Cytokines promote survival of mast cells by inhibiting apoptotic pathways regulated by the Bcl-2 protein family. We previously showed that lymphocyte apoptosis can proceed via a Bcl-2-inhibitable pathway independent of the canonical initiator caspase, caspase-9, and its adaptor, Apaf-1. Here we report that mast cells lacking caspase-9 or Apaf-1 are refractory to apoptosis after cytotoxic insults but still lose effector function and ability to proliferate. In response to cytokine deprivation or DNA damage, fetal liver-derived mast cells lacking Apaf-1 or caspase-9 failed to undergo apoptosis. Nevertheless, the cytokine-starved cells were not functionally alive, because, unlike those overexpressing Bcl-2, they could not degranulate on Fcϵ receptor stimulation or resume proliferation on re-addition of cytokine. Furthermore, mast cells lacking Apaf-1 or caspase-9 had no survival advantage over wild-type counterparts in vivo. These results indicate that the Apaf-1/caspase-9-independent apoptotic pathway observed in lymphocytes is ineffective in cytokine-deprived mast cells. However, although Apaf-1 and caspase-9 are essential for mast cell apoptosis, neither is required for the functional or clonogenic death of the cells, which may be due to mitochondrial dysfunction.
Introduction
Mast cells are hemopoietic cells sparsely dispersed throughout the body that play important roles in immunity to certain infections as well as in allergic responses and some inflammatory conditions.1 Their survival in vivo, as well as differentiation and proliferation, is regulated by cytokines, including stem cell factor (SCF) and interleukin-3 (IL-3).2,3 In many hemopoietic cell types, cytokines mediate survival via the Bcl-2 family of proteins.4 This family comprises both antiapoptotic proteins, like Bcl-2 itself, as well as proapoptotic proteins, such as Bax and Bim.5 The cellular life-versus-death switch is regulated by the balance between the proapoptotic and antiapoptotic factions of the Bcl-2 family. Indeed, in mast cells both SCF6 and IL-37 modulate the ratio between proapoptotic and antiapoptotic Bcl-2 family members. Moreover, mast cells expressing a Bcl2 transgene are almost completely refractory to death in the absence of these cytokines, while mast cells lacking proapoptotic Bim are partially resistant.8
The Bcl-2 family governs apoptosis by controlling the activation of the aspartate-specific cysteine proteases known as caspases. Much evidence suggests that it does so by controlling the release from mitochondria of apoptogenic proteins, such as cytochrome c.9,10 Cytoplasmic cytochrome c cooperates with the adaptor protein Apaf-1 to activate the initiator caspase, caspase-9, in a complex termed the apoptosome. Caspase-9 in turn proteolytically activates effector caspases, which cause cell demolition by cleaving a multitude of substrates.11 However, we previously have shown that Apaf-1 and caspase-9 are not essential for apoptosis of lymphocytes subjected to cytokine deprivation (or certain other apoptotic stimuli); these cells can instead use an alternate caspase-mediated pathway.12,13 Moreover, in contrast to the proapoptotic Bcl-2 family member Bim,14 Apaf-1 and caspase-9 were found to be not essential for suppressing lymphomagenesis induced by Myc transgene expression.15
An apoptosome-independent pathway to apoptosis, however, may not be evident in all cell types. Ekert et al16 showed recently with immortalized promyeloid cells that the absence of Apaf-1 or caspase-9 greatly delayed acquisition of an apoptotic phenotype upon cytokine deprivation. Nevertheless, the starved cells still had lost viability, as they could not resume proliferation after re-addition of growth factor. Thus, in the absence of overt apoptosis, some cells can undergo “clonogenic death,” but whether such cells retain effector functions is an important unresolved question.
Since Apaf-1 and caspase-9 are dispensable for cytokine withdrawal-induced apoptosis of lymphoid cells12 but required for apoptosis (albeit not for clonogenic death) in myeloid progenitor cells,16 we have examined their roles in the apoptosis as well as the clonogenic and “functional” death of mast cells.
Materials and methods
Mice
All experiments with mice were performed according to the guidelines of the Melbourne Directorate Animal Ethics Committee. The breeding and genotyping of the Apaf-1 as well as caspase-9 knock-out mice and the vav-Bcl2 (line 69) transgenic mice have been described previously in detail, as has the protocol for hematopoietic reconstitution of lethally irradiated (2 × 5.5 Gy) C57BL/6-Ly5.1 mice with fetal liver stem cells.12 The vav-Bcl2 transgenic mice were generated on a C57BL/6-Ly5.2 genetic background. The Apaf-1- and caspase-9-deficient mice, which were originally generated on a mixed C57BL/6 × 129Sv background (using 129Sv ES cells for gene targeting), had been backcrossed for at least 10 generations with C57BL/6-Ly5.2 mice before use in this study.
Generation of fetal liver-derived mast cells
Dispersed E14 fetal liver cells were plated and maintained at a concentration of 5 × 105 cells/mL in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 50 μM 2-mercaptoethanol, and IL-3 (as culture supernatant of X63/0 hybridoma cells stably transfected with an IL-3 expression vector) at a concentration of approximately 100 U/mL. Suspension cells in culture were washed and replated weekly for 3 weeks to exclude adherent cells. In the fourth week of culture, 25 ng/mL SCF (gift of Dr H. Martin, Walter and Eliza Hall Institute [WEHI]) was added to these cultures. Cells were subsequently maintained in approximately 100 U/mL IL-3 plus 25 ng/mL SCF. All results are derived from cells that had been in culture for fewer than 4 weeks after the commencement of SCF treatment.
Histology and microscopy
To assess the purity of cultured mast cells, cells were cytospun and stained with 0.5% toluidine blue for 15 minutes to reveal granules. Cytospins of peritoneal lavage were stained with May-Grunwald/Giemsa to reveal mast cells, while sections of skin taken from the right flank of mice were stained with 1% toluidine blue at 60°C for 15 minutes to reveal mast cells, with sequential sections stained with hematoxylin and eosin to show tissue structure.
Western blotting
Preparation of cell lysates, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein separation, and immunoblotting were performed as previously described.12 The antibodies used were specific for Apaf-1 (rat monoclonal 18H2, Alexis Biochemicals; Lausen, Switzerland17 ), actin (AC-40; Sigma, St Louis, MO), caspase-1 (rat monoclonal 1H11 Alexis; L.A.O'R., unpublished results, April 2005), caspase-2 (rat monoclonal 10C6; Alexis18 ), caspase-3 (rabbit polyclonal 586), caspase-7 (mouse monoclonal 1-1-11), caspase-9 (mouse monoclonal 10-1-87; last 3 all gifts from Y. Lazebnik, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY), caspase-6 (New England Biolabs, Ipswich, MA), caspase-8 (rat monoclonal 1G12, Alexis19 ), caspase-11 (rat monoclonal 4E11), caspase-12 (rat monoclonal 11F10) (both Alexis; L.A.O'R., unpublished results, April 2005), inhibitor of caspase-activated DNAse (ICAD; rabbit polyclonal, BD Pharmingen, San Diego, CA), and XIAP (clone 2F1, MBL).
Flow cytometry
Analysis of cell viability by exclusion of propidium iodide (PI) and cell surface marker expression with fluorescein isothiocyanate (FITC)- or biotin-conjugated antibodies were performed as described previously.12 The antibodies used were ACK-4 (anti-c-kit) and MAR-1 (anti-FcϵRIα; eBio-science, San Diego, CA). Viable cells were counted by flow cytometry by adding a known number of beads (Flow Cytometry Reference Beads; Molecular Probes, Eugene, OR) plus 2 μg/mL PI to cultures, followed by FACS analysis and calculation of viable cell number from the ratio of beads to viable (PI-negative) cells. Staining with PI (2 μg/mL) plus FITC- conjugated annexin V (0.1 μg/mL) was used to identify cells early and late in apoptosis.
Mitochondrial cytochrome c was detected by flow cytometric analysis by permeabilizing cells with 120 μg/mL digitonin (Calbiochem, San Diego, CA) in buffer (75 mM KCl, 250 mM sucrose, 1 mM NaH2PO4, and 8 mM Na2HPO4) followed by fixation in formaldehyde at a final concentration of 4% v/v. To preclude nonspecific binding of antibodies used for staining, cells were incubated for a minimum of 1 hour with blocking buffer (3% bovine serum albumin [BSA] and 0.05% saponin). Following overnight incubation with mouse anti-cytochrome c antibody (1:200 dilution; Pharmingen, San Diego, CA), cells were stained with an FITC-conjugated goat anti-mouse IgG secondary antibody (1/100; Southern Biotechnology Associates, Birmingham, AL) and analyzed in a FACScan.
Degranulation assay
Functional mast cell degranulation was assessed by staining exocytosing granules of activated mast cells with annexin V.20 5 × 105 mast cells cultured in the presence of SCF plus IL-3 or deprived of these cytokines for 48 hours were washed once in degranulation medium (DgM = RPMI containing 0.2% BSA). The cells were then incubated for 12 hours at 37°C in 1 mL DgM with or without 15% of anti-TNP (2,4,6-trinitrophenyl) IgE antibody-containing hybridoma supernatant (IgEL-b4, American Type Culture Collection, Manassas, VA). After 2 washes in DgM, the cells were resuspended in 1 mL DgM containing 100 ng/mL TNP-BSA (Sigma) and incubated at 37°C for 30 minutes to induce mast cell degranulation. The cells were then spun and washed twice in phosphate-buffered saline prior to staining with FITC-conjugated annexin V (0.1 μg/mL) plus PI (2 μg/mL) and subsequent analysis in a FACScan (Becton Dickinson, San Jose, CA). The level of granular exocytosis and hence mast cell degranulation was quantified by measuring the annexin V-FITC fluorescence of PI-negative (viable) cells. The C57 mast cell line served as a positive control.
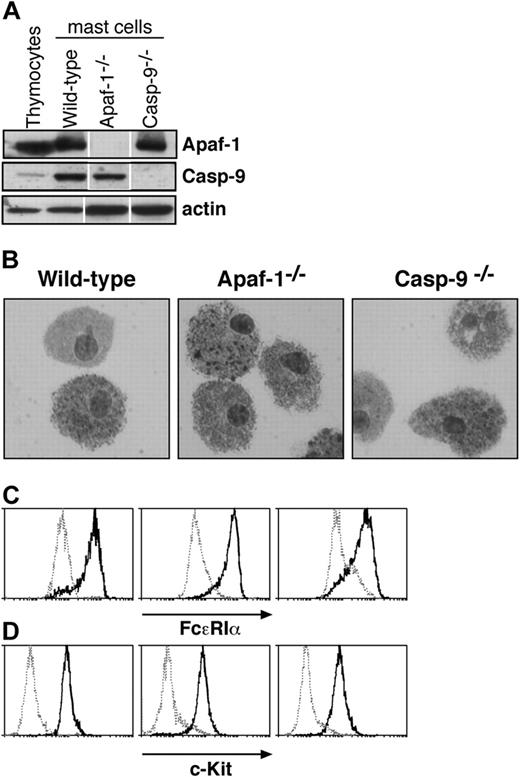
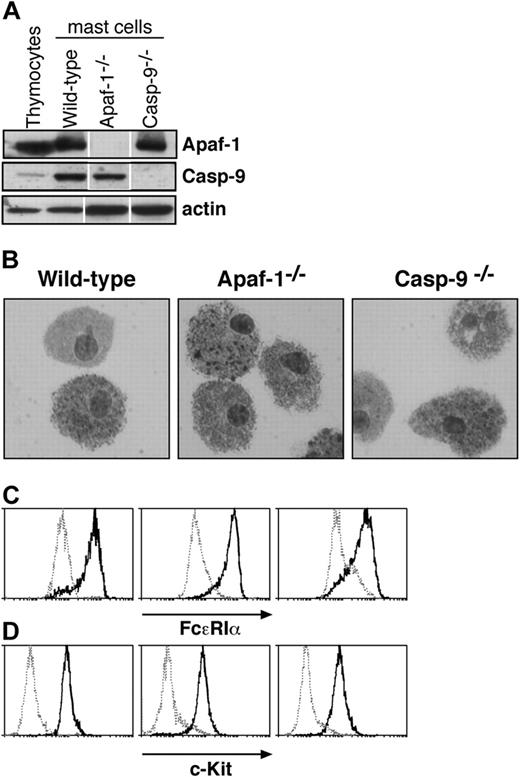
Characterization of cultured mast cells. (A) Fetal liver-derived mast cells express Apaf-1 and caspase-9, as determined by Western blotting. Lysates of thymocytes and of mast cells cultured from wild-type, Apaf-1-/-, or caspase-9-/- fetal liver cells were examined. Probing with an antibody to actin served as a loading control. White lines indicate that lanes of the same exposure of immunoblots have been rearranged for clarity. (B-D) Cells cultured from wild-type, Apaf-1-/-, and caspase-9-/- fetal livers in SCF and IL-3 (as described in “Materials and methods”) resemble mast cells in (B) microscopic appearance of cytospins stained with toluidine blue as well as surface expression of (C) FcϵR1a and (D) c-Kit. In panels C and D, solid histograms represent staining with antibodies specific to FcϵR1a (C) or c-Kit (D), respectively, while broken histograms indicate the staining with isotype-matched control antibodies.
Characterization of cultured mast cells. (A) Fetal liver-derived mast cells express Apaf-1 and caspase-9, as determined by Western blotting. Lysates of thymocytes and of mast cells cultured from wild-type, Apaf-1-/-, or caspase-9-/- fetal liver cells were examined. Probing with an antibody to actin served as a loading control. White lines indicate that lanes of the same exposure of immunoblots have been rearranged for clarity. (B-D) Cells cultured from wild-type, Apaf-1-/-, and caspase-9-/- fetal livers in SCF and IL-3 (as described in “Materials and methods”) resemble mast cells in (B) microscopic appearance of cytospins stained with toluidine blue as well as surface expression of (C) FcϵR1a and (D) c-Kit. In panels C and D, solid histograms represent staining with antibodies specific to FcϵR1a (C) or c-Kit (D), respectively, while broken histograms indicate the staining with isotype-matched control antibodies.
Photography
Mast cells were viewed under a Leica DMLIL microscope (Leica, Wetzlar, Germany) using a 20 × 0.0-0.4 numeric aperture objective. Images were captured using an Axiocam camera and processed using Axiovision software (both from Zeiss, Hallbergmoos, Germany).
Results
Mast cells are sparsely dispersed in tissues, but cells retaining their characteristics can be propagated in vitro with IL-3 and SCF from a tissue rich in hematopoietic stem cells,21 and the cultured cells exhibit mast cell properties in vivo.22 As Apaf-1 or caspase-9 deficiency causes late embryonic lethality in mice (reviewed by Adams11 ), we chose to culture mast cells from livers of E14 embryos that lack Apaf-1 or caspase-9, as well as from wild-type (wt) embryos and ones that bear a vav-Bcl2 transgene expressed throughout the hematopoietic compartment.23 As expected, the cells derived from wild-type embryos expressed Apaf-1 and caspase-9, but the cells from the respective knock-out mice did not (Figure 1A). The cultured cells closely resembled primary mast cells. Microscopy revealed cytoplasmic granules that stained with toluidine blue (Figure 1B), and the cells expressed both the high-affinity receptor for IgE (FcϵR1α chain) and c-Kit, the receptor for SCF (Figures 1C,D). Moreover, they degranulated in response to FcϵR1 stimulation (Figure 2). In all of these respects, wild-type mast cells and those lacking Apaf-1 or caspase-9 were indistinguishable.
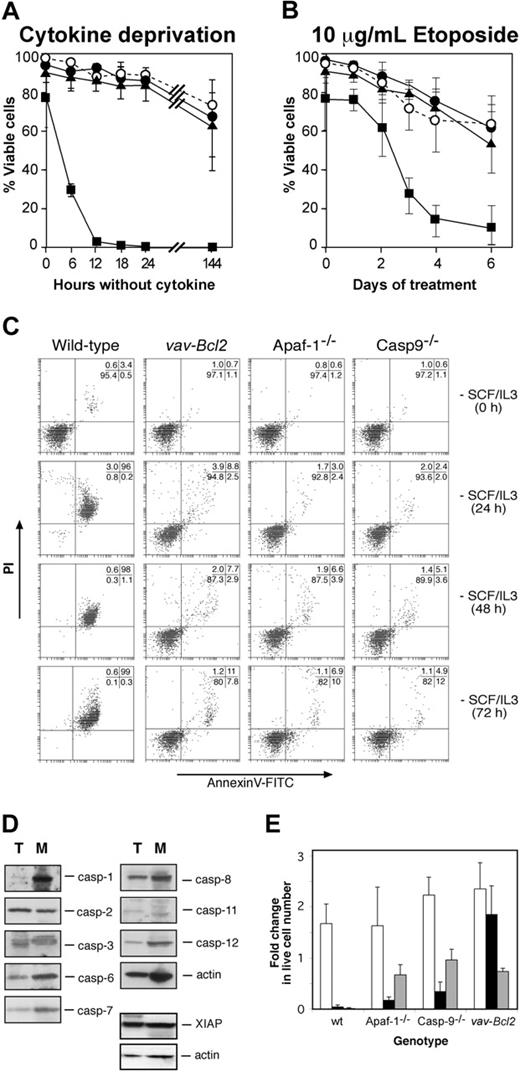
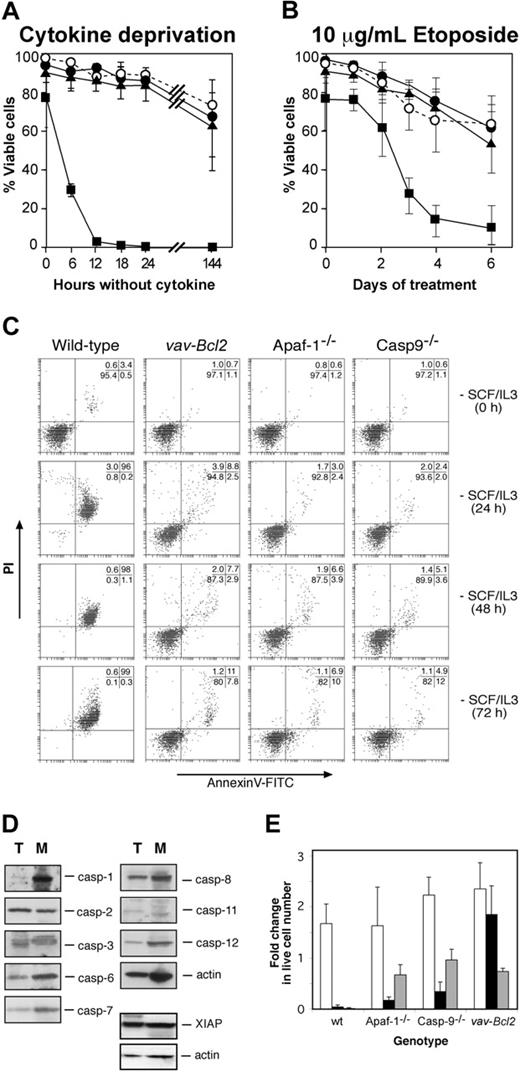
We have shown that lymphocytes can undergo apoptosis upon cytokine deprivation in the absence of Apaf-1 or caspase-9 through a Bcl-2-regulated alternate pathway of caspase activation.12 We therefore examined whether Apaf-1 or caspase-9 is required for cytokine deprivation-induced apoptosis of mast cells, as assessed by binding of annexin V (to surface-exposed phosphatidyl-serine) and PI exclusion.12 Wild-type mast cells deprived of both SCF and IL-3, or deprived of each cytokine independently, died rapidly, and, as previously shown,8 this death could be largely inhibited or even prevented by expression of the Bcl2 transgene (Figure 3A, Supplemental Figure S1A-B; see the Supplemental Figures link at the top of the online article, at the Blood website). Intriguingly, Apaf-1 or caspase-9 deficiency protected mast cells from loss of plasma membrane integrity as efficiently as Bcl-2 overexpression (Figure 3A, Supplemental Figure S1A-B). While more than 95% wild-type cells became permeable to the vital dye PI and exhibited apoptotic morphology after 12 hours without either IL-3 or SCF, even after 72 hours without cytokine, the majority of Bcl2 transgenic, Apaf-1-, and caspase-9-deficient cells retained the morphology of viable cells (not shown), excluded PI, and did not bind annexin V (Figure 3C). Moreover, unlike wild-type cells, cytokine-deprived Apaf-1-/-, caspase-9-/-, and vav-Bcl2 transgenic mast cells did not exhibit processing of caspase-6 and caspase-7 or cleavage of ICAD, the inhibitor of the caspase-activated DNAse, CAD (Supplemental Figure S2). The resistance to apoptosis provided by loss of Apaf-1 or caspase-9 was not restricted to cytokine deprivation, as both proteins also were required for the apoptosis of mast cells treated with the DNA damaging chemotherapeutic agent etoposide (Figure 3B).
The failure of mast cells lacking Apaf-1 or caspase-9 to undergo apoptosis suggests that they do not activate an apoptotic pathway present in lymphocytes. One reason for this might be that lymphocyte apoptosis can occur via caspases that are expressed poorly, or not at all, in mast cells. However, a comparison by Western blotting of the caspases expressed by thymocytes and mast cells indicated that this is not the case. All the murine caspases that have been implicated in apoptosis (caspase-1, -2, -3, -6, -7, -8, -9, -11, and -12) as well as the apoptosis inhibitory protein XIAP were expressed not only in thymocytes but also in mast cells (Figure 3D).
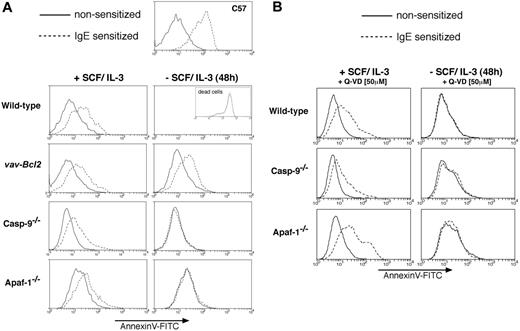
Tests of the ability of mast cells to degranulate. (A) Bcl-2 overexpression but not loss of Apaf-1 or caspase-9 or (B) treatment with the broad-spectrum caspase inhibitor Q-VD allows cytokine-deprived mast cells to retain the ability to degranulate. Mast cell degranulation was determined by staining exocytosing granules with annexin V-FITC. Histograms represent annexin V-FITC fluorescence of PI-negative (viable) cells on the x-axis and relative cell numbers on the y-axis. Mast cells of the indicated genotypes, cultured in the presence or absence (48 hours) of SCF/IL-3, were either left untreated (solid line) or sensitized with IgE anti-TNP (broken line) prior to addition of TNP-BSA to stimulate the Fcϵ receptor and trigger degranulation. FITC-conjugated annexin V was used to stain exocytosing granules of degranulating mast cells and analysis performed on a FACS analyzer, excluding PI-positive (morphologically dead) cells. Note that wild-type mast cells in the absence of SCF/IL-3 are all dead (100% PI positive), and the annexin V-FITC histogram of the dead cell fraction is shown in the inset. The C57 mast cell line served as a positive control for the assay.
Tests of the ability of mast cells to degranulate. (A) Bcl-2 overexpression but not loss of Apaf-1 or caspase-9 or (B) treatment with the broad-spectrum caspase inhibitor Q-VD allows cytokine-deprived mast cells to retain the ability to degranulate. Mast cell degranulation was determined by staining exocytosing granules with annexin V-FITC. Histograms represent annexin V-FITC fluorescence of PI-negative (viable) cells on the x-axis and relative cell numbers on the y-axis. Mast cells of the indicated genotypes, cultured in the presence or absence (48 hours) of SCF/IL-3, were either left untreated (solid line) or sensitized with IgE anti-TNP (broken line) prior to addition of TNP-BSA to stimulate the Fcϵ receptor and trigger degranulation. FITC-conjugated annexin V was used to stain exocytosing granules of degranulating mast cells and analysis performed on a FACS analyzer, excluding PI-positive (morphologically dead) cells. Note that wild-type mast cells in the absence of SCF/IL-3 are all dead (100% PI positive), and the annexin V-FITC histogram of the dead cell fraction is shown in the inset. The C57 mast cell line served as a positive control for the assay.
Analysis of mast cell survival. (A,C) Survival of wild-type, Apaf-1-/-, caspase-9-/-, and Bcl2 transgenic mast cells in the absence of cytokines or (B) during treatment with 10 μg/mL etoposide. Cell viability was measured by exclusion of PI (A,B) or by staining with PI plus annexin V-FITC (C). (A-B)▪ indicates wild type; •, Apaf-1-/-; ▴, caspase-9-/-; and ○, vav-Bcl2. (D) Expression of caspases and the caspase inhibitor XIAP as determined by Western blotting of lysates from wild-type thymocytes (T) and wild-type fetal liver-derived mast cells (M). Probing for actin served as a loading control. (E) Cytokine-induced proliferation of wild-type, Apaf--/-, caspase-9-/-, and Bcl2 transgenic fetal liver-derived mast cells following cytokine deprivation for 24 hours. Cells were enumerated 6 days after re-addition of cytokine or after 6 days of cytokine deprivation, and cell numbers are expressed relative to the initial number of cells in the culture. □ indicates no delay in re-addition; ▪, 24 hours delay; and ▦, cytokines never added. Graphs indicate mean ± SD (in panel A, n = 19, wild type; n = 4, Apaf-1-/-;n = 4, caspase-9-/-;n = 3, vav-Bcl2;in panel B, n = 11, wild type; n = 3, Apaf-1-/-;n = 4, caspase-9-/-;n = 3, vav-Bcl2;in panel E, n = 3 for all genotypes, except n = 4 for caspase-9-/-).
Analysis of mast cell survival. (A,C) Survival of wild-type, Apaf-1-/-, caspase-9-/-, and Bcl2 transgenic mast cells in the absence of cytokines or (B) during treatment with 10 μg/mL etoposide. Cell viability was measured by exclusion of PI (A,B) or by staining with PI plus annexin V-FITC (C). (A-B)▪ indicates wild type; •, Apaf-1-/-; ▴, caspase-9-/-; and ○, vav-Bcl2. (D) Expression of caspases and the caspase inhibitor XIAP as determined by Western blotting of lysates from wild-type thymocytes (T) and wild-type fetal liver-derived mast cells (M). Probing for actin served as a loading control. (E) Cytokine-induced proliferation of wild-type, Apaf--/-, caspase-9-/-, and Bcl2 transgenic fetal liver-derived mast cells following cytokine deprivation for 24 hours. Cells were enumerated 6 days after re-addition of cytokine or after 6 days of cytokine deprivation, and cell numbers are expressed relative to the initial number of cells in the culture. □ indicates no delay in re-addition; ▪, 24 hours delay; and ▦, cytokines never added. Graphs indicate mean ± SD (in panel A, n = 19, wild type; n = 4, Apaf-1-/-;n = 4, caspase-9-/-;n = 3, vav-Bcl2;in panel B, n = 11, wild type; n = 3, Apaf-1-/-;n = 4, caspase-9-/-;n = 3, vav-Bcl2;in panel E, n = 3 for all genotypes, except n = 4 for caspase-9-/-).
When deprived of cytokine, immortalized promyeloid cells lacking Apaf-1 or caspase-9 lose clonogenicity, despite appearing viable by other assays.16 We therefore explored whether Apaf-1- and caspase-9-deficient mast cells could proliferate following a period of cytokine deprivation. We deprived cells of both SCF and IL-3 for 24 hours, then re-administered both cytokines for 6 days and monitored cell numbers to determine whether they had proliferated. As expected, wild-type cells could not be rescued by re-addition of cytokine (Figure 3E), probably because the few remaining viable cells at the time of cytokine re-addition were committed to apoptosis. In contrast, the protection offered by a Bcl2 transgene enabled starved cells to resume proliferation following cytokine re-addition (Figure 3E). Intriguingly, although the majority of cells lacking Apaf-1 or caspase-9 appeared viable in the absence of cytokines and were not stained with PI or annexin V (Figure 3C), when cytokines were resupplied, these cells did not grow; in fact, their number even declined somewhat (Figure 3E). Thus, cytokine deprivation committed mast cells unable to form the apoptosome to die, whereas those overexpressing Bcl-2 could retain clonogenic potential for prolonged periods.
To test whether the Apaf-1- or caspase-9-deficient cells retained mast cell function after cytokine deprivation, we assayed their ability to degranulate in response to stimulation through the FcϵR by analyzing for increased staining with FITC-conjugated annexin V.20 Unlike starved Bcl2 transgenic mast cells, the starved cells lacking Apaf-1 or caspase-9 could not degranulate (Figure 2A). We also attempted to measure degranulation by studying β-hexosaminidase release, but this assay was not reliable due to high background in dead and functionally dead cells that had not been activated (not shown). We conclude that the Apaf-1- and caspase-9-deficient mast cells were not only unable to resume proliferation (clonogenic death), but they also were functionally dead.
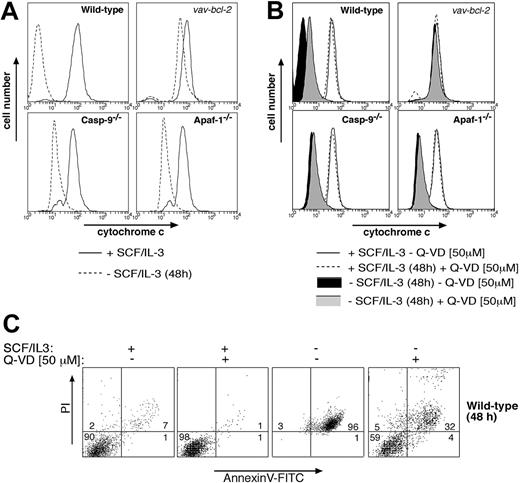
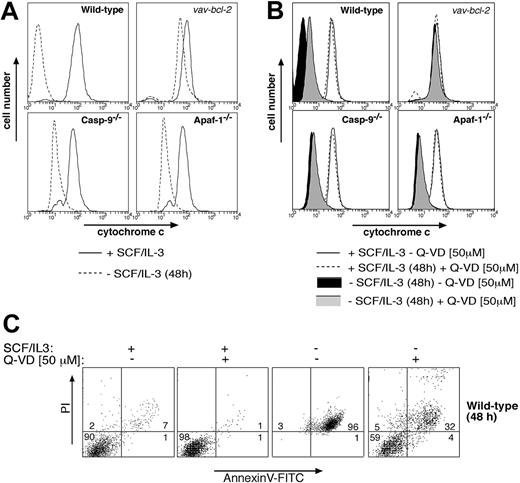
Collectively, these results indicate that mast cells deprived of cytokine possess 2 separate Bcl-2-regulated death pathways: one that mediates apoptotic morphology and requires Apaf-1 and caspase-9, and another that mediates clonogenic and functional death and does not require Apaf-1 or caspase-9. The second pathway might depend on activation of other caspases11 or represent a caspase-independent mode of death, perhaps activated by mitochondrial damage and the resulting release of cytochrome c.10 Consistent with caspase-independent death, we found that cytokine deprivation rapidly caused release of cytochrome c from mitochondria in wild-type, Apaf-1-/-, and caspase-9-/- mast cells (Figure 4A). This cytochrome c release could be prevented by Bcl-2 overexpression but not by addition of the broad-spectrum caspase inhibitor Q-VD (Figures 4A,B), although Q-VD was capable of inhibiting cytokine deprivation-induced exposure of phosphatidylserine and loss of plasma membrane integrity (Figure 4C). Furthermore, addition of Q-VD to cytokine-deprived mast cells did not restore their ability to degranulate in response to FcϵR stimulation (Figure 2B). These results indicate that clonogenic death and functional death may both be due to mitochondrial damage and release of cytochrome c, but a role for Q-VD- insensitive caspases in this process cannot yet be excluded.
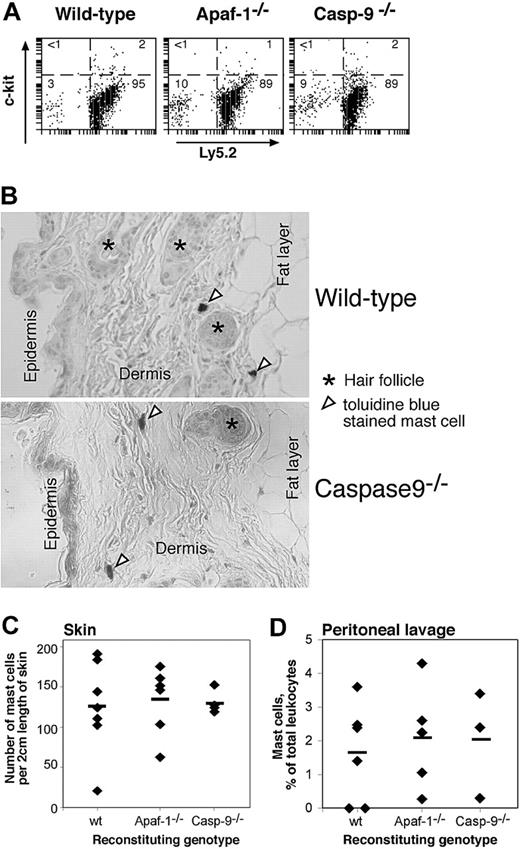
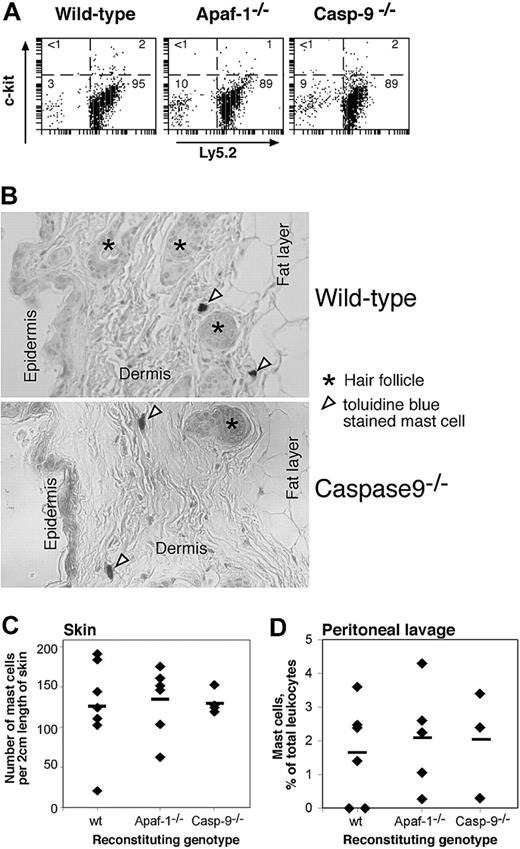
Homeostasis of hemopoietic cells in vivo requires appropriate regulation of apoptosis and is typically mediated by cytokines.4 We reported previously that, in contrast to lymphocytes lacking the proapoptotic BH3-only protein Bim or overexpressing Bcl-2, lymphocytes lacking Apaf-1 or caspase-9 did not accumulate abnormally in vivo, presumably because their programmed cell death does not rely upon the apoptosome.12 As Apaf-1- and caspase-9-deficient mast cells were refractory to apoptosis induced by cytokine deprivation or genotoxic damage in vitro, we wished to determine whether they would accumulate in vivo. Hence, we reconstituted lethally irradiated wild-type mice with Apaf-1-/-, caspase-9-/-, or, as a control, with wild-type fetal liver cells and examined the abundance of mast cells in their peritoneal cavity and skin 3 months later, a period sufficient for any accumulation to occur. Neither the proportion of mast cells in the peritoneal cavity (Figure 5A,D) nor the frequency of mast cells in the skin (Figure 5B,C) differed between mice reconstituted with wild-type or mutant stem cells. As the donor and recipient mice differed at the Ly5 locus, we could confirm by FACS analysis that all the mast cells (c-Kit+) from the peritoneal lavage were derived from the donor (Ly5.2+) stem cells (Figure 5A). These results demonstrate that Apaf-1- and caspase-9-deficient mast cells have no survival advantage over their wild-type counterparts in vivo. Thus, under physiologic stress, the mutant mast cells, like the mutant B and T lymphocytes,12 must be fated to die by a mechanism that does not require the apoptosome.
Analysis of cytochrome c release in cytokine-deprived mast cells. Cytokine deprivation causes release of cytochrome c from mitochondria in mast cells, and this can be blocked by (A) Bcl-2 overexpression but not by loss of Apaf-1 or caspase-9 or (B) by addition of the broad-spectrum caspase inhibitor Q-VD. Mast cells (wild-type, vav-Bcl2 transgenic, Apaf-1-/-, or caspase-9-/-) were either cultured in IL-3 plus SCF or deprived of cytokines for 48 hours. In panel B, wild-type cells were cultured in the presence of the broad-spectrum caspase inhibitor Q-VD. Mitochondrial cytochrome c release was measured by permeabilizing cells, followed by fixation and immunofluorescent staining with antibodies to cytochrome c. In cells with damaged mitochondria, cytochrome c will be washed out, resulting in low-intensity anti-cytochrome c staining, whereas cells with intact outer mitochondrial membranes will show high-intensity staining in this assay. (C) Mast cells (wt) were cultured for 48 hours in SCF plus IL-3 or deprived of both cytokines, either in the presence or absence of the caspase inhibitor Q-VD (50 μM). Cells were then stained with PI plus FITC-conjugated annexin V and analyzed by flow cytometry.
Analysis of cytochrome c release in cytokine-deprived mast cells. Cytokine deprivation causes release of cytochrome c from mitochondria in mast cells, and this can be blocked by (A) Bcl-2 overexpression but not by loss of Apaf-1 or caspase-9 or (B) by addition of the broad-spectrum caspase inhibitor Q-VD. Mast cells (wild-type, vav-Bcl2 transgenic, Apaf-1-/-, or caspase-9-/-) were either cultured in IL-3 plus SCF or deprived of cytokines for 48 hours. In panel B, wild-type cells were cultured in the presence of the broad-spectrum caspase inhibitor Q-VD. Mitochondrial cytochrome c release was measured by permeabilizing cells, followed by fixation and immunofluorescent staining with antibodies to cytochrome c. In cells with damaged mitochondria, cytochrome c will be washed out, resulting in low-intensity anti-cytochrome c staining, whereas cells with intact outer mitochondrial membranes will show high-intensity staining in this assay. (C) Mast cells (wt) were cultured for 48 hours in SCF plus IL-3 or deprived of both cytokines, either in the presence or absence of the caspase inhibitor Q-VD (50 μM). Cells were then stained with PI plus FITC-conjugated annexin V and analyzed by flow cytometry.
Discussion
Experiments with gene knock-out mice have produced conflicting results about the role of Apaf-1 and caspase-9 in programmed cell death and stress-induced apoptosis. Although neuronal hyperplasia and exencephaly were seen in Apaf-1-/- and caspase-9-/- embryos on a mixed C57BL/6 × 129Sv background, this was less obvious on an inbred C57BL/6 background.11 Moreover, although loss of proapoptotic Bim or Bcl-2 overexpression caused progressive lymphoid and myeloid cell hyperplasia, no such effect was seen in the absence of Apaf-1 or caspase-9.12 Experiments with immortalized myeloid progenitors indicated that the apoptosome is required for the development of apoptotic morphology but dispensable for clonogenic death following cytokine withdrawal.16 The role of the apoptosome in functional death of cells has not yet been investigated.
We used mast cells to study in a single cell type the role of Apaf-1 and caspase-9 in apoptosis, clonogenic as well as functional death. These experiments demonstrated that in contrast to the behavior of the mutant lymphocytes in vitro,12 mast cells required Apaf-1 and caspase-9 to undergo apoptosis in response to cytokine deprivation or DNA damage (Figures 3A,B and Supplemental Figures S1-S2). Hence, the caspase-dependent alternative pathway observed in lymphocytes must either be inactive in mast cells or expressed at a level insufficient to evoke an apoptotic phenotype. Although mast cells and thymocytes express the same set of caspases and similar levels of the caspase inhibitor XIAP (Figure 3D), it remains possible that some other apoptotic regulators, such as additional caspase-inhibitory IAP (inhibitor of apoptosis proteins), might be differentially expressed between these 2 cell types.
Enumeration of mast cells in vivo in lethally irradiated mice reconstituted with wild-type, Apaf-1-/-, or caspase-9-/- fetal liver cells. (A,D) The proportion of mast cells (as percent of nucleated cells) in the peritoneal lavage was determined by immunofluorescent staining with antibodies to c-kit and Ly5.2 (donor cell specific) (A) or in cytospins by their deep blue staining with May Grunwald/Giemsa (D). In panel D, ♦ indicates individual mice; horizontal bars, the mean percentage for each genotype (n = 6, wild type; n = 5, Apaf-1-/-; n = 3, caspase-9-/-). (B,C) The frequency of mast cells in the skin of reconstituted animals was determined by counting the number of toluidine blue-staining cells in 2-cm lengths of sectioned skin. ♦ indicates individual mice; horizontal bars, the mean percentage for each genotype (n = 7, wild-type; n = 8, Apaf-1-/-;n = 4, caspase-9-/-).
Enumeration of mast cells in vivo in lethally irradiated mice reconstituted with wild-type, Apaf-1-/-, or caspase-9-/- fetal liver cells. (A,D) The proportion of mast cells (as percent of nucleated cells) in the peritoneal lavage was determined by immunofluorescent staining with antibodies to c-kit and Ly5.2 (donor cell specific) (A) or in cytospins by their deep blue staining with May Grunwald/Giemsa (D). In panel D, ♦ indicates individual mice; horizontal bars, the mean percentage for each genotype (n = 6, wild type; n = 5, Apaf-1-/-; n = 3, caspase-9-/-). (B,C) The frequency of mast cells in the skin of reconstituted animals was determined by counting the number of toluidine blue-staining cells in 2-cm lengths of sectioned skin. ♦ indicates individual mice; horizontal bars, the mean percentage for each genotype (n = 7, wild-type; n = 8, Apaf-1-/-;n = 4, caspase-9-/-).
Although they did not undergo apoptosis, the starved Apaf-1 and caspase-9 mutant mast cells were unable to proliferate, or even survive, when resupplied with cytokines after a period of deprivation, whereas the mast cells overexpressing Bcl-2 readily resumed proliferation (Figure 3E). These results are similar to those obtained in studies with immortalized myeloid progenitors16 and nontransformed hematopoietic progenitor cells.12 Moreover, in contrast to Bcl-2 overexpressing mast cells, those lacking Apaf-1or caspase-9 and even wild-type cells treated with caspase inhibitors were unable to degranulate when cultured in the absence of cytokines (Figure 2). Collectively, these results indicate that mast cells deprived of cytokines possess 2 separate Bcl-2-regulated death pathways: one that mediates apoptotic morphology and requires Apaf-1 and caspase-9, and another that mediates clonogenic and functional death and does not require Apaf-1 or caspase-9. The second pathway might depend on activation of other caspases11 or might represent a caspase-independent mode of death, perhaps activated by mitochondrial damage and the resulting release of cytochrome c.10 Since cytochrome c release was prevented by overexpressed Bcl-2 but not by absence of the apoptosome components or by a caspase inhibitor (Figures 4A,B), the simplest explanation for the functional and clonogenic death of the cytokine-deprived cells would be mitochondrial dysfunction. The role of Q-VD-insensitive caspases can, however, not yet be excluded, and this could be addressed by generating compound mutant mice lacking multiple (initiator) caspases. Remarkably, upon cytokine deprivation wild-type mast cells lost considerably more cytochrome c from their mitochondria than Apaf-1-/- or caspase-9-/- cells. We hypothesize that this may be due to the fact that efficient effector caspase activation, which occurs in wild-type but not in Apaf-1-/- or caspase-9-/- cells, enhances cytochrome c release by cleaving components of the mitochondrial membrane, such as the p75 subunit of complex I of the electron transport chain.24
Some cases of mastocytosis exhibit molecular changes rendering mast cells refractory to cytokine withdrawal-induced apoptosis.25 While our in vivo results (Figure 5) suggest that loss of Apaf-1 or caspase-9 alone is unlikely to cause mastocytosis, loss of apoptosome function might act in combination with deregulation of another antiapoptotic or growth-stimulating pathway to promote mast cell accumulation.
Prepublished online asBlood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2005-05-2160.
Supported by grants and fellowships from the National Health and Medical Research Council (Canberra), the Leukemia and Lymphoma Society (SCOR grant), the National Institutes of Health, the Cancer Council of Victoria (Postdoctoral Cancer Research Fellowship [V.S.M.]), and the Swiss National Science Foundation (Postdoctoral Fellowship [T.K.]).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs S. Cory, P. Gruss, R. Flavell, A. Harris, F. Cecconi, K. Kuida, and J. Silke for gifts of transgenic and gene knock-out mice and antibodies; to A. Naughton, J. Morrow, and K. Pioch for animal care; to Dr S. Mihajlovic for histologic preparation; to Dr H. Martin for SCF; to Dr Y. Lazebnik for gifts of antibodies; and to Prof D. Metcalf, Drs J. Alfredsson, P. Ekert, and D. Vaux for valuable discussions.