Abstract
Heparin-induced thrombocytopenia (HIT) antibodies recognize complexes between heparin and platelet factor 4 (PF4). Heparin and PF4 bind HIT antibodies only over a narrow molar ratio. We explored the involvement of platelet surface–bound PF4 as an antigen in the pathogenesis of experimental HIT. We show that cell-surface PF4 complexes are also antigenic only over a restricted concentration range of PF4. Heparin is not required for HIT antibody binding but shifts the concentration of PF4 needed for optimal surface antigenicity to higher levels. These data are supported by in vitro studies involving both human and murine platelets with exogenous recombinant human (h) PF4 and either an anti–PF4-heparin monoclonal antibody (KKO) or HIT immunoglobulin. Injection of KKO into transgenic mice expressing different levels of hPF4 demonstrates a correlation between the severity of the thrombocytopenia and platelet hPF4 expression. Therapeutic interventions in this model using high-dose heparin or protamine sulfate support the pathogenic role of surface PF4 antigenic complexes in the etiology of HIT. We believe that this focus on surface PF4 advances our understanding of the pathogenesis of HIT, suggests ways to identify patients at high risk to develop HIT upon heparin exposure, and offers new therapeutic strategies.
Introduction
Heparin-induced thrombocytopenia (HIT) is an iatrogenic complication of heparin therapy caused by antibodies that recognize complexes formed between heparin and the endogenous protein platelet factor 4 (PF4).1-3 Approximately half of affected patients develop limb- or life-threatening thrombosis.4-6 Management involves careful monitoring of platelet counts, a high index of clinical suspicion, cessation of heparin exposure, and the introduction of alternative anticoagulants.7,8 These measures have reduced the incidence of new thromboembolic complications but have had less impact on the incidence of amputations and death.9,10 Heparin remains an important anticoagulant in widespread use, and studies that help define the pathophysiology of HIT may lead to better identification of patients at risk and to more targeted intervention strategies.
The antibody response in HIT is unusual in several respects. First, the major complications of HIT are related to thrombosis in contrast to other drug-induced thrombocytopenias.11 This high incidence of thrombosis may be related in part to the ability of HIT antibodies to activate platelets via FcγRIIA.12,13 In a murine model of HIT, only mice with platelets that expressed both human (h) PF4 and FcγRIIA developed thrombocytopenia and thrombosis when given an anti–PF4-heparin monoclonal antibody (mAb), KKO.14 A second unusual feature is the surprisingly high incidence of anti–PF4-heparin antibodies in heparinized patients, exceeding a quarter to half of all exposed patients in some settings.15-17 Why only a small portion of these patients develop HIT is not clear and no unequivocal differences between the vast majority of individuals who remain asymptomatic and the small number who develop HIT have been identified, although differences in immunoglobulin G (IgG) titers have been noted.18-21 A third characteristic of HIT antibodies (including KKO) is that they bind optimally to PF4-heparin complexes over a narrow molar ratio in vitro.1-3,22 In the case of unfractionated heparin, PF4 forms ultralarge complexes (ULCs) of larger than 670 kDa at these same molar ratios.23 These ULCs are stable, are particularly antigenic, bind multiple IgG antibodies per complex, and promote platelet activation.
It is not known whether similar complexes between PF4 and cell-surface glycosaminoglycans (GAGs) form on the surface of platelets or how heparin affects surface complex formation and antigenicity. Based on the knowledge that PF4 can bind to diverse anionic polysaccharides,24 PF4 may form similar antigenic complexes on platelets by binding to GAGs on the surface of platelets independent of heparin. The composition of these antigenic complexes and their capacity to be modulated has not been studied. We examined the effect of the anti-PF4/heparin mAb KKO (and in some studies, HIT IgG) on platelets expressing varied amounts of endogenous or exogenous PF4 on their surface both in vitro and in vivo. The results of these studies provide insight into the importance of the level of surface PF4 expression, the effect of heparin on formation of surface antigenic complexes, and potential new diagnostic and therapeutic approaches to HIT based on these new insights.
Patients, materials, and methods
Preparation of recombinant WT hPF4
Wild-type (WT) hPF4 in pT7-7 plasmid was expressed in BL21DE30 pLysS bacteria, purified, and characterized as described.25 Recombinant protein was isolated from bacterial lysate supernatant by affinity chromatography using a HiTrap Heparin HP column (Amersham Bioscience, Upsala, Sweden). Proteins were purified further by fast protein liquid chromatography (FPLC) using a RESOURCE RPC column (Amersham Bioscience). Protein purity was assessed by 15% (wt/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining.26 Samples were subjected to immunoblotting after electrotransfer to polyvinylidenedifluoride (PVDF) membranes using rabbit anti-hPF4 polyclonal antibody (PeproTech, Rocky Hill, NJ), followed by donkey anti–rabbit secondary antibody conjugated to horseradish peroxidase (HRP; Jackson ImmunoResearch Laboratories, West Grove, PA) and were developed using the enhanced chemiluminescence (ECL) kit (PerkinElmer Life Sciences, Boston, MA). Total protein concentrations were determined using the bicinchoninic acid assay (Pierce, Rockford, IL) as per manufacturer with BSA as standard.
Monoclonal antibodies and HIT immunoglobulins
KKO, the anti-hPF4–specific mAb RTO, and isoimmune control TRA are all mouse IgG2b mAbs.27 Antibodies were fluorescein isothiocyanate (FITC) labeled using an E-Z FITC labeling kit (Pierce) as per the manufacturer. Monoclonal antihuman CD41a PerCP-Cy5 and antimouse CD41 phycoerythrin (PE) antibodies and annexin V–PE were from Pharmingen (San Diego, CA). Polyclonal IgG was isolated from the plasma of 4 patients with clinical HIT7,8,11,28 and a positive HIT enzyme-linked immunosorbent assay (ELISA)3 and from 4 healthy subjects with recombinant protein G–agarose (Invitrogen, Carlsbad, CA), as per the manufacturer. A commercial human IgG preparation (Pierce) was used as an additional control. HIT IgG reactivity with PF4-heparin complexes was confirmed by ELISA.3
Platelet preparation and analyses
Studies were performed using human platelets or platelets from WT and transgenic mice (see below for the description of the mice). Human blood was collected in acid citrate dextrose (ACD; pH 4.5, 10:1 vol/vol) after informed consent from healthy, aspirin-free volunteers under a protocol approved by the Institutional Review Board for Studies involving Human Subjects of the Children's Hospital of Philadelphia. All studies involving mice were approved by the same institution's Institute Animal Care and Use Committee. Blood was centrifuged at 200g for 15 minutes at room temperature (RT) to generate platelet-rich plasma (PRP). PGE1 (final concentration 1 μg/mL; Sigma, St Louis, MO) was added to the PRP to prevent spontaneous platelet activation. PRP was centrifuged at 800g for 10 minutes at RT, and the pellet was washed and resuspended in modified Tyrode buffer (134 mM NaCl, 3 mM KCl, 0.3 mM NaH2PO4, 2 mM MgCl2, 5 mM HEPES, 5 mM glucose, 12 mM NaHCO3, 0.1% BSA; Sigma A7030, fatty acid–free). Mouse PRP and washed platelets were prepared using blood collected from the inferior vena cava in ACD (1:5 vol/vol), immediately diluted 1:3 (vol/vol) in modified Tyrode buffer containing PGE1 (final concentration, 1 μg/mL), and centrifuged at 200g for 4 minutes at RT. PRP was centrifuged at 800g for 10 minutes at RT, and the pellet was washed and resuspended in modified Tyrode buffer. Both human and murine washed platelets were used at 108/mL.
Washed platelets were incubated with varying amounts (0-80 μg) of recombinant hPF4 in a final volume of 100 μL for 45 minutes at RT. In some experiments, increasing amounts of unfractionated heparin (0-40 μg, porcine intestinal mucosa; Sigma) were added to the platelets before the PF4. KKO or another antibody (50 μg) under study was then added to each sample for an additional 15 minutes. Samples were then diluted with Tyrode buffer and enumerated immediately or fixed in 1% paraformaldehyde in PBS (1:10 vol/vol).
Total immunodetectable platelet hPF4 was determined using murine platelets completely deficient in murine PF4 (mPF4null).29 PF4 was cross-linked on the platelet surface by adding 1% paraformaldehyde (1:10 vol/vol) overnight at 4°C. Samples were washed with PBS, and platelets were lysed in NuPage LDS Sample Buffer (Invitrogen). Fractions were separated on a 10% SDS-PAGE gel and immunoblotted after electrotransfer to a PVDF membrane with a rabbit anti–human PF4 (1:5000) primary antibody (PeproTech) followed by an HRP-conjugated donkey anti–rabbit antibody (Jackson ImmunoResearch Laboratories) and developed using an ECL kit. Autoradiographic bands over the linear range of exposure were analyzed on a UMAX Vista-58 scanner (Hsinchu, Taiwan), and the data were analyzed by developing a histogram of the calculated density using the National Institutes of Health (NIH) program ImageJ (http://rsb.info.nih.gov). Total surface PF4 binding was also detected using mPF4null platelets after paraformaldehyde cross-linking, as described above. Samples were then washed 3 times with Tyrode buffer and incubated with polyclonal rabbit anti-hPF4 antibody and followed by FITC-conjugated goat anti–rabbit IgG. Binding of KKO was performed by indirect immunofluorescence as a control. Samples were incubated with unlabeled KKO after a 1-hour incubation with PF4, then fixed with 1% paraformaldehyde overnight at 4°C, washed 3 times with Tyrode buffer, and stained with FITC-conjugated goat anti–mouse IgG.
Washed mPF4null platelets were incubated with various concentrations (0-5 U/mL) of chondroitinase ABC (Sigma) and/or heparinase I (Sigma) at 37°C. After 30 minutes, aliquots containing equal numbers of platelets were incubated with Tyrode buffer containing various concentrations of PF4 (0-400 μg/mL, final concentration) for 60 minutes at RT. FITC-labeled KKO (50 μg/mL) was added for 15 minutes, the sample was diluted 1:10 with Tyrode buffer, and antibody binding was measured by flow cytometry.
Platelet flow cytometry
Binding of FITC-labeled KKO to the platelet was identified using a Becton Dickinson FACSscan calibrated for fluorescence and light scatter using the manufacturer's standard beads (CaliBRITE; Becton Dickinson, San Jose, CA). Data for forward-angle scatter (FSC), side-angle scatter (SSC), and fluorescence were obtained with gain settings in logarithmic mode. Human platelets were identified and gated according to the SSC and immunofluorescence with anti-CD41a mAb. Platelet activation was estimated by annexin V binding.23 To measure the binding of annexin V, the incubated platelets were diluted 1:10 in binding buffer (0.01 M HEPES, 0.14 M NaCl, and 2.5 mM CaCl2) containing annexin V–PE. When annexin V–PE and KKO-FITC binding were measured simultaneously, platelets were size-selected based on FSC and SSC.
Characterization of transgenic mice
Transgenic mice expressing different amounts of hPF4 mRNA per platelet have been described previously.26 Three lines bearing 1, 6, and 22 copy numbers of the human PF4 gene/haplotype were used. Previous analysis of multiple tissues using immunohistochemistry and reverse transcriptase–PCR (polymerase chain reaction) showed that hPF4 was expressed exclusively in megakaryocytes. Transgenic mice expressing FcγRIIA were generously provided by Steven McKenzie, Thomas Jefferson University30 and crossbred with these hPF4 mice. All murine lines were backcrossed onto the C57BL/6J background more than 8 times. Genomic makeup of mice was determined by PCR analysis using oligonucleotide primers described previously.26,30 Controls included littermates transgenic for hPF4 or FcγRIIA only. Mice were 6 to 10 weeks of age at the time of study.
Total platelet hPF4 levels in the various transgenic hPF4 lines were determined using an Asserachrom PF4 kit (Diagnostica Stago, Parsippany, NJ), as per the manufacturer, using recombinant hPF4 as the standard. Mouse blood was obtained by retro-orbital puncture. The plate was read at 450 nm in a THERMOmax microplate reader (Molecular Devices, Sunnyvale, CA). Measurements of surface KKO binding in vivo in WT and the hPF4 transgenic mice were determined after intravenous injection of 20 μg FITC-labeled KKO in 200 μL sterile PBS via the tail vein followed by withdrawal of 50 μL blood from the retro-orbital plexus 10 minutes later. The blood was coimmunostained for CD41, and KKO binding to CD41+ cells was estimated by flow cytometry. In other studies, KKO was injected intraperitoneally in a final volume of 200 μL diluted with sterile PBS. Porcine heparin (200 μL 100 U/mL stock; Abbott Laboratories, Abbott Park, IL) was injected subcutaneously in a subgroup of studied animals beginning at 24 hours for 4 consecutive days. Complete blood counts were measured in 50 μL whole blood obtained by retro-orbital puncture into Safe-T-Fill minicapillary blood collection tubes (Kabe Labortechnik, Nümbrecht-Elsenroth). Platelets were enumerated using an automatic cell counter (HEMAVET; Drew Scientific, Oxford, CT).
In the therapeutic intervention studies, either porcine heparin (100 U/kg) or protamine sulfate (2 mg/kg) was injected intravenously over 2 minutes. KKO (200 μg) was given intraperitoneally 1 hour later (zero time point). Injection of heparin or protamine was repeated 21 and 45 hours later. Blood counts were determined as described for the other murine studies.
Statistics
Platelet counts between groups were compared using the Student t test. Statistical analyses were performed using Graph Pad Prism (GraphPad Software, San Diego, CA). Differences were considered significant at a P value of less than .05.
Results
PF4 bound to the platelet surface forms antigenic complexes on human platelets
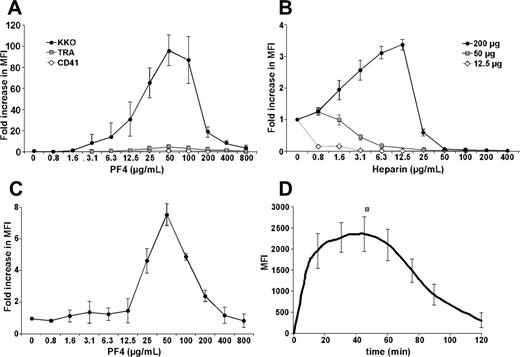
To better understand the pathogenesis of HIT, we asked whether antigenic complexes form between PF4 and GAGs on the platelet surface. KKO bound poorly to unstimulated, washed human platelets (Figure 1A). However, addition of recombinant hPF4 markedly increased binding of KKO in a dose-dependent manner. Binding followed a bell-shaped curve (Figure 1A). Maximal binding of KKO, corresponding to an approximately 100-fold increase in fluorescence intensity, occurred at an hPF4 concentration of 50 μg/mL. This peak was not limited by the amount of KKO added (data not shown). Binding of an isotype control mAb (TRA) and anti-CD41 mAb increased less than 7% compared with KKO over the same range of PF4 concentrations (Figure 1A).
Binding of mAb to human platelets in the presence of added hPF4. (A) The graph shows the fold increase in the mean fluorescence intensity (MFI) of antibody binding in the presence and absence of the noted concentrations of PF4. Gray squares indicate TRA isoimmune control; open diamonds, anti–human CD41 mAb; and filled circles, KKO. Each antibody was added at 50 μg/mL. (B) The fold change in antigenicity for KKO in the presence of PF4 at 12.5 μg/mL (open diamonds), 50 μg/mL (gray squares), and 200 μg/mL (filled circles), with heparin added at the concentrations shown. The y-axis indicates fold change from that at baseline without heparin. (C) Platelet activation by KKO (50 μg/mL) at the indicated PF4 concentrations as measured by annexin V binding. (D) Kinetics of KKO binding (50 μg/mL) in the presence of 50 μg/mL of PF4. The mean ± 1 standard deviation (SD) is shown for the experiments performed 3 times, each in triplicate.
Binding of mAb to human platelets in the presence of added hPF4. (A) The graph shows the fold increase in the mean fluorescence intensity (MFI) of antibody binding in the presence and absence of the noted concentrations of PF4. Gray squares indicate TRA isoimmune control; open diamonds, anti–human CD41 mAb; and filled circles, KKO. Each antibody was added at 50 μg/mL. (B) The fold change in antigenicity for KKO in the presence of PF4 at 12.5 μg/mL (open diamonds), 50 μg/mL (gray squares), and 200 μg/mL (filled circles), with heparin added at the concentrations shown. The y-axis indicates fold change from that at baseline without heparin. (C) Platelet activation by KKO (50 μg/mL) at the indicated PF4 concentrations as measured by annexin V binding. (D) Kinetics of KKO binding (50 μg/mL) in the presence of 50 μg/mL of PF4. The mean ± 1 standard deviation (SD) is shown for the experiments performed 3 times, each in triplicate.
We next examined the effect of heparin on the binding of KKO to surface-bound PF4. Platelet GAGs are composed predominantly of chondroitin and, to a lesser extent, heparan sulfates,31 each of which has a lower affinity for PF4 than high–molecular weight (HMW) heparin.32 At levels of added PF4 where binding of KKO to platelets is suboptimal (left side of the curve in Figure 1A), binding was reduced further or eliminated by addition of heparin. Figure 1B shows this result at a low level of surface hPF4 (12.5 μg/mL added; Figure 1B, diamonds) and for the peak level of surface hPF4 (50 μg/mL added; Figure 1B, squares). However, in the presence of hPF4 concentrations that exceeded peak antigen formation on platelets, addition of heparin enhanced KKO binding. Figure 1B shows this for 200 μg/mL hPF4 (Figure 1B, circles). These studies suggest that in settings associated with high levels of surface-bound PF4, heparin enhances cell surface antigenicity.
Binding of KKO to PF4-coated platelets induced their activation as measured by an increase in surface binding of annexin V (Figure 1C). We then asked whether activation releases additional PF4 from internal stores, which in turns alters the composition of PF4-GAG complexes and KKO binding. To examine this possibility, we incubated human platelets with 50 μg/mL of hPF4 and followed KKO binding over time. KKO binding increased with time, reaching a plateau at 20 to 60 minutes and then decreased (Figure 1D). These dynamic changes suggest that the composition of the surface PF4-GAG complexes had been modified over time, possibly due to release of PF4 from newly recruited FcγRIIA-activated platelets.14 As additional PF4 is incorporated into these complexes, the optimal ratio is exceeded and antibody binding is impaired.
PF4 bound to the platelet surface forms antigenic complexes on murine platelets
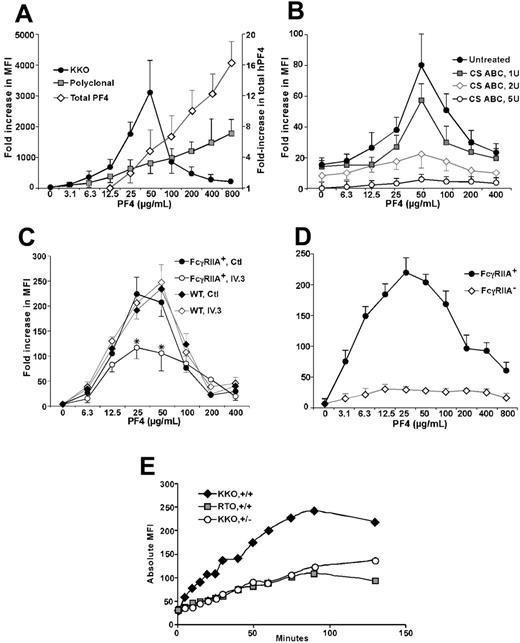
Studies of human platelets are thus confounded by the release of internal stores of hPF4 and the presence of FcγRIIA on their surface. We, therefore, switched to murine platelets that naturally lack the FcγRIIA platelet receptor and studied KKO binding to the surface of mPF4null platelets.29 Addition of hPF4 led to a near doubling in total platelet PF4 for each doubling of hPF4 in the media over the range studied (Figure 2A, diamonds) with a slightly blunted, but similar, proportional increase in total surface immunogenic PF4 detected using a polyclonal anti-hPF4 antibody (Figure 2A, squares). Under similar conditions, binding of KKO to murine platelets followed the same bell-shaped curve seen with human platelets (Figure 2A, circles).
KKO binding to murine platelets in the presence of hPF4. (A) Details are the same as for Figure 1A but mPF4null platelets were studied with increasing amounts of added hPF4. Diamonds indicate total platelet PF4; squares, total surface immunogenic PF4; and circles, KKO-detectable surface PF4. (B) Details are the same as for panel A for KKO but mPF4null platelets pretreated with CS ABC were used. (C) Details are the same as for panel A for KKO but genotypes of the mice are as shown and the platelets were incubated with either the FcγRIIA blocking mAb IV.3 or an isotype control (50 μg/mL) for 30 minutes at RT prior to addition of PF4 and KKO. (D) Details are the same as for panel A for KKO with the genotypes of the mice indicated. Relative annexin binding was measured. (E) Time course of KKO and RTO binding (50 μg/mL) in the whole blood samples to transgenic murine platelets. +/+ indicates hPF4high/FcγRIIA+ double-transgenic mouse platelets; +/–, hPF4high/FcγRIIA– transgenic mouse platelets. MFI is in absolute values. For panels A-D, the mean ± 1 SD is shown. Each experiment was performed 3 times. In panel E, a study representative of 3 is shown.
KKO binding to murine platelets in the presence of hPF4. (A) Details are the same as for Figure 1A but mPF4null platelets were studied with increasing amounts of added hPF4. Diamonds indicate total platelet PF4; squares, total surface immunogenic PF4; and circles, KKO-detectable surface PF4. (B) Details are the same as for panel A for KKO but mPF4null platelets pretreated with CS ABC were used. (C) Details are the same as for panel A for KKO but genotypes of the mice are as shown and the platelets were incubated with either the FcγRIIA blocking mAb IV.3 or an isotype control (50 μg/mL) for 30 minutes at RT prior to addition of PF4 and KKO. (D) Details are the same as for panel A for KKO with the genotypes of the mice indicated. Relative annexin binding was measured. (E) Time course of KKO and RTO binding (50 μg/mL) in the whole blood samples to transgenic murine platelets. +/+ indicates hPF4high/FcγRIIA+ double-transgenic mouse platelets; +/–, hPF4high/FcγRIIA– transgenic mouse platelets. MFI is in absolute values. For panels A-D, the mean ± 1 SD is shown. Each experiment was performed 3 times. In panel E, a study representative of 3 is shown.
We then examined whether KKO recognized PF4 bound to surface GAGs by pretreating the cells with either chondroitinase (CS) ABC or heparinase I or both together. CS ABC alone (Figure 2B) or with heparinase I (data not shown), but not heparinase I alone (data not shown), decreased KKO binding. These data are consistent with platelet membrane GAGs being composed predominantly of chondroitin sulfates.31,32 When GAGs were stripped from the platelet surface, the concentration of PF4 needed for maximal KKO binding was unaltered. This may indicate that clusters of chondroitin remain intact, whereas other areas of the platelet become devoid of GAGs.
We then asked whether FcγRIIA contributed to the binding of KKO. Murine platelets from WT animals or FcγRIIA+ transgenic animals were incubated with increasing concentrations of hPF4. The amount of KKO bound in the presence of IV.3, an FcγRIIA blocking antibody or an isotype control,12 was then measured. Binding of KKO to WT and FcγRIIA+ platelets followed the same bell-shaped curve in the presence of the isotype control, consistent with binding through the Fab end of the molecule (Figure 2C). However, IV.3 did reduce the total amount of KKO that bound to FcγRIIA+ platelets (Figure 2C), suggesting that the presence of the FcγRIIA receptor may also provide stability to bound KKO.
Platelet activation by KKO clearly occurs via FcγRIIA engagement, as WT murine platelets are minimally activated, as measured by the binding of annexin V (Figure 2D). We then studied the effect of platelet activation on KKO binding using murine platelets that were double transgenic for high levels of hPF4 and FcγRIIA, hPF4high/FcγRIIA+ (Figure 2E +/+), compared with hPF4high/FcγRIIA– platelets (Figure 2E +/–). hPF4high/FcγRIIA+ and hPF4high/FcγRIIA– platelets bound the same amount of KKO at time zero. However when FcγRIIA was expressed, binding of KKO increased greatly over time (Figure 2E). A much smaller increase was also seen for the binding of RTO, a mAb that binds to hPF4 independent of heparin27 (Figure 2E). These studies support the concept that platelet activation via FcγRIIA releases additional PF4 that becomes incorporated within antigenic complexes recognized by KKO.
Studies with HIT IgG
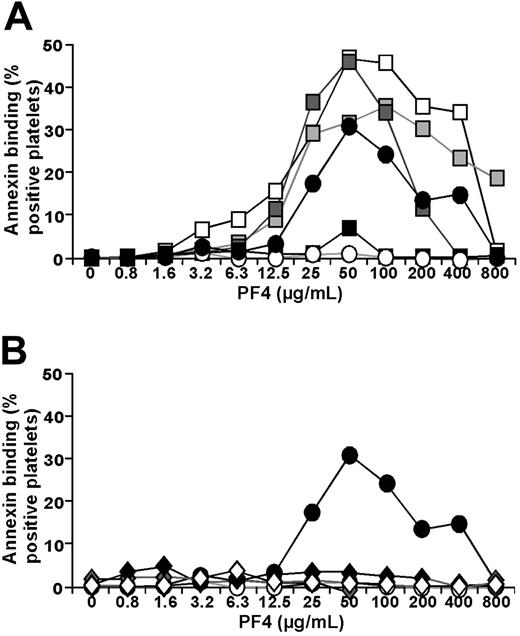
KKO competes with many HIT antibodies for binding to platelets, suggesting a common epitope on PF4-heparin, and activates platelets through similar mechanisms.27 Nevertheless, we extended our studies to determine whether HIT antibodies behaved in a similar manner with respect to platelet surface PF4 levels. Studies based on those shown in Figure 1A were repeated using either IgG isolated from patients with HIT diagnosed by clinical criteria7,8,11,28 and a positive HIT ELISA,3 IgG from healthy volunteers, or a commercial pooled IgG preparation. Three of the 4 HIT IgG samples tested caused strong activation of platelets as measured by binding of annexin V (Figure 3A) in contrast to the 4 healthy controls or a commercial IgG preparation (Figure 3B). Maximal platelet activation occurred at the same concentration of PF4 (50 μg/mL) as was seen with KKO.
In vivo studies in mice expressing different amounts of hPF4
The in vitro data indicate that there is an amount of cell-surface PF4 at which HIT-antibody binding is maximal. This bell-shaped relationship between PF4 concentration and binding of HIT antibody extends previous studies in which a similar relationship was seen when the concentrations of PF4 and heparin in solution were varied.22 However, GAGs appear to fulfill the role of heparin on the platelet surface. If these findings have clinical relevance, then in a murine model of HIT, (1) the severity of thrombocytopenia should parallel endogenous hPF4 expression; (2) if sufficient PF4 has already been released and bound to the cell surface, exogenous heparin would not be required to cause thrombocytopenia once antibody is present; and (3) heparin would exacerbate thrombocytopenia in the setting of high PF4 content.
Platelet activation by HIT IgG. (A) Squares represent IgG isolated from 4 HIT plasmas incubated with human platelets that had been exposed to different amounts of hPF4. Filled circles indicate simultaneously studied KKO; and open circles, simultaneously studied isoimmune control TRA. (B) Details are the same as for panel A using a commercial control IgG preparation (open diamonds) or 4 preparations of IgG from healthy controls (filled and gray diamonds). The mean value is shown for each patient studied on 3 to 5 separate occasions. Each experiment was performed in duplicate. For clarity, standard deviations are not shown.
Platelet activation by HIT IgG. (A) Squares represent IgG isolated from 4 HIT plasmas incubated with human platelets that had been exposed to different amounts of hPF4. Filled circles indicate simultaneously studied KKO; and open circles, simultaneously studied isoimmune control TRA. (B) Details are the same as for panel A using a commercial control IgG preparation (open diamonds) or 4 preparations of IgG from healthy controls (filled and gray diamonds). The mean value is shown for each patient studied on 3 to 5 separate occasions. Each experiment was performed in duplicate. For clarity, standard deviations are not shown.
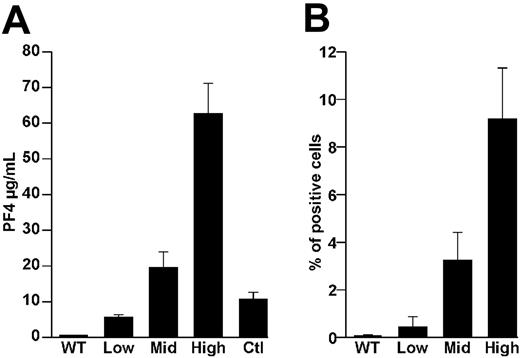
Characterization of hPF4 mice. (A) Total platelet-associated hPF4 expressed per mL of blood in WT animals and the 3 hPF4 transgenic mice lines studied. Controls (Ctl) were platelets from 4 human donors. The mean ± 1 SD is shown for the experiments performed 3 times, each in triplicate. (B) Flow cytometric measurement of CD41+–platelet-bound FITC-KKO in the same animals as in panel A measured 10 minutes after intravenously injected FITC-KKO.
Characterization of hPF4 mice. (A) Total platelet-associated hPF4 expressed per mL of blood in WT animals and the 3 hPF4 transgenic mice lines studied. Controls (Ctl) were platelets from 4 human donors. The mean ± 1 SD is shown for the experiments performed 3 times, each in triplicate. (B) Flow cytometric measurement of CD41+–platelet-bound FITC-KKO in the same animals as in panel A measured 10 minutes after intravenously injected FITC-KKO.
We previously described the creation of transgenic mouse lines expressing various levels of hPF4 RNA.26 We now measured total platelet hPF4 compared with the average hPF4 content of 4 human platelet controls. hPF4 levels varied from approximately 0.5 times the content of human platelets in hPF4low mice (which have one copy of the hPF4 transgene/haploid genome) to approximately 2 times the level in hPF4mid mice (which have 6 copies/haploid genome) to approximately 6 times in hPF4high mice (which have 22 copies/haploid genome; Figure 4A). Flow cytometric studies of platelets from these transgenic lines demonstrate that all have detectable surface-bound hPF4 in vivo measured 10 minutes after intravenous injection of KKO, with antibody binding proportional to platelet PF4 expression (Figure 4B).
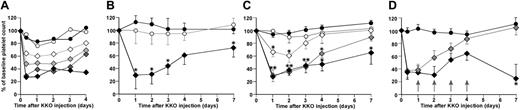
By 3 hours after an intraperitoneal injection of KKO, hPF4high/FcγRIIA mice developed severe antibody dose-dependent thrombocytopenia, which persisted for longer than 7 days (Figure 5A). Mice that were hPF4high or FcγRIIA alone did not develop thrombocytopenia (Figure 5A). The severity of thrombocytopenia correlated with the dose of injected antibody. Thrombocytopenia was not seen with an equivalent amount of the isoimmune control TRA or RTO (Figure 5B). The severity of the thrombocytopenia also correlated with the genetically determined level of hPF4 (Figure 5C). Daily injections of 20 U of subcutaneous heparin into hPF4mid/FcγRIIA mice as used in the previously described HIT murine model14 did not lower the initial nadir platelet count further but did prolong the duration of severe thrombocytopenia for longer than 2 weeks (Figure 5D; data not shown). There was no unexpected loss of animals in these studies, and specific histologic studies for thrombotic events were not pursued.
KKO-induced thrombocytopenia in hPF4 mice. (A) Platelet counts in mice after intraperitoneal injection of KKO. The first time point is at 3 hours after injection. Filled circles indicate hPF4high mice, 200 μg KKO; and open circles, FcγRIIA transgenic mice, 200 μg KKO. Diamonds indicate hPF4high/FcγRIIA double-transgenic mice. Open diamonds, light gray diamonds, dark gray diamonds, and filled diamonds indicate 50, 100, 200, and 400 μg KKO intraperitoneally, respectively. The mean of 3 experiments, each performed in triplicate, is shown. (B) Animals were all hPF4high/FcγRIIA double-transgenic mice. ○ indicates 200 μg TRA, intraperitoneally; ♦, 200 μg RTO, intraperitoneally; and •, 200 μg KKO, intraperitoneally. The mean ± 1 SD of 3 experiments, each in triplicate is shown. *P < .05 (C) All animals received 200 μg KKO. • indicates hPF4high mice, 200 μg KKO; ○, FcγRIIA transgenic mice, 200 μg KKO; ⋄, hPF4low/FcγRIIA;  , hPF4mid/FcγRIIA; and •, hPF4high/FcγRIIA. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 from baseline value. (D) All animals received 200 μg KKO, intraperitoneally. • indicates hPF4high mice, 200 μg KKO;
, hPF4mid/FcγRIIA; and •, hPF4high/FcγRIIA. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 from baseline value. (D) All animals received 200 μg KKO, intraperitoneally. • indicates hPF4high mice, 200 μg KKO;  , hPF4mid mice; and •, hPF4mid mice that also received 20 U heparin subcutaneously daily for 4 days as indicated by arrows. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 of heparin treated from untreated hPF4mid/FcγRIIA mice.
, hPF4mid mice; and •, hPF4mid mice that also received 20 U heparin subcutaneously daily for 4 days as indicated by arrows. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 of heparin treated from untreated hPF4mid/FcγRIIA mice.
KKO-induced thrombocytopenia in hPF4 mice. (A) Platelet counts in mice after intraperitoneal injection of KKO. The first time point is at 3 hours after injection. Filled circles indicate hPF4high mice, 200 μg KKO; and open circles, FcγRIIA transgenic mice, 200 μg KKO. Diamonds indicate hPF4high/FcγRIIA double-transgenic mice. Open diamonds, light gray diamonds, dark gray diamonds, and filled diamonds indicate 50, 100, 200, and 400 μg KKO intraperitoneally, respectively. The mean of 3 experiments, each performed in triplicate, is shown. (B) Animals were all hPF4high/FcγRIIA double-transgenic mice. ○ indicates 200 μg TRA, intraperitoneally; ♦, 200 μg RTO, intraperitoneally; and •, 200 μg KKO, intraperitoneally. The mean ± 1 SD of 3 experiments, each in triplicate is shown. *P < .05 (C) All animals received 200 μg KKO. • indicates hPF4high mice, 200 μg KKO; ○, FcγRIIA transgenic mice, 200 μg KKO; ⋄, hPF4low/FcγRIIA;  , hPF4mid/FcγRIIA; and •, hPF4high/FcγRIIA. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 from baseline value. (D) All animals received 200 μg KKO, intraperitoneally. • indicates hPF4high mice, 200 μg KKO;
, hPF4mid/FcγRIIA; and •, hPF4high/FcγRIIA. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 from baseline value. (D) All animals received 200 μg KKO, intraperitoneally. • indicates hPF4high mice, 200 μg KKO;  , hPF4mid mice; and •, hPF4mid mice that also received 20 U heparin subcutaneously daily for 4 days as indicated by arrows. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 of heparin treated from untreated hPF4mid/FcγRIIA mice.
, hPF4mid mice; and •, hPF4mid mice that also received 20 U heparin subcutaneously daily for 4 days as indicated by arrows. The mean ± 1 SD of 3 experiments, each performed in triplicate, is shown. *P < .05 of heparin treated from untreated hPF4mid/FcγRIIA mice.
Therapeutic intervention in the murine model
The above studies suggest that interventions that skew the GAG/PF4 ratio toward either extreme may protect against formation of HIT antigenic complexes on the platelet surface. We employed 2 such strategies to test this hypothesis. (1) Based on the data in Figure 1B, we inferred that a marked excess of heparin would reduce surface antigenicity and prevent HIT even in the presence of a pathogenic anti-PF4/heparin antibody. (2) The data suggest a similar outcome would be expected from an excess of a cationic moiety that binds to platelets and prevents incorporation of PF4 into the antigenic complexes. Protamine sulfate is a small positively charged molecule that competes with PF4 for binding to GAGs33 and has been used clinically to neutralize heparin.34 Although cardiovascular side effects have been reported rarely,35,36 protamine sulfate has the advantage over infusing large amounts of hPF4 by not chancing a transient increase in surface antigenicity.
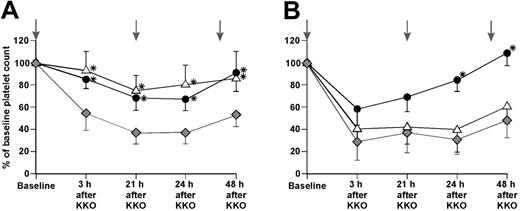
Transgenic hPF4mid/FcγRIIA mice were given an intravenous infusion of either 100 U/kg unfractionated heparin or 2 mg/kg protamine sulfate 1 hour prior to an intraperitoneal injection of 200 μg KKO. Both prevented thrombocytopenia at 3 hours and decreased the severity of thrombocytopenia at 24 hours (Figure 6A). Repeat doses given on the second and third days maintained platelet counts above the level in mice that had received KKO alone. In hPF4high/FcγRIIA mice, these treatment regimens gave different results (Figure 6B). High-dose heparin was ineffective in preventing KKO-induced thrombocytopenia. In contrast, platelet counts were significantly higher in protamine sulfate–treated mice than in mice receiving antibody alone at 24 and 48 hours.
Therapeutic intervention in HIT model. KKO (200 μg) was given intraperitoneally at baseline preceded by either intravenous heparin (100 U/kg) or protamine sulfate (2 mg/kg). Platelet counts were measured at the times noted. Subsequent therapeutic interventions at 21 and 45 hours are denoted by vertical gray arrows. (A) hPF4mid/FcγRIIA animals. (B) hPF4high/FcγRIIA animals.  indicate animals that received only KKO; ▵, KKO plus heparin; and ♦, KKO plus protamine sulfate. At least 4 animals were studied per time point. The means ± 1 SD are shown. *P < .05 versus animals receiving KKO alone.
indicate animals that received only KKO; ▵, KKO plus heparin; and ♦, KKO plus protamine sulfate. At least 4 animals were studied per time point. The means ± 1 SD are shown. *P < .05 versus animals receiving KKO alone.
Therapeutic intervention in HIT model. KKO (200 μg) was given intraperitoneally at baseline preceded by either intravenous heparin (100 U/kg) or protamine sulfate (2 mg/kg). Platelet counts were measured at the times noted. Subsequent therapeutic interventions at 21 and 45 hours are denoted by vertical gray arrows. (A) hPF4mid/FcγRIIA animals. (B) hPF4high/FcγRIIA animals.  indicate animals that received only KKO; ▵, KKO plus heparin; and ♦, KKO plus protamine sulfate. At least 4 animals were studied per time point. The means ± 1 SD are shown. *P < .05 versus animals receiving KKO alone.
indicate animals that received only KKO; ▵, KKO plus heparin; and ♦, KKO plus protamine sulfate. At least 4 animals were studied per time point. The means ± 1 SD are shown. *P < .05 versus animals receiving KKO alone.
Discussion
Surface-bound PF4 is antigenic for HIT antibodies and KKO over a narrow range of PF4 concentrations, leading to platelet activation through FcγRIIA. Our data suggest that PF4 forms antigenic complexes with endogenous GAGs on the surface of platelets similar to ULCs that form between HMW heparin and PF4 in solution.23 These data could explain why only a subgroup of heparinized patients with HIT antibodies develop HIT. Platelets vary widely in PF4 content (unpublished data) and perhaps in released PF4 and surface PF4 levels. Those individuals with the highest levels of surface PF4 prior to heparinization may be most susceptible to continue to express surface HIT antigenic complexes after heparinization and develop HIT. In addition, the proposed model may also help explain why HIT can develop after heparin therapy has been stopped37 and why HIT can occur in a delayed fashion long after infused heparin has been cleared.38
PF4 is a member of the CXC subfamily of chemokines that possesses high affinity for heparin and other large, anionic molecules.39 PF4 is expressed in megakaryocytes and stored in platelet α-granules from which it is released upon activation.40,41 After its release, PF4 binds to GAG on vascular cell surfaces.42 HIT IgG and the mAb KKO bind to Chinese hamster ovarian (CHO) cells in the presence of exogenous PF4 but not to CHO cells lacking heparan sulfate– or chondroitin sulfate–containing proteoglycans.27 Similarly, these antibodies bind directly to monocytes43 and cultured endothelial cells,44 and binding is reduced by pretreating with heparanases.44 Under certain experimental circumstances, heparin has been shown to promote the binding of HIT IgG and KKO to activated platelets, which act in a feed-forward manner to perpetuate platelet activation and more IgG binding.22,45
The concentration of PF4 that optimized KKO platelet binding (50 μg/mL) is the same as proved optimal for activation by HIT IgG (Figures 1A and 3A, respectively) and is well within what is attained in the immediate environ of activated platelets after platelet α-granular release (unpublished data). Moreover, the heparin concentrations (2-4 μg/mL; Figure 1B) that enhanced KKO binding to platelets at 200 μg/mL of PF4 fall within the therapeutic range of heparinization (0.4-0.7 U/mL),46 so that the conditions we analyzed are achievable in vivo.
To study the in vivo relevance of our observation, we used the previously described murine HIT model.14 We, as others, had assumed that heparin would be a necessary component for thrombocytopenia to develop in this model. Contrary to expectation, heparin is not required to induce thrombocytopenia. The pathogenic relevance of surface PF4 expression was supported by in vivo studies in transgenic mice expressing varying amounts of PF4 in which the severity of KKO-induced thrombocytopenia was proportionate to total platelet (and surface) hPF4 content (Figure 5C). The reason for the presence of hPF4 on the surface of these platelets is unclear. Unlike patients with HIT, mice have little vascular disease that would sustain platelet activation leading to PF4 release and surface-bound PF4. Transgenic expression of hPF4 in the presence of the full complement of murine PF4 may have exceeded the storage content of serglycins47 inside the α-granules of their platelets, resulting in the observed “leak” of hPF4 and allowing the murine platelets to simulate patients with ongoing activation and partial degranulation of their platelets that may predispose to HIT.
Based on our findings, we reasoned that we could interfere with the development of thrombocytopenia in the double-transgenic mice by altering the surface PF4-GAG ratio on the platelets. In support of this concept, infusing either high doses of heparin or protamine sulfate prevented KKO-induced thrombocytopenia in hPF4mid/FcγRIIA mice (Figure 6). In the hPF4high/FcγRIIA mice, the same heparin dose was ineffective, in contrast to the protamine sulfate, which retained its efficacy (Figure 6). The dose of heparin we used is often exceeded in clinical settings48 and we have used higher doses of both agents safely in mice.29 However, these interventions were intended to test our model and the role of surface platelet PF4 in HIT antigenicity. Moreover, we have yet to determine whether similar strategies can reverse established thrombocytopenia or thrombosis. Clinically, direct thrombin inhibitors block the explosive amplification of thrombin on platelet activation and coagulation but have not eliminated the occurrence of amputations and death in affected patients. It is possible that antibody-mediated platelet activation promotes thrombosis through additional mechanisms involving platelet adhesion to the vasculature49 and platelet-leukocyte aggregation50 that would be better addressed by an intervention that acts proximal to thrombin generation. Thus, we envisage similar strategies to those in Figure 6 that could target these proximal HIT mechanisms and be used in combination with direct thrombin inhibitors.
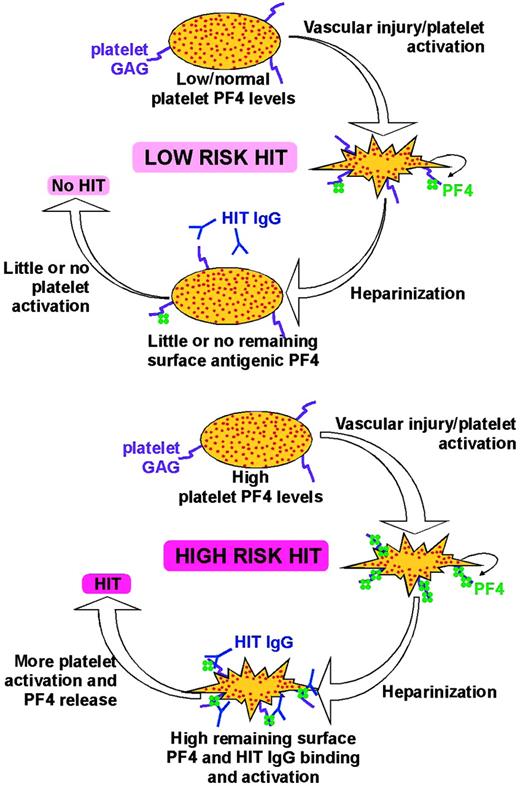
Schematic representation of HIT model. The situation shown in the top panel is more common. Patients have low or normal levels of total platelet PF4, and if they have atherosclerosis or other causes of vascular injury leading to platelet activation and PF4 release, they have relatively low levels of surface PF4 expression. When these patients are heparinized, PF4 is removed, fewer antigenic complexes remain, and there is less likelihood of platelet activation if HIT antibodies develop. These patients are at low risk of HIT. The bottom panel shows the smaller subset of patients with high levels of total PF4 who have suffered significant vascular injury and/or significant platelet activation and have high surface PF4 levels. Upon heparinization, they form and retain significant amounts of antigenic complexes on the platelet surface. If they develop HIT antibodies, they are at high risk of developing HIT.
Schematic representation of HIT model. The situation shown in the top panel is more common. Patients have low or normal levels of total platelet PF4, and if they have atherosclerosis or other causes of vascular injury leading to platelet activation and PF4 release, they have relatively low levels of surface PF4 expression. When these patients are heparinized, PF4 is removed, fewer antigenic complexes remain, and there is less likelihood of platelet activation if HIT antibodies develop. These patients are at low risk of HIT. The bottom panel shows the smaller subset of patients with high levels of total PF4 who have suffered significant vascular injury and/or significant platelet activation and have high surface PF4 levels. Upon heparinization, they form and retain significant amounts of antigenic complexes on the platelet surface. If they develop HIT antibodies, they are at high risk of developing HIT.
In Figure 7, we propose a model for the onset of HIT based on our findings and on published literature. Patients who develop HIT are typically older and likely to have underlying cardiovascular disease and/or have undergone surgical manipulation. We propose that platelet activation in these patients leads to PF4 release and rebinding. In the vast majority of individuals and clinical settings, endogenous PF4 is low and surface PF4 expression does not exceed the equivalent of adding 50 μg/mL PF4 (Figure 7, top). Therapeutic heparinization markedly reduces platelet surface PF4. Heparinization would induce HIT antibody formation in up to half of these patients but there would be little surface HIT antigen available and little risk of developing HIT. On the other hand, in the small number of individuals with high platelet PF4 content and sufficient platelet activation leading to high surface PF4 levels, therapeutic heparinization would not eliminate surface antigenicity (Figure 7, bottom). These patients are at least as likely as other patients to develop HIT antibodies after heparinization. However in these individuals, the antibodies can activate a large number of platelets because of the high level of remaining platelet surface antigen, leading to more PF4 release and repetitive cycles of platelet activation. These patients are at high risk of developing HIT.
The model we propose offers the testable hypothesis that patients with high total and/or surface PF4 are at significant risk of developing HIT and that they can be identified prospectively and offered alternative management. Identifying such high-risk patients will require additional studies to measure HIT antigen using an antibody like KKO and studying its binding in the presence of heparin (Figure 1B) or subsequent to platelet activation (Figure 1C).
Our studies and model focus on the events on the platelet surface but there is little reason to suppose that similar events are not concurrently happening on the surface of the endothelial lining, circulating monocytes, and other vascular cells. The binding of HIT antibodies to the PF4 antigenic complexes on these cells would contribute not only to the developing thrombocytopenia but also to the inflammatory state and to the thrombosis by expressing tissue factor and releasing procoagulant microparticles accelerating thrombin formation, which are recognized components of HIT.43,51,52
Finally, we believe that surface PF4 may have a biologic role as well. We have previously shown that platelet PF4 content affects thrombogenicity in a bell-shaped curve fashion.29 We propose that both thrombogenicity and HIT antigenicity are greatest when formation of stable PF4-GAG ULCs on cell surfaces is maximal. How such complexes contribute to thrombosis needs further study, but, if true, patients whose platelets retain surface antigenic ULCs after heparinization are not only targets for HIT antibodies but also intrinsically prothrombotic.
In summary, the formation of HIT antigen on platelets occurs at specific concentrations of reactants. This can be demonstrated for binding of the mAb KKO to platelets and for FcγRIIA activation of platelets by KKO and by HIT IgG. When surface-bound PF4 exceeds this level, heparinization increases antigen formation. Murine models support the role of platelet surface PF4 complexes in the development of thrombocytopenia and show that severity of thrombocytopenia depends on the level of platelet hPF4. Infusions of either high-dose heparin or protamine sulfate prevent the development of the thrombocytopenia in most settings, but heparin is ineffective when the concentration of platelet PF4 is high. These data suggest that patients with high total and surface platelet PF4 expression may be at the highest risk of developing HIT when exposed to heparin, and strategies to identify such patients and avoid heparin may be warranted. Novel strategies to interfere with the formation of surface PF4-GAG complexes suggested by this model may prove useful in the prevention and treatment of HIT.
Prepublished online as Blood First Edition Paper, November 22, 2005; DOI 10.1182blood-2005-08-3122.
Supported by National Institutes of Health (NIH) grants HL54500 (M.P., D.B.C.), HL054749 (M.P.), HL68631 (M.P.), and HL69471 (D.B.C.); American Heart Association (AHA) grants (L.R., postdoctoral grant; G.M.A., Beginning Grant-in-Aid); and a grant from the Gustavus and Louise Pfeiffer Research Foundation (G.M.A.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Steven McKenzie and Michael Reilly (Cardeza Research Institute, Thomas Jefferson University School of Medicine) for having generously shared the FcγRIIA mouse with our group.