center (USOC), Tacoma, WA; Craig Hurwitz, Maine Children's Cancer Program, Scarborough, ME; Howard Weinstein, Massachusetts General Hospital, Boston, MA; Sharon Abish, McGill Univ Health Ctr - Montreal Children's Hosp, Montreal, Canada; Carol Portwine, McMaster University, Hamilton, Canada; Julio Barredo, Medical University of South Carolina, Charleston, SC; Enrique Escalon, Miami Children's Hospital, Miami, FL; Bruce Camitta, Midwest Children's Cancer Center, Milwaukee, WI; Orren Beaty III, Mission Hospitals, Asheville, NC; Elizabeth Raetz, Mount Sinai Medical Center, New York, NY; Wanda Salzer, Naval Medical Center/Portsmouth (USOC), Portsmouth, VA; Eric Sandler, Nemours Children's Clinic-Jacksonville, Jacksonville, FL; Vincent Giusti, Nemours Children's Clinic-Orlando, Orlando, FL; Carl Lenarsky, North Texas Hosp for Children at Med City Dallas, Dallas, TX; Patricia Shearer, Ochsner Clinic, New Orleans, LA; Mark Mogul, Presbyterian Hospital, Charlotte, NC; Sam Lew, Rhode Island Hospital, Providence, RI; Martin Brecher, Roswell Park Cancer Institute, Buffalo, NY; Helen Irving, Royal Children's Hospital, Brisbane, Herston, Brisbane, Australia; John Heath, Royal Children's Hospital, University of Melbourne, Parkville, Australia; Allen Korenblit, Rush-Presbyterian St. Luke's Medical Center, Chicago, IL; John Kelleher, Sacred Heart Hospital, Pensacola, FL; Luis Clavell, San Jorge Children's Hospital, Santurce, PR; Arlene Redner, Schneider Children's Hospital, New Hyde Park, NY; Dick Suh, Scott & White Memorial Hospital, Temple, TX; Hadi Sawaf, St John Hospital and Medical Center, Grosse Point Woods, MI; Gregory Halligan, St. Christopher's Hospital for Children, Philadelphia, PA; Wayne Furman, St. Jude Children's Research Hospital Memphis, Memphis, TN; John McCallister, St. Jude Midwest Affiliate, Peoria, IL; Narayana Gowda, St. Mary's Hospital, West Palm Beach, FL; Jon Brandt, St. Vincent Hospital - Wisconsin, Green Bay, WI; Neyssa Marina, Stanford University Medical Center, Palo Alto, CA; Robert Parker, State University of New York at Stony Brook, Stony Brook, NY; Paul Grundy, Stollery Children's Hospital, Edmonton, Canada; Ronald Dubowy, SUNY Upstate Medical University, Syracuse, NY; Yisheng Lee, Sutter Medical Center, Sacramento, Sacramento, CA; Annette Ridolfi-Luethy, Swiss Pediatric Oncology Group Bern, Bern, Sweden; Hulya Ozsahin, Swiss Pediatric Oncology Group Geneva, Geneva, Sweden; Maja Beck Popovic, Swiss Pediatric Oncology Group Lausanne, Lausanne, Sweden; Cameron Tebbi, Tampa Children's Hospital, Tampa, FL; C. Steuber, Texas Children's Cancer Center at Baylor College of Medicine, Houston, TX; Geoffrey McCowage, The Children's Hospital at Westmead, Westmead, Australia; Emad Salman, The Children's Hospital of Southwest Florida Lee Memorial Health System, Ft. Myers, FL; Wanda Salzer, Tripler Army Medical Center (USOC), Tripler AMC, HI; Marshall Schorin, Tulane Univ./Tulane Univ. Hospital and Clinic, New Orleans, LA; Wanda Salzer, United States Air Force Med Ctr, Keesler AT (USOC), Keesler AFB, MS; Willem Kamps, University Medical Center Groningen, Groningen, Netherlands; Robert Castleberry, University of Alabama, Birmingham, AL; Rochelle Bagatell, University of Arizona Health Sciences Center, Tucson, AZ; David Becton, University of Arkansas, Little Rock, AR; Theodore Zwerdling, University of California, Davis, Sacramento, CA; Stephen Hunger, University of Florida, Gainesville, FL; Robert Trueworthy, University of Kansas Medical Center, Kansas City, KS; Neil Grossman, University of Maryland at Baltimore, Baltimore, MD; Ghazala Usmani, University of Massachusetts Medical School, Worcester, MA; Stuart Toledano, University of Miami School of Medicine, Miami, FL; Gail Megason, University of Mississippi Medical Center Children's Hospital, Jackson, MS; Barbara Gruner, University of Missouri - Columbia, Columbia, MO; Jami Frost, University of New Mexico School of Medicine, Albuquerque, NM; William Meyer, University of Oklahoma Health Sciences Center, Oklahoma City, OK; Barbara Asselin, University of Rochester Medical Center, Rochester, NY; Felicia Wilson, University of South Alabama, Mobile, AL; Paul Thomas, University of Texas Health Science Center at San Antonio, San Antonio, TX; Frederick Huang, University of Texas Medical Branch, Galveston, TX; Alan Homans, University of Vermont College of Medicine, Burlington, VT; Kimberly Dunsmore, University of Virginia Health Sciences Center, Charlottesville, VA; Naomi Winick, UT Southwestern Medical Center, Dallas, TX; David Rosen, Via Christi Regional Medical Center, Wichita, KS; Kamar Godder, Virginia Commonwealth Univ Health System-MCV, Richmond, VA; Thomas McLean, Wake Forest University School of Medicine, Winston-Salem, NC; Wanda Salzer, Walter Reed Army Medical Center (USOC), Washington, DC; Martina Hum, Warren Clinic, Inc., Tulsa, OK; Robert Hayashi, Washington University Medical Center, St. Louis, MO; David Rosen, Wesley Medical Center, Wichita, KS; Elizabeth Kurczynski, West Virginia University HSC - Charleston, Charleston, WV; Frank Keller, West Virginia University HSC - Morgantown, Morgantown, WV; David Rosen, Wichita CCOP, Wichita, KS; Aaron Pitney, Wilford Hall Medical Center, Lackland AFB, TX; and Jack van Hoff, Yale University School of Medicine, New Haven, CT.
Prepublished online as Blood First Edition Paper, October 27, 2005; DOI 10.1182/blood-2004-08-3218.
Supported in part by National Institutes of Health grant RO1 CA90916 (G.V.D., N.J.L., and B.I.S.). A complete listing of grant support for research conducted by the Children's Cancer Group (CCG) and the Pediatric Oncology Group (POG) before initiation of the Children's Oncology Group (COG) grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
David Becton was principal investigator for the POG Protocol 9421. Gary Dahl co-developed the POG Protocol #9421 along with Drs Weinstein, Sikic, Arceci, and Ravindranath, and wrote the manuscript. Y. Ravindranath co-developed the POG Protocol 9421 along with Drs Weinstein, Sikic, Arceci, and Dahl. In addition, Dr Ravindranath performed MDR studies and helped write the manuscript. Myron Chang was statistician for the POG 9421 study. Fred Behm was central reviewer of bone marrow or blood for all patients enrolled into the study. In addition, he directed the laboratory part of the study. Susana Raimondi performed many cytogenetic studies and was the central reviewer for all cytogenetic data. David Head, along with Dr Behm, performed all morphology categorizations. Kimo Stine was responsible for managing BMT data. Norman Lacayo assisted in data analysis, performed pharmacokinetic studies on patients, and writing. Branimir I. Sikic co-developed the POG Protocol 9421 along with Drs Weinstein, Dahl, Arceci, and Ravindranath. Dr Sikic developed the concept of P-glycoprotein inhibition with CSA in leukemia. Dr Sikic was instrumental in development and input of this protocol and ancillary biologic studies. Robert Arceci co-developed the POG Protocol 9421 along with Drs Weinstein, Sikic, Dahl, and Ravindranath. Dr Arceci conceived the study and participated in analysis. Howard Weinstein was the AML Disease Committee Chair in POG during the study and co-architect of study design.