Abstract
Chk1 and Akt signaling facilitate survival of cells treated with nucleoside analogues. Activation of Chk1 in response to cytarabine (ara-C) induced an S-phase checkpoint characterized by the inhibition of Cdk2, cell cycle arrest, no change in constitutively active Akt, or low-stress kinase signaling in ML-1 cells. However, inhibition of Chk1 by UCN-01 in S-phase-arrested cells resulted in an abrogation of the checkpoint, inhibition of Akt, activation of JNK, and a rapid induction of apoptosis. Similarly, primary acute myelogenous leukemia (AML) blasts exposed to ara-C and UCN-01 demonstrated a selective loss in cloning potential when compared with normal progenitors. Therefore, we evaluated a pilot clinical trial of ara-C in combination with UCN-01 in patients with relapsed AML. Blasts from some patients demonstrated a previously activated Chk1-Cdk2 DNA damage response pathway that decreased during therapy. Constitutively phosphorylated Akt kinase declined on addition of UCN-01 to the ara-C infusion, an action accompanied by an activation of JNK and reduction in absolute AML blast counts. Thus, use of UCN-01 in combination with ara-C decreases Chk1 phosphorylation, inhibits the Akt survival pathway, and activates JNK during the course of therapy, offering a rationale for the cytotoxic action of this combination during AML treatment. (Blood. 2006;107:2517-2524)
Introduction
The nucleoside analogue cytarabine (ara-C), an effective and widely used agent for the treatment of acute myelogenous leukemia (AML), induces high initial rates of complete remissions.1 However, only a fraction of these individuals continue to have a long-term disease-free survival.2-4 Accordingly, there is considerable interest in understanding the mechanisms of resistance to ara-C and devising strategies for overcoming them.5 The Chk1 DNA damage kinase is activated by phosphorylation on Ser345 and Ser317 in response to replicative arrest due to the misincorporation of nucleoside analogues into DNA.6-9 This event activates an S-phase-specific checkpoint pathway whereby Chk1 inhibits the activity of the Cdc25A phosphatase. In turn, Cdk2 accumulates with elevated levels of phosphorylation on Tyr15 causing cell cycle arrest.7,10 Thus, the ability of cells to enact a delay in cell cycle progression in response to stalled replication forks and resultant inhibition of DNA synthesis may permit removal of the analogue and repair of damage associated with these processes. In this sense, S-phase arrest may constitute a defense mechanism that spares cells potential toxicity.
Chk1 kinase has an analogous role in the G2/M checkpoint pathway that is activated in response to agents that cause DNA breaks, such as radiation11,12 and strand-breaking nucleosides.13 Abrogation of either checkpoint by inhibition of Chk1 with pharmacologic intervention or by siRNA knockdown strategies sensitizes cells to S/G2-phase-acting agents.7-9,14-16 Prominent among pharmacologic agents that directly inhibit Chk1 and abrogate the checkpoint pathways is 7-hydroxystauroporine (UCN-01). Abrogation of the nucleoside analogue-induced S-phase checkpoint by UCN-01 was associated with an inhibition of Chk1 kinase activity.7,8 This was measured as a reactivation of Cdc25A phosphatase, the consequent reduction in Tyr15 phosphorylation on Cdk2, restoration of its kinase activity, and induction of apoptosis within 3 to 6 hours.7,14 These events, which have been demonstrated in cell culture systems, suggest a mechanistic rationale for combining these agents. Several phase 1 and 2 trials of UCN-01, either alone17-19 or in combination with established cytotoxic agents, are in progress.20,21
Akt/PKB, an important downstream mediator of phosphoinositol 3-kinase (PI3K), has also been implicated in modulating survival in response to chemotherapeutic agents.22-24 For instance, primary cells from patients with AML demonstrate constitutive activation of Akt23,24 ; in model systems, this is associated with the induction of a downstream signaling cascade such as phosphorylation of BAD,25 caspase-9,26 NF-κB,24 and Forkhead proteins.22 Phosphorylation of these Akt substrates results in antiapoptotic effects22,24-26 and is associated with resistance to ara-C in vitro.24 The phosphoinositol-dependent kinase 1 (PDK1), which functions downstream of PI3K, and DNA-PK (a putative PDK2) are implicated in activating Akt by phosphorylating Thr308 and Ser473, respectively.27,28 Both PDK127,29 and DNA-PK30 and therefore Akt27,31 are subject to inhibition by UCN-01. Thus, it follows that agents that interfered with the PI3K/Akt signal transduction pathway led to growth inhibition in cell lines as well as AML samples and were associated with the induction of apoptosis in these cells.23,24,32 Conversely, constitutively activated forms of Akt partially attenuated the cytotoxic effects of UCN-01 in combination regimens.33
The c-jun kinase (JNK) regulates survival in response to nucleoside analogues and other DNA-damaging agents by translating cellular stress into signals for cell death. It functions by phosphorylating 14-3-3 proteins to promote the nuclear accumulation of the c-Abl tyrosine kinase, which is associated with a proapoptotic response after DNA damage.34 Additionally, JNK signaling can induce the transcriptional activation of death receptor-dependent apoptosis35,36 as well as phosphorylation-dependent regulation of the mitochondrial pathway to apoptosis.37,38 Inhibition of JNK attenuated cell death in response to the nucleoside analogue fludarabine39,40 as well as to UCN-01 in combination with other signal transduction inhibitors.33
In this report we initially established in human acute myelogenous leukemia ML-1 cells a model for the activation of an S-phase checkpoint by ara-C and its abrogation by UCN-01. The model identified a mechanistic role for cell cycle checkpoint dysregulation, abrogation of Akt survival pathways, and activation of stress kinase signaling that enabled UCN-01 to augment the cytotoxicity of ara-C in S-phase-arrested cells. This model was further investigated in clonogenic assays of primary AML blasts and compared with normal myeloid progenitors. Finally, we have conducted a pilot clinical trial of ara-C in combination with UCN-01 for the therapy of patients with relapsed AML. Chk1 was phosphorylated in most AML samples obtained prior to treatment. Inhibition of Chk1 and Akt signaling was paralleled by an activation of the JNK pathway during therapy, suggesting that targeting the Chk1 and Akt pathway may have therapeutic implications in the treatment of AML.
Patients, materials, and methods
Materials
The ara-C used for in vitro investigations was purchased from SigmaAldrich (St Louis, MO). UCN-01 (NSC 638850) for use in vitro was kindly provided by the Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute (NCI; Bethesda, MD). Aliquots of UCN-01 (10 mM in DMSO) were stored at -20°C and diluted in RPMI immediately prior to each experiment. UCN-01 used in the clinical trial was supplied by the Cancer Therapy and Evaluation Program, NCI. All other chemicals were reagent grade.
Patients and methods
A pilot clinical trial was designed where ara-C was administered at 1 g/m2/d for 4 days as a continuous infusion with UCN-01 administered at 45 mg/m2/d starting day 2 as a continuous infusion. A detailed analysis of the clinical aspects of this trial will be reported separately. After informed consent was obtained under the aegis of the University of Texas MD Anderson Institutional Review Board-approved protocols, blood samples were collected in heparin-containing vacutainer tubes. Mononuclear cells were isolated on Ficoll-Hypaque gradients as described.41 Two patients received 1.5 g/m2/d ara-C followed by UCN-01 administered as described above (Table 1 patients 10 and 13). Intracellular concentration of ara-CTP and inhibition of DNA synthesis were quantitated in 13 samples as described previously.42 Reduction of absolute blast counts, the levels of pSer345Chk1 and pTyr15Cdk2, as well the status of activation of JNK and inhibition of Akt during the course of therapy were evaluated in cohorts of samples from 8 to 10 patients. Median blast percentage was 91% in pretreatment samples (range, 38%-99%). The percent blast value did not change appreciably during therapy for 7 of 8 samples; the exception was patient 2.
Cell culture and antibodies
ML-1 human leukemia cells were a gift from Dr Michael B. Kastan (St Jude Children's Research Hospital, Memphis, TN). Cells were resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum and incubated at 37°C in a humidified incubator with 5% CO2. Rabbit polyclonal antibodies to Cdk2 (sc-163), cyclin A (sc-751), Chk1 (sc-7898 and sc-8408), and JNK1 (sc-474) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies to pTyr15Cdk2 (9111), pSer317Chk1 (2344), pSer345Chk1 (2341), Chk1 (7536), pSer473Akt (9271), pSer308Akt (9275), and Akt (9272) and the JNK assay kit (9810) were purchased from Cell Signaling Technology (Beverly, MA).
Cell cycle distribution analysis and TUNEL assay
Cell pellets were washed with cold phosphate-buffered saline (PBS) and split into 2 portions. One portion was fixed in 70% ethanol to determine cell cycle distribution, whereas the second portion was fixed in 1% paraformaldehyde for 20 minutes on ice and stored in 70% ethanol to assay for DNA nicks by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). Briefly, cell suspensions were washed with PBS and resuspended in 0.1% sodium citrate, 0.1% Triton X100, and 50 μg/mL propidium iodide (PI) for 20 minutes at room temperature in the presence of 250 μg/mL RNAse. Monovariate distributions of cell number versus DNA content (PI) were analyzed using a BD FACS Calibur flow cytometer. At least 10 000 events were collected in each histogram. Cell cycle analysis was performed using the BD Cell Quest Pro Acquisition and analysis software (BD Biosciences, San Jose, CA).
The cells were also analyzed for DNA ends using the APO-DIRECT kit for the terminal TUNEL assay (PharMingen, San Diego, CA). Briefly, cells were prepared to simultaneously assess DNA nicks by TUNEL assay and DNA content by PI staining, which were analyzed simultaneously by flow cytometry using an BD FACS Calibur (BD Biosciences). The percentages of TUNEL-positive and -negative cells were obtained by standard analysis techniques.
Immunodepletion of Cdk1, immunoblotting, and JNK kinase assays
Cell protein (200 mg) was mixed with anti-Cdk1 on a rocker for 3 hours at 4°C. The immune complexes were then mixed with 100 mL of 20% protein A/G Sepharose beads (Oncogene Research Products, San Diego, CA) on a rocker for another 1 to 2 hours at 4°C, and the beads were washed twice with lysis buffer. Beads containing Cdk1 immunoprecipitates were discarded and the lysates frozen. This procedure enabled us to investigate the action of Chk1-mediated signaling on Tyr15Cdk2 levels while excluding Tyr15 Cdk1. For all immunoblots, proteins were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-page) gels and the membranes were probed variously with antibodies specific to pSer317 and pSer345Chk1 (Chk1), pTyr15Cdk2 and Cdk2, pSer473 and pSer308Akt and Akt; washed in TBS-T; and incubated with horseradish peroxidase-conjugated goat anti-rabbit for 1 hour. The blots were visualized by enhanced chemiluminescence according to the manufacturer's instructions (Pierce, Rockford, IL). The radioactive JNK assays were performed as described in Sampath and Plunkett.40 Alternatively, JNK activity was measured using the nonradioactive JNK assay kit.
AML blast colony assay and colony-forming unit-granulocyte-macrophage assay
A method described previously was used to measure AML blast colony formation.43 Briefly, 1 × 105 T-cell-depleted, nonadherent, low-density bone marrow cells were plated in 0.8% methylcellulose in Iscove modified Dulbecco medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum, recombinant human granulocyte-macrophage (hGM)-colony-stimulating factor (50 ng/mL; Immunex, Seattle, WA), and recombinant stem cell factor (50 ng/mL; Amgen, Thousand Oaks, CA). Duplicate cultures were incubated in 35-mm Petri dishes for 7 days at 37°C in a humidified atmosphere of 5% CO2 in air. AML blast colonies were microscopically evaluated on day 7. A blast colony was defined as a cluster of 40 or more cells. Individual colonies were plucked, smeared on glass slides, and stained to microscopically confirm leukemic cellular composition. In 2 experiments, 2 × 105 CD34+ cells isolated from normal bone marrow were plated in 0.8% methylcellulose with Iscove modified Dulbecco medium supplemented with 30% fetal bovine serum, recombinant human erythropoietin (1 unit/mL; Terry Fox Laboratories, Vancouver, BC, Canada), and recombinant hGM-colony-stimulating factor (50 ng/mL). All cultures were evaluated after 14 days for the number of colony-forming unit (CFU)-GM colonies defined as a cluster of at least 40 granulocytes, monocyte-macrophages, or both.
Results
A model of S-phase checkpoint activation in response to ara-C in human leukemia cells: consequences of abrogation
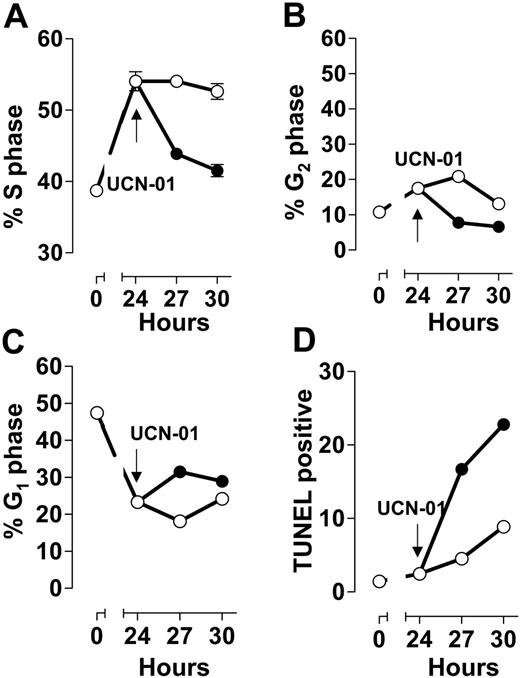
The human myelogenous leukemia line ML-1 arrested growth in S phase during a 24-hour incubation with 50 nM ara-C (Figure 1A) and to a much lesser extent in G2/M (Figure 1B), at the expense of the G1 population (Figure 1C). Similar to populations arrested with gemcitabine14 and fludarabine,7,44 addition of the Chk1 kinase inhibitor UCN-01 to ara-C-arrested cells resulted in a rapid decrease in the S-phase fraction (Figure 1A), consistent with abrogation of the checkpoint. This was paralleled by a reciprocal increase in the TUNEL-positive fraction (Figure 1D). The TUNEL-positive fraction resultant to the addition of UCN-01 to S-phase-arrested cells has been previously demonstrated by us to be associated with cells with an S-phase DNA content by 2-parameter flow cytometry,7,14 indicating cell death prior to progression into G2/M (Figure 1D). Further, inhibition of Chk1 resulted in a decrease in the G2/M population (Figure 1B), with a corresponding increase in the percentage of cells with a G1 DNA content (Figure 1C). Although this was a minor portion of the population, the rapid transit out of G2/M suggests that some of these cells, while damaged, may have escaped S phase only to have been blocked at the G2 checkpoint. In parallel, colony-forming assays demonstrated that ML-1 cells arrested in S phase in response to 50 nM ara-C had clonogenic viability similar to controls. In contrast, S-phase-arrested cells exposed to 100 nM UCN-01 demonstrated a significant loss in cloning potential (35% and 40% of control value; data not shown).
S-phase arrest by ara-C: abrogation by UCN-01. Exponentially growing ML-1 cells were incubated with 50 nM ara-C for 24 hours (○), at which time the culture was split and UCN-01 (50 nM) was added to one portion (•). Cells were sampled from each culture at the indicated times. DNA content (A, S phase; B, G2/M; C, G1) and TUNEL reactivity (D) were analyzed by flow cytometry. Symbols represent mean ± SD.
S-phase arrest by ara-C: abrogation by UCN-01. Exponentially growing ML-1 cells were incubated with 50 nM ara-C for 24 hours (○), at which time the culture was split and UCN-01 (50 nM) was added to one portion (•). Cells were sampled from each culture at the indicated times. DNA content (A, S phase; B, G2/M; C, G1) and TUNEL reactivity (D) were analyzed by flow cytometry. Symbols represent mean ± SD.
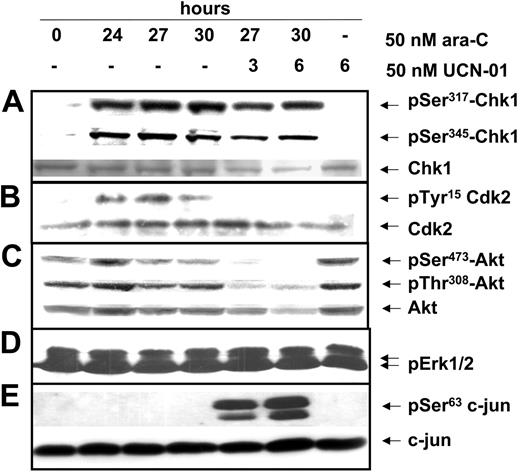
Molecular pharmacology studies have demonstrated that phosphorylation of Chk1 on Ser345 and Ser317 in response to DNA damage is associated with activation of the S-phase checkpoint pathway; Chk1 kinase-mediated inhibition of Cdc25A phosphatase leads to an accumulation of catalytically inactive Tyr15-phosphorylated Cdk2. Evaluation of these parameters in ML-1 cells arrested with 50 nM ara-C demonstrated an increase in the phosphorylation of Ser317 and Ser345 on the Chk1 protein (Figure 2A), indicating its activation. Correspondingly, Tyr15-phosphorylated Cdk2 increased in the S-phase-arrested population (Figure 2B). Addition of 50 nM UCN-01 to ara-C-arrested cells did not alter the phosphorylation levels of Ser317 or Ser345 on Chk1 (Figure 2A), likely because it is the substrate for upstream phosphoinositol kinases45 that might sense the damage caused in response to the actions of UCN-01. However, there was a significant decrease in the levels of pTyr15 phosphorylation on Cdk2 within 3 to 6 hours of UCN-01 addition (Figure 2B), demonstrating that the S-phase checkpoint was dysregulated in response to the UCN-01-induced inhibition of Chk1 kinase.
Activation of an S-phase checkpoint in response to ara-C: dysregulation by UCN-01 in ML-1 cells. Exponentially growing ML-1 cells were treated as in Figure 1. (A) pSer317Chk1 (top), pSer345Chk1 (middle), and total Chk1 (bottom) in response to ara-C and UCN-01; (B) pTyr15Cdk2 (top) and total Cdk2 (bottom) in cells depleted for Cdk1 protein. (C) pSer473Akt (top), pSer308Akt (middle), and total Akt (bottom) and (D) p42/44Erkkinase activity and (E) c-jun kinase activation as measured by endogenous c-jun phosphorylation (top) normalized to the levels of total c-jun (bottom).
Activation of an S-phase checkpoint in response to ara-C: dysregulation by UCN-01 in ML-1 cells. Exponentially growing ML-1 cells were treated as in Figure 1. (A) pSer317Chk1 (top), pSer345Chk1 (middle), and total Chk1 (bottom) in response to ara-C and UCN-01; (B) pTyr15Cdk2 (top) and total Cdk2 (bottom) in cells depleted for Cdk1 protein. (C) pSer473Akt (top), pSer308Akt (middle), and total Akt (bottom) and (D) p42/44Erkkinase activity and (E) c-jun kinase activation as measured by endogenous c-jun phosphorylation (top) normalized to the levels of total c-jun (bottom).
The sudden loss of viability upon abrogation of the S-phase checkpoint suggests that survival pathways may have been compromised. The Akt kinase provides prosurvival signals in leukemia cell lines and in primary AML.23,24 Consistent with this function in ML-1 cells, Akt was constitutively phosphorylated on both Ser473 and Thr308 in untreated populations as well as in S-phase-arrested cells (Figure 2C). Exposure of exponentially growing cells to 50 nM UCN-01 alone for 6 hours did not affect the phosphorylation of Akt on Thr308 or Ser473 (Figure 2C), indicating its lack of effect on Akt-activating kinases in unperturbed ML-1 cells. However, addition of UCN-01 to ara-C-arrested cells markedly decreased phosphorylation of Akt on both Ser473 and Thr308 between 3 to 6 hours (Figure 2C). In contrast, there was no effect on the kinase activity of extracellular signal-regulated kinases, thus supporting the specificity of this effect on the Akt pathway (Figure 2D). Thus, the observed loss of constitutively activated Akt when UCN-01 was added to S-phase-arrested cells may be partly responsible for the ability of UCN-01 to target this population for cell death.
Conversely, activation of the JNK pathway is implicated in translating cellular stress into signals for cell death in response to a variety of chemotherapeutic agents.37,40,46 Exponentially growing ML-1 cells demonstrated low levels of JNK activity and this was not changed in populations arrested in S phase with ara-C (Figure 2E). However, exposure of S-phase-arrested cells to UCN-01 resulted in a significant increase in the kinase activity of JNK, suggesting that the JNK kinase was activated as part of the cellular death response subsequent to the dysregulation of the S-phase checkpoint by UCN-01 (Figure 2E).
UCN-01 in combination with ara-C selectively inhibits the colony-forming ability of primary AML blasts
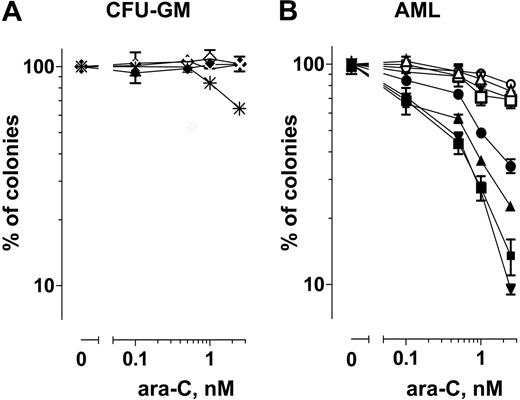
The effect of ara-C alone was compared with that of the combination (ara-C + UCN-01) on the colony-forming ability of normal CFU-GM hematopoietic progenitor cells from 2 individuals versus that of AML blasts obtained from 4 patients (Figure 3). The aim of these experiments was to determine whether individually minimally toxic concentrations of ara-C and UCN-01 could combine to kill AML blasts in a manner similar to cells in culture. Preliminary studies (not shown) demonstrated that 0.1 to 2.5 nM cytarabine produced similar toxicity in blasts as did 50 nM cytarabine in ML-1 cells. The UCN-01 concentration was chosen based on the level of the free drug reported in clinical trials.17,18 Exposure to ara-C alone or in combination with 100 nM UCN-01 had minimal toxicity on CFU-GM progenitor cells. At 2.5 nM ara-C the mean colonies were 104% and 102% of controls, whereas the ara-C+UCN-01 combination were 102% and 65% of controls. Similarly, exposure of AML blasts to 2.5 nM ara-C (mean ± SD, 74% ± 7%; n = 4) or UCN-01 (99% ± 6%; n = 4) alone minimally inhibited their colony-forming ability. In contrast, exposure of AML blasts to 2.5 nM ara-C in combination with UCN-01 significantly inhibited colony-forming cells (mean ± SD, 20% ± 10%; n = 4; Figure 3B). These data suggest that ara-C in combination with UCN-01 could selectively target AML blasts while sparing normal hematopoietic progenitor cells from toxicity.
Based on these findings and the results with the ML-1 cell model described, we designed a pilot clinical trial of ara-C in combination with UCN-01 in patients with relapsed or refractory acute myelogenous leukemia. Ara-C (1.0 g/m2/d) was administered as a continuous intravenous infusion for 96 hours. At cytotoxic concentrations, ara-C causes a time-dependent arrest as well as death of leukemia blasts in S phase.47-49 To facilitate laboratory correlative investigations, the UCN-01 infusion (45 mg/m2/d) was begun 24 hours after the start of ara-C and maintained for 72 hours. This design permitted evaluation of the effects of UCN-01 on ara-C-induced changes in survival, cell cycle parameters, and signaling pathways in AML blasts during therapy.
Clonogenic assays. (A) Effect of ara-C (⋄, ×) and ara-C in combination with UCN-01 (♦, *) on the clonogenic survival of CFU-GM progenitor cells from normal bone marrow. (B) Effect of ara-C alone (○, ▵, □, ▿), UCN-01 alone (*, +, ×, |), and ara-C in combination with UCN-01 (•, ▴, ▪, ▾) on the clonogenic survival of AML blasts. Symbols indicate the mean of 2 separate determinations from individual patients.
Clonogenic assays. (A) Effect of ara-C (⋄, ×) and ara-C in combination with UCN-01 (♦, *) on the clonogenic survival of CFU-GM progenitor cells from normal bone marrow. (B) Effect of ara-C alone (○, ▵, □, ▿), UCN-01 alone (*, +, ×, |), and ara-C in combination with UCN-01 (•, ▴, ▪, ▾) on the clonogenic survival of AML blasts. Symbols indicate the mean of 2 separate determinations from individual patients.
Pharmacodynamics of ara-C in the absence and presence of UCN-01 in AML blasts
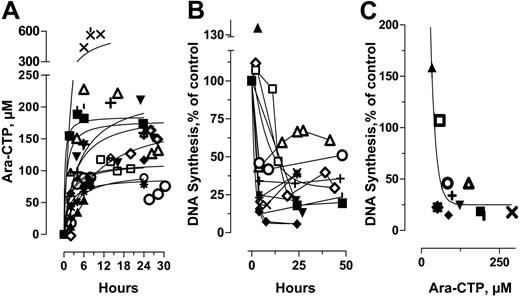
The steady-state concentrations of ara-CTP (median, 130 μM; range, 52-440 μM) were achieved within the first 4 to 6 hours following the start of infusion in the majority of samples and were maintained at a plateau thereafter (Figure 4A). These findings are consistent with earlier studies that demonstrated that ara-CTP levels were maintained constant within an individual for several days.50,51 Accumulation of ara-CTP is related to its incorporation into DNA which in turn results in the inhibition of DNA synthesis.52 To determine the effect of ara-CTP on DNA synthesis, blasts were isolated before and during therapy and incubated ex vivo with [3H]dThd. The patterns of DNA synthesis inhibition showed reciprocity with ara-CTP accumulation (Figure 4B-C). DNA synthesis was maximally inhibited in most samples by 4 to 6 hours but was inhibited by greater than 85% in only 2 of 12 samples (Figure 4B). The extent of DNA synthesis inhibition, although heterogeneous among individuals, was relatively unperturbed upon the infusion of UCN-01 at 24 hours. The absolute blast count in blood was reduced in all patients during infusion of ara-C alone for 24 hours (median reduction, 73% of pretreatment value; range, 22%-242%; Table 1) and continued to decrease upon the addition of UCN-01 to the ara-C regimen (median reduction, 22% of 24-h value; range, 9%-45%).
Pharmacodynamics of ara-C in combination with UCN-01. (A) Cellular accumulation of ara-CTP during therapy. (B) Inhibition of DNA synthesis during therapy. (C) Relationship between cellular concentrations of ara-CTP in circulating leukemic blasts 4 hours after ara-C infusion and extent of DNA synthesis inhibition relative to pretreatment values. ▴ indicates patient 1; ▪, patient 2; ▾, patient 3; ♦, patient 4; •, patient 5; □, patient 6; ⋄, patient 7; ○, patient 8;▵, patient 9; *, patient 10; ¦, patient 11; +, patient 12; and ×, patient 13.
Pharmacodynamics of ara-C in combination with UCN-01. (A) Cellular accumulation of ara-CTP during therapy. (B) Inhibition of DNA synthesis during therapy. (C) Relationship between cellular concentrations of ara-CTP in circulating leukemic blasts 4 hours after ara-C infusion and extent of DNA synthesis inhibition relative to pretreatment values. ▴ indicates patient 1; ▪, patient 2; ▾, patient 3; ♦, patient 4; •, patient 5; □, patient 6; ⋄, patient 7; ○, patient 8;▵, patient 9; *, patient 10; ¦, patient 11; +, patient 12; and ×, patient 13.
Response of the S-phase checkpoint pathway to ara-C/UCN-01 therapy
The absolute levels of Chk1 and Cdk2 kinase and their activated (phosphorylated) forms are shown in a representative patient during therapy (Figure 5 top 2 panels). We expressed the levels of Chk1 and pSer345Chk1 that were quantitated for each patient as a ratio to evaluate their responses during therapy. In pretreatment samples, Chk1 kinase was quantifiable in 7 of the 8 samples analyzed, whereas, surprisingly, pSer345Chk1 was evident in 6 of the pretreatment samples (Figure 6A). These results, which are in contrast to the low levels of pSer345Chk1 from untreated controls in cell culture systems (Figure 2A), suggest the existence of an activated DNA damage checkpoint prior to therapy. In 2 of the 5 samples, pSer345Chk1 was maintained during 24 hours of ara-C infusion, but the ratio decreased after 24 hours of UCN-01 administration (Figure 6A). Levels of pSer345Chk1 in 2 additional samples decreased between 75% and 50% after 24 hours of ara-C therapy and were diminished to less than 10% of pretreatment values following addition of UCN-01. In one other sample, levels of pSer345Chk1 decreased to less than 25% of the pretreatment value after ara-C infusion; the levels were not further affected by addition of UCN-01. Thus, there was a trend for the loss of pSer345Chk1 as blast counts decrease in response to therapy.
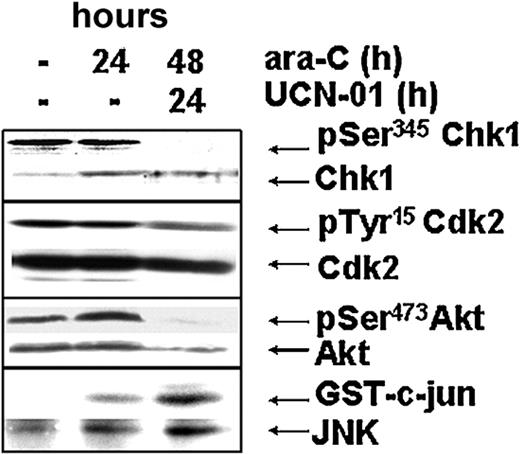
Response of the checkpoint, survival, and stress-activated kinases within a cell sample from patient 4. Effect of ara-C and UCN-01 on levels of pSer345Chk1, total Chk1, pTyr15Cdk2, total Cdk2, pSer473Akt, total Akt, and JNK activation from AML blasts of patient 4 during therapy.
Response of the checkpoint, survival, and stress-activated kinases within a cell sample from patient 4. Effect of ara-C and UCN-01 on levels of pSer345Chk1, total Chk1, pTyr15Cdk2, total Cdk2, pSer473Akt, total Akt, and JNK activation from AML blasts of patient 4 during therapy.
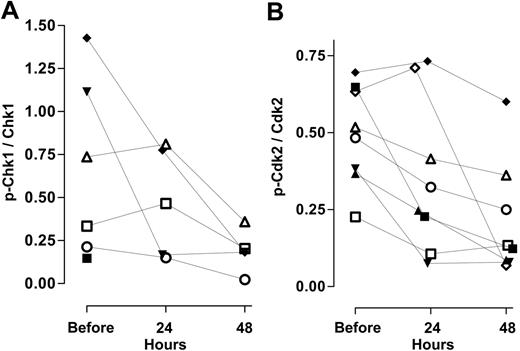
Effect of ara-C alone and in combination with UCN-01 on cell cycle checkpoint pathways. (A) Levels of pSer345Chk1 relative to total Chk1 and (B) pTyr15Cdk2 relative to total Cdk2 in samples from 8 individuals during therapy. See Figure 4 for explanation of symbols.
Effect of ara-C alone and in combination with UCN-01 on cell cycle checkpoint pathways. (A) Levels of pSer345Chk1 relative to total Chk1 and (B) pTyr15Cdk2 relative to total Cdk2 in samples from 8 individuals during therapy. See Figure 4 for explanation of symbols.
A similar analysis was conducted for Cdk2 and its inactive phosphorylated protein prior to and during the course of therapy. Immunoblot assays were positive in 8 patients for both forms; the levels of pTyr15Cdk2 relative to total Cdk2 exhibited a small range, between 0.25 and 0.75 (Figure 6B). This may reflect the fact that only a small percentage of these pretreatment peripheral AML blasts were cycling; such populations would be expected to have Cdk2 that was phosphorylated on Tyr15. Following 24 hours of ara-C infusion, the relative amount of pTyr15Cdk2 decreased between 25% and 75% in 6 of 8 patients (Figure 6B). There was pronounced loss of the inhibited form in 2 samples following UCN-01 administration (24 to 48 hours).
Inhibition of survival pathways with concomitant activation of the stress kinase during ara-C and UCN-01 therapy
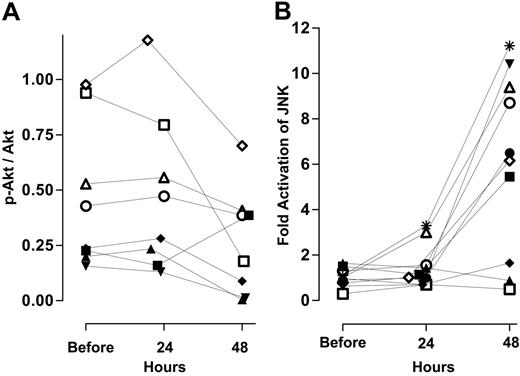
The absolute levels of phosphorylated Akt (p-Akt), Akt, and JNK activation are shown in a representative patient during therapy (Figure 5 bottom 2 panels). The ratio of pSer473Akt to total Akt in 8 individuals prior to therapy was heterogeneous (Figure 7A). Infusion of ara-C for 24 hours had little effect on this ratio (Figure 7A). In contrast, the relative level of pSer473 decreased in 7 of 8 samples following UCN-01 administration. Unlike Akt, there was little variation among samples with regard to the ratio of activated JNK to total JNK before treatment (Figure 7B). Samples from 10 patients analyzed in this fashion demonstrated a minor increase in the activity of JNK following 24 hours after infusion of ara-C in comparison to untreated controls. However, 24 hours after initiation of UCN-01 to the ara-C infusion there was a 5- to 12-fold induction in JNK activity in 6 of the 10 samples (Figure 7B). Thus, decreases in pSer473Akt along with concomitant activation of JNK were associated with decreases in the circulating leukemia blasts following addition of UCN-01 to the ara-C infusion.
Effect of ara-C alone and in combination with UCN-01 on survival pathways and stress-activated kinases. (A) pSer473Akt relative to total Akt levels in 8 individuals during therapy. (B) Change in JNK activity in 8 individuals during therapy measured as described.40 See Figure 4 for explanation of symbols.
Effect of ara-C alone and in combination with UCN-01 on survival pathways and stress-activated kinases. (A) pSer473Akt relative to total Akt levels in 8 individuals during therapy. (B) Change in JNK activity in 8 individuals during therapy measured as described.40 See Figure 4 for explanation of symbols.
Discussion
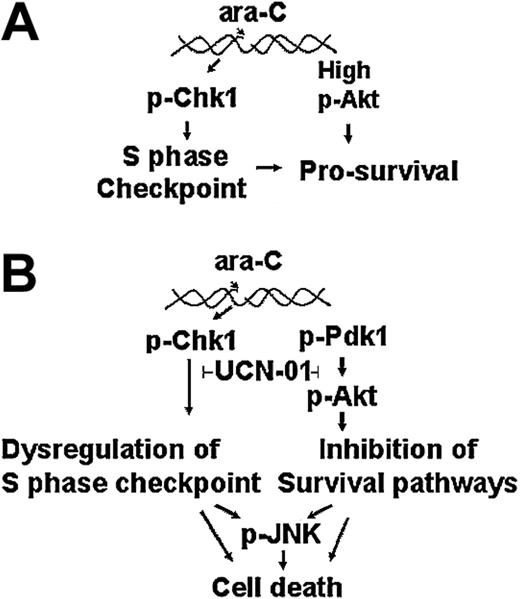
Our model study demonstrated that the response to ara-C is governed by the activation of an S-phase checkpoint mediated by Chk1 kinase. As such, this checkpoint pathway may provide the cells a resistance mechanism by limiting further incorporation of the analog and provide time for repair to occur (Figure 8A). This observation is supported by our results where S-phase-arrested ML-1 cells were spared the toxicity due to ara-C in colony-forming assays. Abrogation of the S-phase checkpoint may generate signals that result in cell death, thereby circumventing the cellular defense mechanism (Figure 8B). Thus, inhibiting Chk1 enhanced the toxicity of ara-C as measured by decreases in cell viability (Figure 1) and loss of cloning potential. Further, ML-1 cells maintained high levels of phospho-Akt during S-phase arrest. It is possible that prosurvival signals from Akt may augment the protective function of Chk1, thus contributing to cell viability. Upon checkpoint abrogation, the levels of both pSer473Akt and pSer308Akt were reduced as cells lost viability. Additionally, there was some decrease in the levels of total Akt by 6 hours of exposure to the combination in our model system (Figure 2) and in the patient sample after 24 hours of UCN-01 infusion (Figure 5). This result may represent a consequence of caspase-3-mediated cleavage of total Akt as seen in other systems.53 The concomitant activation of JNK upon Chk1 inhibition and decrease of Akt phosphorylation is consistent with the response of this stress pathway to the loss of these survival mechanisms. Thus, the strategy of checkpoint dysregulation augments cell death by compromising a survival pathway and activating stress kinase responses.
Clonogenic survival assays of primary AML samples generated results supporting this mechanism-based approach. Leukemia colony formation was decreased 5-fold relative to ara-C alone when samples were simultaneously incubated with UCN-01. In contrast, the clonogenic viability of normal myeloid progenitor cells was minimally affected by ara-C alone or in combination with UCN-01. This selective action of the combination suggests a biologic basis for a favorable therapeutic index.
During cytotoxic ara-C therapy, in addition to death of leukemic blasts in S phase, there is a time-dependent cytarabine-induced arrest,47-49 thus providing a basis for the combination of UCN-01 with ara-C in this pilot clinical study. Ara-CTP accumulated to at least 100 μM in blasts of most samples. As a result DNA synthesis was maximally inhibited in most samples by 4 to 6 hours, maintained constant thereafter, and not affected by initiation of UCN-01 infusion, which reflects the cellular response to the continual maintenance of ara-CTP levels over 96 hours. The absolute blast count in blood was reduced in all patients during infusion of ara-C alone for 24 hours and continued to decrease upon the addition of UCN-01 to the ara-C regimen. However, only one patient achieved a complete response, which may reflect the fact that clinical response is governed by multiple factors associated with poor prognosis (Table 1) in patients with relapsed/refractory AML. Therefore, we compared cytoreduction (Table 1) during the first 2 days of therapy to the observed biochemical changes in checkpoint proteins Chk1 and Cdk2.
Chk1 was phosphorylated in most AML samples obtained prior to treatment, although the ratio of pSer345Chk1 relative to total Chk1 varied considerably among samples. In contrast, exponentially growing cells (Figure 2) and indolent normal lymphocytes (data not shown) lack phosphorylation of these proteins. Thus, the status of Chk1 activation is different in primary blasts from patients with refractory AML and may indicate the presence of a previously activated DNA damage response, which is not surprising given that this cohort of individuals had undergone prior intensive chemotherapy. Tyr15Cdk2 was also phosphorylated in pretreatment blasts. Consequently, it was not possible to detect the activation of a classical cell cycle checkpoint as measured by activation of the Chk1-Cdk2 pathway in response to ara-C infusion. Given that only a small fraction of blasts are cycling54,55 at any one time, it is possible that the S-phase checkpoint was activated within those cells but was undetectable due to the high levels of phosphorylated Chk1-Cdk2 overall in these samples. Therefore, cell culture model systems may be poor predictors of the behavior of refractory AML blasts to ara-C in combination with UCN-01. It remains to be seen whether blasts from chemo-naive patients will activate Chk1 and evoke S-phase arrest in response to these or lower doses of ara-C, thus allowing effective combinations with cell cycle dysregulating agents such as UCN-01.
Response of leukemia cells to ara-c and UCN-01. Action of ara-C alone (A) or in combination with UCN-01 (B) on the Chk1, Akt, and JNK pathways in leukemia cells.
Response of leukemia cells to ara-c and UCN-01. Action of ara-C alone (A) or in combination with UCN-01 (B) on the Chk1, Akt, and JNK pathways in leukemia cells.
High levels of p-Akt have been shown to be necessary for the survival of AML blasts and are associated with resistance.23,24,56 At doses of above 42 mg/m2/d, the plasma concentrations of UCN-01 were about 35 μM,17,57 whereas the achievable free concentrations of UCN-01 were only 111 to 144 nM.17,18 Although PDK1 appears to be a direct target of UCN-01,58 exposure of leukemia cells to this agent either singly or in combination also induced the dephosphorylation of Ser473Akt, inhibited Akt activity, and caused cytotoxicity in cell culture systems.27,59 Ser473Akt was reported to be phosphorylated by DNA-PKcs,28 and this activity was inhibited by 10 μM UCN-01,30 a concentration of free drug well beyond that achieved in the clinic.17,18 Further, unlike in cell culture preparations (Figure 2), antibodies against pSer308Akt, the substrate of PKD1, did not identify discrete protein species in protein lysates from primary AML blasts. Thus, while the mechanism requires further investigation, the observed diminution of pSer473Akt in AML blasts in response to the combination of ara-C and UCN-01 is indicative of an abrogation of this survival pathway. Inhibition of the Akt pathway was paralleled by a concomitant activation of JNK in leukemic blasts after infusion of UCN-01 in addition to ara-C for 24 hours. Suppression of Akt signaling has been associated with a JNK-mediated induction of Fas ligand ex vivo which then participates in a positive feedback loop to promote cell death.60 Other reports demonstrate that inhibition of JNK attenuated cell death in response to inhibition of PI3K-Akt.39 Thus, the cellular stress that activates JNK generates proapoptotic signals that cause cell death. The phosphorylation of JNK in response to cellular damage is a strong pharmacodynamic signal in AML, the prognostic potential of which requires further investigation. Thus while UCN-01 was intended to dysregulate the S-phase checkpoint during therapy, a second mechanistic basis for the observed decreases in AML blast counts may be the inhibitory action of UCN-01 on Akt signaling.
In conclusion, the present results indicate that UCN-01 enhances the cytotoxicity of ara-C in leukemia cell lines and in primary AML blasts in vitro. In addition, our studies provide evidence that Akt phosphorylation is diminished by UCN-01 during therapy, giving credence to the notion that UCN-01 may have multiple targets of therapeutic relevance.15,33,59 Potent and specific inhibitors of the PI3K-Akt pathway are currently in development.61-63 Future clinical trials might consider infusion of low-dose ara-C in combination with UCN-01 in chemo-naive patients to maximize the therapeutic potential of this combination. Also, future strategies aimed at dysregulating cell cycle checkpoints may consider combinations with agents that target the Akt survival pathway.
Prepublished online as Blood First Edition Paper, November 17, 2005; DOI 10.1182/blood-2005-08-3351.
Supported in part by grants CA28596, CA32839, CA55164, CA62461, and Cancer Center Support Grant P50 CA16672 from the National Cancer Institute, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.