Abstract
The physiologic role of CXCR4 on hematopoietic stem/progenitor cells (HSPCs) is not fully understood. Here, we show that radioprotection of lethally irradiated mice by embryonic day 14.5 (E14.5) CXCR4–/– fetal liver (FL) cells was markedly impaired when compared with CXCR4+/+ counterparts, but this defect was rescued when hosts were engrafted with high cell numbers. This quantitative defect contrasted with a similar content in hematopoietic colony-forming cells (CFCs), splenic colony-forming units (CFUs-S), and Lin– Sca-1+ c-kit+ cells in E14.5 CXCR4–/– and CXCR4+/+ livers. In addition, the homing of HSPCs in the bone marrow was not altered as detected with a CFSE-staining assay. In contrast, a 30-fold increase in CFCs was seen in the circulation of mice stably reconstituted with CXCR4–/– FL cells and this increment was already observed before hematopoiesis had reached a steady-state level. Together, the data strongly suggest that impaired retention may, at least in short-term hematopoietic reconstitution, lead to a diminution in the number of available progenitors required for radioprotection.
Introduction
The CXC chemokine ligand 12 (CXCL12)/stromal cell–derived factor-1 (SDF-1)/pre-B cell growth-stimulating factor (PBSF)1,2 mediates the homing of hematopoietic stem/progenitor cells (HSPCs) to the bone marrow (BM) by binding to its receptor CXCR4.3 SDF-1/CXCR4 interactions play pleiotropic roles during development, as revealed by the phenotype of mice with a homozygous targeted gene disruption that die from defects in multiples organs.4-7 In fetal hematopoiesis, CXCR4–/– or SDF-1–/– embryos display more profound defects in the marrow than in the fetal liver (FL), suggesting a major role for the CXCR4/SDF-1 axis in marrow colonization.4-7 In adult hematopoiesis, CXCR4 receptors are involved in numerous biologic processes, including cell migration,8 proliferation, and survival.9-14 Several data suggest that SDF-1 and CXCR4 play a critical role in the homing of HSPCs with SDF-1 acting as a major chemoattractant for severe combined immunodeficiency (SCID)–repopulating cells and leukemic cells.8,15,16 Blocking CXCR4 function on CD34+ cells with anti-CXCR4 antibodies prevented hematopoietic reconstitution in nonobese diabetic (NOD)/SCID mice,17 whereas CXCR4 overexpression increased their abilities to engraft NOD/SCID mice.9 In mice, it has been reported that CXCR4–/– FL cells can successfully reconstitute hematopoiesis of lethally irradiated mice,18-20 whereas secondary transplantation experiments showed defective reconstitution.20
Acritical role for CXCR4 in mobilization, the egress of HSPCs from BM to the peripheral blood, was recently suggested. For instance, a rapid mobilization of HSPCs was obtained by treatment with the CXCR4 antagonist AMD3100,21,22 as well as by induction of SDF-1 plasma elevation.23,24 In addition, G-CSF–induced HSPC mobilization coincides in vivo with a decrease in BM SDF-1 concentration related to SDF-1 cleavage by marrow serine proteases25,26 and dipeptidylpeptidase IV (CD26).27,28 Furthermore, treating HSPCs by pertussis toxin (PT), which causes an interruption of G-protein signaling, did not affect their BM engraftment29 but induced their release in the peripheral blood,30 suggesting that a Gαi-protein signaling identical to that mediated by CXCR4 receptor or other chemokine receptors must be required for HSPC retention in BM microenvironment.8,30,31 Consistent with the hypothesis that the CXCR4/SDF-1 axis plays a major role in the retention of hematopoietic cells, it has been shown that elevated numbers of granulocytic precursors were present in the peripheral blood of mice reconstituted with CXCR4–/– FL cells.19,20 Nevertheless, it is not clear whether the production and/or anchorage of HSPCs is normal in these mice or whether the reported data were simply due to the impaired retention of cells within the BM cellular niches. Actually, we do not know precisely the mechanisms by which CXCR4–/– FL cells were able to engraft adult irradiated hosts.
We took advantage of the CXCR4–/– mouse syngenic transplantation model to further dissect the role of CXCR4 on hematopoiesis. The data revealed that CXCR4 is required for the retention of HSPCs and short-term reconstituting cells within the BM microenvironment, whereas CXCR4 was dispensable for their homing. Impaired retention led to a marked defect in short-term hematopoietic reconstitution when mice received transplants of limited numbers of CXCR4–/– FL cells.
Materials and methods
Mice and genotyping
The generation of CXCR4–/– C57BL/6-Ly5.2 mice was described previously.6 Heterozygous animals were genotyped as previously described.6 Fetuses at E14.5 were individually collected from heterozygous pregnant females humanely killed. After genotyping, FL cells from either CXCR4–/– or CXCR4+/+ embryos were pooled. Transplantation assays were performed with adult (6 to 8 weeks old) C57BL/6-Ly5.2 and C57BL/6-Ly5.1 mice purchased from Janvier CERJ (Le Genest-St-Isle, France) and Charles River Laboratories (Les Oncins, France), respectively.
Adoptive and secondary transfer
Recipient C57BL/6-Ly5.1 mice were exposed to 11 Gy total body X-ray irradiation administered in 2 doses of 5.5 Gy separated by 3 hours. For primary transplantation, FL cells suspended in PBS were injected intravenously (0.2 mL/mouse) via the retro-orbital sinus of anesthetized mice. For secondary transplantation, blood cells from C57BL/6-Ly5.1 mice primarily engrafted with 5 × 106 FL cells were taken at 8 weeks after transplantation and injected into secondary lethally irradiated C57BL/6-Ly5.1 mice.
Mouse peripheral blood hematology
A sample of peripheral blood (50 μL) was obtained from the retro-orbital sinus of anesthetized mice with a heparinized microcapillary tube. Total nucleated blood cells, erythrocyte and platelet counts, and number of lymphocytes and granulocytes were automatically measured with an MS9 counter (Melet Schloesing Technologies, Cergy-Pontoise, France) calibrated for mouse blood.
Antibodies and preparation of fetal liver cells for fluorescence-activated cell sorter (FACS)
Donor-specific anti-CD45.2–FITC was used to determine chimerism in adoptive and secondary transfer experiments. To quantify primitive Lin–Sca-1+c-Kit+ cells, FL cells were incubated for 20 minutes at 4°C with lineage-specific monoclonal antibodies (mAbs): anti–Gr-1 (RB6-8C5), antierythroid lineage cells (TER119), anti-B220 (RA3-6B2), anti-CD4 (GK1.5), and anti-CD8 (Lyt-1) followed by phycoerythrin (PE)–conjugated anti–rat Ig (Clinisciences, Montrouge, France). After washing, cells were incubated with 5% rat serum followed by allophycocyanin (APC)–conjugated anti–c-Kit and biotinylated anti–Sca-1 revealed by Steptavidin-PE-Cy7. Hoechst 33342 HSPC staining and side population (SP) cell analysis were performed according to procedures given by Goodell et al.32 When verapamil was used, FL cells were incubated in the presence of 75 μM verapamil (Sigma Chemical, St Louis, MO). Data were collected either on FACSort and analyzed with CELLQuest Pro v4.0.2 software or on BD LSRII and analyzed using FACSDiva software (BD Biosciences, Strasbourg, France). All antibodies, except for GK1.5 and Lyt-1, were purchased from BD Biosciences.
In vivo homing assay
Freshly isolated E14.5 FL cells were labeled with the cytoplasmic dye carboxyfluorescein diacetate succinimidyl diester (CFSE) according to manufacturer's instructions (Molecular Probes, Eugene, OR). After this step, greater than 99% of cells were positively stained and the fluorescence intensity ranged between 103 and 104 (data not shown). Lethally irradiated C57BL/6 mice were injected with 20 × 106 to 30 × 106 CXCR4–/– and CXCR4+/+ CFSE–stained cells via the left retro-orbital sinus. Three and 24 hours after injection, mice were bled from the right retro-orbital sinus. Determination of the percentage of CFSE+ cells in BM, spleen, and blood was scored by flow cytometry. The frequency of CFSE+ donor cells was determined based on the background fluorescence of cells from control-uninjected mice. The BM homing was calculated based on the assumption that 2 femurs and 2 tibiae represent 25% of total BM.33 Since recipients were lethally irradiated 24 hours before transplantation, BM, spleen, and blood cellularities were different at 3 and 24 hours after injection (Figure 3B legend). The formula is based on the percentage of CFSE+ donor cells that homed in the specific tissue determined by flow cytometry (A), multiplied by the cellularity of recovered organ from the lethally irradiated recipients at 3 and 24 hours after transplantation (B), and divided by the number of CFSE+ cells injected per mice (N): % homing = (A × B)/N.
In vitro colony-forming cell assay
The colony-forming cell (CFC) potential was assayed in semisolid medium containing 1 mL MethoCult M3234 supplemented with recombinant cytokines according to manufacturer's instructions (Stem Cell Technologies, Vancouver, BC, Canada). Total colonies were counted 8 to 10 days later using an inverted microscope (Zeiss TELAVAL 31; AFT Micromecanique, Fillinges, France) in cultures performed in duplicate.
Splenic colony-forming units assay
Day 12 splenic colony-forming unit (CFU-S) content in the FL from CXCR4+/+ and CXCR4–/– E14.5 embryos was determined by injection of 5 × 104 and 105 cells. Circulating CFUs-S of chimeric mice engrafted with 5 × 106 FL cells were assessed by the injection of 20 μL total peripheral blood diluted into 180 μL of PBS. Cells were injected into the retro-orbital sinus of lethally irradiated C57BL/6 mice killed 12 days after injection. Spleens were fixed in Bouin solution and colonies were scored manually.
Statistics
Results of experimental points obtained from 3 to 4 repeated experiments are reported as the mean plus or minus standard deviation (SD). Statistical analysis was performed using the 2-tailed Student t test for paired data.
Results
HSPC content in E14.5 CXCR4–/– and CXCR4+/+ livers
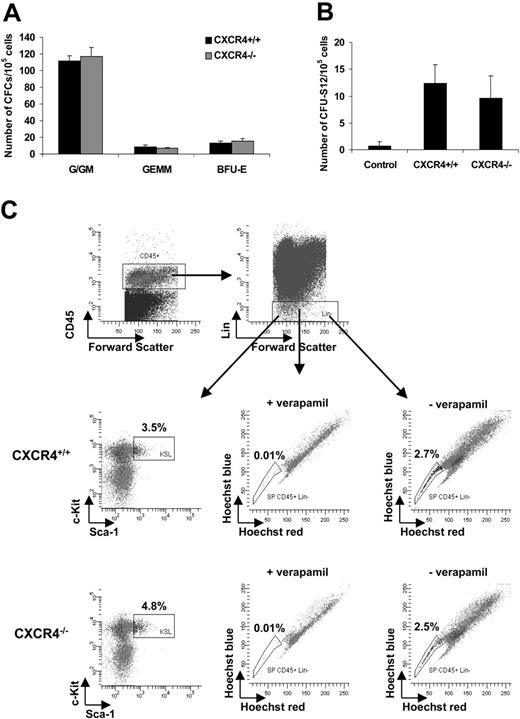
Previous studies have shown that CXCR4–/– hematopoietic stem cells have a defect in their ability to reconstitute hematopoiesis,18-20 but this defect was not fully characterized. To further address its mechanism, we first examined the hematopoietic cell content of E14.5 CXCR4–/– and CXCR4+/+ livers. Total number counts of nucleated cells were comparable in both genotypes but with phenotypic differences in the content of hematopoietic maturing cells as previously described.4-7 The HSPC compartment was subsequently evaluated by functional assays and flow cytometry analysis. Indeed, as previously shown in SDF-1–/– FLs,4 we observed that the frequency of CFCs as well as the number of CFUs-S in E14.5 CXCR4+/+ and CXCR4–/– livers were in the same order of magnitude (Figure 1A-B). No significant differences in the percentage of primitive CD45+ Lin–Sca-1+c-Kit+ and committed CD45+ Lin– Sca-1–c-Kit+ progenitor cells were observed between the 2 genotypes (Figure 1C). Previous studies have shown that a small subset within primitive CD45+ Lin–Sca-1+c-Kit+ cells, the SP, exhibits lymphoid and myeloid hematopoietic reconstituting activity in vivo. This population is characterized by its ability to efflux the Hoechst 33342 DNA dye. SP formation is blocked by incubation with verapamil.32,34 We used flow cytometric analysis of Hoechst staining to investigate SP stem cells. The data show that SP formation is blocked by verapamil incubation and that E14.5 CXCR4–/– and CXCR4+/+ livers contained similar percentages of CD45+ Lin– SP cells (Figure 1C). Collectively, these results indicate that E14.5 CXCR4–/– FL contains similar numbers of HSPCs compared with wild-type FL.
HSPC content in E14.5 CXCR4+/+ and CXCR4–/– livers. (A) CFC number in CXCR4+/+ and CXCR4–/– FL cells. Data represent the mean ± SD number of colonies for each genotype and for 2 dilutions of FL cells (105 and 2 × 105 cells plated per dish) in 3 independent experiments performed in duplicate. Colony numbers are expressed for 105 cells. G indicates granulocyte; GM, granulocyte macrophage; GEMM, granulocyte erythrocyte megakaryocyte macrophage; and BFU-E, erythroid burst-forming unit. (B) The day 12 CFU-S potential of CXCR4+/+ and CXCR4–/– FL determined by injecting 5 × 104 and 105 cells for each genotype per recipient. The data represent the mean ± SD number of colonies scored for each genotype and for control uninjected mice. Results are expressed for 105 cells (n = 3 independent experiments with 10 mice injected per dilution). (C) Representative flow cytometry analysis of hematopoietic stem cells staining using Hoechst 33342 as described by Goodell et al.32 Afterward, CXCR4+/+ and CXCR4–/– FL cells were stained with lineage-specific antibodies and the stem cell markers Sca-1 and c-Kit. The dead cells were previously excluded by 7-AAD staining. The primitive Sca-1+ c-Kit+ and SP cells were analyzed in the CD45+ Lin– gate and their relative percentages are shown.
HSPC content in E14.5 CXCR4+/+ and CXCR4–/– livers. (A) CFC number in CXCR4+/+ and CXCR4–/– FL cells. Data represent the mean ± SD number of colonies for each genotype and for 2 dilutions of FL cells (105 and 2 × 105 cells plated per dish) in 3 independent experiments performed in duplicate. Colony numbers are expressed for 105 cells. G indicates granulocyte; GM, granulocyte macrophage; GEMM, granulocyte erythrocyte megakaryocyte macrophage; and BFU-E, erythroid burst-forming unit. (B) The day 12 CFU-S potential of CXCR4+/+ and CXCR4–/– FL determined by injecting 5 × 104 and 105 cells for each genotype per recipient. The data represent the mean ± SD number of colonies scored for each genotype and for control uninjected mice. Results are expressed for 105 cells (n = 3 independent experiments with 10 mice injected per dilution). (C) Representative flow cytometry analysis of hematopoietic stem cells staining using Hoechst 33342 as described by Goodell et al.32 Afterward, CXCR4+/+ and CXCR4–/– FL cells were stained with lineage-specific antibodies and the stem cell markers Sca-1 and c-Kit. The dead cells were previously excluded by 7-AAD staining. The primitive Sca-1+ c-Kit+ and SP cells were analyzed in the CD45+ Lin– gate and their relative percentages are shown.
Impaired hematopoietic reconstitution by E14.5 CXCR4–/– FL cells
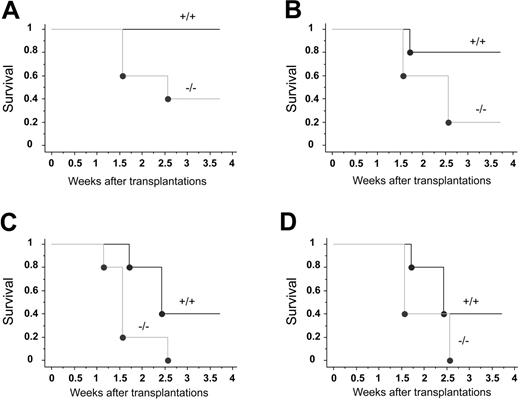
To assess the function of CXCR4 in hematopoiesis, we tested the capacity of E14.5 CXCR4–/– and CXCR4+/+ FL cells to radioprotect lethally irradiated congenic recipient mice. Radioprotection is defined as the ability to rapidly reconstitute an ablated blood-forming system in order to maintain the viability of the host for a limited period of time, generally 30 days in the mouse.35 Lethally irradiated mice were engrafted with increasing numbers of FL cells from each genotype and the hosts' survival was monitored during 4 weeks. At the highest dose injected (5 × 106 cells) and whatever the graft origin, all mice were rescued from irradiation and were alive after 4 weeks (Table 1). However, when mice were injected with 2 × 106 CXCR4–/– FL cells or less, a high rate of mortality was observed (Figure 2; Table 1). This was in sharp contrast with the group of mice engrafted with CXCR4+/+ FL cells (Figure 2A-D; Table 1). The mortality occurred at earlier time points and was more drastic in hosts injected with CXCR4–/– FL cells than in wild types (Figure 2A-D). Based on these limiting-dilution transplantation analyses, the frequency of radioprotective cells can be estimated as 3- to 4-fold lower in CXCR4–/– FL (between 1/2 × 106 and 1/3 × 106 FL cells) than in CXCR4+/+ FL (between 1/0.5 × 106 and 1/106 FL cells) at 4 weeks. These results suggest that CXCR4 is needed for short-term radioprotection. In addition, we performed reconstitution analyses in surviving animals. The chimerism observed with 2 × 106 CXCR4–/– FL cell–injected mice was notably lower compared with CXCR4+/+ cells at both weeks 5 and 16 (Table 2). When 5 × 106 cells were engrafted, the early defective reconstitution ability of CXCR4–/– FL cells was not observed and chimerism levels were similar whether cells originated from CXCR4+/+ or CXCR4–/– donors at 5 weeks (Table 2). In contrast, the chimerism at week 16 was significantly decreased with CXCR4–/– donors even when 5 × 106 cells were infused (Table 2). Altogether, these data indicate that CXCR4–/– FL cells exhibit both an early and a long-term hematopoietic repopulation defect.
Homing analysis of FL hematopoietic progenitor cells
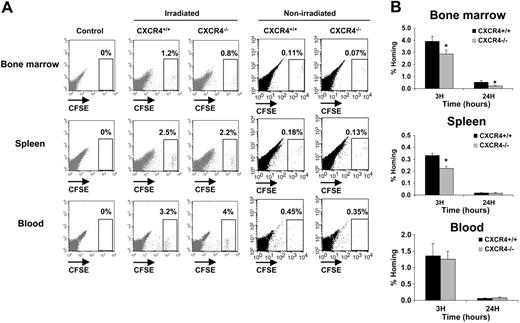
In order to study the homing properties of CXCR4–/– and CXCR4+/+ FL cells, cells were stained with the CSFE dye before being transplanted into lethally irradiated hosts. Homing into BM and spleen was evaluated by flow cytometry at 3 and 24 hours after injection. For the initial studies, 2 × 106 to 107 cells were injected but the number of CFSE+ cells in the bone marrow and spleen was in the order of uninjected negative controls (data not shown). We also examined the distribution of donor cells in vivo following transplantation in nonirradiated animals, but since the frequency of donor cells was typically less than 0.2% in the BM and the spleen, these analyses have been omitted (Figure 3A). These results suggest that FL cells exhibit very low homing efficiency. Similar results were already reported in previous studies.36,37 Based on these data and previous studies showing that the frequency of donor cells in BM and spleen is linearly related to the number of injected cells between 106 and 108 cells,38 20 × 106 to 30 × 106 CFSE-stained cells were injected per animal. In these conditions, a well-individualized CFSE+ cell population could be distinguished in BM, spleen, and blood samples harvested from CXCR4+/+- and CXCR4–/– CFSE–injected mice (Figure 3A). No staining was detected in mice that were not injected (Figure 3A). When the homing percentage of CFSE+ cells was calculated (see “In vivo homing assay”), we observed that both CXCR4+/+ CFSE+ and CXCR4–/– CFSE+ cells homed rapidly within the BM and the spleen (3 hours after injection), but their number was decreased after 24 hours (Figure 3B). However, significantly fewer CXCR4–/– CFSE+ cells were found in the BM at both 3 hours (1.4-fold; P < .01) and 24 hours (2-fold; P < .01). Similarly, the homing of CXCR4–/– cells in the spleen was significantly reduced (1.5-fold; P < .05) at 3 hours compared with CXCR4+/+ cells but was similar at 24 hours (Figure 3B). The levels of CFSE+ cells remaining in the circulation at the same times were not significantly different between CXCR4+/+ and CXCR4–/– (Figure 3B).
Hematopoietic radioprotection deficiency in CXCR4–/– FL cells. Lethally irradiated recipient mice received transplants of 4 limiting dilutions of E14.5 CXCR4+/+ and CXCR4–/– FL cells: 2 × 106 (A), 106 (B), 0.5 × 106 (C), and 0.25 × 106 (D) cells injected per mouse; n = 5 mice per cell dose and per genotype. Recipients were monitored daily during the first 2 weeks and weekly until week 4 after transplantation. Survival data were analyzed using a log-rank nonparametric test and the results are expressed as Kaplan-Meier survival curves.
Hematopoietic radioprotection deficiency in CXCR4–/– FL cells. Lethally irradiated recipient mice received transplants of 4 limiting dilutions of E14.5 CXCR4+/+ and CXCR4–/– FL cells: 2 × 106 (A), 106 (B), 0.5 × 106 (C), and 0.25 × 106 (D) cells injected per mouse; n = 5 mice per cell dose and per genotype. Recipients were monitored daily during the first 2 weeks and weekly until week 4 after transplantation. Survival data were analyzed using a log-rank nonparametric test and the results are expressed as Kaplan-Meier survival curves.
In vivo homing analysis of CXCR4+/+ and CXCR4–/– FL cells. Freshly isolated FL cells were stained with the CFSE dye as described in “In vivo homing assay.” A range of 20 × 106 to 30 × 106 CFSE+ cells for each genotype were injected in lethally syngenic irradiated and nonirradiated mice. Recipients were killed 3 and 24 hours after transplantation and BM, spleen, and blood were harvested and analyzed for the percentage of homed CFSE+ cells by flow cytometry. (A) Representative flow cytometry analysis of BM, spleen, and blood from control uninjected mice, CXCR4+/+ CFSE+–injected mice, and CXCR4–/– CFSE+–injected mice after 3 hours' homing in either irradiated or nonirradiated recipients. CFSE+ cell populations are shown in a gate and their relative numbers are indicated. (B) Histograms showing the percentage of recovered CFSE+ cells per organ (% Homing) in the BM, spleen, and blood 3 and 24 hours after transplantation. The results are calculated based on the formula described in “In vivo homing assay.” The formula is based on the percentage of homed CFSE+ donor cells multiplied by the cellularities of each organ recovered from mice after being lethally irradiated 24 hours before injected by CFSE+ donor cells and divided by the number of CFSE+ donor cells injected per mouse. Data representing mean ± SD of BM, spleen, and blood (n = 24 for each) cellularities (B as mentioned in the formula) were as follows: at 3 hours after injections Bmarrow = 171.8 × 106 ± 20.8 × 106 cells, Bspleen = 4.12 × 106 ± 1.4 × 106 cells, and Bblood = 15.86 × 106 ± 2.15 × 106 cells; and at 24 hours after injections Bmarrow = 30 × 106 ± 10.7 × 106 cells, Bspleen = 1.86 × 106 ± 0.5 × 106 cells, and Bblood = 1.2 × 106 ± 0.3 × 106 cells. Each histogram bar represents the mean ± SD of the percentage of homing obtained in 4 independent experiments (3 mice/genotype/time). *P < .01.
In vivo homing analysis of CXCR4+/+ and CXCR4–/– FL cells. Freshly isolated FL cells were stained with the CFSE dye as described in “In vivo homing assay.” A range of 20 × 106 to 30 × 106 CFSE+ cells for each genotype were injected in lethally syngenic irradiated and nonirradiated mice. Recipients were killed 3 and 24 hours after transplantation and BM, spleen, and blood were harvested and analyzed for the percentage of homed CFSE+ cells by flow cytometry. (A) Representative flow cytometry analysis of BM, spleen, and blood from control uninjected mice, CXCR4+/+ CFSE+–injected mice, and CXCR4–/– CFSE+–injected mice after 3 hours' homing in either irradiated or nonirradiated recipients. CFSE+ cell populations are shown in a gate and their relative numbers are indicated. (B) Histograms showing the percentage of recovered CFSE+ cells per organ (% Homing) in the BM, spleen, and blood 3 and 24 hours after transplantation. The results are calculated based on the formula described in “In vivo homing assay.” The formula is based on the percentage of homed CFSE+ donor cells multiplied by the cellularities of each organ recovered from mice after being lethally irradiated 24 hours before injected by CFSE+ donor cells and divided by the number of CFSE+ donor cells injected per mouse. Data representing mean ± SD of BM, spleen, and blood (n = 24 for each) cellularities (B as mentioned in the formula) were as follows: at 3 hours after injections Bmarrow = 171.8 × 106 ± 20.8 × 106 cells, Bspleen = 4.12 × 106 ± 1.4 × 106 cells, and Bblood = 15.86 × 106 ± 2.15 × 106 cells; and at 24 hours after injections Bmarrow = 30 × 106 ± 10.7 × 106 cells, Bspleen = 1.86 × 106 ± 0.5 × 106 cells, and Bblood = 1.2 × 106 ± 0.3 × 106 cells. Each histogram bar represents the mean ± SD of the percentage of homing obtained in 4 independent experiments (3 mice/genotype/time). *P < .01.
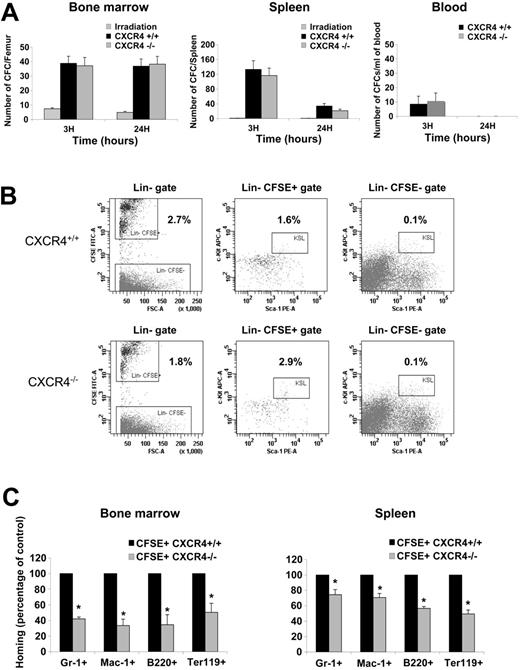
The homing experiments indicated that CXCR4–/– FL cells displayed a reduced homing efficiency. To more specifically investigate the cell types that are affected in CXCR4–/– FL, we first determined the absolute number of CFCs found in BM, spleen, and blood of irradiated hosts after 3 and 24 hours homing. The number of CFCs present in BM and spleen at 3 hours was similar between CXCR4–/– and CXCR4+/+ FL cell–injected animals. At 24 hours, the number of homed CFCs remained constant in the BM but was decreased in the spleen (Figure 4A). In addition, only a small fraction of circulating CFCs was recovered at 3 hours and these cells were not detectable at 24 hours (Figure 4A).
Multiparametric flow cytometry analyses using several cell surface markers were performed on homed CXCR4+/+ CFSE+ and CXCR4–/– CFSE+ FL cells to identify the cell populations that exhibit altered homing. As shown in Figure 4B, the percentage of Lin– CFSE+ mononuclear cells that homed to BM was significantly decreased in CXCR4–/–-injected mice (1.8% ± 0.2%) compared with CXCR4+/+-injected mice (2.7% ± 0.6%). Interestingly, as shown in Figure 4B, in contrast to that observed for the Lin–CFSE––irradiated cells, the percentage of primitive Lin–Sca-1+c-Kit+ within the homed Lin–CFSE+ was significantly higher in CXCR4–/–-injected animals (2.9% ± 0.63%) compared with CXCR4+/+-injected animals (1.6% ± 0.4%). Notably, however, the absolute number of Lin–Sca-1+c-Kit+ CFSE+ cells recovered in the BM was similar (CXCR4–/–, 3480 ± 435; CXCR4+/+, 3272 ± 510). These results suggest that a population within CXCR4-null Lin– cells displays an altered homing to the BM. The nature of this population remains to be characterized. Furthermore, a significant decrease in the homing of CXCR4–/– cells expressing Gr-1 (granulocytes), Mac-1 (granulocytes and monocytes), B220 (B-lymphocytes), and Ter-119 (erythroid cells) was also observed (Figure 4C). Overall, these results indicate that CXCR4 function is necessary for the homing of mature cells to both BM and spleen although it is dispensable for HSPCs homing to both these organs.
Analysis of homed CFSE+ cells. (A) BM, spleen, and blood were harvested after 3 and 24 hours homing and assayed for the CFC potential in a semisolid medium. The homing of hematopoietic progenitors was assayed by histogram profiles showing either the absolute number of CFCs recovered from one femur, the absolute number of CFCs recovered per spleen, or the number of CFCs obtained per mL of blood harvested from irradiated uninjected mice, CXCR4+/+ CFSE+–injected mice, and CXCR4–/– CFSE+–injected mice. BM and spleen histogram bars represent the mean ± SD of 4 independent experiments (3 mice/genotype/time), whereas blood histogram bar represents the mean ± SD of 2 independent experiments (3 mice/genotype/time). (B) Representative multiparametric flow cytometry analysis of CXCR4+/+ CFSE+–and CXCR4–/– CFSE+–homed cells in the BM after 3 hours homing. Cells have been first gated in a large morphologic gate including lymphocytes, monocytes, and granulocytes then viable cells (excluding 7AAD dye) were analyzed for lineage markers staining in order to draw a Lin– gate. The percentage of Lin– cells was similar between CXCR4+/+- and CXCR4–/–-injected animals and represented about 10% of viable cells. After that, Lin– gate served for the further analysis as shown in the figure. Percentages are shown and represented means of 4 independent experiments. The differences observed for Lin–CFSE+ and Lin–Sca-1+c-Kit+CFSE+ cell populations between CXCR4+/+- and CXCR4–/–-injected mice were statistically significant (P = .019 and P = .006, respectively). (C) BM and spleen were harvested after 3 hours' homing, and multiparametric flow cytometry analyses were performed in order to study CXCR4–/– populations that exhibited homing deficiency. Results are represented as percentage of control homed CXCR4+/+ cells in each organ of 2 independent experiments. *P < .01.
Analysis of homed CFSE+ cells. (A) BM, spleen, and blood were harvested after 3 and 24 hours homing and assayed for the CFC potential in a semisolid medium. The homing of hematopoietic progenitors was assayed by histogram profiles showing either the absolute number of CFCs recovered from one femur, the absolute number of CFCs recovered per spleen, or the number of CFCs obtained per mL of blood harvested from irradiated uninjected mice, CXCR4+/+ CFSE+–injected mice, and CXCR4–/– CFSE+–injected mice. BM and spleen histogram bars represent the mean ± SD of 4 independent experiments (3 mice/genotype/time), whereas blood histogram bar represents the mean ± SD of 2 independent experiments (3 mice/genotype/time). (B) Representative multiparametric flow cytometry analysis of CXCR4+/+ CFSE+–and CXCR4–/– CFSE+–homed cells in the BM after 3 hours homing. Cells have been first gated in a large morphologic gate including lymphocytes, monocytes, and granulocytes then viable cells (excluding 7AAD dye) were analyzed for lineage markers staining in order to draw a Lin– gate. The percentage of Lin– cells was similar between CXCR4+/+- and CXCR4–/–-injected animals and represented about 10% of viable cells. After that, Lin– gate served for the further analysis as shown in the figure. Percentages are shown and represented means of 4 independent experiments. The differences observed for Lin–CFSE+ and Lin–Sca-1+c-Kit+CFSE+ cell populations between CXCR4+/+- and CXCR4–/–-injected mice were statistically significant (P = .019 and P = .006, respectively). (C) BM and spleen were harvested after 3 hours' homing, and multiparametric flow cytometry analyses were performed in order to study CXCR4–/– populations that exhibited homing deficiency. Results are represented as percentage of control homed CXCR4+/+ cells in each organ of 2 independent experiments. *P < .01.
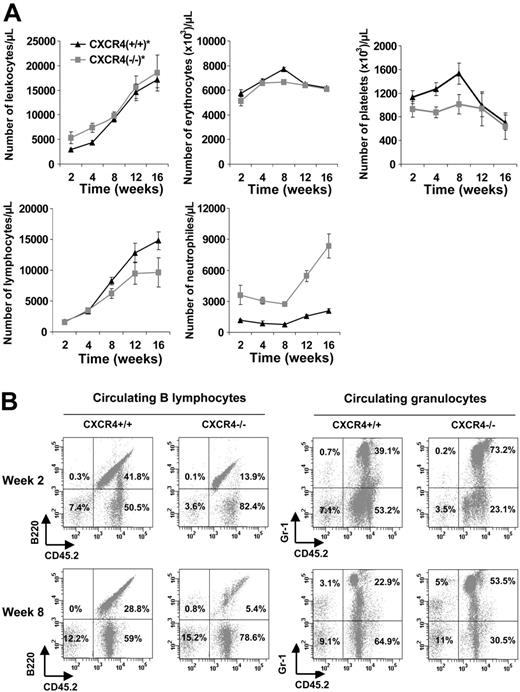
Analysis of the hematopoietic cells in the blood of CXCR4–/– chimeras
Although we cannot exclude the homing defect in more primitive HSCs, this is unlikely because homing of CFCs and primitive Lin–Sca-1+c-Kit+ cells was normal. An alternative explanation of the radioprotection defect of CXCR4–/– FL cells was a retention defect in the bone marrow microenvironment. In order to better characterize the hematopoietic defect observed in CXCR4–/– chimeric mice, we analyzed the peripheral blood reconstitution in CXCR4+/+ and CXCR4–/– chimeras that received transplants of 5 × 106 cells, to avoid selection of the mice on their survival. No significant difference was observed on the total number of either circulating leukocytes or circulating erythrocytes until week 16 (Figure 5A). Interestingly, CXCR4+/+ chimeric mice exhibited an early thrombocytosis (1.5- to 2-fold the platelet count of CXCR4–/–-engrafted mice) before week 12 that was not observed in CXCR4–/– chimeric mice and then the count returned to normal. Flow cytometry analysis of blood cells from CXCR4+/+ and CXCR4–/– chimeras revealed a lesser proportion of donor-derived B220-positive cells in CXCR4–/– chimeras compared with wild types (Figure 5A-B). In addition, the number of donor-derived Gr-1–positive cells was increased in CXCR4–/– chimeras (Figure 5A-B). Similar results were reported in other studies,18-20 highlighting the role of CXCR4 receptor in B lymphopoiesis and in the retention of maturing granulocytes.
Presence of HSPC in the blood of CXCR4–/– chimeras
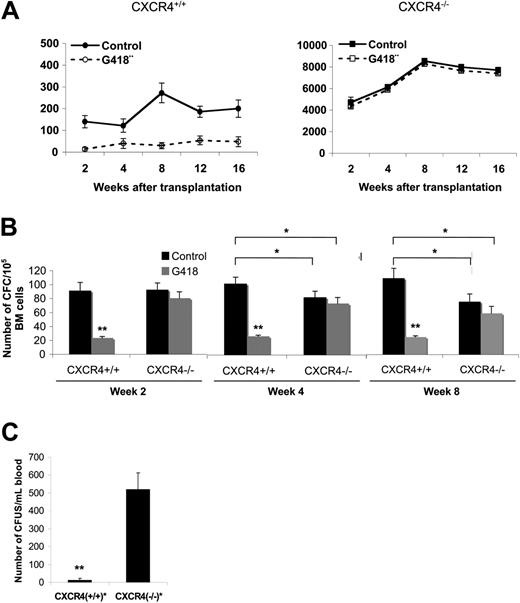
We also analyzed whether CFCs were circulating in CXCR4–/– chimeras engrafted with 5 × 106 FL cells. The CFC potential of CXCR4–/– blood cells in semisolid medium at different time points after transplantation was measured. As shown in Figure 6A, blood cells from CXCR4–/– mice contained a high number of CFCs compared with CXCR4+/+ chimeras (P < .001). This number increased between weeks 2 to 8 and then remained stable. As CXCR4–/– FL cells carry a neomycin insertion cassette, we also added 600 μg/mL G418 to the semisolid culture medium to validate the origin of circulating CFCs. Addition of G418 did not significantly modify the growth of CFCs from CXCR4–/– blood cells, whereas it induced a significant decrease in the number of colonies from CXCR4+/+ blood cells (Figure 6A). In parallel, the BM progenitor cell content was determined at weeks 2, 4, and 8 after transplantation. As shown in Figure 6B, the number of donor-derived BM CFCs was similar for CXCR4+/+ and CXCR4–/– chimeras at 2 weeks but was slightly decreased in CXCR4-null mice from 4 weeks (Figure 6B). No difference in the progenitor content was observed between CXCR4–/– and CXCR4+/+ chimeras' spleen (data not shown). In addition, extramedullary hematopoiesis was not detected in the liver, lung, brain, muscle, or heart of CXCR4–/– chimeras (data not shown). In some experiments, we also analyzed the number of CFUs-S in the circulation of CXCR4+/+ and CXCR4–/– chimeras by injecting blood cells into secondary lethally irradiated hosts. We observed that blood cells from CXCR4–/– chimeras contained a 40-fold (P < .001) enrichment in CFUs-S compared with wild types (Figure 6C). We also phenotypically characterized these circulating CXCR4-null progenitor cells using flow cytometry analysis. All blood samples from CXCR4–/– chimeras revealed the presence of committed Lin–Sca-1–c-Kit+ and primitive Lin–Sca-1+c-Kit+ progenitors (10% and 6% of CD45.2+ Lin––gated cells, respectively), whereas a similar circulating population was almost absent in blood from CXCR4+/+ chimeras (0.6% and 1.3% of CD45.2+ Lin––gated cells, respectively; Figure 7A).
Hematology parameters of mice reconstituted with CXCR4+/+ and CXCR4–/– FL cells. Lethally irradiated C57BL/6-Ly5.1 mice received transplants of 5 × 106 cells per mouse. CXCR4+/+ and CXCR4–/– mice displayed similar levels of peripheral chimerisms. (A) Blood samples were analyzed from 2 to 16 weeks after transplantation for the hematology parameters using an MS9 Counter. Each point represents the mean ± SD of absolute numbers scored from mice that received CXCR4+/+ transplants (n = 6; ▴, CXCR4+/+) and mice that received CXCR4–/– transplants (n = 5; ▦, CXCR4–/–). (B) Representative flow cytometry analysis of peripheral blood leukocytes in CXCR4+/+ and CXCR4–/– mice 2 and 8 weeks after transplantation. Cells were dually labeled with either mAb Gr-1 to detect granulocytic cells or mAb B220 to detect B lymphocytes and anti-CD45.2 to detect donor-derived cells. The same animals were followed at 2 and 8 weeks after transplantation. The percentages are calculated based on total viable blood cells.
Hematology parameters of mice reconstituted with CXCR4+/+ and CXCR4–/– FL cells. Lethally irradiated C57BL/6-Ly5.1 mice received transplants of 5 × 106 cells per mouse. CXCR4+/+ and CXCR4–/– mice displayed similar levels of peripheral chimerisms. (A) Blood samples were analyzed from 2 to 16 weeks after transplantation for the hematology parameters using an MS9 Counter. Each point represents the mean ± SD of absolute numbers scored from mice that received CXCR4+/+ transplants (n = 6; ▴, CXCR4+/+) and mice that received CXCR4–/– transplants (n = 5; ▦, CXCR4–/–). (B) Representative flow cytometry analysis of peripheral blood leukocytes in CXCR4+/+ and CXCR4–/– mice 2 and 8 weeks after transplantation. Cells were dually labeled with either mAb Gr-1 to detect granulocytic cells or mAb B220 to detect B lymphocytes and anti-CD45.2 to detect donor-derived cells. The same animals were followed at 2 and 8 weeks after transplantation. The percentages are calculated based on total viable blood cells.
Finally, we performed secondary transplantation experiments into lethally irradiated CD45.1 mice using blood cells. Secondary irradiated mice were injected with a mixture of either 100 μL CXCR4+/+ or 100 μL CXCR4–/– blood and 1.5 × 105 nonfractionated BM CD45.1+ cells. We observed a significant percentage of CD45.2+ cells in the periphery of CXCR4–/– blood–injected mice (6.4% ± 3.1% of circulating donor-derived CD45.2+ cells) in contrast to mice injected with blood cells from CXCR4+/+ donors (0.7% ± 0.6% of circulating donor-derived CD45.2+ cells) at 5 weeks after transplantation (Figure 7B-C).
Altogether, these results suggest that CXCR4-null FL cells have a defect in retention of both maturing cells and primitive stem cells. This reduced retention may be responsible for both short-term and long-term altered reconstitution of CXCR4–/– chimeras.
Discussion
Engraftment and successful hematopoietic reconstitution by transplanted HSPCs depends on 3 important functions. First, transplanted cells must home to appropriate niches in the BM microenvironment.39 Second, HSPCs must settle in these niches. Third, HSPCs must proliferate and differentiate along the different hematopoietic lineages.40 CXCR4, the receptor for SDF-1, plays an important role in hematopoiesis.8,15,41 The SDF-1/CXCR4 axis seems to play a dual role in hematopoiesis by regulating the homing and the retention of hematopoietic cells within the BM microenvironment. Phenotypic and functional analysis of CXCR4–/– or SDF-1–/– embryos revealed abnormalities in B lymphopoiesis and BM myelopoiesis during embryogenesis.4-7 In addition, adoptive transfer experiments have revealed a deficiency in long-term lymphoid and myeloid reconstitution of adult BM by CXCR4–/– FL cells, although the mechanisms involved in these defects were not clearly identified.18-20
Hematopoietic progenitor cell mobilization in unperturbed CXCR4–/– chimeras. Animals engrafted with 5 × 106 FL cells were analyzed between 2 and 16 weeks after transplantation. (A) CFC potential in the peripheral blood of CXCR4+/+ and CXCR4–/– chimeras from 2 to 16 weeks after transplantation. Red cells were lysed and blood cells were plated in semisolid medium for CFC assay in standard conditions (control, solid line) or in the presence of 600 μg/mL G418 (G418, broken line) in duplicate. Data represent the mean ± SD number of colonies scored for CXCR4+/+ (n = 6) and CXCR4–/– (n = 5) blood samples in 3 independent experiments performed in duplicate. (B) CFC potential in the bone marrow of CXCR4+/+ and CXCR4–/– chimeras from 2 to 8 weeks after transplantation. BM cells were plated in semisolid medium for CFC assay in standard conditions (control, ▪) or in the presence of 600 μg/mL G418 (G418, ▦) in duplicate. Data represent the mean ± SD number of colonies scored for CXCR4+/+ (n = 5) and CXCR4–/– (n = 5) BM samples in 2 independent experiments performed in duplicate. *P < .01; **P < .001. (C) Number of CFUs-S in the peripheral blood of CXCR4+/+ and CXCR4+/+ chimeras by injections of 20 μL total blood for each genotype in syngeneic lethally irradiated mice (10 mice injected per blood donor). Data represent the mean ± SD number of colonies of scored CFUs-S in 2 independent experiments. **P < .001.
Hematopoietic progenitor cell mobilization in unperturbed CXCR4–/– chimeras. Animals engrafted with 5 × 106 FL cells were analyzed between 2 and 16 weeks after transplantation. (A) CFC potential in the peripheral blood of CXCR4+/+ and CXCR4–/– chimeras from 2 to 16 weeks after transplantation. Red cells were lysed and blood cells were plated in semisolid medium for CFC assay in standard conditions (control, solid line) or in the presence of 600 μg/mL G418 (G418, broken line) in duplicate. Data represent the mean ± SD number of colonies scored for CXCR4+/+ (n = 6) and CXCR4–/– (n = 5) blood samples in 3 independent experiments performed in duplicate. (B) CFC potential in the bone marrow of CXCR4+/+ and CXCR4–/– chimeras from 2 to 8 weeks after transplantation. BM cells were plated in semisolid medium for CFC assay in standard conditions (control, ▪) or in the presence of 600 μg/mL G418 (G418, ▦) in duplicate. Data represent the mean ± SD number of colonies scored for CXCR4+/+ (n = 5) and CXCR4–/– (n = 5) BM samples in 2 independent experiments performed in duplicate. *P < .01; **P < .001. (C) Number of CFUs-S in the peripheral blood of CXCR4+/+ and CXCR4+/+ chimeras by injections of 20 μL total blood for each genotype in syngeneic lethally irradiated mice (10 mice injected per blood donor). Data represent the mean ± SD number of colonies of scored CFUs-S in 2 independent experiments. **P < .001.
Circulation of short-term hematopoietic reconstituting cells in CXCR4–/– chimeras. Week-8 posttransplantation animals that were engrafted with 5 × 106 FL cells were used. (A) Blood cells were stained with anti-CD45.2 mAb to identify donor-derived cells (chimerism), a cocktail of specific lineage mAbs (Lin), 7-AAD for cell viability, and the stem cell markers Sca-1 and c-Kit. Two different gates are shown in the figure: one represented CD45.2+ donor–derived cells and the other represented CD45.2+ Lin– populations. Arrows indicate that the Sca-1/c-Kit profiles were analyzed within the CD45.2+ Lin– gates. Data are representative profiles obtained for each CXCR4+/+ (n = 6) and CXCR4–/– (n = 5) chimera. (B) Peripheral blood was harvested from CXCR4+/+ and CXCR4–/– chimeras from the retro-orbital sinus. One hundred microliters of whole blood sample was mixed with 1.5 × 105 Ly5.1 host–derived BM cells and transplanted in lethally irradiated C57BL/6-Ly5.1 mice (500 μL blood volume from either CXCR4+/+ or CXCR4–/– mice was used to engraft 5 mice per genotype). The recipients were analyzed 5 weeks after transplantation. Representative FACS profiles assayed from both groups are shown. Blood was stained with anti-CD45.2 to identify donor-derived cells. (C) These results are summarized as histogram profiles showing the mean ± SD of percentages of circulating CD45.2+ cells in 2 independent experiments (5 mice per genotype and per experiment). *P < .01.
Circulation of short-term hematopoietic reconstituting cells in CXCR4–/– chimeras. Week-8 posttransplantation animals that were engrafted with 5 × 106 FL cells were used. (A) Blood cells were stained with anti-CD45.2 mAb to identify donor-derived cells (chimerism), a cocktail of specific lineage mAbs (Lin), 7-AAD for cell viability, and the stem cell markers Sca-1 and c-Kit. Two different gates are shown in the figure: one represented CD45.2+ donor–derived cells and the other represented CD45.2+ Lin– populations. Arrows indicate that the Sca-1/c-Kit profiles were analyzed within the CD45.2+ Lin– gates. Data are representative profiles obtained for each CXCR4+/+ (n = 6) and CXCR4–/– (n = 5) chimera. (B) Peripheral blood was harvested from CXCR4+/+ and CXCR4–/– chimeras from the retro-orbital sinus. One hundred microliters of whole blood sample was mixed with 1.5 × 105 Ly5.1 host–derived BM cells and transplanted in lethally irradiated C57BL/6-Ly5.1 mice (500 μL blood volume from either CXCR4+/+ or CXCR4–/– mice was used to engraft 5 mice per genotype). The recipients were analyzed 5 weeks after transplantation. Representative FACS profiles assayed from both groups are shown. Blood was stained with anti-CD45.2 to identify donor-derived cells. (C) These results are summarized as histogram profiles showing the mean ± SD of percentages of circulating CD45.2+ cells in 2 independent experiments (5 mice per genotype and per experiment). *P < .01.
The aim of this study is to further investigate the role of CXCR4 during hematopoiesis. We examined the repopulating potential of FL cells from CXCR4–/– and CXCR4+/+ E14.5 embryos in a syngeneic transplantation model and we investigated the trafficking of hematopoietic progenitors after their engraftment in lethally irradiated recipient mice. The data show that CXCR4–/– FL cells display reduced radioprotection capacities. One possible explanation is that the content in HSPCs with short-term and long-term reconstituting activity was decreased in CXCR4–/– FL. However, our results indicate that the numbers of CFCs, CFUs-S, and Lin–Sca-1+c-kit+ cells contained in CXCR4–/– FL were similar to CXCR4+/+ FL. Similarly, it has been described that the content in HSPCs with long-term reconstituting activity was normal or only slightly decreased in SDF-1–/– FL.42 In addition, reintroduction of CXCR4 in CXCR4–/– FL cells allows normal survival of engrafted mice (data not shown). Thus the absence of CXCR4 expression does not seems to quantitatively alter the HSPC compartment in the embryo. As CXCR4 plays a major role in both homing and retention of hematopoietic cells, this defect may be due to abnormal homing or retention of hematopoietic cells within the BM microenvironment. In agreement with this hypothesis, we observed that all maturing lineages (B lymphocytes, granulocytes, monocytes, and erythroid cells) are less represented in the BM and spleen of CXCR4–/– FL-injected mice.
Previous studies have shown that deficiencies of red blood cells, platelets, or both are responsible for mortality from hematopoietic failure following total body irradiation in mice. Interestingly, it has been shown that committed myeloerythroid progenitors (MEPs) and common myeloid progenitors (CMPs) are responsible for radioprotection by providing immediate precursors of erythrocytes and platelets.35 These MEP and CMP cells have been phenotypically defined as Lin–IL-7Rα–Sca-1– c-Kit+FcγRlow CD34– and as Lin–IL-7Rα–Sca-1–c-Kit+FcγRlow CD34+, respectively, and showed dose-dependent rescue of irradiated mice probably due to their lack of self-renewal capacity and relatively short life spans.35 Interestingly, we observed that in contrast to primitive Lin–Sca-1+c-Kit+ HSPCs, a population within CXCR4-null Lin– cells displays an altered homing to the BM. Further experiments are needed to determine whether CXCR4-null MEP and CMP cells display a reduced homing. Therefore, our results showing that when high numbers of cells were engrafted, mice were totally radioprotected and survived with a hematopoiesis predominantly of donor origin, may be explained by a defect in homing or retention of these precursors.
In contrast to maturing cells, we could not detect any defect in the early homing of committed progenitors in BM and spleen. We also observed normal homing of CXCR4-null primitive Lin–Sca-1+c-kit+ cells to the BM, suggesting that HSPCs exhibit normal homing in the absence of CXCR4 receptor. However, even if HSPCs have been highly enriched in Lin–Sca-1+c-kit+ cell fraction, only 20% of intravenously injected cells gave long-term multilineage reconstitution. Alternatively, the role of CXCR4 on CFC homing to the BM could be undervalued in our results because we performed total body irradiation (TBI) for host conditioning. Indeed, it has been shown that TBI was able to induce expression of several proteins including homing receptor,43 V-CAM-1,44 SDF-1,45 or matrix metalloproteinase-9,46 permitting large-scale entry of transplanted hematopoietic cells into BM compartments thus explaining the less efficient engraftment of hematopoietic cells in nonablated recipients.47,48 However, it has recently been shown that the ability of BM cells to home to the BM microenvironment was not different regardless of whether the cells were transplanted into ablated or nonablated recipients.49,50 Therefore, we cannot exclude that long-term reconstituting cells display a reduced homing in steady-state conditions. In accordance with this, previous studies have documented a role of CXCR4 for long-term repopulating activities in NOD-SCID mice.9,17,51,52 At a first glance, these findings seem to contradict previous studies that have documented a role of CXCR4 for HSC homing in NOD-SCID mice.9,17,51,52 These studies warrant further investigation of the fate and homing patterns of transplanted HSPCs in nonmyeloablated or minimally myeloablated recipients.
Our results are consistent with a dispensable role of CXCR4 receptor on HSPCs homing in irradiated animals. These data are in line with those previously reported29,31,52 that demonstrated that inhibition of Gαi-coupled chemokine receptor by PT does not induce an altered homing to the BM. However, more recent studies are controversial indicating that PT also inhibited the homing of HSPCs to the BM microenvironment.50 These results suggest that another Gαi-protein–coupled receptor may be implicated in HPC homing to the BM. Recently, the orphan receptor RDC1 was reported to bind and mediate SDF-1 signaling in T lymphocytes.53 Alternatively to a role in mediating the homing, SDF-1/CXCR4 interactions are involved in the retention of hematopoietic cells within the BM microenvironment. It was previously reported that mice reconstituted with CXCR4–/– FL had a high number of mature granulocytes and precursors in the peripheral blood.19,20 Interestingly, our results describe the presence of a high number of CFCs (30-fold increase), CFUs-S (40-fold increase), and Lin–Sca-++c-kit+ cells in the peripheral blood of mice stably engrafted with CXCR4–/– FL cells. In addition, 100 μL blood from CXCR4–/– chimeras contained enough HSPCs to give rise to a significant level of chimerism in all engrafted recipients at week 5. In SDF-1–/– embryos, an increase in circulating HSPCs has also been observed.42 Indeed, a recent study demonstrated that SDF-1–expressing stromal cells are involved in the attachment of the earliest B-lymphocyte precursors, pre–pro-B cells, in cellular niches within BM microenvironment, implying that the SDF-1/CXCR4 axis is crucial in their anchorage in cellular niches.54 Moreover, this lower retention ability of CXCR4–/– HSPCs in the BM is in agreement with the mechanisms that are involved in the mobilization of hematopoietic progenitors by cytokines and/or chemotherapy.25,26 A low level of CXCR4 expression is observed on mobilized CD34+ cells, due to its cleavage by different proteases.26,55 Similarly, disruption of the CXCR4/SDF-1 interactions is associated with CD34+ cell mobilization.26,30 Our results strongly support that the SDF-1/CXCR4 predominant role concerns HSPC mobilization. Particularly, SDF-1/CXCR4 seemed to be implied at the first steps of the mechanisms involved in the circulation of HSPCs, being that the number of circulating CFCs noticed in CXCR4–/– chimeric mice was always more important than in wild-type mice treated by a combination of G-CSF and cyclophosphamide (data not shown).
The abnormal retention of hematopoietic progenitors and of granulocyte precursors within the BM, which already occurred at 2 weeks after transplantation, may have a considerable negative impact on hematopoietic reconstitution, especially by slowing hematopoietic recovery. Indeed, in the absence of CXCR4, 2 major steps would be impaired: first, the attachment and the contact with extracellular matrix proteins that are known to be regulated by CXCR4/SDF-1 signaling, and second, proliferation and differentiation mediated by microenvironment components. By the way, a recent study has indicated that SDF-1 is an important megakaryocytic-active chemokine and has shown that CXCR4 receptor can promote survival, maturation, and platelet release into vascular niches.56 Our results are in line with these data and those of Hodohara et al,57 since mice engrafted with CXCR4–/– cells exhibited a milder platelet recovery than wild-type chimeras.
In this study, we observed that mice, which have a marked short-term reconstitution, keep a high level of chimerism at week 16 after transplantation, suggesting that the short-term and long-term reconstitution activities were parallely altered by the absence of CXCR4 expression. Thus, it seems possible that a retention defect also plays an important role in the long-term hematopoietic reconstitution defect of CXCR4–/– FL cells. Indeed, determination of long-term reconstitution is only based on transplantation assays that are dependent on homing, retention, and proliferation. Thus, in case of a cell autonomous defect as for CXCR4–/– HSPCs, determination of a long-term reconstitution defect would be extremely difficult and could be biased.
Altogether, our results demonstrate that the defect in hematopoietic reconstitution of CXCR4–/– FL cells is more related to an altered anchorage in the BM than a real defect in the homing of primitive hematopoietic cells. This further underlines the role of CXCR4 and SDF-1 in the circulation and mobilization of hematopoietic precursors, progenitors, and stem cells. These studies provide an experimental model suitable for further investigation aiming to pinpoint the role of CXCR4 in hematopoiesis.
Prepublished online as Blood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2005-02-0581.
Supported by grants from INSERM, the Association de la Recherche contre le Cancer (ARC; grant no. 4309 [F.L.]), the Gustave Roussy Institute (IGR; grant CRI-SPS-2003-02 [F.L.]), and the French ministry of research (ACI). A.F. and Y.Z. were supported by grants from the French Society of Hematology (SFH) and the Association de la Recherche contre le Cancer.
A.F. designed and performed experiments, analyzed data, and wrote the paper; P.J. and Y.Z. designed and performed experiments; M.W. and J.-F.G. performed experiments; Y.L. assisted in flow cytometry experiments; T.N. provided CXCR4+/– mice and helped in analyzing data; W.V. assisted in writing the paper; and F.L. designed experiments, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Françoise Wendling for discussion and critical reading of the manuscript. We thank Patrice Ardouin and Annie Rouchès (SCEA IGR, Villejuif) for the in vivo experiments. We also thank Frederic Larbret (IFR54, Villejuif) for FACS technical assistance.